Abstract
Autosomal dominant retinitis pigmentosa (ADRP) is frequently caused by mutations in RHO, the gene for rod photoreceptor opsin. Earlier, a study on mice carrying mutated rhodopsin transgenes on either RHO + / + or RHO + /– backgrounds suggested that the amount of wild-type rhodopsin affected survival of photoreceptors. Therefore, we treated P23H RHO transgenic mice with adeno-associated virus serotype 5 (AAV5) expressing a cDNA clone of the rhodopsin gene (RHO301) that expressed normal opsin from the mouse opsin promoter. Analysis of the electroretinogram (ERG) demonstrated that increased expression of RHO301 slowed the rate of retinal degeneration in P23H mice: at 6 months, a-wave amplitudes were increased by 100% and b-wave amplitudes by 79%. In contrast, nontransgenic mice injected with AAV5 RHO301 demonstrated a decrease in the ERG, confirming the damaging effect of rhodopsin overproduction in normal photoreceptors. In P23H mice, the increase in the ERG amplitudes was correlated with improvement of retinal structure: the thickness of the outer nuclear layer in RHO301-treated eyes was increased by 80% compared with control eyes. These findings suggest that the wild-type RHO gene can be delivered to rescue retinal degeneration in mice carrying a RHO mutation and that increased production of normal rhodopsin can suppress the effect of the mutated protein. These findings make it possible to treat ADRP caused by different mutations of RHO with the expression of wild-type RHO.
In this study, Mao and colleagues examine the efficacy of AAV5-mediated gene transfer of the wild-type rhodopsin (RHO) gene in a mouse model of autosomal dominant retinitis pigmentosa. The authors find that this approach leads to the production of normal RHO protein and to the rescue of retinal degeneration.
Introduction
Retinitis Pigmentosa (RP) is a neurodegenerative disorder with the prevalence of 1 in 4,000 and almost 1.5 million patients in the world (Phelan and Bok, 2000; Hartong et al., 2006; Daiger et al., 2007). It is characterized by apoptotic death of rod photoreceptor cells. There are three forms of RP: autosomal dominant RP (ADRP), autosomal recessive RP, and X-linked RP, with ADRP accounting for 40% of clinical cases. Although the pathological progression is variable in different individuals diagnosed with ADRP, the primary symptoms are gradual loss of vision in the dark followed by loss of peripheral vision. Eventually, central vision is diminished by cone-cell degeneration late in the disease course (Van Soest et al., 1999; Farrar et al., 2002).
Pioneering work by geneticists and ophthalmologists over the past two decades has identified mutations in 20 genes that lead to ADRP, paving the way for the possible gene-specific treatments of this complex disease (Bok, 2007). Rhodopsin is encoded by one of these genes (RHO), and mutations in RHO are associated with over 25% of ADRP cases (Daiger et al., 2007). The first identified RHO mutation, P23H (proline 23 substituted by histidine), is associated with 12% of ADRP patients in the United States (Dryja et al., 1990; Van Soest et al., 1999; Daiger et al., 2007). Different models have been proposed for the mechanism of photoreceptor death resulting from RHO mutations, and these vary based on the amino acid residue affected (Dryja et al., 2000; Stojanovic et al., 2003; Lewis and Kono, 2006). To study the underlying pathology of ADRP, several P23H transgenic animal models, including mouse, rat, fly, and frog models, have been developed (Olsson et al., 1992; Roof et al., 1994; Organisciak et al., 2003; Galy et al., 2005; Gorbatyuk et al., 2005b; Tam and Moritz, 2006). In the rat model, Saito and colleagues have shown that P23H rhodopsin exhibits delayed dephosphorylation, and they suggest that abnormal levels of cytosolic Ca2+ are responsible for cell death (Saito et al., 2008).
Protein misfolding caused by the P23H mutation may also contribute to pathogenesis by interfering with the transport or function of normal rhodopsin and by initiating endoplasmic reticulum (ER) stress (Illing et al., 2002; Lin et al., 2007). One point of discussion concerns whether RHO mutations cause photoreceptor death by a toxic gain-of-function mechanism or by a dominant negative mechanism. Stimulation of unregulated phototransduction would suggest the former, but photoreceptor damage in P23H transgenic frogs does not require activation of transducin, suggesting that phototransduction is not required for rod cell death (Tam and Moritz, 2006). Study of the GHL allele of rhodopsin in transgenic mice (the H in this case represents P23H) suggests that the mutation behaves in a dominant negative fashion: the rate of retinal degeneration observed in the presence of one wild-type endogenous allele (RHO + /–) was significantly greater than when two normal copies were present (RHO + / + ) (Frederick et al., 2001).
Animal models of ADRP have also been useful in testing therapies for RP. Despite the complexity and genetic heterogeneity of ADRP, several research groups have been involved in the development of a gene therapy in animal models. Our group and two others have explored “a cut and replace” therapy using RNA interference (RNAi) or catalytic RNA enzymes (ribozymes) (Lewin et al., 1998; Sullivan et al., 2002; Gorbatyuk et al., 2005a, 2007b, 2008; Kiang et al., 2005). Both allele-dependent and allele-independent strategies have been attempted. For the allele-dependent method, specific ribozymes or small interfering RNAs (siRNAs) were designed to silence the mutant gene, but leave the original mRNA uncut to obtain a knockdown of the mutant rhodopsin in ADRP animal models. Some of these studies showed rescue of retinal structure and function in the animal models (Lewin et al., 1998; Gorbatyuk et al., 2004). In contrast, an allele-independent approach was designed to suppress expression of both mutant and wild-type genes in order to treat ADRP patients with different mutations (Sullivan et al., 2002; Cashman et al., 2005; Kiang et al., 2005; Tessitore et al., 2006; Gorbatyuk et al., 2007a,b, 2008). In this case, a gene for wild-type rhodopsin that is resistant to the RNA knockdown agent is supplied in the same vector. This approach has led to rescue of vision when tested in ADRP mice (Palfi et al., 2006; O'Reilly et al., 2008; Smith et al., 2009).
