Abstract
R5 and X4 HIV strains use CCR5 or CXCR4 chemokine receptors (CKRs), respectively, for entry. Preferential growth of X4 vs. R5 HIV in cell lines reflects constitutive expression of CXCR4, but not CCR5 (in contrast to dual expression on primary T cells), and CXCR4 is the predominant CKR found on most tumors. Non-Hodgkin's B cell lymphomas (NHL) are increased among HIV+ patients, and interactions between HIV envelope and CKRs may contribute to lymphomagenesis. Despite strong evidence for a CXCR4–SDF-1 oncogenic axis, no in vitro evaluation of CXCR4-mediated normal lymphocyte transformation has been published. Exposure of normal B cells to EBV in the presence of X4 gp120 (but not R5 gp120) increased proliferation and BLCL outgrowth, comparable to anti-CD40 mAb costimulation. This suggests a role for X4 tropic viral envelope signaling via CXCR4 and/or CXCR7 in HIV-associated lymphomagenesis.
R5 and X4 HIV strains bind CCR5 or CXCR4 chemokine receptors (CKRs), respectively. Growth of X4, but not R5, HIV in cell lines reflects constitutive expression of CXCR4, but not CCR5, in immortalized CD4+ T cells. Predominant CXCR4 vs. other CKR expression on long-term cell lines and cancers has led to the CXCR4-CXCL12 (SDF-1) “oncogenic axis” concept.1–6 Most studies implicate CXCR4 in metastatic invasiveness, rather than proliferation and transformation, but there is evidence for transformation-related signaling, allowing proliferating cells to escape apoptotic control.4 Thus, in the absence of other CKRs, CXCR4 mediated antiapoptotic Akt phosphorylation in response to SDF-1 on 13 of 16 glioma lines.5,6 Also, stimulation with α-CD28 + α-CD3 + interleukin (IL)-2 reversibly blocked T cell surface expression of CCR5, but not CXCR4, and allowed continuous exponential growth.7 Although not previously emphasized, this is the best published in vitro evidence of dichotomous roles for these two CKRs in apoptosis. In vivo, Piovan et al. interrupted early lymphoma development and metastatic growth in SCID mice transfused with EBV+ peripheral blood mononuclear cells (PBMCs) by blocking CXCR4 signaling.8
We9 and others10 showed that X4 gp120 can bind CXCR4 on PBMCs and transduce chemotactic signals, independent of CD4. Subsequently, gp120 engagement of CXCR4 on T cells was found to trigger Rho and cyclophilin-dependent cytoskeletal phosphorylation and nuclear translocation.11 Another SDF-1 receptor, CXCR7, is necessary for sustained EBV+ B lymphoblastoid cell line (BLCL) proliferation, and is more strongly induced by EBNA2 from Type 1 vs. Type 2 EBV, apparently explaining the latter's poorer transforming efficiency.12 Thus, gp120 signaling via CXCR4 or CXCR7 could be implicated in the increased B cell lymphomagenesis seen among HIV+ patients.13
Cross-sectional studies in HIV+ adults14 showed a correlation between the SDF-1-3'A variant and NHL. Intriguingly, prospective studies15 correlated increased SDF-1 mRNA with subsequent NHL among HIV+, but not uninfected, children. Conversely, CXCR4, can mediate apoptotic signals, depending on the kinase pathways concomitantly activated,16 and X4 HIV strains and envelope have been implicated in Fas-mediated and Fas independent autophagy-mediated CD4 cell death of uninfected cells.16–20 However, the latter pathway requires fusogenic gp41 rather than gp120 CKR signaling, and is efficiently mediated by both R5 and X4 virus after CD4 binding.20 CCR5 may also mediate HIV-induced Fas-dependent lymphocyte apoptosis, as Vlahakis et al. demonstrated R5 envelope activation of caspase-8.16,17 Thus, published data support a proapoptotic role for CCR5, and both proapoptotic and antiapoptotic functions for CXCR4, depending on the in vitro system employed.
