Abstract
Hexavalent chromium (CrVI) has been widely used in industries throughout the world. Increased usage of CrVI and atmospheric emission of CrVI from catalytic converters of automobiles, and its improper disposal causes various health hazards including female infertility. Recently we have reported that lactational exposure to CrVI induced a delay/arrest in follicular development at the secondary follicular stage. In order to investigate the underlying mechanism, primary cultures of rat granulosa cells were treated with 10 μM potassium dichromate (CrVI) for 12 and 24 h, with or without vitamin C pre-treatment for 24 h. The effects of CrVI on intrinsic apoptotic pathway(s) were investigated. Our data indicated that CrVI: (i) induced DNA fragmentation and increased apoptosis, (ii) increased cytochrome c release from the mitochondria to cytosol, (iii) downregulated anti-apoptotic Bcl-2, Bcl-XL, HSP70 and HSP90; upregulated pro-apoptotic BAX and BAD, (iv) altered translocation of Bcl-2, Bcl-XL, BAX, BAD, HSP70 and HSP90 to the mitochondria, (v) upregulated p-ERK and p-JNK, and selectively translocated p-ERK to the mitochondria and nucleus, (vi) activated caspase-3 and PARP, and (vii) increased phosphorylation of p53 at ser-6, ser-9, ser-15, ser-20, ser-37, ser-46 and ser-392, increased p53 transcriptional activation, and downregulated MDM-2. Vitamin C pre-treatment mitigated CrVI effects on apoptosis and related pathways. Our study, for the first time provides a clear insight into the effect of CrVI on multiple pathways that lead to apoptosis of granulosa cells which could be mitigated by vitamin C.
Keywords: Chromium, Ovary, Apoptosis, p53, Bcl-2
Introduction
Chromium (Cr) exists in a series of oxidation states from −2 to +6 valences; the most important stable states are elemental metal (0), trivalent (CrIII) and hexavalent (CrVI) compounds (Barceloux, 1999; Shi et al., 2004; Zhitkovich, 2005; Valko et al., 2006). CrVI is commonly used in numerous industrial processes and as emission or erosion byproducts of Cr-based catalytic converters, asbestos brake linings, cement dust, as well as in tobacco and food additives (Nriagu, 1988). Non-occupational sources of CrVI include contaminated soil, air and water (O’Brien et al., 2003). Occupational exposure to Cr is found among approximately half a million industrial workers in the United States and several millions worldwide. Significant contamination with CrVI has been found in approximately 30% of the drinking water in California (Salnikow and Zhitkovich, 2008). According to Environmental Working Group (EWG) water utility tests from 48,000 communities in 42 states (Sutton, 2010), at least 74 million people in nearly 7000 communities drink tap water polluted with CrVI. The USEPA has set a legal limit in tap water for total chromium of 100 ppb. However, chromium levels in the drinking water measured by EWG shows that total chromium is 1700 times higher than California’s proposed public health goal for CrVI (Sutton, 2010). This disparity could indicate significant cancer risk and other health hazard for communities drinking chromium-containing tap water. The deposition of CrVI wastes in landfills and waterways by chromate industries affects millions of people residing in the vicinity of dangerously polluted sites who drink Cr containing water (BlacksmithInstitute, 2007). CrVI causes dermatitis, skin, lung and throat cancers, and infertility. Increased incidences of birth and developmental defects among children living around tanneries and chrome and leather industries are clearly evident in the developing world (BlacksmithInstitute, 2007).
Women working in Cr industries and living around Cr contaminated areas experience abnormal menses (Makarov and Shimtova, 1978), postnatal hemorrhage and birth complications with high levels of chromium in blood and urine (Shmitova, 1978, 1980). Cr is transported to offspring through milk in lactating women exposed to CrVI (Barceloux, 1999). CrVI can traverse the placental barrier in rodents (Tipton and Shafer, 1964; Barceloux, 1999). Within the pregnant uterus, CrVI alters early development and hatching of blastocysts (Jacquet and Draye, 1982), decreases the number of implantation sites and viable fetuses (Junaid et al., 1996; Kamath et al., 1997; Kanojia et al., 1998), produces embryotoxic and fetotoxic effects, and increases conceptus resorption in rodents (Junaid et al., 1996). Cr exposure through drinking water impairs ovarian follicular maturation and differentiation and promotes follicular atresia (Murthy et al., 1996), delays puberty, lengthens interestrus intervals and reduces number of ovulation (Kanojia et al., 1998) in rodents.
CrVI can escape from primary contact organs and blood erythrocytes and reach different organs (Barceloux, 1999; Dayan and Paine, 2001). In biological systems, after entry into cells, CrVI is rapidly detoxified/reduced to CrIII by an intracellular defensive reductant system that includes ascorbate (vitamin C), glutathione (GSH) and cysteine (Valko et al., 2005, 2006). CrIII is also a very popular nutritional supplement consumed by many people (Kirpnick-Sobol et al., 2006). Exposing yeast and mice via drinking water to CrVI and CrIII significantly increased the frequency of DNA deletions. Surprisingly, CrIII is a more potent inducer of DNA deletions than CrVI once CrIII is absorbed (Kirpnick-Sobol et al., 2006). Thus, both the environmental contaminant CrVI and the nutritional supplement CrIII increase DNA deletions in vitro and in vivo, when ingested via drinking water. Vitamin C accounts for ~80% of CrVI metabolism in target tissues such as lung, liver and kidney, being the fastest reducer of CrVI in vitro (Zhitkovich, 2005; Zhitkovich et al., 2005). Unlike rodents, human beings are unable to synthesize L-ascorbic acid because of their deficiency in t-gulono-g-lactone oxidase, the enzyme catalyzing the terminal step in L-ascorbic acid biosynthesis (Nishikimi et al., 1994). Therefore, the potential risk for CrVI exposure in humans might be more severe than what is reported in rodent models.
We have recently reported that lactational exposure to CrVI decreased primordial, primary, secondary, and antral follicles and thus delayed follicular development, decreased steroidogenesis, extended estrous cycle and pubertal onset in postnatal rat ovaries. Vitamin C supplementation protects ovary from these deleterious effects of CrVI (Banu et al., 2008a). However, the specific mechanism(s) responsible for CrVI-induced follicular arrest/atresia on follicular development are not yet understood. Follicular granulosa cell apoptosis or follicular atresia governs follicular growth and development in the ovary (Hirshfield, 1997; Hoyer, 2005). Metal toxins including CrVI and cadmium alter programmed granulosa cell death and follicular apoptosis (Blankenship et al., 1997; Matsuda-Minehata et al., 2006). In metal-induced apoptosis, mitochondria are reported to be the most pertinent target (Rana, 2008). Both mitochondrial damage and genotoxic effects determine the fate of CrVI-exposed cells to either growth arrest or apoptosis (Ye et al., 1999).
Therefore, we hypothesize that CrVI induces follicular atresia through apoptosis of granulosa cells by activating multiple cell signaling pathways. The objectives of the present study were to: (i) determine the effects of CrVI on activation of intrinsic apoptotic pathways and suppression of cell survival pathways in primary cultures of granulosa cells; (ii) understand the involvement of p53 and MAP-kinases in granulosa cell apoptosis; and (iii) evaluate the mitigative effects of vitamin C on CrVI-induced changes on the molecular end-points in granulosa cell apoptosis. Our results for the first time reveal that CrVI induces apoptosis of granulosa cells through activation of mitochondria-mediated intrinsic pathways, suppression of AKT pathways, and phosphorylation / activation of p53 through sustained and delayed activation of ERK1/2 pathways. Vitamin C partially mitigated these adverse effects of CrVI and protects granulosa cells from apoptosis.
Materials and methods
Chemicals
The reagents used in this study were purchased from the following suppliers: Antibiotic-antimycotic, Trypsin-EDTA (Invitrogen Life Technologies Inc., Carlsbad, CA); fetal bovine serum (Hyclone, Logan, UT); and tissue culture dishes and plates (Corning Inc., Corning, NY); potassium dichromate (K2Cr2O7) and ascorbate (Sigma-Aldrich, St. Louis, MO); The other chemicals used were molecular biologic grade available from Fisher Scientific (Pittsburgh, PA) or Sigma-Aldrich (St. Louis, MO). Antibodies were from Cell Signaling Technology (Danvers, MA).
Animals
Immature female Sprague–Dawley rats (22–25 days old) were euthanized by CO2 asphyxiation followed by cervical dislocation and ovaries were collected in DMEM-F12 media. Animal use protocols were approved by the Animal Care and Use Committee of Texas A&M University and were in accordance with the standards established by Guiding Principles in the Use of Animals in Toxicology and Guidelines for the Care and Use of Experimental Animals by National Institute of Health.
Granulosa cell isolation and culture
Granulosa cells from 80 ovaries collected from 40 rats were harvested as described (Kayampilly and Menon, 2007). Briefly, 80 ovaries were cleared from the surrounding fat under a Stereo dissection microscope and punctured with 25-gauge needles. Cells were collected in phenol red free DMEM-F12 containing 0.2% BSA, 10 mM HEPES, and 6.8 mM EGTA, incubated for 15 min at 37 °C, and centrifuged for 5 min at 250g. The pellets were suspended in a solution containing 0.5 M sucrose, 0.2% BSA, and 1.8 mM EGTA in DMEM-F12 and incubated for 5 min. After incubation, the suspension was diluted with 3 vol DMEM-F12, centrifuged at 250g, and treated sequentially with trypsin (20 μg/ml) for 1 min, 300 μg/ml soybean trypsin inhibitor for 5 min, and DNase I (100 μg /ml) for 5 min at 37 °C. The cells were washed with media and suspended in DMEM-F12. Cells obtained from the 80 ovaries were cultured in 18 (100 mm) dishes in DMEM-F12 supplemented with 20 mM HEPES (pH 7.4), 4 mM glutamine, 100 IU penicillin/ml, and 100 μg/ml streptomycin. To perform the CrVI in vitro experiments, these dishes were then divided into six groups with three dishes per group. Each group represents treatment as described below.
In vitro experimental design for CrVI treatment
At 70% confluency, cells were serum-starved for 24 h with or without vitamin C in the media, and divided into six treatment groups. (1) Control: cells were treated with media; (2) CrVI-12 h: cells were treated with 10 μM potassium dichromate for 12 h; (3) CrVI-24 h: cells were treated with 10 μM potassium dichromate for 24 h; (4) Vitamin C: cells were treated with 1 mM ascorbate for 24 h; (5) vitamin C+CrVI-12 h: cells were pre-treated with 1 mM ascorbate for 24 h and treated with 10 μM potassium dichromate for 12 h; (6) vitamin C+CrVI-24 h: cells were pre-treated with 1 mM ascorbate for 24 h and treated with 10 μM potassium dichromate for 24 h. After the treatment, cells were harvested using 0.1% trypsin-EDTA and total RNA was isolated using TriZol (Banu et al., 2008). All treatments were performed in triplicates on the same day and each experiment was repeated three times on different days.