In earlier work, we demonstrated that gene transfer of siRNA301, which is specific for mouse RHO, to photoreceptors leads to long-term rescue of photoreceptors in rats bearing a mutant mouse RHO transgene (Gorbatyuk et al., 2007b). We began the present studies to optimize the expression of an allele of RHO (RHO301) that encodes the normal protein, but is resistant to cleavage by this siRNA. In the course of these experiments, we determined that the increased production of normal rhodopsin rescued photoreceptor function in P23H RHO mice, suggesting that gene therapy with normal gene is possible for treatment of many different ADRP mutations affecting rhodopsin.
Materials and Methods
Cloning of the RHO301 gene
The mouse RHO (GenBank accession number BC013125) cDNA we used contained a 109-bp 5’-untranslated region (UTR) and a 159-bp 3’-UTR. It also contains five silent mutations to eliminate the siRNA301 recognition site (wild-type: CTTCCTCACGCTCTACGTC to RHO301: CTTCTTAACCTTGTACGTC). The production of the opsin protein was confirmed by immunohistochemistry of 293 cells transfected with a plasmid expressing RHO301 from the cytomegalovirus immediate-early promoter (data not shown). For in vivo studies, RHO301 was embedded in an adeno-associated virus (AAV) vector under the control of a mouse proximal opsin promoter (Flannery et al., 1997). Recombinant vector then was packaged in AAV serotype 5 (AAV5), resulting in a final titer of 2 × 1012 viral genomes/ml (Zolotukhin et al., 2002).
Animal model
All experimental procedures with mice were approved by the University of Florida Institutional Animal Care and Use Committee in accordance with the NIH Guide for Care and Use of Laboratory Animals and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. P23H transgenic mice (line 37) contains a human P23H RHO transgene comprised of the entire rhodopsin gene transcriptional unit plus 4.2 kb of upstream and 8.4 kb of downstream DNA. Founder P23H RHO mice (on an FVB background) were backcrossed with C57BL/6J mice for 10 generations to obtain human transgenic mice on a uniform B6 genetic background. This line contains an equal number of copies of the human RHO transgene and the endogenous mouse RHO gene (Supplementary Fig. S1; supplementary data are available online at www.liebertonline.com/hum). As the wild-type animals, we used C57BL/6J mice that were purchased from Jackson Laboratories (Bar Harbor, ME). Consistent with the nomenclature of Dryja and colleagues, and to indicate that the mice have two copies of the endogenous RHO gene, we term these mice hP23H RHO + /–, mRHO + / + (Dryja et al., 1990; Olsson et al., 1992). All mice were housed in a 12-hr light/12-hr dark cycle under specific pathogen-free conditions.
Subretinal AAV injections were performed as described by Timmers et al. (2001). Wild-type and P23H RHO mice were injected at postnatal day 15 (P15). For this purpose, mice were anesthetized by ketamine/xylazine injection. Pupils were dilated with one drop of 1% atropine sulfate and 2.5% phenylephrine. Right eyes were injected in the superior hemisphere with 1 μl of AAV RHO301 (2 × 109 vector genomes), and left eyes were kept as untreated controls. For injection controls, other mice were injected with AAV of the same titer expressing green fluorescent protein (GFP) from the mouse opsin promoter.
RNA analysis
Total RNA was extracted from retinas of three P23H mice treated with AAV-RHO301 in their right eyes and uninjected in their left eyes. TRIzol reagent (Invitrogen, Carlsbad, CA) was used to isolate RNA from fresh retinas according to the manufacturer's procedure. Extracted RNA samples were treated with RNase free DNase I (Ambion, Austin, TX) to remove DNA contamination. RNA concentration was estimated by A260 using a NanoDrop N-1000 spectrophotometer (NanoDrop, Wilmington, DE). Human RHO, mouse RHO and mouse RHO301 mRNA were converted into cDNA by RT-PCR with a first-strand cDNA synthesis kit (GE Healthcare, Piscataway, NJ). Gene-specific primers were used for reverse transcription of mouse and human RHO and β-actin: 5′-TTCTCCCCGAAGCGGAAGTT-3′ (RHO exon 2), 5′-TGGCCATCTCCTGCTCGAAGTC-3′ (β-actin). Forward PCR primers were: 5′-CCATGGCAGTTCTCCATGCT-3′ for both human and mouse RHO exon 1 and 5′-TGAGACCTTCAACACCCCAGCC-3′ for β-actin. After PCR amplification, PCR products were purified using a GenElute PCR Clean-Up Kit (Sigma–Aldrich, St. Louis, MO), and RHO PCR products were digested with the endonuclease MseI (Promega, Madison, WI), which does not cleave within the human P23H and mouse RHO PCR products, but does cleave within RHO301. Treatment of these PCR products gave one band of 353 bp in the samples of uninjected retinas. The RHO301 PCR digested with MseI gave two bands of 70 bp and 283 bp. After digestion, PCR products from left and right eyes were loaded on 12% polyacrylamide gels run in Tris-borate EDTA buffer for measurement of both RHO PCR products and β-actin PCR products. SYBR Green (Invitrogen) was used to stain PCR product bands. The intensity of each band was detected with a scanner and analyzed using “Quantity One” software (Bio-Rad, Hercules, CA) to quantify the volume of each band of interest. Expression of RHO mRNA was normalized based on β-actin content.