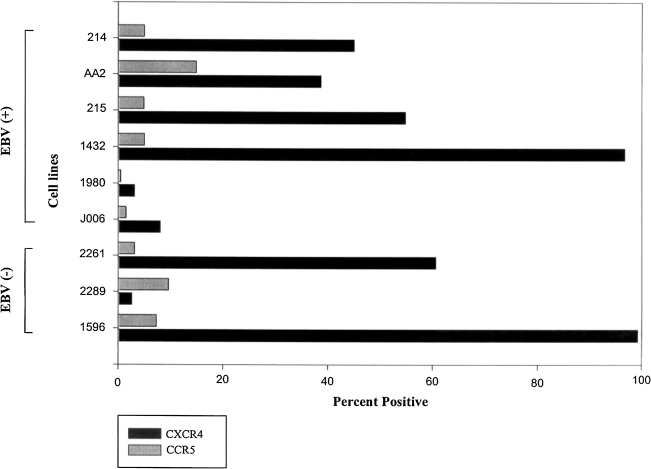
We first asked whether long-term NHL patient-derived BLCLs would conform to the pattern of CXCR4 overexpression described for tumors of neural and other tissue origin. Flow cytometric analysis of surface CXCR4 and CCR5 using monoclonal antibodies (mAbs) 12G5 and 2D7, respectively (R&D, MN), revealed that four of six EBV+ and two of three EBV− lines strongly expressed surface CXCR4 (Fig. 1). In contrast, most BLCLs failed to express high surface CCR5, consistent with published mRNA studies.21,22 Confirmation of an apparent CXCR4 dependency for patient-derived BLCLs raised the question of differential impacts of CXCR4 vs. CCR5 signaling in the context of normal B cell transformation by EBV. In particular, we were interested in the effects of X4 gp120 engagement of CXCR4 vs. R5 gp120 engagement of CCR5 in the context of EBV-mediated B cell transformation.
FIG. 1.
Overexpression of surface CXCR4 vs. CCR5 on long-term B cell lymphoblast cell lines. CXCR4 exceeded CCR5 surface expression on all EBV+ BLCLs tested, with strongly positive cells exceeding 30% in four of six EBV+ lines. Two of three EBV− lines tested were strongly positive for CXCR4, with minimal CCR5 expression. The one BLCL (#2289) with CCR5 > CXCR4 expression had relatively low levels of both CKRs.
To gain insight into possible roles of gp120-induced CKR signal transduction in B cell lymphomagenesis, we assessed the impact of X4 vs. R5 gp120 on in vitro EBV transformation of normal B cells, defined by colony outgrowth in the fourth week (day 23). At this time point, no detectable replicating cells remained in control wells unexposed to EBV, indicating undetectable levels of endogenous EBV-induced transformation under our experimental conditions. Normal adult donor PBMCs, depleted of T cells by two rounds of immunomagnetic α-CD2 bead-negative selection (Dynal, Lake Success, NY) to eliminate EBV-specific T cells (<1% CD3+ by flow cytometry), were cultured in RPMI 1640 + 20% fetal calf serum (FCS) at 2 × 105 cells/200 μl in 96-well flat bottom plates. To increase the sensitivity for detection of enhancement, we used inefficiently transforming Type 2 AG876 EBV as well as the Type 1 B95.8 EBV usually employed for in vitro studies of EBV-induced transformation.
Replicate groups of 10 microwells were exposed to AG876 or B95.8 EBV in the presence or absence of 0.5 μg/ml gp120 from either R5 HIV-1 BaL or X4 HIV-1 IIIB, produced in the baculovirus SF9 cell system (Protein Sciences, Meriden, CT). In some wells 100 ng/ml SDF-1α and/or β (R&D) was added to EBV or EBV plus gp120-treated cells. Various controls received no EBV (10 wells), EBV in complete media only (20 replicates), or EBV plus SF9 cell extract subjected to the same purification steps as the envelope preparations (10 replicate wells). Positive controls received EBV plus α-CD40 mAb (0.1 μg/ml, BioSource, Camarillo, CA), a known costimulus for B cell transformation. Wells were microscopically inspected for blastoid colony clusters at 7, 14, and 21 days for the presence of expanded clones, and pulsed on day 23 with tritiated thymidine for 6 h to measure proliferation. Results of thymidine incorporation were analyzed by Kruskal–Wallis one-way ANOVA.