TUNEL assay
TUNEL assay was performed to assess apoptosis of granulosa cells as described (Banu et al., 2009). Briefly, non-adherent and adherent cells were harvested and resuspended at the concentration of 1×106 cells/ml. Nicks in DNA were determined by terminal deoxynucleotidyl transferase and 5-bromo-2′-deoxyuridine (BrdU) 5′-triphosphate labeling using an APOBrdU TUNEL assay kit (Molecular Probes Inc., Eugene, OR) as recommended by the manufacturer. Detection of BrdU incorporation at DNA break sites was achieved using Alexa Fluor 488 dye-labeled anti-BrdU antibody. Numbers of apoptotic cells were analyzed by flow cytometry (FACSCaliber; Becton Dickinson, San Jose, CA) using Cell Quest software.
Protein extraction and immunoblotting
After the CrVI treatment with or without vitamin C pre-treatment, total protein from granulosa cells was isolated and immunoblotting/western blotting was performed as we described previously (Banu et al., 2008b). Briefly, the cells were harvested using 1% Trypsin-EDTA and pelleted. The cell lysates were sonicated in sonication buffer which consisted of 20 mM Tris–HCl, 0.5 mM EDTA, 100 μM DEDTC, 1% Tween, 1 mM phenylmethylsulfonyl fluoride, and protease inhibitor cocktail tablets: complete EDTA-free (1 tablet/50 ml) and PhosStop (1 tablet/10 ml). Sonication was performed using a Microson ultrasonic cell disruptor (Microsonix Incorporated, Farmingdale, NY). Protein concentration was determined using the Bradford method and a Bio-Rad Protein Assay kit. Protein samples (75 μg) were resolved using 7.5%, 10% or 12.5% SDS-PAGE. Chemiluminescent substrate was applied according to the manufacturer’s instructions (Pierce Biotechnology, Rockford, IL). The blots were exposed to Blue X-Ray film and densitometry of autoradiograms was performed using an Alpha Imager (Alpha Innotech Corporation, San Leandro, CA).
Cytosolic, mitochondrial and nuclear protein fractionation
Granulosa cells were harvested after CrVI treatment with or without vitamin C pretreatment. Cytoplasmic, mitochondrial and nuclear protein fractions were isolated using a Cell Fractionation kit from MitoSciences, (Eugene, OR) according to manufacturer’s instructions. The distribution of proteins between cytosolic (C), mitochondrial (M) and nuclear (N) fractions was calculated as percentage of the protein present in a fraction of the sum of the protein present in C and M fractions. For example, the determination of cytosolic cytochrome c is indicated by the formula below: cytochrome c fraction C (%)=100×cytochrome c fraction C/(cytochrome c fraction C+cytochrome c fraction M). Western blots of Rho-GD1, pyruvate dehydrogenase and histone H2B proteins were used as loading controls for cytosolic, mitochondrial and nuclear protein fractions, respectively.
p53 Luciferase reporter assay
The Cignal p53 Reporter kit (CCS-004L, SA Biosciences) was used to monitor the activity of the p53-regulated signal transduction pathway in granulosa cells treated with CrVI as per the protocol from the manufacturer. Briefly, granulosa cells were seeded at a density of 3×105 per well in 6-well plates and grown to ~60% confluency. For each well, a mixture of inducible p53-responsive firefly luciferase construct and constitutively expressing Renilla luciferase construct were cotransfected into the cells using the oligofectamine reagent (Invitrogen). After 24 h incubation with or without vitamin C (1.0 mM), cells were treated with CrVI (10 μM) for 24 h. The preparation of cell extracts and measurement of luciferase activities were carried out using the Dual-Luciferase Reporter Kit according to recommendations of the manufacturer (Promega). The firefly luciferase activity and Renilla luciferase activity were measured. Changes in firefly luciferase activity were calculated and plotted after normalization with changes in Renilla luciferase activity within the same sample.
Statistical analyses
All numerical data were subjected to one-way ANOVA to detect the effects of treatment and time interactions. Tukey–Kramer HSD test was used to adjust for multiple pair-wise comparisons of means. Least squares regression analysis was used to determine effects of treatment (Control, CrVI, Vitamin C, CrVI+ Vitamin C); time (CrVI 12 h, CrVI 24 h) and treatment×time interactions. Each value is the mean±SEM from 3 different plates per treatment, cultured using 80 ovaries collected from 40 immature rats. Similar results were obtained in three different experiments performed on three different days/time. P<0.05 was considered to be significant. Statistical analyses were performed using general linear models of Statistical Analysis System (SAS, Cary, NC).
Results
CrVI induced apoptosis of granulosa cells through intrinsic apoptotic pathway
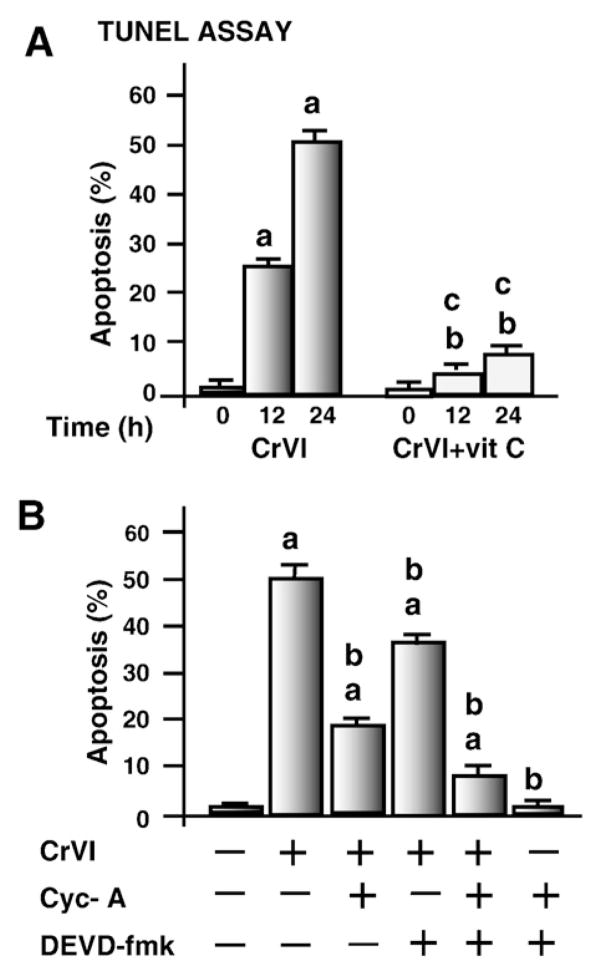
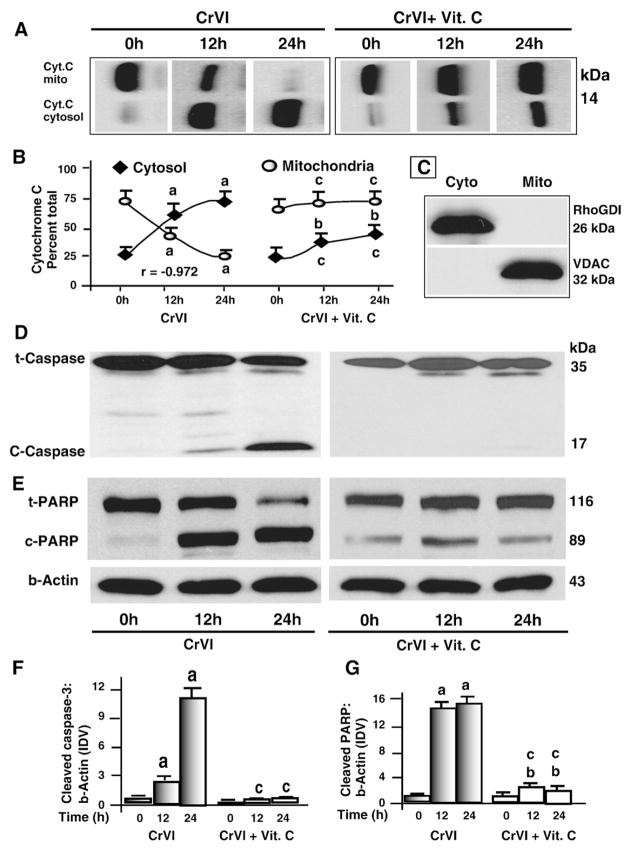
Effects of CrVI on DNA fragmentation was measured using the TUNEL assay. Results indicated that CrVI induced DNA fragmentation and apoptosis of granulosa cells in a time-dependent manner (P<0.05) whereas pre-treatment of cells with vitamin C mitigated (P<0.05) the effect of CrVI (Fig. 1A). Release of cytochrome c from mitochondria into the cytosol and activation of caspase-3 and nuclear poly (ADP-ribose) polymerase (PARP) enzymes are important terminal events which promote apoptosis of cells (Jiang and Wang, 2004). Therefore we examined whether CrVI-induced apoptosis of granulosa cells is mediated through release of cytochrome c and activation of caspase-3. Results indicated that CrVI induced release of cytochrome c from the mitochondria into cytosol in a time-dependent manner. The increase of cytochrome c protein in the cytosol was negatively correlated (r=−0.97) with a concurrent decrease in the mitochondria whereas vitamin C prevented this translocation (Fig. 2A and B). Down-stream of cytochrome c, CrVI increased cleavage of caspase-3 and PARP proteins. Vitamin C mitigated the effect of CrVI on cleavage of caspase-3 and PARP (Fig. 2D–G). CrVI-induced apoptosis of granulosa cells was inhibited by a cytochrome c inhibitor (cyclosporine-A) or caspase-3 inhibitor (DEVD-fmk) (Fig. 1B). These results together indicate that CrVI induces apoptosis of granulosa cells through cytochrome c and caspase-3 dependent intrinsic apoptotic pathways.
Fig. 1.