Protein extraction and immunoblots
At 1 month after injection, three P23H mice or wild-type mice were euthanized by carbon dioxide inhalation, and the retinas were dissected. Protein extracts of retinas were prepared by sonication in electrophoresis sample buffer (Laemmli, 1970). Concentration of total retinal proteins from each sample was detected by the Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL). For immunoblotting, 20 μg of total protein from each sample was loaded on 12% sodium dodecyl sulfate–polyacrylamide gels run in Tris-glycine buffer. Proteins were transferred to polyvinylidene fluoride membranes by iBlot dry blotting system (Invitrogen). After transferring, the blots were treated with either a monoclonal antibody against an N-terminal bovine rhodopsin fragment (B6-30) or a monoclonal antibody against the C-terminus of mouse rhodopsin (1D4). The second antibody detects all rhodopsin proteins, including the P23H RHO. However, it is possible that B6-30 might have a lower affinity to P23H opsin due to the P23H mutation. For secondary antibodies, we used infrared dye-tagged anti-mouse (for rhodopsin) and anti-rabbit (for β-actin) IgG from LI-COR Biosciences (Lincoln, NE). Stained blots were scanned and analyzed for intensity by using the LI-COR Odyssey scanner and Odyssey analysis software. Levels of rhodopsin protein were normalized to the endogenous β-actin.
Electroretinography (ERG)
At 1, 2, 3, and 6 months following subretinal injection, mice were analyzed by simultaneous full-field ERG. For ERG, mice were dark-adapted overnight, then anesthetized with ketamine/xylazine, and their eyes dilated in dim red light with 2.5% phenylephrine solution. Small contact lenses with gold wire loops were placed on each cornea with a drop of 2.5% methylcellulose to maintain corneal hydration. A silver wire reference electrode was placed subcutaneously between the eyes, and a ground electrode was placed subcutaneously in a hind leg. Responses from both eyes were recorded simultaneously using a UTAS-E 2000 Visual Electrodiagnostic System (LKC Technologies, Gaithersburg, MD). Scotopic ERGs, which primarily measure rod function, were elicited with 10-msec flashes of white light at −30, −20, −10, and 0 dB with appropriate delay between flashes. Five to 10 scans were averaged at each light intensity. The a-wave amplitudes were measured from baseline to the peak in the cornea-negative direction, and b-wave amplitudes were measured from cornea-negative peak to major cornea-positive peak. The results from each group of mice were averaged, and the means were compared statistically by using Student's t test for paired data, when comparing treated and control eyes from the same mice. For comparison of the mean amplitudes of multiple groups, we used ANOVA.
Histological analysis
For morphometric analysis, the retinas were fixed and prepared for plastic sectioning after perfusion in 2% paraformaldehyde plus 2.5% glutaraldehyde. Dissected tissues were postfixed in 1% osmium tetroxide at 4°C for 4 hr and then maintained in 0.1 M cacodylate buffer overnight. After dehydration, tissues were embedded in an epoxy resin. Tissue sections (1 μm) were made along the vertical meridian through the optic disc and stained with toluidine blue. The thickness of the outer nuclear layer (ONL) was measured at five equally spaced superior loci and five inferior loci using the MBF Stereo Investigator (MicroBrightField, Inc., Williston, VT) connected to a Zeiss microscope. Ten measurements were averaged at each site with system software by an operator who was unaware of the identity of the samples. The distribution of the ONL thickness of superior and inferior retina was determined by the mean thickness from each section. Analysis was described by Faktorovitch et al. (1990). Differences between the ONL thickness of left control eyes and right treated eyes were analyzed by using Student's t test for paired samples. A p value of <0.05 was considered significant.
Quantification of apoptosis
Six P23H transgenic mice were injected in their right eyes with AAV-RHO301on P15; 1 month later, the retinas from both eyes were extracted to detect the apoptotic cells by a nucleosome release assay, using the cell death detection ELISA kit (Roche Diagnostics, Indianapolis, IN). In brief, based on the manufacturer's protocol, each retinal sample was placed in 200 μl of lysis buffer on ice. RHO301-injected and uninjected retinas were homogenized for 3 sec with a tissue homogenizer (Polytron; PT1200). Insoluble material was sedimented by centrifugation at full speed in a microcentrifuge, and 10 μl of the supernatant was diluted 1:100 in lysis buffer. Twenty microliters of the final solution was used to measure the nucleosome release.
Results
Expression of AAV-delivered RHO301
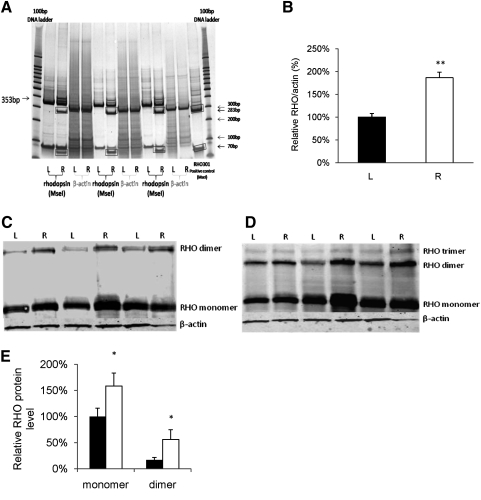
We constructed the RHO301 allele by introducing five silent mutations into a normal mouse RHO cDNA clone to eliminate the cleavage site for a previously designed siRNA (Gorbatyuk et al., 2007b, 2008). The gene was delivered by subretinal injection of an AAV2 vector pseudotyped with AAV5 capsids on P15. Expression was driven by the mouse opsin proximal promoter (Supplementary Fig. S2). To confirm the expression of RHO301 in P23H retinas, we analyzed the mRNA level of the transferred gene 1 month following subretinal injection with AAV-RHO301. To distinguish RHO301 mRNA from endogenous mouse RHO mRNA and the mRNA produced by the human P23H transgene, we used a restriction site that was created in the RHO301 allele by the silent mutations. Total amplified PCR products were digested with MseI, and digestion products were normalized to β-actin PCR amplification products from the same samples (Fig. 1A). Compared with rhodopsin mRNA levels in untreated left eyes, we obtained an almost twofold increase of total RHO mRNA in treated right eyes (Fig. 1B), indicating the successful delivery and expression of RHO301 in right eyes (p < 0.005).
FIG. 1.
