Abstract
The spatial structure of genetic diversity underlying social variation is a critical determinant of how cooperation and conflict evolve. Here we investigated whether natural social groups of the cooperative soil bacterium Myxococcus xanthus harbor internal genetic and phenotypic variation and thus the potential for social conflict between interacting cells. Ten M. xanthus fruiting bodies isolated from soil were surveyed for variation in multiple social phenotypes and genetic loci, and patterns of diversity within and across fruiting body groups were examined. Eight of the 10 fruiting bodies were found to be internally diverse, with four exhibiting significant variation in social swarming phenotypes and five harboring large variation in the number of spores produced by member clones in pure culture. However, genetic variation within fruiting bodies was much lower than across fruiting bodies, suggesting that migration across even spatially proximate groups is limited relative to mutational generation of persisting endemic diversity. Our results simultaneously highlight the potential for social conflict within Myxococcus social groups and the possibility of social coevolution among diverse related lineages that are clustered in space and cotransmitted across generations.
Keywords: bacterial population structure, micro-scale variation
Social evolution research seeks to explain the origin, maintenance, and diversification of both cooperative and competitive social traits. This goal requires understanding the character of social environments that mediate selection on these traits. The distribution of behavioral and genetic diversity within and across groups of social animals has received much attention (1–3). In contrast, relatively little is known about the structure of diversity among natural groups of social microbes (4–10). However, detailed knowledge of group composition is necessary for understanding the roles of mutation, migration, lateral gene transfer, genetic drift, and various forms of selection in shaping the evolution of social microbes in natural habitats.
Microbes engage in a wide range of social behaviors, both cooperative and antagonistic, that affect the evolutionary fitness of others (11–13). Some of the most biologically complex forms of prokaryotic cooperation occur in the myxobacteria (order Myxococcales, δ-proteobacteria), which are best known for social development of multicellular, spore-bearing fruiting bodies in response to starvation (14). In particular, the predatory soil bacterium Myxococcus xanthus has become a model organism for the study of microbial sociality, including cooperative motility (15), social predation (16) and fruiting body formation (14), and its population biology (17).
M. xanthus cells swarm in a coordinated manner through soil habitats in cohesive groups using two genetically distinct motility systems, one of which is obligately social [type IV pili-driven “S-motility” (15, 18)] and one of which allows individual cell movement (“A-motility”) (19, 20). Swarms of M. xanthus in the soil kill and lyse prey cells of other microbial species with secreted antibiotics and lytic enzymes (21). Upon starvation, swarming cells aggregate and develop into multicellular fruiting bodies (14). In these fruiting body aggregates a minority of cells convert to metabolically quiescent spores, whereas many other cells within the fruiting body lyse, possibly to the benefit of sporulating cells (22). The precise advantages of sporulation within fruiting bodies are unknown, although several hypotheses have been proposed, including enhanced dispersal, increased germination and/or growth rates in high-density groups, and protection from predation and/or environmental insults (summarized in greater detail in ref. 17). Here “fruiting body group” and “group” generally refer to either all cells that compose a particular fruiting body or a set of laboratory strains isolated from the same fruiting body.
Although bacterial growth by binary fission in structured habitats inherently generates clonal cell pockets (23), cell groups forming Myxococcus fruiting bodies are not expected to be entirely clonal owing to mutation. The M. xanthus genome is large [>9 Mb (24)] and fruiting bodies are thought to be constructed by ≈100,000 cells (14). If the mean M. xanthus mutation rate is roughly similar to that of Escherichia coli [≈5.4 × 10−10 per base pair per generation in one estimate (25)], any given fruiting body should contain at least dozens of mutational variants, even if the entire fruiting body group originated from a single cell. Although most mutations are deleterious (26) and are lost by selection or genetic drift, a small minority will persist and rise to high frequency either because they confer a selective advantage or nonadaptively by hitchhiking (27) or genetic drift (28). Persisting mutants might be socially defective cheaters that increase owing to a frequency-dependent advantage within groups (29–32). Alternatively, such mutants may be socially proficient strains that outcompete dominant genotypes owing to increased intrinsic fitness (33) or the ability to socially exploit majority genotypes (34–36). Assessing the degree to which such persisting mutants migrate across social groups—within which cells interact during motility, predation, and development—is critical for understanding social evolution in the myxobacteria.
If intergroup migration is low, within-group diversity should derive primarily from endemic mutation and be lower than diversity across groups. In this scenario, relatedness values for social loci among interacting variants may often be high, thus promoting the maintenance of cooperation by kin selection (37–39). Under low migration, cotransmission of within-group social diversity across generations will be high (40, 41), and lineages that repeatedly and preferentially interact may coevolve to reduce within-group conflict. Even if spatially proximate, distinct lineage groups among which migration is low may diversify via differential trajectories of adaptation and drift.