Effect of CrVI on apoptosis of granulosa cells. Apoptosis was assayed by TUNEL assay as described in Materials and methods. Cells were pre-treated with or without vitamin C and treated with CrVI (10 μM) for 0 h, 12 h and 24 h period and harvested and processed for TUNEL assay. Each value is the mean±SEM of three experiments, P<0.05. A, Effect of CrVI on apoptosis. a: CrVI-treatment, 0 h vs 12 h or 24 h; b: CrVI+Vitamin C-treatment, 0 h vs 12 h or 24 h; c: CrVI (0 h or 12 h or 24 h) vs CrVI+Vitamin C (0 h or 12 h or 24 h; B, Effects of cytochrome c inhibitor cyclosporine A and/or caspase-3 inhibitor DEVD-fmk on apoptosis. Cells were serum deprived and pre-treated with or without cyclosporine A (cyc A) (5 μM) and/or DEVD-fmk (50 μM) for 1 h, and then treated with CrVI (10 μM) for a 24 h period, and processed for TUNEL assay. a: Control vs CrVI-treatment with cyclosporine A and/or DEVD-fmk; b: CrVI vs CrVI+cyclosporine A and/or DEVD-fmk.
Fig. 2.
Time-course effect of CrVI on cytochrome c release from mitochondria, cleavage of caspase-3 and PARP in granulosa cells. Cells were pre-treated with or without vitamin C for 24 h and treated with CrVI (10 μM) for 0 h, 12 h and 24 h period. Protein (50 μg of either total protein or mitochondrial or cytosolic fraction) from each sample was subjected to western blot analysis as described in Materials and methods. A, Western blots of cytochrome c; B, Quantification of cytochrome c in cytosol and mitochondria with time; C, Western blots of mitochondria-specific voltage-dependent anion channel (VDAC) and cytosol-specific RhoGDI in mitochondrial and cytosolic protein fractions. Western blots of D total (t) and cleaved (c) caspase-3; and E total (t) and cleaved (c) PARP, representative blots are shown. Quantification of average protein levels of F caspase-3 and G PARP from three individual experiments is shown. Each value is the mean±SEM of three experiments, P<0.05; a: CrVI-treatment, 0 h vs 12 h or 24 h; b: CrVI+Vitamin C-treatment, 0 h vs 12 h or 24 h; c: CrVI (0 h or 12 h or 24 h) vs CrVI+Vitamin C (0 h or 12 h or 24 h). Cyt.C—cytochrome c; Vit.C—Vitamin C; mito—mitochondrial protein fraction; cyto—cytosolic protein fraction; t-PARP—total PARP; c-PARP—cleaved PARP; t-caspase-3—total caspase-3; c-caspase-3—cleaved caspase-3.
CrVI altered expression of Bcl-2, Bcl-XL, Bax, Bad, HSP70 and HSP90 proteins in granulosa cells
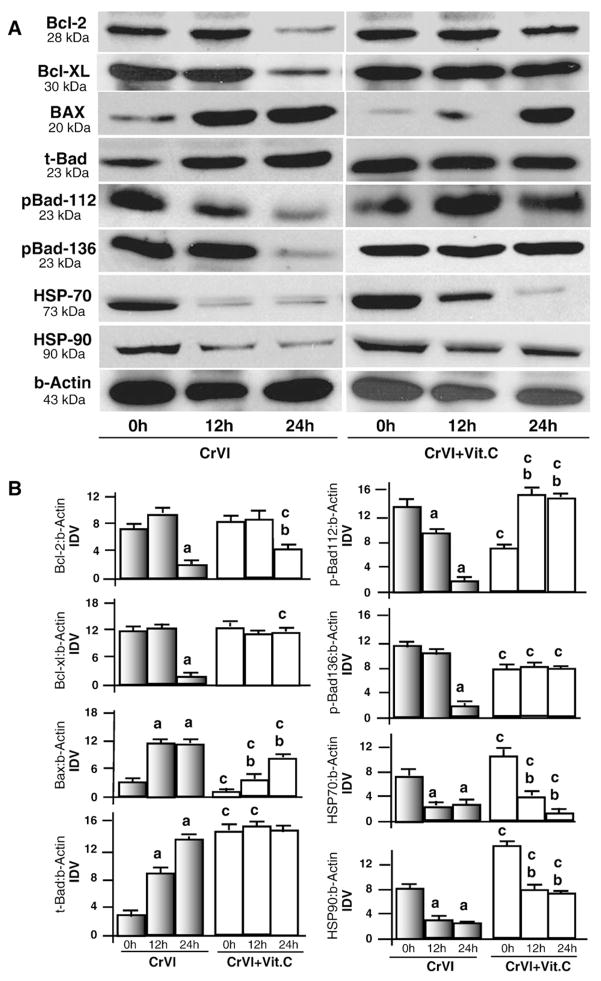
The Bcl-2 family proteins (Bcl-2, Bcl-XL Bad, and Bax) constitute critical components to regulate activation of intrinsic apoptotic pathways by governing mitochondrial membrane permeabilization and subsequent release of cytochrome c (Danial and Korsmeyer, 2004). In addition to Bcl-2 family, heat shock proteins HSP70 or HSP90 are anti-apoptotic by preventing mitochondrial membrane permeabilization (Joly et al., 2010; Stankiewicz et al., 2005; Bivik et al., 2007). Down-regulation or inhibition of HSP70 or HSP90 protein is sufficient to sensitize a cell for apoptosis (Lanneau et al., 2007; Kang et al., 2007; Neckers et al., 2007). Therefore, we studied the effects of CrVI on expression of antiapoptotic proteins Bcl-2 and Bcl-XL, proapoptotic proteins Bax and Bad, as well as HSP70 and HSP90 proteins. Results indicated that CrVI decreased (P<0.05) expression of Bcl-2 and Bcl-XL proteins, increased (P<0.05) total Bad and Bax proteins, and decreased (P<0.05) HSP70 and HSP90 proteins. Vitamin C mitigated effects of CrVI on the levels of Bcl-2, Bcl-XL, Bax and Bad, HSP70 and 90 proteins temporally at 12 and 24 h. (Fig. 3A and B). In the absence of apoptotic stimuli, Bad protein is phosphorylated at serine 112 and 136 by MAPK and AKT pathways. Phosphorylated Bad proteins bind with 14-3-3 proteins and are sequestered in the cytosol (Zha et al., 1996). Dephosphorylation of Bad is important for its translocation into mitochondria to initiate intrinsic apoptotic pathways. Therefore, we determined the effect of CrVI on phosphorylation of Bad 112 and 136. Results indicated that CrVI decreased (P<0.05) phosphorylation of Bad protein at ser-112 (p-Bad-112) and ser-136 (pBad-136) within 24 h, and interestingly, vitamin C mitigated CrVI effects on phosphorylation of Bad protein. Together, these results indicate that CrVI decreases expression of antiapoptotic proteins and increases expression of pro-apoptotic proteins in granulosa cells.
Fig. 3.
Effect of CrVI on Bcl-2 family proteins and heat shock proteins 70 and 90 in granulosa cells. Cells were pre-treated with or without vitamin C for 24 h and treated with CrVI (10 μM) for 0 h, 12 h and 24 h period. Western blots were performed with 75 μg of protein, as described in Materials and methods. A, Representative western blots of Bcl-2, Bcl-XL, BAX, total Bad (t-Bad), phosphorylated Bad (pBad-112, pBad-136), HSP-70, HSP-90, and b-actin. B, Histograms of Integrated Density Value (IDV) for each protein, normalized to beta-actin. Each value is the mean±SEM of three experiments, P<0.05; a: CrVI-treatment, 0 h vs 12 h or 24 h; b: CrVI+Vitamin C-treatment, 0 h vs 12 h or 24 h; c: CrVI (0 h or 12 h or 24 h) vs CrVI+Vitamin C (0 h or 12 h or 24 h).
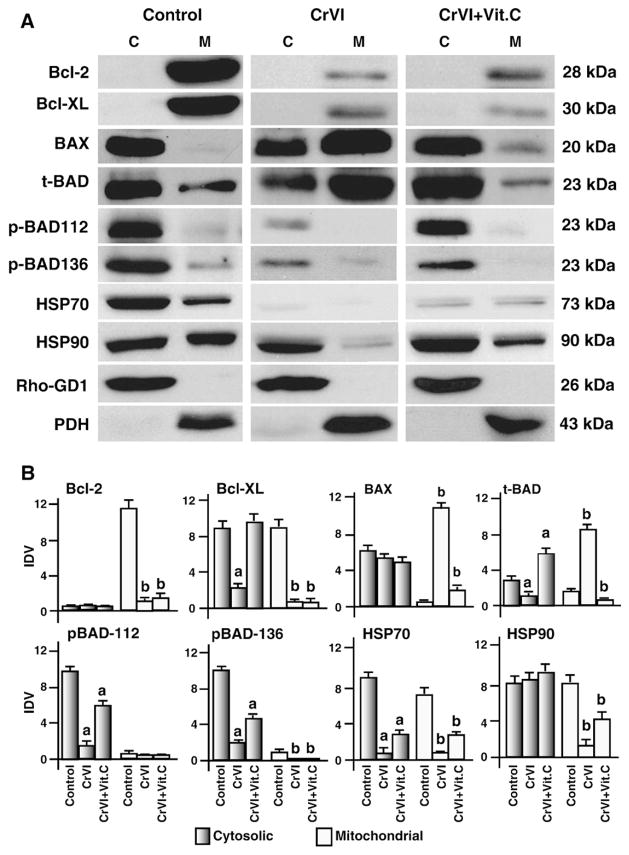
CrVI altered mitochondrial translocation of BAX, BAD, HSP70 and HSP90 in granulosa cells
Translocation of Bax and Bad proteins from cytoplasm into mitochondria is critical to execute apoptotic cell death in response to oxidative stress and DNA damage (Youle and Strasser, 2008). Therefore the effect of CrVI on the translocation of Bax and Bad was studied. In control cells, Bcl-2 and Bcl-XL proteins were abundantly localized in the mitochondria, CrVI decreased (P<0.05) mitochondrial levels of Bcl-2 and Bcl-XL proteins and vitamin C partially mitigated the effects of CrVI on Bcl-2 but not Bcl-XL protein. CrVI increased (P<0.05) translocation of Bax and Bad from the cytosol to the mitochondria and vitamin C mitigated (P<0.05) the effect of CrVI on the translocation of Bax and Bad to mitochondria. Once dephosphorylated, Bad translocates to the mitochondria and interacts with Bcl-2 or Bcl-XL proteins to neutralize their activity (Rapp et al., 2007). Therefore, the effect of CrVI on translocation of Bad was studied. In control cells, as expected, p-Bad-112 and p-Bad-136 proteins are sequestered (P<0.05) in the cytosol but not in the mitochondria. CrVI decreased (P<0.05) phosphorylation p-BAD-112 and p-BAD-136 in the cytosol, and vitamin C mitigated this effect. HSP-70 and HSP-90 are localized in the cytosol and mitochondria of control cells. CrVI decreased (P<0.05) cytosolic and mitochondrial expression of HSP70 and mitochondrial HSP90 proteins; and vitamin C mitigated the effect of CrVI on HSP90 but not on HSP70 proteins (Fig. 4A and B). These results together indicate that CrVI translocates Bax and Bad proteins from the cytosol into mitochondria in granulosa cells.