Increased expression of rhodopsin following AAV delivery of RHO301. Expression of RHO mRNA was elevated nearly twofold by AAV5-RHO301 gene transfer in P23H transgenic mice (p < 0.005). (A) Image of a polyacrylamide gel with MseI digestion products of PCR products from left uninjected and right injected eyes of three randomly selected mice. To differentiate the RHO301 transcript from endogenous RHO mRNA, total RNA extraction was amplified by specific rhodopsin primers, and PCR products were digested by MseI. Both mouse RHO and human transgenic P23H RHO products were uncut by MseI (353 bp), but RHO301 PCR products were digested into 283-bp and 70-bp fragments (rectangular boxes) in right, RHO301-injected eyes (labeled R), but not in uninjected left eyes (labeled L). MseI-digested RHO301 PCR products were applied as a positive control. A 100-bp DNA ladder was used as size marker. (B) Densitometric quantification of the scanned gel. RHO PCR products were normalized by the intensity of the endogenous β-actin PCR product. The ratio of the intensity of the 353-bp fragment to the β-actin fragment in uninjected left eyes (black bar) was set to 100%, and the ratio of injected right eyes (white bar) was significantly increased (**p < 0.005). (C and D) Elevation of rhodopsin protein levels in eyes treated with AAV-RHO301. Western blot analysis of rhodopsin protein production in three P23H mice injected with RHO301 in their right eyes using 1D4 (C) or B6-30 (D) monoclonal antibodies. (E) Densitometric quantification of the blot in panel C reveals an elevated production of opsin protein in injected right eyes (white bars) compared with uninjected left eyes (black bars). The data were normalized to endogenous β-actin, and the ratio of intensity of the untreated opsin monomer was set as 100% (*p < 0.05).
Proteins were also extracted from P23H retinas of mice 1 month after subretinal injection, and expression of rhodopsin was detected by immunoblot using mouse anti-rhodopsin monoclonal antibodies 1D4, which recognizes a C-terminal epitope, and B6-30, which recognizes an N-terminal epitope (Fig. 1C and D). For data analysis, the rhodopsin band was normalized using the intensity of the endogenous β-actin band. A 58% increase of monomer protein level and a threefold increase in the dimer was found in the RHO301-injected eyes (p < 0.05) (Fig. 1E). Similar results were found using both monoclonal antibodies. Under native conditions, rhodopsin forms dimers and higher-order species in rod outer segments (Fotiadis et al., 2003). As we saw no increase in endogenous mouse RHO transcripts (data not shown), we conclude that the elevation in opsin protein is attributable to expression of RHO301 rather than to increased survival of rods at this early stage (6 weeks of age).
Rescue of retinal function in AAV-RHO301-treated P23H eyes in mouse and rat models
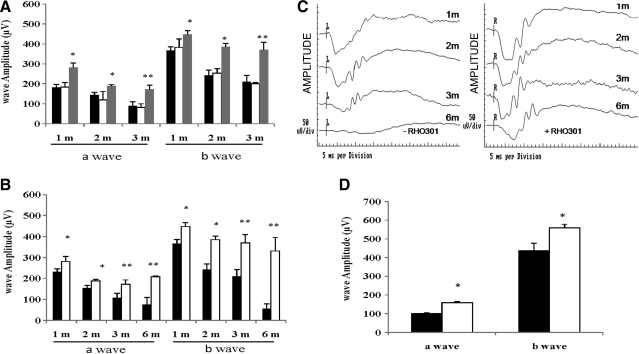
To assess retinal function in treated eyes, the full-field electroretinogram (ERG) was measured in dark-adapted mice at increasing intervals following injection. To control for the impact of injection (Wen et al., 1995), we injected additional P23H transgenic mice with AAV5-expressing GFP from the mouse opsin promoter. Analysis of ERG amplitudes showed that there was no difference in retinal function between uninjected and GFP-injected mice over the course of 3 months (Fig. 2A). However, the a-wave and b-wave amplitudes for RHO301-injected eyes were higher compared with those for GFP-injected animals at 1 month after injection. At 3 months post injection, we observed an almost twofold increase for both a-wave and b-wave amplitudes in the RHO301-injected eyes versus the GFP-injected contralateral eyes (p < 0.05).
FIG. 2.
Increased expression of rhodopsin protects P23H retinas in both P23H mice and rats. Both a-wave and b-wave amplitudes of scotopic (dark-adapted) full-field ERG were evaluated in nine P23H mice injected with AAV-RHO301 (gray bars) and compared with nine mice injected with AAV-GFP control virus (white bars) or with an uninjected control group (n = 9) (black bars) over a time course of 3 months following injection (A). The ERG a-wave and b-wave amplitudes of the RHO301-treated group were significantly elevated relative to GFP-injected or uninjected eyes at all time points (*p < 0.05 at 1 and 2 months post injection; **p < 0.005 at 3 months post injection). (B) AAV-RHO301 protects the retina for up to 6 months. The a-wave and b-wave amplitudes of injected right eyes were elevated (white bars) in nine P23H transgenic mice at 1, 2, 3, and 6 months following injection, compared with uninjected left eyes (black bars) (*p < 0.05 at 1 and 2 months post injection; **p < 0.005 at 3 and 6 months). (C) Representative waveforms of P23H transgenic mice in uninjected eyes (left panel) and AAV-RHO301-injected eyes (right panel). (D) The scotopic ERG response in six P23H-RHO line 3 rats injected with AAV-RHO301 in their right eyes (white bars). A 54% increase in a-wave amplitude relative to control uninjected left eyes (black bars) and a 27% increase in b-wave amplitude were observed in RHO301-injected P23H-RHO rats at 2 months post injection (*p < 0.05).