For donor “A,” both α-CD40 and X4 IIIB rgp120 added at the time of EBV exposure significantly enhanced proliferation 23 days later (p < 0.01, Fig. 2A). Five of five randomly chosen X4 gp120 wells were successfully expanded into long-term immortalized cell lines. Using another donor's cells, similar day 23 proliferative enhancement was detected whether IIIB gp120 was added with EBV, or up to 4 days later, whereas α-CD40 had only a modest effect (Fig. 2B). The R5 BaL envelope had no enhancing effect. Rather, in some experiments with a additional donors and Type 1 B 95.8 EBV, with high day 23 proliferation in the EBV exposed, untreated control cultures, BaL gp120 was actually inhibitory (Fig. 2C).
FIG. 2.
Enhancement of EBV-induced continuous B cell proliferation by X4 gp120 but not R5 gp120. (A) Proliferation of T cell-depleted normal (Donor A) PBMCs 23 days after infection with AG876 Type 2 EBV was significantly enhanced by simultaneous exposure to 0.5 μg/ml X4 IIIB rgp120 (p < 0.1) but not equal amounts of R5 BaL gp120 or the equivalent amount of SF9 cell eluate from the same insect cell baculovirus expression system. The known costimulus, anti-CD40 mAb, also enhanced week 4 proliferation when added at time of EBV exposure. Addition of SDF-1α, SDF-1β, or both at 100 ng/ml in combination with IIIB gp120 had no additional positive effect on cell growth and, in fact, blunted the effect (p < 0.5 vs. IIIB gp120 alone). (B) In normal T-depleted cells from a different donor (Donor B), AG876 EBV alone was inefficient at transforming cells for continuous day 23 proliferation, and greater proliferative enhancement was seen with IIIB gp120 than with anti-CD40, even when the envelope protein was added up to 96 h after EBV. (C) For Type I B95.8 EBV exposures in a third (representative) donor, day 23 proliferation to virus alone was roughly 10-fold greater than for Type 2 EBV-induced proliferation, but potentiation by IIIB gp120, as well as by α-CD40 mAb, was highly significant (p < 0.01). By contrast, added R5 BaL gp120 significantly inhibited the proliferation induced by EBV alone (p < 0.01).
Neither SDF-1α nor SDF-1β enhanced proliferation induced by EBV (not shown) or EBV + IIIB gp120 (Fig. 2A). In fact, there was a significant reduction of gp120 enhancement when these CXCL12 species were present (p < 0.05). Differences between SDF-1 and gp120 in specific residue binding may account for differences in signal transduction and proliferative outcome.23,24 CXCR4-specific 12G5 mAb also failed to enhance growth, but agonistic 12G5 mAb effects might not occur at the saturating concentrations used. Also, recent work12 indicates that the CXCL12 receptor, CXCR7, is upregulated and crucial during EBV transformation. Absent cross-reactivity, 12G5 would not mimic any gp120 effects mediated by CXCR7 binding. Our use of normal B cells, rather than transformed gliomas,21 established Type 1 lymphoma lines,25 or in vivo-generated lymphomas8 may explain why we also did not confirm previous reports of SDF-1 promoting growth. Indeed, it would have been surprising to find that a natural ligand for CXCR4 was tumorigenic for normal B cells in the presence of EBV.
B cell lymphomagenesis in the HIV+ setting is multifactorial. These experiments suggest that HIV CKR tropism may be one piece of the puzzle, and are consistent with the increased appearance of X4 virus and NHL in advanced disease. These are the first studies to implicate gp120-induced signaling as a factor in the earliest stages of normal cell transformation. Inefficient transformation by Type 2 EBV may help reveal X4 gp120-enhancing effects, whereas much more robust Type 1 transformation may provide the context necessary for detecting R5 inhibition (Fig. 2C). The relative contributions of gp120 signaling through CXCR4 vs. CXCR7 remain to be explored.