Fig. 4.
Effect of CrVI on translocation of Bcl-2 family proteins and heat shock proteins 70, and 90 in the mitochondria and cytosol. Cells were pre-treated with or without vitamin C for 24 h and treated with CrVI (10 μM) for 0 h, 12 h and 24 h. Western blots were performed with 75 μg of protein, as described in Materials and methods. A, Representative western blots of Bcl-2, Bcl-XL, BAX, total Bad (t-Bad), phosphorylated Bad (pBad-112, pBad-136), HSP-70 and HSP-90 in the cytosolic and mitochondrial protein fractions. M- mitochondrial protein fraction; C-cytosolic protein fraction. B, Histograms of Integrated Density Value (IDV) for each protein. Each value is the mean±SEM of three experiments, P<0.05; a: CrVI-treatment, 0 h vs 12 h or 24 h; b: CrVI+Vitamin C-treatment, 0 h vs 12 h or 24 h. Vit.C—Vitamin C.
CrVI increased phosphorylation of ERK and JNK, and decreased PI3K/AKT in granulosa cells
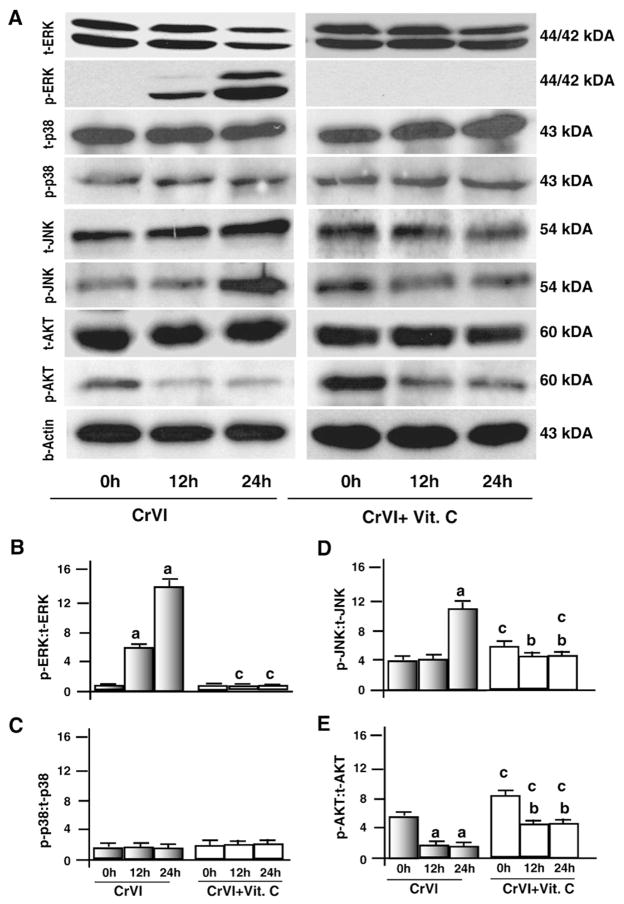
MAP-kinase and AKT pathways are the major intracellular cell survival pathways and are associated with expression of Bcl-2 and Bcl-XL proteins and phosphorylation of Bad protein. Therefore, we determined the effects of CrVI on phosphorylation of MAP-kinases and AKT. Our data showed that CrVI increased (P<0.05) phosphorylation of ERK1/2 at 12 h and 24 h, increased JNK at 24 h. However, CrVI decreased (P<0.05) AKT at 12 h and 24 h and did not alter p38MAPK. Vitamin C inhibited (P<0.05) effects of CrVI on phosphorylation of ERK1/2 protein. Furthermore, vitamin C mitigated effects of CrVI by decreasing (P<0.05) phosphorylation of JNK and increasing (P<0.05) phosphorylation of AKT (Fig. 5A–E). These results together indicate that CrVI suppresses the AKT pathway but activates the EKR1/2 pathway in granulosa cells.
Fig. 5.
Effect of CrVI on phosphorylation of ERK1/2, p38, JNK and AKT. Cells were pre-treated with or without vitamin C for 24 h and treated with CrVI (10 μM) for 0 h, 12 h and 24 h. Western blots were performed with 75 μg of protein, as described in Materials and methods, and probed with the antibodies for total and phosphorylated forms of ERK, p38, JNK, and AKT/PI3K. A, Representative western blots of total and phosphorylated forms of p38, ERK, p38, JNK, and AKT/PI3K. B–E, Histograms showing the ratio between phosphorylated to total forms of each protein expressed as Integrated Density Value (IDV). Each value is mean±SEM of three experiments, P<0.05. a: Control vs CrVI-treatment, 0 h vs 12 h or 24 h; b: Vitamin C vs CrVI+Vitamin C 12 h or 24 h; c: CrVI (0 h or 12 h or 24 h) vs CrVI+Vitamin C (0 h or 12 h or 24 h).
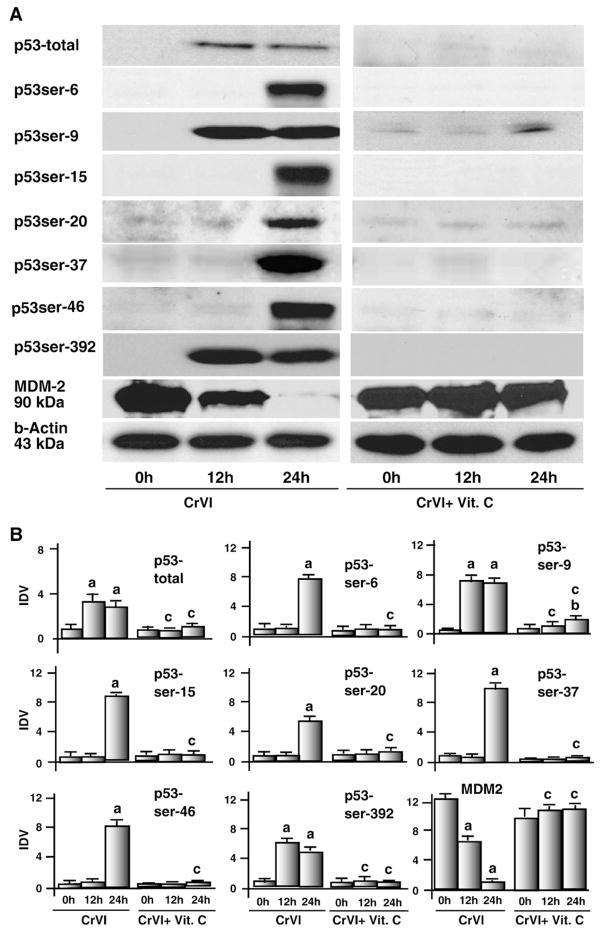
CrVI increased phosphorylation of p53 at multiple serine sites and decreased MDM-2 expression
DNA damage promotes phosphorylation and subsequent stabilization of p53 leading to apoptotic cell death (Meek, 2009). Apoptosis induced by oxidative stress involves p53 phosphorylation at ser-6, ser-9, ser-15, ser-20, ser-37, ser-46 and ser-392 in various cells (Meek, 1998b; Dumaz and Meek, 1999; Jardine et al., 1999; Brooks and Gu, 2003; Meek and Anderson, 2009). MDM2 promotes p53 degradation through an ubiquitin-dependent pathway (Moll and Petrenko, 2003). However, effects of CrVI on p53 phosphorylation resulting in apoptosis of granulosa cells are not understood. Therefore, this study attempted to understand the effect of CrVI on the phosphorylation of p53 at various serine sites. Interestingly, CrVI increased levels of total p53 protein in granulosa cells at 12 h and 24 h and induced phosphorylation of p53 protein at ser-9 and ser-392 at 12 h and 24 h and at ser-6, ser-15, ser-20, ser-37 and ser-46 at 24 h. Interestingly, vitamin C mitigated effects of CrVI on expression and phosphorylation of p53 protein at ser-6, ser-9, ser-15, ser-20, ser-37, ser-46 and ser-392. MDM-2 protein was abundantly expressed in untreated granulosa cells and CrVI decreased (P<0.05) its expression level in a time-dependent manner at 12 h and 24 h. Vitamin C mitigated CrVI-induced effects on expression of MDM-2 protein in granulosa cells (Fig. 6A and B). These results together indicate that CrVI phosphorylates p53 protein at multiple serine sites in granulosa cells.
Fig. 6.
Effect of CrVI on total p53 protein, phosphorylation of p53 at ser-6, ser-15, ser-20, ser-37, ser-46, ser-392 and on MDM-2. Cells were pre-treated with or without vitamin C and treated with CrVI (10 μM) for 0 h, 12 h and 24 h. Western blots were performed with 75 μg of protein, as described in Materials and methods. A, Representative western blots of total p53 protein, phosphorylated p53 at ser-6, ser-15, ser-20, ser-37, ser-46, ser-392 and MDM-2. B, Histograms of Integrated Density Value (IDV) for each protein. Each value is the mean±SEM of three experiments, P<0.05; a: CrVI-treatment, 0 h vs 12 h or 24 h; b: CrVI+Vitamin C-treatment, 0 h vs 12 h or 24 h; c: CrVI (0 h or 12 h or 24 h) vs CrVI+Vitamin C (0 h or 12 h or 24 h). Vit.C—Vitamin C.