Over a longer time course, RHO301 gene transfer consistently improved both the a-wave and b-wave responses of P23H mice compared with those of untreated control eyes (Fig. 2B and C). At 6 months post injection, there was a 2.8-fold increase of a-wave amplitude in injected eyes compared with untreated eyes (p < 0.005). Although the a-wave amplitudes from the treated eyes declined gradually compared with the value at 1 month post injection, they remained relatively constant (around 200 μv) from 2 months to 6 months (Fig. 2B). Moreover, there was significant increase in the latency (implicit time) of the a-wave in untreated P23H retinas. (Implicit time was measured from the light flash to the negative peak of the a-wave.) In RHO301-injected P23H mice, the implicit time of the injected right eyes was shorter than that of the uninjected left eyes at 3 months and 6 months (Supplementary Fig. S3A). The b-wave amplitudes of RHO301-treated eyes were also substantially improved (sixfold) compared with those of untreated P23H eyes (Fig. 2B) at the 6-month time point. At this stage, untreated eyes demonstrated less than 20% of the b-wave amplitude of the 1-month time point. In contrast, the P23H eyes treated with AAV-RHO301 injection maintained 80% of the b-wave response compared with their amplitudes at 1 month. For sake of illustration, representative ERG waveforms from treated and untreated eyes of P23H mice are shown in Fig. 2C. This experiment demonstrated that the decline in retinal function in P23H transgenic mice was dramatically reduced by delivery of functional RHO.
To determine if the therapeutic effect of RHO delivery is limited to a single mouse model, we performed a pilot project in which we injected transgenic rats expressing P23H-RHO (line 3) with AAV-RHO301. This is a well characterized model of ADRP caused by RHO mutation (Organisciak et al., 2003; Lin et al., 2007). At 2 months post injection, gene transfer of RHO301 to photoreceptors resulted in an increase in ERG a-wave and b-wave amplitudes in this ADRP model as well (Fig. 2D).
AAV-RHO301 delivery damages wild-type retinas
Overexpression of wild-type rhodopsin can be detrimental in transgenic mice (Olsson et al., 1992; Tan et al., 2001). To determine if overexpression of rhodopsin from viral delivery damaged the retinas of wild-type mice, we injected AAV-RHO301 in C57BL/6J mice, which accumulate more rhodopsin than the P23H transgenic mice (Noorwez et al., 2009). Proteins were also extracted from wild-type C57BL/6J mice retinas 1 month after subretinal injection, and expression of rhodopsin was detected by anti-rhodopsin monoclonal antibody 1D4. There was a 45% increase in the monomer form of rhodopsin and a twofold increase in the dimer form in the RHO301-injected eyes compared with uninjected eyes (p < 0.05) (Supplementary Fig. S4). From 1 month to 6 months post treatment, both a-wave and b-wave amplitudes remained constant in the control eyes (Fig. 3A). However, by 1 month post injection, delivery of RHO301 led to a slight (15%) reduction of the a-wave response. By 2 months after injection, the a-wave amplitude in RHO301-injected eyes dropped to half the value of that in untreated eyes. At 3 and 6 months post injection, the a-wave amplitude dropped at a slower rate. RHO301 gene transfer led to a similar decline of b-wave amplitudes. The b-wave peaks were gradually reduced to 80% and 70% after 1 and 2 months post injection, whereas at 3 and 6 months post injection, the b-wave response remained at 33% of the untreated amplitude (Fig. 3A). In contrast to the P23H transgenic mice, wild-type mice had longer implicit time in ERG a-wave after RHO301 injection in right eyes compared with left untreated wild-type eyes (Supplementary Fig. S3B). Therefore, injection with AAV-expressing normal rhodopsin reduced retinal function in wild-type mice. For illustration, wave forms of the treated and untreated ERG responses are presented in Fig. 3B.
FIG. 3.
AAV-RHO301delivery impairs the function of wild-type retinas. (A) Retinal degeneration was observed in seven wild-type mice injected with AAV5-RHO301 by a reduction in both a-wave and b-wave amplitudes in injected right eyes (white bars) compared with the uninjected contralateral eyes (black bars) over a time course of 6 months. For the a-wave amplitudes, **p < 0.005 at 2, 3, and 6 months post injection. For b-wave amplitudes, *p < 0.05 at 1 month and **p < 0.005 at 2, 3, and 6 months post injection. (B) ERG wave forms from wild-type mice either uninjected (left panel) or injected with AAV-RHO301 (right panel).
AAV-RHO301 preserves retinal structure in P23H mice
The thickness of the ONL was measured as a metric of surviving photoreceptors in both the superior and inferior retinas of the P23H mice (Fig. 4A and B). Although the distribution of the ONL measurements showed regional differences between superior and inferior, there was better survival of photoreceptors after RHO301 treatment of P23H transgenic mice, with approximately double the ONL thickness in most regions of the retina (Fig. 4E). Moreover, the thickness of the ONL from RHO301 expression was remarkably elevated in the inferior retina close to the optic nerve head: 42.5 ± 3.3 μm in RHO301-treated P23H eyes compared with the ONL thickness of 23.8 ± 7.5 μm in untreated eyes (p < 0.005). Averaging across the entire retina, ONL thickness was 80% greater in RHO301-treated P23H eyes than in untreated P23H degenerated eyes (Fig. 4F). Higher magnification micrographs also showed longer and more normal appearing rod outer segments in RHO301-treated retinas compared with untreated control retinas (Supplementary Fig. S5). This difference was statistically significant (p < 0.05) in both the superior and inferior posterior retina. Increased length of rod outer segments is consistent with the increased rhodopsin content we measured immunologically (Fig. 1).
FIG. 4.