Previous work has indicated that some M. xanthus genotypes disperse far, despite the overall differentiation of meter-scale populations isolated by distance across large spatial scales [e.g., >2,000 km (8)]. Across smaller scales (<300 km), local meter-scale populations were not differentiated at the genetic loci examined, and dispersal appears to be extensive (8). In another study, the spatial distribution of diverse multi-locus genotypes among 78 cm-scale isolates did not appear to be significantly clustered, consistent with the possibility of extensive intergroup migration at this scale (7). However, near the cellular (μm) scale, genetic variation in natural M. xanthus populations must be nonrandomly distributed due to the nature of bacterial colony growth by asexual binary fission in viscous environments (23). Further work is required to better resolve patterns of genetic structure and degrees of dispersal and intergroup migration across a wide range of spatial scales.
A high level of phenotypic and genetic diversity has been documented among cm-scale M. xanthus isolates (7, 36, 42–45), despite the fact that genetic diversity at this scale was found to be much lower than at only slightly larger sampling scales (8). For example, genetically similar centimeter-scale isolates were found to show extremely divergent competitive abilities during fruiting body development in forcibly mixed pairings (36). However, the degree to which such diverse clones migrate across fruiting body-forming groups—either passively or by active motility—has remained unclear. In Myxococcus, cell–cell adhesion (46) and territorial kin discrimination (36) may limit intergroup migration.
Using a new collection of natural isolates, here we have tested whether diverse social phenotypes coexist within the most discrete social unit of the Myxococcus life cycle, the fruiting body group. We then tested for group-level structure in genetic and phenotypic diversity to discriminate between scenarios of low vs. high migration among social groups residing in forest soils. Ten natural fruiting body groups were harvested from soil collected at three Indiana woodland locations separated by several kilometers. Fruiting bodies from a given km-scale location originated from cm-scale (MC fruiting bodies; see SI Methods) or m-scale (GH and KF fruiting bodies, see SI Methods) sites along sample transects. Forty-eight clones were independently isolated from each fruiting body and screened for diversity at several genetic loci and in several social phenotypes during group swarming and fruiting body development. Patterns of diversity within and across fruiting body groups were then analyzed. Detailed descriptions of the methods used can be found in SI Methods.
Results
Variation in Swarming Phenotypes.
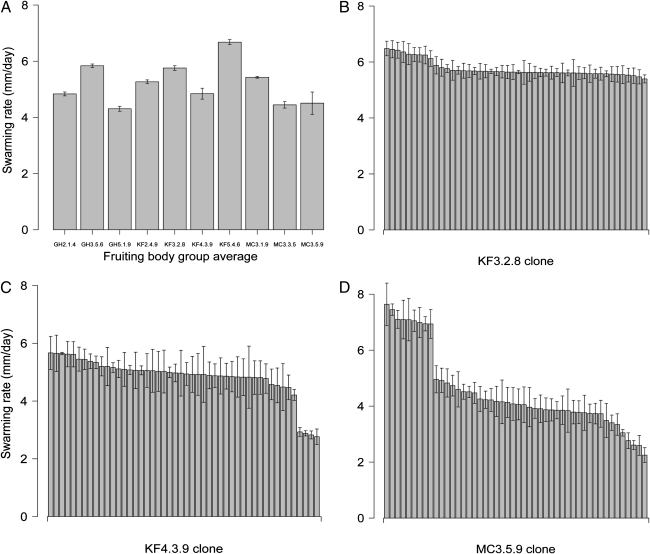
Myxococcus group swarming on soft agar is a social trait that is driven by the type IV pili-based S-motility system in standard laboratory strains (47). Natural fruiting body groups, each represented by 48 clones, varied significantly in their mean swarming rate on soft agar (Fig. 1A and Table S1; Kruskal-Wallis test: P < 0.001). This result is consistent with previous work that documented extensive variation in soft-agar swarming among other natural isolates (45).
Fig. 1.
Swarming rates on soft agar. (A) Average swarming rates of all clones from each fruiting body group (n = 46–48 per fruiting body). (B–D) Average swarming rates for individual clones from fruiting bodies KF3.2.8, KF4.3.9, and MC3.5.9, respectively. Error bars represent 95% confidence intervals. Swarming rates for each clone can be found in Table S1.