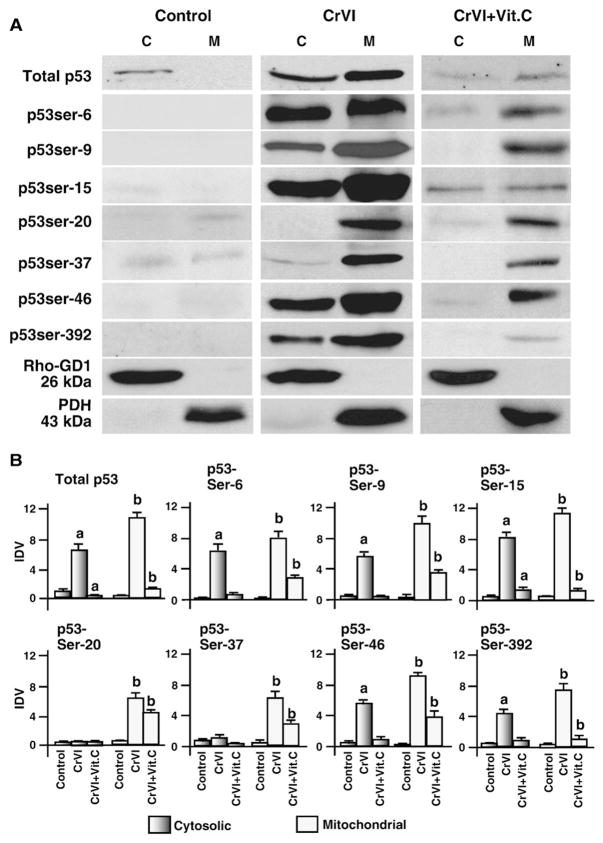
CrVI increased mitochondrial translocation of p53 in granulosa cells
Recent studies have shown that translocation of p53 from cytosol to the mitochondria is important for its interactions with antioxidants and apoptotic proteins (Pani et al., 2004; Siu et al., 2009; Galluzzi et al., 2010; Holley et al., 2010a,b,c). Mitochondrial translocation of p53 triggers a rapid proapoptotic response (Erster and Moll, 2004). Therefore, we determined whether CrVI induces translocation of p53 protein from cytosol to the mitochondria. CrVI increased accumulation of phosphorylated p53 protein at ser-9, ser-15, ser-20, ser-37, ser-46 and ser-392 in the mitochondria compared to cytosol. Vitamin C mitigated effects of CrVI on translocation of p53 to the mitochondria (Fig. 7A and B). These results indicate that CrVI induces translocation of p53 protein from cytosol to the mitochondria.
Fig. 7.
Effect of CrVI on translocation of total and phosphorylated p53 from cytosol to the mitochondria. Cells were pre-treated with or without vitamin C and treated with CrVI (10 μM) for 0 h, 12 h and 24 h. Western blots were performed with 75 μg of protein, as described in Materials and methods. A, Representative western blots of total p53 and phosphorylated p53 in the cytosolic and mitochondrial protein fractions. M-mitochondrial protein fraction; C-cytosolic protein fraction. B, Histograms of Integrated Density Value (IDV) for each protein. Each value is the mean±SEM of three experiments, P<0.05; a: CrVI-treatment, 0 h vs 12 h or 24 h; b: CrVI+Vitamin C-treatment, 0 h vs 12 h or 24 h. Vit.C—Vitamin C.
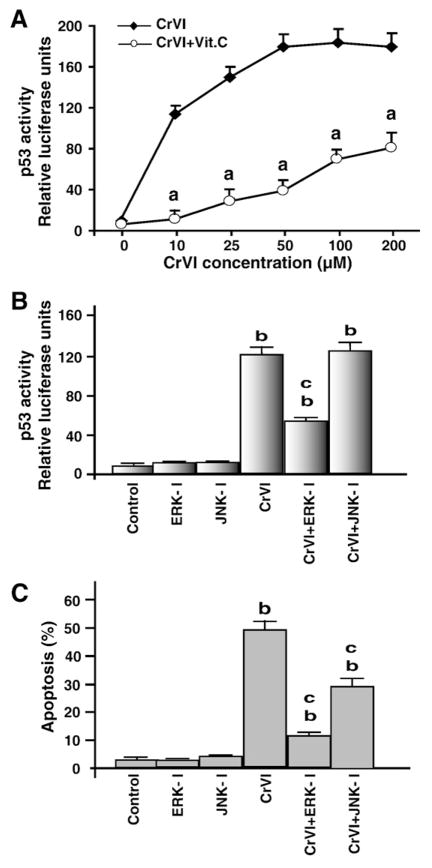
CrVI increased p53 transcriptional activity through ERK1/2 pathway
MAP-kinases have been identified as upstream kinases that activate p53 phosphorylation in several cell types (Meek et al., 1997; Milczarek et al., 1997; Meek, 1998a). In Fig. 5, we have shown that CrVI increases phosphorylation of ERK1/2 and JNK proteins. Therefore, we determined interaction between p53 and ERK1/2 or JNK. Transcriptional activity of p53 was measured in cells exposed to increasing concentrations of CrVI (0–200 μM). Results showed a dose-dependent increase (P<0.05) in p53 transcriptional activity and that reached (P<0.05) maximal levels at 50 μM CrVI. Vitamin C pre-treatment mitigated (P<0.05) the effect of CrVI on p53 transcriptional activity even at the highest dose (200 μM) of CrVI used (Fig. 8A). To determine whether ERK1/2 or JNK is involved in the activation of p53 and apoptosis, granulosa cells were treated with ERK1/2 inhibitor (U0126) or JNK inhibitor (SP600125) in the presence or absence of CrVI, and p53 transcriptional activity and apoptosis were measured. Results indicated that inhibition of ERK1/2 decreased (P<0.05) p53 activity and apoptosis (Fig. 8B and C). Inhibition of JNK did not inhibit p53 transcriptional activity but decreased apoptosis. These results indicate that CrVI activates p53 through ERK1/2 pathway in granulosa cells.
Fig. 8.
Effect of CrVI on the transcriptional activity of p53 through activation of ERK1/2. Cells were pre-treated with or without vitamin C for 24 h, and transfected with p53 reporter according to the manufacturer’s protocol. After 24 h of transfection, cells were treated with CrVI (10 μM). Dual Luciferase assay was performed 24 h after treatment, and promoter activity values are expressed as arbitrary units using a Renilla reporter for internal normalization. A, Effect of CrVI on p53 activity in the presence or absence of vitamin C; B, Effect of CrVI on p53 activity in the presence or absence of ERK-inhibitor and/ or JNK-inhibitor; C, Effect of CrVI on apoptosis in the presence or absence of ERK-inhibitor and/or JNK-inhibitor. Each value is the mean±SEM of three experiments, P<0.05. a: CrVI vs CrVI+Vitamin C-treatment. b: Control vs CrVI with or without ERK-I or JNK-I; c: CrVI vs CrVI+ERK-I or CrVI+JNK-I. ERK-I, ERK inhibitor; JNK-I, JNK inhibitor.
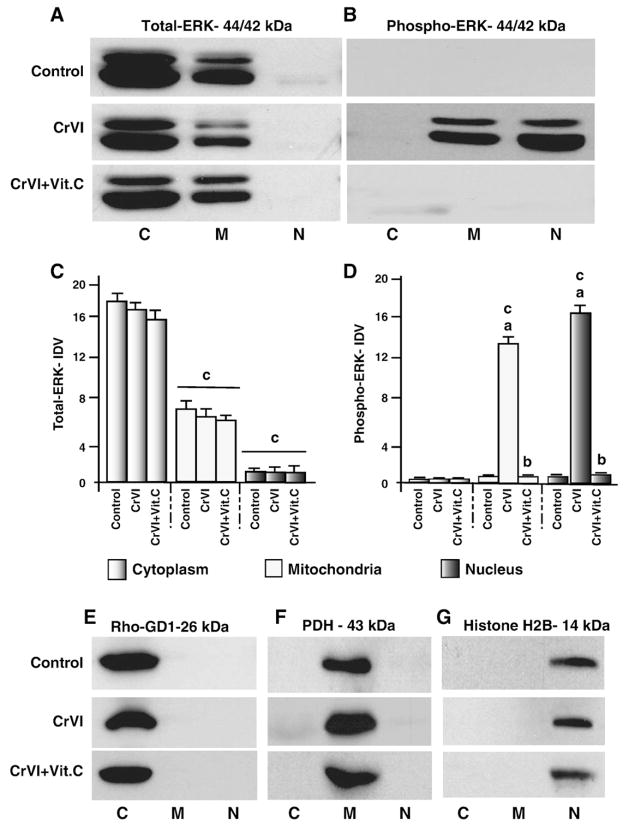
CrVI treatment leads to persistent nuclear and mitochondrial translocation of activated ERK1/2 in granulosa cells
ERK has been traditionally viewed as a mitogenic factor; however, sustained and/or delayed activation of ERK is associated with apoptosis (Kulich and Chu, 2001; Kulich and Chu, 2003); (Stadheim et al., 2001; Brantley-Finley et al., 2003). Besides the important mitogenic activity of ERK1/2 in the nucleus, ERK1/2 is localized in the mitochondria and plays roles in cell survival/apoptosis (Dagda et al., 2008; Poderoso et al., 2008). Therefore, we determined translocation of total (t-ERK1/2) and phosphorylated or active (p-ERK1/2) forms of ERK1/2 in the cytosol, mitochondria and nucleus. Results indicated that t-ERK1/2 protein is constitutively expressed in the cytosol and mitochondria and not in the nucleus; the expression level of t-ERK1/2 protein was lower in the mitochondria compared to that in cytosol; and p-ERK1/2 was not detected in cytosol, mitochondria or nucleus in untreated granulosa cells. CrVI treatment did not alter levels of t-ERK1/2 proteins in cytosol, mitochondria, and nucleus (Fig. 9A and C). By contrast, CrVI increased (P<0.05) translocation of p-ERK1/2 from cytosol into the mitochondria and nucleus. Vitamin C pretreatment mitigated effects of CrVI and prevented translocation of pERK1/2 from cytosol into the mitochondria and nucleus (Fig. 9B and D). These results indicate that CrVI accelerates selective translocation of active ERK1/2 into nucleus in granulosa cells.
Fig. 9.
Effect of CrVI on translocation of total and phosphorylated ERK from cytosol to the mitochondria and the nucleus. Cells were pre-treated with or without vitamin C for 24 h and treated with CrVI (10 μM) for 0 h, 12 h and 24 h. Western blots were performed with 75 μg of protein, as described in Materials and methods. Representative western blots of A, total ERK (t-ERK); and B, phospho-ERK (p-ERK) in the cytosolic, mitochondrial and nuclear protein fractions. Histogram showing Integrated Density Value (IDV) of C, total ERK, and D, phospho-ERK for each protein fraction. M—mitochondrial protein fraction; C—cytosolic protein fraction; N—Nuclear protein fraction. Each value is mean±SEM of three experiments, P<0.05. a: Control vs CrVI treatment; b: CrVI vs CrVI+Vit.C; c: t-ERK or p-ERK in the cytosol vs mitochondria or nucleus. Rho-GD1—loading control for cytosolic protein; pyruvate dehydrogenase (PDH) —loading control for mitochondrial protein; histone H2B—loading control for nuclear protein.
Discussion
Lactational exposure to CrVI during the postnatal days 1–21 decreased development of antral follicles and arrested follicular development at the secondary follicular stage in rat (Banu et al., 2008b; Samuel et al., 2010). The underlying molecular and cellular mechanisms that regulate CrVI-induced follicular atresia/apoptosis are not known. Results of the present study for the first time showed that CrVI induces apoptosis of granulosa cells through multiple mechanisms.