Acknowledgments
This work was funded in part by American Cancer Society Institutional Research Grant IRG11-36 to S.I. and NIH Grant N01-AI45207. We thank Dr. Richard Ambinder for the gift of patient-derived BLCLs. Both authors contributed equally to the design, performance, and interpretation of this work.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Luker KE. Luker GD. Functions of CXCL12 and CXCR4 in breast cancer. Cancer Lett. 2006;238(1):30–41. doi: 10.1016/j.canlet.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Gelmini S. Mangoni M. Serio M. Romagnani P. Lazzeri E. The critical role of SDF-1/CXCR4 axis in cancer and cancer stem cells metastasis. Endocrinol Invest. 2008;31(9):809–819. doi: 10.1007/BF03349262. [DOI] [PubMed] [Google Scholar]
- 3.Otsuka S. Bebb G. The CXCR4/SDF-1 chemokine receptor axis: A new target therapeutic for non-small cell lung cancer. J Thorac Oncol. 2008;3(12):1379–1383. doi: 10.1097/JTO.0b013e31818dda9d. [DOI] [PubMed] [Google Scholar]
- 4.Croker AK. Allan AL. Cancer stem cells: Implications for the progression and treatment of metastatic disease. Cell Mol Med. 2008;12(2):374–390. doi: 10.1111/j.1582-4934.2007.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Y. Larsen PH. Hao C. Yong VW. CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. J Biol Chem. 2002;277(51):49481–49487. doi: 10.1074/jbc.M206222200. [DOI] [PubMed] [Google Scholar]
- 6.Sehgal A. Keener C. Boynton AL. Warrick J. Murphy GP. CXCR-4, a chemokine receptor, is overexpressed in and required for proliferation of glioblastoma tumor cells. J Surg Oncol. 1998;69(2):99–104. doi: 10.1002/(sici)1096-9098(199810)69:2<99::aid-jso10>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Carroll RG. Riley JL. Levine BL. Feng Y. Kaushai S. Ritchey DS. Bernstein W. Weislow OS. Brown CR. Berger EA. June CH. St Louis DC. Differential regulation of HIV-1 fusion cofactor expression by CD28 costimulation of CD4+ T cells. Science. 1997;276(5310):273–276. doi: 10.1126/science.276.5310.273. [DOI] [PubMed] [Google Scholar]
- 8.Piovan E. Tosello V. Indraccolo S. Cabrelle A. Baesso I. Trentin L. Zamarchi R. Tamamura H. Fujii N. Semenzato G. Chieco-Bianchi L. Amadori A. Chemokine receptor expression in EBV-associated lymphoproliferation in hu/SCID mice: Implications for CXCL12/CXCR4 axis in lymphoma generation. Blood. 2005;105(3):931–939. doi: 10.1182/blood-2004-03-0799. [DOI] [PubMed] [Google Scholar]
- 9.Iyengar S. Schwartz DH. Hildreth JEK. T-cell tropic gp120 mediates CD4 and CD8 cell chemotaxis through CXCR4 independent of CD4: Implications for HIV pathogenesis. J Immunol. 1999;162(10):6263–6267. [PubMed] [Google Scholar]
- 10.Misse D. Cerutti M. Noraz N. Jourdan P. Favero J. Devauchell G. Yssel H. Taylor N. Veas F. A CD4-independent interaction of human immunodeficiency virus-1 gp120 with CXCR4 induces their cointernalization, cell signaling, and T-cell chemotaxis. Blood. 1999;93(8):2454–2462. [PubMed] [Google Scholar]
- 11.Pan H. Luo C. Li R. Qiao A. Zhang L. Mines M. Nyanda AM. Zhang J. Fan GH. Cyclophilin A is required for CXCR4-mediated nuclear export of heterogeneous nuclear ribonucleoprotein A2, activation and nuclear translocation of ERK1/2, and chemotactic cell migration. J Biol Chem. 2008;283(1):623–637. doi: 10.1074/jbc.M704934200. [DOI] [PubMed] [Google Scholar]
- 12.Lucchesi W. Brady G. Dittrich-Breiholz O. Kracht M. Russ R. Farrell PJ. Differential gene regulation by Epstein-Barr virus type 1 and type 2 EBNA2. J Virol. 2008;82(15):7456–7466. doi: 10.1128/JVI.00223-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbaro G. Barbarini G. HIV infection and cancer in the era of highly active antiretroviral therapy. Oncol Rep. 2007;17(5):1121–1126. [PubMed] [Google Scholar]