Seven fruiting bodies did not exhibit significant within-group variation in swarming rate according to Kruskal-Wallis tests (P > 0.05), but two of these groups included stark variation in another visual phenotype (colony color in fruiting body GH5.1.9 and degree of cell–cell adhesion in MC3.3.5). (Phenotypic variation for traits other than swarming rate during soft-agar motility assays is summarized in Table S2.) Moreover, although swarming rate variation within GH5.1.9 was not quite significant according to the Kruskal-Wallis test (P = 0.08), comparison of the mean swarming rates of the two color types revealed an extremely significant difference (yellow vs. orange, Wilcoxon rank-sum test, P < 0.001).
Three fruiting bodies (KF3.2.8, KF4.3.9, and MC3.5.9) harbored clones exhibiting significant within-group variation in soft-agar swarming rate according to Kruskal Wallis tests (Figs. 1 B–D and 2; P < 0.001 in all cases). Among KF3.2.8 clones, k-means clustering into two groups reveals one majority rate phenotype [mean cluster swarming rate 5.63 mm/d; 95% confidence interval (CI) = 0.23] and a faster minority type (mean cluster swarming rate = 6.32 mm/d; 95% CI = 0.3) (Fig. 1B). Post hoc testing revealed a significant difference between the swarming rates of those phenotype clusters (Wilcoxon rank sum test on cluster means, P < 0.001), suggesting that they represent two distinct motility genotypes.
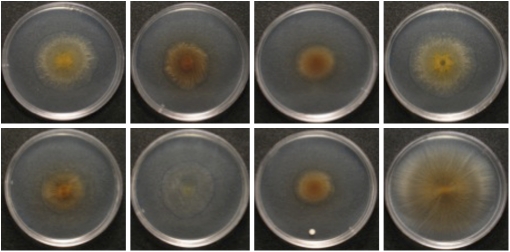
Fig. 2.
Swarming phenotypes of fruiting body MC3.5.9 isolates after 5 d of growth on soft agar plates. Upper: Left to right, c16, c19, c22, and c25; Lower: Left to right: c31, c35, c36, and c48.
Cluster analysis of the other two fruiting bodies with significant variation among clones (KF4.3.9 and MC3.5.9; Fig. 1 C and D) suggested the existence of three swarming rate phenotype classes in each group. The KF4.3.9 fruiting body is characterized by a fast majority type (mean cluster swarming rate 5.54 mm/d, 95% CI = 0.37) and two slower minority variants (Fig. 1C) (mean cluster swarming rates of 4.89 and 2.85 mm/d, respectively; 95% CI = 0.54 and 0.16, respectively). In contrast, the MC3.5.9 clones grouped into a majority phenotype with an intermediate swarming rate (mean cluster swarming rate 4.15, 95% CI = 0.47) as well as faster and slower minority types (Fig. 1D) (mean cluster swarming rates of 7.14 and 2.94 mm/d, respectively; 95% CI = 0.49 and 0.31, respectively). Post hoc testing revealed that the mean swarming rates among the clusters within both KF4.3.9 and MC3.5.9 differ significantly (Wilcoxon rank-sum tests of all possible within-fruiting-body combinations of cluster means: P < 0.001 in all cases). In two cases, within-group variation in swarming rate was accompanied by variation in one or more additional phenotypes (colony color in KF4.3.9 and colony swarm pattern, color, opacity and degree of cell–cell adhesion in MC3.5.9; Fig. 2, Table 1, and Table S2).
Table 1.
Allelic and phenotypic variation within and across fruiting bodies
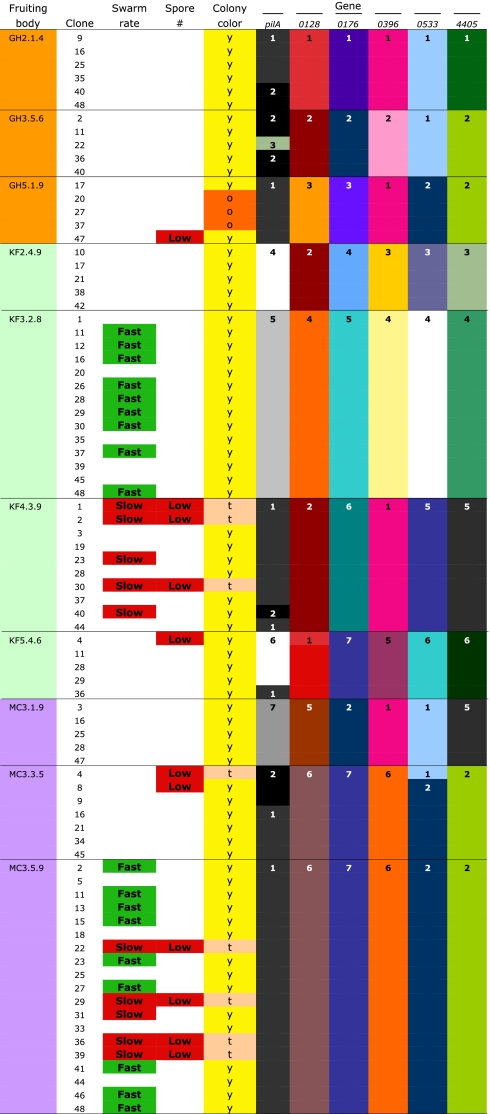 |
Qualitative phenotype categories and allelic states at sequenced loci are shown for several clonal isolates from each fruiting body, including all clones that exhibited clear minority phenotypes for swarming and/or development. Distinct alleles at each locus are distinguished by unique number–color combinations (with numbers shown for only one representative of each allele). “Fast” and “Slow” designate individuals with minority swarming phenotypes in a given fruiting body, and “Low” designates individuals within low spore production clusters as identified by k-means cluster analysis (Methods). Colony color phenotypes are also indicated (y, yellow; o, orange; t, tan).