Bcl-2 family members Bcl-2, Bcl-XL, Bax and Bad proteins are the key mediators of intrinsic apoptotic pathway. In addition, HSP70 protects the cells against apoptosis by inhibiting translocation of BAX protein from the cytosol to the mitochondria, release of cytochrome c from the mitochondria into the cytosol, and activation of caspase-3 and PARP proteins (Stankiewicz et al., 2005; Bivik et al., 2007; Joly et al., 2010). HSP90 protein located in the mitochondria regulates mitochondrial membrane permeabilization and release of cytochrome c (Kang et al., 2007; Neckers et al., 2007). Results of the present study indicated that CrVI decreased expression of antiapoptotic and cell survival proteins Bcl-2, Bcl-XL, HSP70 and HSP90 proteins, translocated BAX and BAD proteins from cytosol to the mitochondria, increased mitochondrial membrane permeability, facilitated the release of cytochrome c, and activated caspase-3 and PARP proteins, and thus induced apoptosis of granulosa cells. These results suggest that CrVI attenuates antiapoptotic pathways in order to stabilize pro-apoptotic members to execute apoptosis of granulosa cells.
The fate of cells to die or survive depends on balance between survival and apoptosis signaling (Matsuzawa et al., 2002). Further, expression of Bcl-2 and Bcl-XL proteins are regulated by MAPK, JNK and AKT pathways (Matsuzawa et al., 2002). Therefore, we determined effects of CrVI on ERK1/2, AKT, p38MAPK, and JNK pathways in granulosa cells. Interestingly, CrVI inhibited phosphorylation of AKT proteins, and in contrast, increased phosphorylation of ERK1/2 and JNK proteins, and did not alter activation of p38MAPK protein. ERK1/2 pathways are mainly associated with mitogenesis and cell survival (Meloche and Pouyssegur, 2007). Inactive ERKs are bound to anchoring proteins in resting cells, mostly confined to the cytosol. Upon phosphorylation, ERK becomes active, translocates to the nucleus, and activates transcription of several proteins (Lidke et al., 2010). Interestingly, recent findings have documented a role for delayed and sustained ERK activation in apoptosis (Gladys et al., 1999; Stanciu and DeFranco, 2002). ERK can be activated often in the same cell type by pro-survival factors and toxic/apoptotic stimuli (Hetman et al., 1999) and thus ERK activation alone may not be predictive of subsequent cellular survival responses (Stanciu et al., 2000; Stanciu and DeFranco, 2002). It has been shown that activated JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3, a cytoplasmic anchor of Bax (Tsuruta et al., 2004). It is evident from the present data that CrVI activates ERK1/2 temporally in a delayed and sustained manner and activates JNK in granulosa cells.
DNA damage promotes phosphorylation and subsequent stabilization of p53 and leads to apoptosis (Meek, 2009). Phosphorylation of p53 protein at one serine residue is not sufficient to induce apoptosis, whereas phosphorylation at multiple serine residues is required (Kurihara et al., 2007). Site specific phosphorylation of p53 is induced by activation of diverse cell signaling pathways and DNA damage (Kurihara et al., 2007). Phosphorylation of p53 at ser-392 is required for p53-mediated growth arrest (Cox and Meek, 2010). Phosphorylation of p53 at ser-15 can be induced by oxidative stress (Long et al., 2007), H2O2 (Verschoor et al., 2010), and ionization (Sluss et al., 2010) and UV irradiation (Milne et al., 1995). In addition, function of p53 is regulated by its negative regulator MDM2 (Shieh et al., 1997). In order to understand the role of p53 in CrVI-induced apoptosis of granulosa cells we determined phosphorylation of p53 protein at multiple serine sites and expression of MDM2 protein. Our results indicate that CrVI increased phosphorylation of p53 protein at ser-6, ser-9, ser-15, ser-20, ser-37, ser-46 and ser-392 and decreased expression of MDM-2 protein in granulosa cells in a time-dependent manner. These results suggest that CrVI increases p53 phosphorylation at multiple serine sites, decreases its interaction with its negative regulator MDM2 and thereby stabilizes p53 and promotes apoptosis of granulosa cells.
One of the interesting findings of the present study is that CrVI selectively translocated active p53 protein into mitochondria in granulosa cells. p53-mediated cell death is primarily routed through the mitochondrial pathways (Schuler and Green, 2001) which require translocation of p53 protein into mitochondria (Zhao et al., 2005). Recent studies showed translocation of p53 from the cytosol to mitochondria and its association with antioxidants and apoptotic proteins (Pani et al., 2004; Siu et al., 2009; Galluzzi et al., 2010; Holley et al., 2010a,b,c). Mitochondrial translocation of p53 triggers a rapid pro-apoptotic response (Erster and Moll, 2004). After translocation into mitochondria, p53 protein could interact with endogenous antiapoptotic Bcl-XL and/or Bcl-2 protein, induce oligomerization of Bak protein, increase permeabilization of the outer mitochondrial membrane in order to facilitate cytochrome c release (Mihara et al., 2003), or interact with MnSOD and inhibit its ability to scavenge free radicals (Holley et al., 2010a). The results of the present study along with available information suggest that p53 could play a central role in CrVI-induced apoptosis by inhibiting association or balance between pro-and anti-apoptotic proteins.
p53 regulates transcription of several genes (Meek, 1998a) that regulate cell cycle, growth arrest, and apoptosis (Agarwal et al., 1998; Giaccia and Kastan, 1998). However, cell signaling associated with phosphorylation of p53 is complex and largely unknown. Our data indicated that CrVI activated ERK1/2 and JNK pathways. Therefore, we tested whether the inhibition of ERK1/2 or JNK decreases CrVI-induced p53 transcriptional activity and apoptosis. Our data showed that inhibition of ERK1/2 decreased CrVI-induced apoptosis of granulosa cells through suppression of transcriptional activity of p53. By contrast, inhibition of JNK did not decrease transcriptional activity of p53 although it decreased apoptosis of granulosa cells. These results indicate that ERK1/2 might be a potential upstream kinase that activates p53 and mediates CrVI-induced apoptosis of granulosa cells through p53.
ERK1/2 proteins are localized in several microenvironments of mitochondria and regulate survival or apoptosis of cells or modulate steroid synthesis (Dagda et al., 2008; Poderoso et al., 2008). Phosphorylation of p53 by ERK1/2 is important for doxorubicin-induced p53 activation and cell death (Yeh et al., 2004). Therefore, we hypothesized that CrVI translocates active ERK1/2 proteins into mitochondria in addition to nucleus in granulosa cells. Our results indicated that CrVI translocated active ERK1/2 proteins not only into the nucleus but also to the mitochondria. The present study indicates that CrVI translocates active p53 protein into mitochondria. Based on these data, we propose that sustained activation of ERK1/2 by CrVI could phosphorylate p53, which in turn, interacts with other mitochondrial proteins of cell survival pathways and or antioxidants, and thus promotes apoptosis. In addition, CrVI translocates active ERK1/2 to the nucleus in granulosa cells and induces apoptosis. This finding is consistent with other evidence that prolonged nuclear retention of activated ERK promotes cell death (Stanciu et al., 2000; Stanciu and DeFranco, 2002). Moreover, association of ERK1/2 activation with granulosa cell apoptosis in the present study supports the recent finding that ERK1/2 is not essential for the active proliferation of granulosa cells from preovulatory follicles; rather ERK1/2 plays an essential role to cease granulosa cell proliferation and to initiate the terminal differentiation response to LH in preovulatory follicles (Fan et al., 2009).
In the present study, vitamin C exhibited a selective and time-dependent molecular intervention of CrVI effects in several signaling pathways that lead to granulosa cell apoptosis. Vitamin C was more effective in mitigating CrVI effects at 12 h of treatment compared to 24 h in most of the end-points studied. This suggests that with short-time (12 h) CrVI exposure, the cells may still retain DNA repair machinery and operational survival signals. However, after 24 h of CrVI treatment, the DNA damage may have exceeded native DNA repair mechanisms so that vitamin C can not rescue granulosa cells from apoptosis. In conclusion, the novel findings of the present study are that CrVI: (i) decreased expression or activity of Bcl-2, Bcl-XL, and AKT proteins; (ii) increased activation and mitochondrial translocation of pro-apoptotic BAD, BAX, (iii) increased sustained activation of ERK1/2 and its sub-cellular translocation into nucleus and mitochondria; (iv) increased phosphorylation of p53 at multiple serine sites and thereby induced apoptosis of granulosa cells. Vitamin C partially mitigated the adverse effects of CrVI on granulosa cells; therefore, vitamin C could be a potential intervention to prevent or reduce the toxic effects of CrVI on the ovary to preserve the fertility.
Acknowledgments
This work was supported by National Institute of Health (NIH)/ National Institute of Environmental Health Sciences (NIEHS) Grants ES016605-01A2 to S.K.B. The authors acknowledge Dr. K.M.J. Menon and Dr. Palaniappan, Department of Obstetrics and Gynecology and Biological Chemistry, University of Michigan Medical School, Ann Arbor, Michigan, for providing the laboratory training in granulosa cell primary culture for the authors. We acknowledge Ryan Byrd, Crystal Page and Andrea Taylor, Comparative Medicine Program, Texas A&M University for their help in maintaining and handling rats. We acknowledge Dr. Rola Barhoumi, Dept of Integrative Biosciences, Texas A&M University for assistance with statistical analysis.
Abbreviations
- CrVI
Hexavalent chromium
- CrIII
Trivalent chromium
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- Bcl-2
B-cell lymphoma 2
- Bcl-XL
B-cell lymphoma-extra large
- BAX
BCL2-associated X protein
- BAD
Bcl-xL/ Bcl-2-Associated Death Promoter
- HSP70/HSP90
Heat shock proteins-70 and -90
- MAP Kinase
Mitogen-activated protein (MAP) kinase
- ERK
Extracellular-signal-regulated kinase
- JNK
c-Jun N-terminal kinase
- PARP
Poly (ADP-ribose) polymerase
Footnotes
Conflict of interest statement
None.