RHO301 gene delivery preserves the structural integrity of P23H retinas. The thickness of the ONL was measured in RHO301-injected P23H retinas (A) and compared with untreated contralateral retinas (B) from the same four mice at 6 months post injection. At most sites along the vertical meridian, the thickness of the ONL was greater in the RHO301-treated retinas. In contrast, the ONL from the RHO301-injected wild-type retinas (C) was thinner than that of untreated wild-type retinas (D). Calibration bar is 20 μm. Analysis was made as described by Lewin et al. (1998). Data are summarized from P23H mice and wild-type mice (E). Significant increase of the ONL thickness was detected in the RHO301-injected (right) eyes of P23H mice (circles) compared with the ONL of untreated (left) eyes (triangles) of the same animals (*p < 0.05 in the inferior retina). The ONL thickness in the RHO301-injected (right) retinas of wild-type mice (squares) was significantly reduced compared with the uninjected contralateral eyes (rectangles) (*p < 0.05 in the superior retina) (E). (F) Averaged data with overall ONL thickness from the same animal groups shown as a bar graph in both superior and inferior areas. For the P23H mice, both superior and inferior ONL from RHO301-injected retinas (black bars) were significantly thicker than that of the left untreated group (white bars) (*p < 0.05). In contrast, wild-type mice showed significant decrease in the ONL thickness only in the inferior area of the RHO301-injected eyes (small grid bars) compared with the uninjected wild-type eyes (gray bars) (*p < 0.05).
In contrast, in wild-type mice treated with RHO301, the ONL was thinner compared with that of control eyes (Fig. 4C and D). Although some superior and inferior regions had no significant changes, almost 40% reduction was detected in the central superior regions. The ONL thickness dropped from 48.3 ± 2.9 μm (central superior) and 49.2 ± 3.8 μm (central inferior) to 32.3 ± 7.9 μm and 30.0 ± 11.0 μm, respectively (p < 0.05) (Fig. 4E) (Faktorovich et al., 1990).
AAV delivery of RHO301 reduced apoptosis in P23H mice
To determine if increased ONL thickness was caused by suppression of apoptosis in P23H transgenic mice, we performed a nucleosome release assay using retinas 1 month following unilateral injection of AAV-RHO301. Apoptosis was reduced by 25% in injected eyes compared with that in uninjected eyes (p < 0.05) (Supplementary Fig. S6). This result was consistent with the rescued function of RHO301-injected retinas indicated by improvement in the ERG response and by preservation of retinal structure measured by increased ONL thickness (Figs. 2 and 4 and Supplementary Fig. S5).
Discussion
Frederick and colleagues determined that retinal degeneration was slower in P23H transgenic mice in the presence of two copies of the mouse RHO gene than in the presence of a single copy, suggesting that damage to photoreceptors is mitigated by increasing the ratio of wild-type/mutant rhodopsin (Frederick et al., 2001). We have converted their result into an application for gene therapy using viral delivery of wild-type RHO. We used AAV5 to transfer a gene encoding wild-type rhodopsin to P23H RHO transgenic mice to test the hypothesis that supplementation with functional rhodopsin would reduce the rate of photoreceptor demise. One month post injection, we observed a twofold increase of total RHO mRNA in treated eyes and a 58% increase in the monomer form of rhodopsin (Fig. 1). As we routinely obtain 70% transduction of the mouse retina following subretinal injection with AAV5, the rhodopsin increase might have been even greater on a per cell basis. The elevation in rhodopsin synthesis that we observed was sufficient to preserve photoreceptors and maintain their function in P23H RHO transgenic mice. At 6 months post treatment, P23H RHO mice had a twofold increase in a-wave amplitude and a sixfold increase in b-wave amplitude relative to that of untreated transgenic eyes (Fig. 2) and corresponding to a 1.8-fold increase in survival of photoreceptors in the central retina of treated eyes (Fig. 4). This level of photoreceptor survival did not reach that of age-matched wild-type (C57BL/6) eyes, but we injected on P15 and, using single-stranded AAV, we could not expect vigorous expression of rhodopsin before 1 month of life. Based on the ERG amplitudes, delivery of RHO arrests the progression of the disease at about this stage. In contrast, the overexpression of normal rhodopsin in the retinas of wild-type mice led to a progressive reduction of scotopic ERG amplitudes between 1 month and 6 months post injection (Fig. 3). The damage to retinal structure was more substantial in the superior hemisphere, which is where we directed our injection (Fig. 4).
At this stage in our experiments, we cannot propose a precise mechanism for photoreceptor rescue by augmentation of rhodopsin synthesis in P23H rodents. In the absence of wild-type rhodopsin, P23H opsin accumulates in the ER and stimulates the unfolded protein response, leading to apoptosis (Lin et al., 2007; Gorbatyuk et al., 2010). However, in the presence of normal rhodopsin, some P23H rhodopsin traffics to the outer segment (Frederick et al., 2001). Increased expression of wild-type rhodopsin did not substantially affect markers of ER stress, such as GrP78 and CHOP (data not shown), but elevation of wild-type rhodopsin suppresses apoptosis (Supplementary Fig. S6). Even though rhodopsin is thought to be active as a monomer, it is organized as a lattice of dimers within the disc membranes of rod outer segments (Fotiadis et al., 2003). Consequently, we speculate that the P23H rhodopsin in the outer segment may interfere with function of normal rhodopsin, and the proportion of wild-type and mutant rhodopsin therefore determines the function and the stability of rod photoreceptors. This hypothesis was previously advanced by Wilson and Wensel (2003).
Tan et al. (2001) demonstrated that even modest overexpression of rhodopsin in transgenic mice can lead to retinal degeneration. In their experiments and those of Olsson et al. (1992), rhodopsin overexpression occurred during development of the retina, whereas our gene-delivery approach increased rhodopsin expression in mature photoreceptors. The question then arises: how much rhodopsin expression is too much? We found that an increase in rhodopsin expression was damaging to wild-type photoreceptors, but was protective to photoreceptors bearing the P23H transgene on the same background. However, the level of rhodopsin protein in the P23H transgenic mice is much lower than that in C57Bl/6 mice (Noorwez et al., 2009). As even wild-type opsin can misfold and aggregate, it is not surprising that a little extra is too much.