Variation in Spore Production.
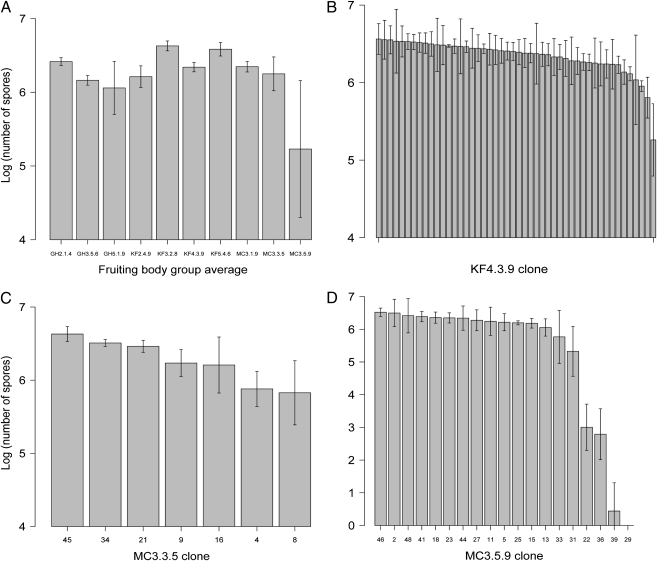
Fruiting body groups, each represented by a subset of the 48 clones per fruiting body examined for motility, varied significantly in their mean levels of spore production (Fig. 3A and Table S3; Kruskal Wallis-test: P < 0.001). This result is consistent with previous work that documented extensive variation in several developmental phenotypes among natural M. xanthus isolates (34, 36, 42, 48).
Fig. 3.
Spore production. (A) Average spore production of examined clones from each fruiting body group (n = 5–48 per fruiting body). (B–D) Average spore production by individual clones from fruiting bodies KF4.3.9, MC3.3.5, and MC3.5.9, respectively. Error bars represent 95% confidence intervals about means calculated from log10-transformed spore counts. Spore counts for each clone can be found in Table S3.
Five of the 10 fruiting body groups examined were found to harbor significant within-group variation in spore production (Fig. 3 B–D and Table S3). In all five cases clones that sporulated poorly were in the minority. Spore production by the lowest sporulator within each of these five groups ranged from ≈10-fold below the level of the dominant phenotype cluster (MC3.3.5; Fig. 3C) to complete inability to produce viable spores (MC3.5.9; Fig. 3D). The isolates from fruiting bodies that harbored variation in spore production clustered into two distinct groups within each respective fruiting body by k-means cluster algorithms based on the criteria described in Methods. In two cases (GH5.1.9 and KF5.4.6), post hoc tests to compare cluster means were not possible because one cluster contained only a single clone. In the three remaining groups, the differences in spore production between the two clusters were either highly significant (KF4.3.9 and MC3.5.9; Wilcoxon rank-sum test, P < 0.001 in both cases) or marginally nonsignificant (MC3.3.5; Wilcoxon rank-sum test, P = 0.09).
Sporulation efficiency correlated significantly with swarming rate across all clones from all sampling locations (Spearman's rank correlation ρ: S = 3758, ρ = 0.56, P < 0.001).
Genetic Structure of Fruiting Body Groups.