References
- Agarwal ML, Agarwal A, Taylor WR, Chernova O, Sharma Y, Stark GR. A p53-dependent S-phase checkpoint helps to protect cells from DNA damage in response to starvation for pyrimidine nucleotides. Proc Natl Acad Sci USA. 1998;95:14775–14780. doi: 10.1073/pnas.95.25.14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banu SK, Lee J, Starzinski-Powitz A, Arosh JA. Gene expression profiles and functional characterization of human immortalized endometriotic epithelial and stromal cells. Fertil Steril. 2008;90:972–987. doi: 10.1016/j.fertnstert.2007.07.1358. [DOI] [PubMed] [Google Scholar]
- Banu SK, Lee J, Satterfield MC, Spencer TE, Bazer FW, Arosh JA. Molecular cloning and characterization of prostaglandin transporter in ovine endometrium: role of mitogen activated protein kinase pathways in release of prostaglandin F2 alpha. Endocrinology. 2008a;149:219–231. doi: 10.1210/en.2007-1087. [DOI] [PubMed] [Google Scholar]
- Banu SK, Samuel JB, Arosh JA, Burghardt RC, Aruldhas MM. Lactational exposure to hexavalent chromium delays puberty by impairing ovarian development, steroidogenesis and pituitary hormone synthesis in developing Wistar rats. Toxicol Appl Pharmacol. 2008b;232:180–189. doi: 10.1016/j.taap.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Banu SK, Lee J, Speights VO, Jr, Starzinski-Powitz A, Arosh JA. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 induces apoptosis of human endometriotic cells through suppression of ERK1/2, AKT, NFkappaB, and beta-catenin pathways and activation of intrinsic apoptotic mechanisms. Mol Endocrinol. 2009;23:1291–1305. doi: 10.1210/me.2009-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barceloux DG. Chromium. J Toxicol Clin Toxicol. 1999;37:173–194. doi: 10.1081/clt-100102418. [DOI] [PubMed] [Google Scholar]
- Bivik C, Rosdahl I, Ollinger K. Hsp70 protects against UVB induced apoptosis by preventing release of cathepsins and cytochrome c in human melanocytes. Carcinogenesis. 2007;28:537–544. doi: 10.1093/carcin/bgl152. [DOI] [PubMed] [Google Scholar]
- BlacksmithInstitute. Top 10 Worst Polluted Sites. The Blacksmith Institute; New York: 2007. [Google Scholar]
- Blankenship LJ, Carlisle DL, Wise JP, Orenstein JM, Dye LE, III, Patierno SR. Induction of apoptotic cell death by particulate lead chromate: differential effects of vitamins C and E on genotoxicity and survival. Toxicol Appl Pharmacol. 1997;146:270–280. doi: 10.1006/taap.1997.8237. [DOI] [PubMed] [Google Scholar]
- Brantley-Finley C, Lyle CS, Du L, Goodwin ME, Hall T, Szwedo D, Kaushal GP, Chambers TC. The JNK, ERK and p53 pathways play distinct roles in apoptosis mediated by the antitumor agents vinblastine, doxorubicin, and etoposide. Biochem Pharmacol. 2003;66:459–469. doi: 10.1016/s0006-2952(03)00255-7. [DOI] [PubMed] [Google Scholar]
- Brooks CL, Gu W. Ubiquitination, phosphorylation and acetylation: the molecular basis for p53 regulation. Curr Opin Cell Biol. 2003;15:164–171. doi: 10.1016/s0955-0674(03)00003-6. [DOI] [PubMed] [Google Scholar]
- Cox ML, Meek DW. Phosphorylation of serine 392 in p53 is a common and integral event during p53 induction by diverse stimuli. Cell Signal. 2010;22:564–571. doi: 10.1016/j.cellsig.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Dagda RK, Zhu J, Kulich SM, Chu CT. Mitochondrially localized ERK2 regulates mitophagy and autophagic cell stress: implications for Parkinson’s disease. Autophagy. 2008;4:770–782. doi: 10.4161/auto.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Dayan AD, Paine AJ. Mechanisms of chromium toxicity, carcinogenicity and allergenicity: review of the literature from 1985 to 2000. Hum Exp Toxicol. 2001;20:439–451. doi: 10.1191/096032701682693062. [DOI] [PubMed] [Google Scholar]
- Dumaz N, Meek DW. Serine15 phosphorylation stimulates p53 transactivation but does not directly influence interaction with HDM2. EMBO J. 1999;18:7002–7010. doi: 10.1093/emboj/18.24.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erster S, Moll UM. Stress-induced p53 runs a direct mitochondrial death program: its role in physiologic and pathophysiologic stress responses in vivo. Cell Cycle. 2004;3:1492–1495. doi: 10.4161/cc.3.12.1318. [DOI] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Morselli E, Kepp O, Vitale I, Pinti M, Kroemer G. Mitochondrial liaisons of p53. Antioxid Redox Signal. 2010 Jan 7; doi: 10.1089/ars.2010.3504. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Giaccia AJ, Kastan MB. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 1998;12:2973–2983. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- Gladys S, Van Meerbeek B, Lambrechts P, Vanherle G. Evaluation of esthetic parameters of resin-modified glass-ionomer materials and a polyacid-modified resin composite in Class V cervical lesions. Quintessence Int. 1999;30:607–614. [PubMed] [Google Scholar]
- Hetman M, Kanning K, Cavanaugh JE, Xia Z. Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J Biol Chem. 1999;274:22569–22580. doi: 10.1074/jbc.274.32.22569. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Overview of ovarian follicular development: considerations for the toxicologist. Environ Mol Mutagen. 1997;29:10–15. [PubMed] [Google Scholar]
- Holley AK, Dhar SK, St Clair DK. Manganese superoxide dismutase versus p53: the mitochondrial center. Ann NY Acad Sci. 2010a;1201:72–78. doi: 10.1111/j.1749-6632.2010.05612.x. [DOI] [PubMed] [Google Scholar]
- Holley AK, Dhar SK, St Clair DK. Manganese superoxide dismutase vs. p53: regulation of mitochondrial ROS. Mitochondrion. 2010b;10:649–661. doi: 10.1016/j.mito.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Holley AK, Dhar SK, Xu Y, St Clair DK. Manganese superoxide dismutase: beyond life and death. Amino Acids. 2010c May 8; doi: 10.1007/s00726-010-0600-9. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer P. Impact of Metals on Ovarian Function. In: Golub MS, editor. Metals, Fertility, and Reproductive Toxicity. Taylor & Francis; 2005. pp. 155–169. [Google Scholar]
- Jacquet P, Draye JP. Toxicity of chromium salts to cultured mouse embryos. Toxicol Lett. 1982;12:53–57. doi: 10.1016/0378-4274(82)90198-9. [DOI] [PubMed] [Google Scholar]
- Jardine LJ, Milne DM, Dumaz N, Meek DW. Phosphorylation of murine p53, but not human p53, by MAP kinase in vitro and in cultured cells highlights species-dependent variation in post-translational modification. Oncogene. 1999;18:7602–7607. doi: 10.1038/sj.onc.1203137. [DOI] [PubMed] [Google Scholar]
- Jiang X, Wang X. Cytochrome c-mediated apoptosis. Annu Rev Biochem. 2004;73:87–106. doi: 10.1146/annurev.biochem.73.011303.073706. [DOI] [PubMed] [Google Scholar]
- Joly AL, Wettstein G, Mignot G, Ghiringhelli F, Garrido C. Dual role of heat shock proteins as regulators of apoptosis and innate immunity. J Innate Immun. 2010;2:238–247. doi: 10.1159/000296508. [DOI] [PubMed] [Google Scholar]
- Junaid M, Murthy RC, Saxena DK. Embryo- and fetotoxicity of chromium in pregestationally exposed mice. Bull Environ Contam Toxicol. 1996;57:327–334. doi: 10.1007/s001289900194. [DOI] [PubMed] [Google Scholar]
- Kamath SM, Stoecker BJ, Davis-Whitenack ML, Smith MM, Adeleye BO, Sangiah S. Absorption, retention and urinary excretion of chromium-51 in rats pretreated with indomethacin and dosed with dimethylprostaglandin E2, misoprostol or prostacyclin. J Nutr. 1997;127:478–482. doi: 10.1093/jn/127.3.478. [DOI] [PubMed] [Google Scholar]
- Kang BH, Plescia J, Dohi T, Rosa J, Doxsey SJ, Altieri DC. Regulation of tumor cell mitochondrial homeostasis by an organelle-specific Hsp90 chaperone network. Cell. 2007;131:257–270. doi: 10.1016/j.cell.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Kanojia RK, Junaid M, Murthy RC. Embryo and fetotoxicity of hexavalent chromium: a long-term study. Toxicol Lett. 1998;95:165–172. doi: 10.1016/s0378-4274(98)00034-4. [DOI] [PubMed] [Google Scholar]
- Kayampilly PP, Menon KM. Follicle-stimulating hormone increases tuberin phosphorylation and mammalian target of rapamycin signaling through an extracellular signal-regulated kinase-dependent pathway in rat granulosa cells. Endocrinology. 2007;148:3950–3957. doi: 10.1210/en.2007-0202. [DOI] [PubMed] [Google Scholar]
- Kirpnick-Sobol Z, Reliene R, Schiestl RH. Carcinogenic Cr(VI) and the nutritional supplement Cr(III) induce DNA deletions in yeast and mice. Cancer Res. 2006;66:3480–3484. doi: 10.1158/0008-5472.CAN-05-3944. [DOI] [PubMed] [Google Scholar]
- Kulich SM, Chu CT. Sustained extracellular signal-regulated kinase activation by 6-hydroxydopamine: implications for Parkinson’s disease. J Neurochem. 2001;77:1058–1066. doi: 10.1046/j.1471-4159.2001.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulich SM, Chu CT. Role of reactive oxygen species in extracellular signal-regulated protein kinase phosphorylation and 6-hydroxydopamine cytotoxicity. J Biosci. 2003;28:83–89. doi: 10.1007/BF02970136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara A, Nagoshi H, Yabuki M, Okuyama R, Obinata M, Ikawa S. Ser46 phosphorylation of p53 is not always sufficient to induce apoptosis: multiple mechanisms of regulation of p53-dependent apoptosis. Genes Cells. 2007;12:853–861. doi: 10.1111/j.1365-2443.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- Lanneau D, de Thonel A, Maurel S, Didelot C, Garrido C. Apoptosis versus cell differentiation: role of heat shock proteins HSP90, HSP70 and HSP27. Prion. 2007;1:53–60. doi: 10.4161/pri.1.1.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidke DS, Huang F, Post JN, Rieger B, Wilsbacher J, Thomas JL, Pouyssegur J, Jovin TM, Lenormand P. ERK nuclear translocation is dimerization-independent but controlled by the rate of phosphorylation. J Biol Chem. 2010;285:3092–3102. doi: 10.1074/jbc.M109.064972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long X, Goldenthal MJ, Marin-Garcia J. Oxidative stress enhances phosphorylation of p53 in neonatal rat cardiomyocytes. Mol Cell Biochem. 2007;303:167–174. doi: 10.1007/s11010-007-9470-1. [DOI] [PubMed] [Google Scholar]