The most promising aspect of our results is that gene transfer of RHO may be applicable to other rhodopsin mutations that lead to RP by mechanisms similar to the P23H mutation. These include the largest class of RHO mutations that affect opsin folding and transport (Mendes et al., 2005). Therefore, the increase of rhodopsin expression by wild-type gene delivery is a potential treatment for a large fraction of ADRP patients. Human clinical trials of gene therapy for a recessive retinal degeneration have shown promise in restoring vision (Bainbridge et al., 2008; Cideciyan et al., 2009; Simonelli et al., 2010) and suggest that gene transfer to the retina is safe. Because of the toxicity of excess rhodopsin expression in wild-type mice (Fig. 4), a precise understanding of the dose response is required before RHO gene transfer should be attempted, and the response may depend on the amount of rhodopsin produced by the patient.
Supplementary Material
Acknowledgments
This work was supported by grant (TA-GT-0507-0384) from the Foundation Fighting Blindness and by the Vision Core Grant (NEI P30 08571) from the National Eye Institute. It was also supported by the Shaler Richardson Professorship endowment.
Author Disclosure Statement
William Hauswirth and the University of Florida own equity in AGTC, Inc., which could commercialize some aspect of this work.
References
- Bainbridge J.W.B. Smith A.J. Barker S.S., et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Bok D. Contributions of genetics to our understanding of inherited monogenic retinal diseases and age-related macular degeneration. Arch. Ophthalmol. 2007;125:160–164. doi: 10.1001/archopht.125.2.160. [DOI] [PubMed] [Google Scholar]
- Cashman S.M. Binkley E.A. Kumar-Singh R. Towards mutation-independent silencing of genes involved in retinal degeneration by RNA interference. Gene Ther. 2005;12:1223–1228. doi: 10.1038/sj.gt.3302512. [DOI] [PubMed] [Google Scholar]
- Cideciyan A.V. Hauswirth W.W. Aleman T.S., et al. Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum. Gene Ther. 2009;20:999–1004. doi: 10.1089/hum.2009.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiger S.P. Bowne S.J. Sullivan L.S. Perspective on genes and mutations causing retinitis pigmentosa. Arch. Ophthalmol. 2007;125:151–158. doi: 10.1001/archopht.125.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja T.P. McGee T.L. Reichel E., et al. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990;343:364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- Dryja T.P. McEvoy J.A. McGee T.L. Berson E.L. Novel rhodopsin mutations Gly114Val and Gln184Pro in dominant retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 2000;41:3124–3127. [PubMed] [Google Scholar]
- Faktorovich E.G. Steinberg R.H. Yasumura D., et al. Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth-factor. Nature. 1990;347:83–86. doi: 10.1038/347083a0. [DOI] [PubMed] [Google Scholar]
- Farrar G.J. Kenna P.F. Humphries P. On the genetics of retinitis pigmentosa and on mutation-independent approaches to therapeutic intervention. EMBO J. 2002;21:857–864. doi: 10.1093/emboj/21.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery J.G. Zolotukhin S. Vaquero M.I., et al. Efficient photoreceptor-targeted gene expression in vivo by recombinant adeno-associated virus. Proc. Natl. Acad. Sci. U.S.A. 1997;94:6916–6921. doi: 10.1073/pnas.94.13.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotiadis D. Liang Y. Filipek S., et al. Atomic-force microscopy: rhodopsin dimers in native disc membranes. Nature. 2003;421:127–128. doi: 10.1038/421127a. [DOI] [PubMed] [Google Scholar]
- Frederick J.M. Krasnoperova N.V. Hoffmann K., et al. Mutant rhodopsin transgene expression on a null background. Invest. Ophthalmol. Vis. Sci. 2001;42:826–833. [PubMed] [Google Scholar]
- Galy A. Roux M.J. Sahel J.A., et al. Rhodopsin maturation defects induce photoreceptor death by apoptosis: a fly model for RhodopsinPro23His human retinitis pigmentosa. Hum. Mol. Genet. 2005;14:2547–2557. doi: 10.1093/hmg/ddi258. [DOI] [PubMed] [Google Scholar]
- Gorbatyuk M. Justilien V. Liu J., et al. Preservation of photoreceptor morphology and function in P23H rats using an allele independent ribozyme. Exp. Eye Res. 2007a;84:44–52. doi: 10.1016/j.exer.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbatyuk M. Justilien V. Liu J., et al. Suppression of mouse rhodopsin expression in vivo by AAV mediated siRNA delivery. Vision Res. 2007b;47:1202–1208. doi: 10.1016/j.visres.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbatyuk M.S. Pang J. Hauswirth W. Lewin A. Ribozyme knockdown of endogenous mouse rhodopsin by AAV-delivered ribozymes. Invest. Ophthalmol. Vis. Sci. 2004;45:U518–U518. [Google Scholar]
- Gorbatyuk M.S. Pang J.J. Thomas J., et al. Knockdown of wild-type mouse rhodopsin using an AAV vectored ribozyme as part of an RNA replacement approach. Mol. Vis. 2005a;11:648–656. [PubMed] [Google Scholar]
- Gorbatyuk M.S. Timmers A.M.M. Pang J.J., et al. Rescue of vision in P23H rats with an rAAV delivered ribozyme targeting mouse opsin. Invest. Ophthalmol. Vis. Sci. 2005b;46:4692. [Google Scholar]
- Gorbatyuk M.S. Hauswirth W.W. Lewin A.S. Gene therapy for mouse models of ADRP. Adv. Exp. Med. Biol. 2008;613:107–112. doi: 10.1007/978-0-387-74904-4_11. [DOI] [PubMed] [Google Scholar]
- Gorbatyuk M.S. Knox T. Lavail M.M., et al. Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proc. Natl. Acad. Sci. U.S.A. 2010;107:5961–5966. doi: 10.1073/pnas.0911991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartong D.T. Berson E.L. Dryja T.P. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- Illing M.E. Rajan R.S. Bence N.F. Kopito R.R. A rhodopsin mutant linked to autosomal dominant retinitis pigmentosa is prone to aggregate and interacts with the ubiquitin proteasome system. J. Biol. Chem. 2002;277:34150–34160. doi: 10.1074/jbc.M204955200. [DOI] [PubMed] [Google Scholar]