- 14.Rabkin CS. Yang Q. Goedert JJ. Nguyen G. Mitsuya H. Sei S. Chemokine and chemokine receptor gene variants and risk of non-Hodgkin's lymphoma in human immunodeficiency virus-1-infected individuals. Blood. 1999;93(6):1838–1842. [PubMed] [Google Scholar]
- 15.Sei S. O'Neill DP. Stewart SK. Yang Q. Kumagai M. Boler AM. Adde MA. Zwerski SL. Wood LV. Venzon DJ. Magrath IT. Increased level of stromal cell-derived factor-1 mRNA in peripheral blood mononuclear cells from children with AIDS related lymphoma. Cancer Res. 2001;61(13):5028–5037. [PubMed] [Google Scholar]
- 16.Vlahakis SR. Villasis-Keever A. Gomez T. Venegas M. Vlahakis N. Paya CV. G protein-coupled chemokine receptors induce both survival and apoptotic signaling pathways. J Immunol. 2002;169(10):5546–5554. doi: 10.4049/jimmunol.169.10.5546. [DOI] [PubMed] [Google Scholar]
- 17.Vlahakis SR. Algeciras-Schimmnich A. Bou G. Heppelmann CJ. Villasis-Keever A. Collman RG. Paya CV. Chemokine-receptor activation by env determines the mechanism of death in HIV-infected and uninfected T lymphocytes. J Clin Invest. 2001;107(2):207–215. doi: 10.1172/JCI11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berndt C. Barbara M. Angermuller S. Gierschik P. Krammer L. CXCR4 and CD4 mediate a rapid CD95-independent cell death in CD4+ T cells. Proc Natl Acad Sci USA. 1998;95:12556–12561. doi: 10.1073/pnas.95.21.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biard-Piechazyk M. Robert-Hebmann V. Richard V. Roland J. Hipskind RA. Devauz C. Caspase-dependent apoptosis of cells expressing the chemokine receptor CXCR4 is induced by cell membrane-associated human immunodeficiency virus type 1 envelope glycoprotein (gp120) Virology. 2000;268(2):329–344. doi: 10.1006/viro.1999.0151. [DOI] [PubMed] [Google Scholar]
- 20.Espert L. Vebanov M. Robert-Hebmann V. Sagnier S. Robbins I. Sandhez F. Lafont V. Biard-Pieschaczyk M. Differential role of autophagy in CD4 T cells and macrophages during X4 and R5 HIV-1 infection. PLoS ONE. 2009;4(6):e5787. doi: 10.1371/journal.pone.0005787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raport CJ. Gosling J. Schweickart VL. Gray PW. Charo IF. Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1beta, and MIP-1alpha. J Biol Chem. 1996;271(29):17161–17166. doi: 10.1074/jbc.271.29.17161. [DOI] [PubMed] [Google Scholar]
- 22.Samson CJ. Labbe O. Mollereau C. Vassart G. Parmentier M. Molecular cloning and functional expression of a new human CC-chemokine receptor gene. Biochemistry. 1996;35(11):3362–3367. doi: 10.1021/bi952950g. [DOI] [PubMed] [Google Scholar]
- 23.Atchison RE. Gosling J. Monteclaro FS. Franci C. Digilio L. Charo IF. Goldsmith MA. Multiple extracellular elements of CCR5 and HIV-1 entry: Dissociation from response to chemokines. Science. 1996;274(5294):1924–1926. doi: 10.1126/science.274.5294.1924. [DOI] [PubMed] [Google Scholar]
- 24.Lee B. Sharron M. Blanpain C. Doranz BJ. Vakili J. Setoh P. Berg E. Liu G. Guy HR. Durell SR. Parmentier M. Chang CN. Price K. Tsang M. Doms RW. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J Biol Chem. 1999;274(14):9617–2626. doi: 10.1074/jbc.274.14.9617. [DOI] [PubMed] [Google Scholar]
- 25.Bertolini F. Dell'Agnola C. Mancuso P. Rabascio C. Berlini A. Monestrioli S. Gobbi A. Pruneri G. Martinelli G. CXCR4 neutralization, a novel therapeutic approach for non-Hodgkin's lymphoma. Cancer Res. 2002;62(11):3106–3112. [PubMed] [Google Scholar]