- Makarov Y, Shimtova LA. Occupational conditions and gynecological illness in workers engaged in the production of chromium compounds. Environ Health Perspect. 1978;24:1–128. [Google Scholar]
- Matsuda-Minehata F, Inoue N, Goto Y, Manabe N. The regulation of ovarian granulosa cell death by pro- and anti-apoptotic molecules. J Reprod Dev. 2006;52:695–705. doi: 10.1262/jrd.18069. [DOI] [PubMed] [Google Scholar]
- Matsuzawa A, Nishitoh H, Tobiume K, Takeda K, Ichijo H. Physiological roles of ASK1-mediated signal transduction in oxidative stress- and endoplasmic reticulum stress-induced apoptosis: advanced findings from ASK1 knockout mice. Antioxid Redox Signal. 2002;4:415–425. doi: 10.1089/15230860260196218. [DOI] [PubMed] [Google Scholar]
- Meek DW. Multisite phosphorylation and the integration of stress signals at p53. Cell Signal. 1998a;10:159–166. doi: 10.1016/s0898-6568(97)00119-8. [DOI] [PubMed] [Google Scholar]
- Meek DW. New developments in the multi-site phosphorylation and integration of stress signalling at p53. Int J Radiat Biol. 1998b;74:729–737. doi: 10.1080/095530098141005. [DOI] [PubMed] [Google Scholar]
- Meek DW. Tumour suppression by p53: a role for the DNA damage response? Nat Rev Cancer. 2009;9:714–723. doi: 10.1038/nrc2716. [DOI] [PubMed] [Google Scholar]
- Meek DW, Anderson CW. Posttranslational modification of p53: cooperative integrators of function. Cold Spring Harb Perspect Biol. 2009;1:a000950. doi: 10.1101/cshperspect.a000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek DW, Campbell LE, Jardine LJ, Knippschild U, McKendrick L, Milne DM. Multi-site phosphorylation of p53 by protein kinases inducible by p53 and DNA damage. Biochem Soc Trans. 1997;25:416–419. doi: 10.1042/bst0250416. [DOI] [PubMed] [Google Scholar]
- Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–3239. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Milczarek GJ, Martinez J, Bowden GT. p53 Phosphorylation: biochemical and functional consequences. Life Sci. 1997;60:1–11. doi: 10.1016/s0024-3205(96)00479-1. [DOI] [PubMed] [Google Scholar]
- Milne DM, Campbell LE, Campbell DG, Meek DW. p53 is phosphorylated in vitro and in vivo by an ultraviolet radiation-induced protein kinase characteristic of the c-Jun kinase, JNK1. J Biol Chem. 1995;270:5511–5518. doi: 10.1074/jbc.270.10.5511. [DOI] [PubMed] [Google Scholar]
- Moll UM, Petrenko O. The MDM2-p53 interaction. Mol Cancer Res. 2003;1:1001–1008. [PubMed] [Google Scholar]
- Murthy RC, Junaid M, Saxena DK. Ovarian dysfunction in mice following chromium (VI) exposure. Toxicol Lett. 1996;89:147–154. doi: 10.1016/s0378-4274(96)03803-9. [DOI] [PubMed] [Google Scholar]
- Neckers L, Kern A, Tsutsumi S. Hsp90 inhibitors disrupt mitochondrial homeostasis in cancer cells. Chem Biol. 2007;14:1204–1206. doi: 10.1016/j.chembiol.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Nishikimi M, Fukuyama R, Minoshima S, Shimizu N, Yagi K. Cloning and chromosomal mapping of the human nonfunctional gene for L-gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. J Biol Chem. 1994;269:13685–13688. [PubMed] [Google Scholar]
- Nriagu J. Chromium in the Natural and Human Environments. Wiley Inter-Science; 1988. [Google Scholar]
- O’Brien TJ, Ceryak S, Patierno SR. Complexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanisms. Mutat Res. 2003;533:3–36. doi: 10.1016/j.mrfmmm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Pani G, Colavitti R, Bedogni B, Fusco S, Ferraro D, Borrello S, Galeotti T. Mitochondrial superoxide dismutase: a promising target for new anticancer therapies. Curr Med Chem. 2004;11:1299–1308. doi: 10.2174/0929867043365297. [DOI] [PubMed] [Google Scholar]
- Poderoso C, Converso DP, Maloberti P, Duarte A, Neuman I, Galli S, Maciel FC, Paz C, Carreras MC, Poderoso JJ, Podesta EJ. A mitochondrial kinase complex is essential to mediate an ERK1/2-dependent phosphorylation of a key regulatory protein in steroid biosynthesis. PLoS ONE. 2008;3:e1443. doi: 10.1371/journal.pone.0001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana SV. Metals and apoptosis: recent developments. J Trace Elem Med Biol. 2008;22:262–284. doi: 10.1016/j.jtemb.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Rapp UR, Fischer A, Rennefahrt UE, Hekman M, Albert S. BAD association with membranes is regulated by Raf kinases and association with 14-3-3 proteins. Adv Enzyme Regul. 2007;47:281–285. doi: 10.1016/j.advenzreg.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Salnikow K, Zhitkovich A. Geneticandepigeneticmechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic, and chromium. Chem Res Toxicol. 2008;21:28–44. doi: 10.1021/tx700198a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel JB, Stanley JA, Roopha DP, Vengatesh G, Anbalagan J, Banu SK, Aruldhas MM. Lactational hexavalent chromium exposure-induced oxidative stress in rat uterus is associated with delayed puberty and impaired gonadotropin levels. Hum Exp Toxicol. 2010 Mar 4; doi: 10.1177/0960327110364638. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Schuler M, Green DR. Mechanisms of p53-dependent apoptosis. Biochem Soc Trans. 2001;29:684–688. doi: 10.1042/0300-5127:0290684. [DOI] [PubMed] [Google Scholar]
- Shi H, Hudson LG, Liu KJ. Oxidative stress and apoptosis in metal ion-induced carcinogenesis. Free Radic Biol Med. 2004;37:582–593. doi: 10.1016/j.freeradbiomed.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- Shmitova L. The course of pregnancy in women engaged in the production of chromium and its compounds. Sverdlovsk. 1978;19:108–111. [Google Scholar]
- Shmitova L. Content of hexavalent chromium in the biological substrates of pregnant woen and women in the immediate postnatal period engaged in the manufacture of chromium compounds. Gig Trud Prof Zabol. 1980;2:32–35. [PubMed] [Google Scholar]
- Siu PM, Wang Y, Alway SE. Apoptotic signaling induced by H2O2-mediated oxidative stress in differentiated C2C12 myotubes. Life Sci. 2009;84:468–481. doi: 10.1016/j.lfs.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluss HK, Gannon H, Coles AH, Shen Q, Eischen CM, Jones SN. Phosphorylation of p53 serine 18 upregulates apoptosis to suppress Myc-induced tumorigenesis. Mol Cancer Res. 2010;8:216–222. doi: 10.1158/1541-7786.MCR-09-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadheim TA, Xiao H, Eastman A. Inhibition of extracellular signal-regulated kinase (ERK) mediates cell cycle phase independent apoptosis in vinblastine-treated ML-1 cells. Cancer Res. 2001;61:1533–1540. [PubMed] [Google Scholar]
- Stanciu M, DeFranco DB. Prolonged nuclear retention of activated extracellular signal-regulated protein kinase promotes cell death generated by oxidative toxicity or proteasome inhibition in a neuronal cell line. J Biol Chem. 2002;277:4010–4017. doi: 10.1074/jbc.M104479200. [DOI] [PubMed] [Google Scholar]
- Stanciu M, Wang Y, Kentor R, Burke N, Watkins S, Kress G, Reynolds I, Klann E, Angiolieri MR, Johnson JW, DeFranco DB. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J Biol Chem. 2000;275:12200–12206. doi: 10.1074/jbc.275.16.12200. [DOI] [PubMed] [Google Scholar]
- Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem. 2005;280:38729–38739. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- Sutton R. In: Chromium-6 in US Tap Water. Jane Houlihan RS, Bruzelius Nils, editors. Environmental Working Group; Washington, DC: 2010. pp. 1–23. [Google Scholar]
- Tipton IH, Shafer JJ. Statistical Analysis of Lung Trace Element Levels. Arch Environ Health. 1964;8:58–67. doi: 10.1080/00039896.1964.10663632. [DOI] [PubMed] [Google Scholar]
- Tsuruta F, Sunayama J, Mori Y, Hattori S, Shimizu S, Tsujimoto Y, Yoshioka K, Masuyama N, Gotoh Y. JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO J. 2004;23:1889–1899. doi: 10.1038/sj.emboj.7600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Verschoor ML, Wilson LA, Singh G. Mechanisms associated with mitochondrial-generated reactive oxygen species in cancer. Can J Physiol Pharmacol. 2010;88:204–219. doi: 10.1139/Y09-135. [DOI] [PubMed] [Google Scholar]
- Ye J, Wang S, Leonard SS, Sun Y, Butterworth L, Antonini J, Ding M, Rojanasakul Y, Vallyathan V, Castranova V, Shi X. Role of reactive oxygen species and p53 in chromium(VI)-induced apoptosis. J Biol Chem. 1999;274:34974–34980. doi: 10.1074/jbc.274.49.34974. [DOI] [PubMed] [Google Scholar]
- Yeh PY, Chuang SE, Yeh KH, Song YC, Chang LL, Cheng AL. Phosphorylation of p53 on Thr55 by ERK2 is necessary for doxorubicin-induced p53 activation and cell death. Oncogene. 2004;23:3580–3588. doi: 10.1038/sj.onc.1207426. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Chaiswing L, Velez JM, Batinic-Haberle I, Colburn NH, Oberley TD, St Clair DK. p53 translocation to mitochondria precedes its nuclear translocation and targets mitochondrial oxidative defense protein-manganese superoxide dismutase. Cancer Res. 2005;65:3745–3750. doi: 10.1158/0008-5472.CAN-04-3835. [DOI] [PubMed] [Google Scholar]
- Zhitkovich A. Importance of chromium-DNA adducts in mutagenicity and toxicity of chromium(VI) Chem Res Toxicol. 2005;18:3–11. doi: 10.1021/tx049774+. [DOI] [PubMed] [Google Scholar]
- Zhitkovich A, Peterson-Roth E, Reynolds M. Killing of chromium-damaged cells by mismatch repair and its relevance to carcinogenesis. Cell Cycle. 2005;4:1050–1052. [PubMed] [Google Scholar]