- Kiang A.S. Palfi A. Ader M., et al. Toward a gene therapy for dominant disease: validation of an RNA interference-based mutation-independent approach. Mol. Ther. 2005;12:555–561. doi: 10.1016/j.ymthe.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewin A.S. Drenser K.A. Hauswirth W.W., et al. Ribozyme rescue of photoreceptor cells in a transgenic rat model of autosomal dominant retinitis pigmentosa. Nat. Med. 1998;4:967–971. doi: 10.1038/nm0898-967. [DOI] [PubMed] [Google Scholar]
- Lewis M.R. Kono M. Rhodopsin deactivation is affected by mutations of Tyrl91. Photochem. Photobiol. 2006;82:1442–1446. doi: 10.1562/2006-02-20-RA-804. [DOI] [PubMed] [Google Scholar]
- Lin J.H. Li H. Yasumura D., et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes H.F. van der Spuy J. Chapple J.P. Cheetham M.E. Mechanisms of cell death in rhodopsin retinitis pigmentosa: implications for therapy. Trends Mol. Med. 2005;11:177–185. doi: 10.1016/j.molmed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Noorwez S.M. Sama R.R.K. Kaushal S. Calnexin improves the folding efficiency of mutant rhodopsin in the presence of pharmacological chaperone 11-cis-retinal. J. Biol. Chem. 2009;284:33333–33342. doi: 10.1074/jbc.M109.043364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson J.E. Gordon J.W. Pawlyk B.S., et al. Transgenic mice with a rhodopsin mutation (Pro23His): a mouse model of autosomal dominant retinitis-pigmentosa. Neuron. 1992;9:815–830. doi: 10.1016/0896-6273(92)90236-7. [DOI] [PubMed] [Google Scholar]
- O'Reilly M. Millington-Ward S. Palfi A., et al. A transgenic mouse model for gene therapy of rhodopsin-linked retinitis pigmentosa. Vision Res. 2008;48:386–391. doi: 10.1016/j.visres.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Organisciak D.T. Darrow R.A. Barsalou L., et al. Susceptibility to retinal light damage in transgenic rats with rhodopsin mutations. Invest. Ophthalmol. Vis. Sci. 2003;44:486–492. doi: 10.1167/iovs.02-0708. [DOI] [PubMed] [Google Scholar]
- Palfi A. Ader M. Kiang A.S., et al. RNAi-based suppression and replacement of rds-peripherin in retinal organotypic culture. Hum. Mutat. 2006;27:260–268. doi: 10.1002/humu.20287. [DOI] [PubMed] [Google Scholar]
- Phelan J.K. Bok D. A brief review of retinitis pigmentosa and the identified retinitis pigmentosa genes. Mol. Vis. 2000;6:116–124. [PubMed] [Google Scholar]
- Roof D.J. Adamian M. Hayes A. Rhodopsin accumulation at abnormal sites in retinas of mice with a human P23H rhodopsin transgene. Invest. Ophthalmol. Vis. Sci. 1994;35:4049–4062. [PubMed] [Google Scholar]
- Saito Y. Ohguro H. Ohguro I., et al. Misregulation of rhodopsin phosphorylation and dephosphorylation found in P23H rat retinal degeneration. Clin. Ophthalmol. 2008;2:821–828. doi: 10.2147/opth.s4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonelli F. Maguire A.M. Testa F., et al. Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol. Ther. 2010;18:643–650. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.J. Bainbridge J.W. Ali R.R. Prospects for retinal gene replacement therapy. Trends Genet. 2009;25:156–165. doi: 10.1016/j.tig.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Stojanovic A. Hwang I. Khorana H.G. Hwa J. Retinitis pigmentosa rhodopsin mutations L125R and A164V perturb critical interhelical interactions: new insights through compensatory mutations and crystal structure analysis. J. Biol. Chem. 2003;278:39020–39028. doi: 10.1074/jbc.M303625200. [DOI] [PubMed] [Google Scholar]
- Sullivan J.M. Pietras K.M. Shin B.J. Misasi J.N. Hammerhead ribozymes designed to cleave all human rod opsin mRNAs which cause autosomal dominant retinitis pigmentosa. Mol. Vis. 2002;8:102–113. [PubMed] [Google Scholar]
- Tam B.M. Moritz O.L. Characterization of rhodopsin P23H-induced retinal degeneration in a Xenopus laevis model of retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 2006;47:3234–3241. doi: 10.1167/iovs.06-0213. [DOI] [PubMed] [Google Scholar]
- Tan E. Wang Q. Quiambao A.B., et al. The relationship between opsin overexpression and photoreceptor degeneration. Invest. Ophthalmol. Vis. Sci. 2001;42:589–600. [PubMed] [Google Scholar]
- Tessitore A. Parisi F. Denti M.A., et al. Preferential silencing of a common dominant rhodopsin mutation does not inhibit retinal degeneration in a transgenic model. Mol. Ther. 2006;14:692–699. doi: 10.1016/j.ymthe.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Timmers A.M. Zhang H. Squitieri A. Gonzalez-Pola C. Subretinal injections in rodent eyes: effects on electrophysiology and histology of rat retina. Mol. Vis. 2001;7:131–137. [PubMed] [Google Scholar]
- Van Soest S. Westerveld A. De Jong P.T.V.M., et al. Retinitis pigmentosa: defined from a molecular point of view. Surv. Ophthalmol. 1999;43:321–334. doi: 10.1016/s0039-6257(98)00046-0. [DOI] [PubMed] [Google Scholar]
- Wen R. Song Y. Cheng T., et al. Injury-induced upregulation of bFGF and CNTF mRNAs in the rat retina. J. Neurosci. 1995;15:7377–7385. doi: 10.1523/JNEUROSCI.15-11-07377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J.H. Wensel T.G. The nature of dominant mutations of rhodopsin and implications for gene therapy. Mol. Neurobiol. 2003;28:149–158. doi: 10.1385/MN:28:2:149. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S. Potter M. Zolotukhin I., et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28:158–167. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.