All clones assayed for spore production were screened for genotypic variation within ≈500 base pair windows of six loci that contain above-average levels of variation among the laboratory strain DK1622 (49) and two M. xanthus isolates from Tübingen, Germany [A23 and A47 (7)]. Genetic variation within fruiting body groups was found to be extremely low relative to variation across fruiting bodies. Seven pilA alleles were found across all clones (Table 1), but only five of the 10 fruiting bodies harbored pilA polymorphisms, and no more than two pilA alleles were present in any fruiting body group. Each of the five other loci was highly polymorphic across fruiting bodies, with either six or seven alleles detected at each locus (Table 1). However, only one of these loci was found to vary among clones from the same fruiting body, in which instance a minority allele of Mxan_0533 was present in one clone of fruiting body MC3.3.5.
The vast majority of within-group phenotypic variation for swarming rate and spore production occurred between clones that are genetically identical at all (most cases) or most (a minority of cases) of the six loci examined (e.g., the swarming variants within fruiting body KF3.2.8 share the same alleles) (Table 1).
Phylogenetic Relationships.
An unrooted maximum-likelihood tree and a Baysian inference tree were constructed with a sequence concatemer of the five loci other than pilA (Fig. S1). Both phylograms had similar topologies. Only one of the KF haplotypes (4.3.9) was found to group within the highly supported clade containing all of the GH and MC location haplotypes, with the KF5.4.6, KF2.4.9, and KF3.2.8 haplotypes branching more deeply. As reflected by this deep branching pattern, the KF3.2.8, KF2.4.9, and KF5.4.6 haplotypes were found to have only 0, 1, and 2 loci, respectively, that share an allele with one or more other fruiting body haplotypes (Table 1). KF3.2.8 is most similar to the laboratory strain DK1622 (24, 49).
Control for a Laboratory Origin of Minority Phenotypes.
We tested whether the phenotypic variation demonstrated among isolated clones might have arisen during growth in the laboratory rather than in natural populations before soil collection. We examined four clones isolated from a fruiting body group (MC3.5.9) that exhibited a high degree of variation in both swarming rate and spore production. Specifically, we tested whether cultures from these four clones subjected to development and growth regimes similar to those experienced by the original fruiting body culture would generate an array of diverse phenotypes similar to that found among the 48 original MC3.5.9 clones. After cultures derived from these four clones had undergone development, heat, and sonication treatment and subsequent growth in CTT liquid, 48 clones were randomly selected from each culture after dilution plating and were screened for variation in swarming rate and fruiting body morphology. No significant variation was observed in either of these traits within any of the four clone sets, suggesting that the phenotypic variation documented here arose before fruiting body isolation.
Discussion
The genetic and social diversity pervading natural Myxococcus populations is highly structured across local social groups within which cooperative—and likely antagonistic—interactions occur. This study and others have together shown that representatives of distinct but spatially proximate fruiting body groups vary starkly in social motility (Fig. 1A) (45) and several developmental phenotypes, including spore production (Fig. 3A), the rate of development, responsiveness to nutrient depletion in triggering development (42), and competitiveness in forced isolate pairings (36). Here we have now also shown that pronounced social variation is present at high frequencies within many natural fruiting bodies—indeed within a majority of those fruiting bodies sampled here. Thus, diversity within fruiting bodies is high despite the fact that it was found to be much lower than diversity among fruiting bodies (Table 1 and Fig. S1). These results indicate that single clone isolates are likely to misrepresent the social phenotypes of other members of the groups from which they are isolated.
Possible Laboratory Effects.
Control experiments strongly suggest that phenotypic variants isolated from the same fruiting body were already present at substantial frequencies in natural groups before sampling and did not arise in laboratory cultures. However, our data do not necessarily reflect accurately the frequencies of these variants in the soil at the time of sampling owing to possible variation in growth rate under laboratory conditions. Natural isolates of bacteria might vary in their degree of “preadaptation” to laboratory conditions (50), and some degree of phenotypic variation may be specific to laboratory settings. Nonetheless, it is plausible that the large trait differences documented here reflect heritable variation that is also manifested during motility and/or development in natural habitats.
Phase Variation.
The patterns of variation among fruiting body group-mates documented here are not explicable by a previously documented form of “phase variation” in M. xanthus. In phase variation, bacterial cells switch between discrete phenotypic states at much higher rates than would be generated by the genome-wide average mutation rate (51–53). In M. xanthus laboratory strain DK1622, color phase variation occurs in which ≈1% of cells derived from a yellow colony grow into tan colonies, whereas ≈25% of cells from a tan colony grow into yellow colonies (52). Although it has been suggested that tan and yellow cells may have different functional roles during development (53), the genetic basis and population-level effects of M. xanthus phase variation remain poorly understood.
Most minority variants in swarming rate and sporulation among our fruiting body isolates did not exhibit minority color phenotypes (Table 1). The one exception is that all seven clones identified as having both unusually slow swarming and unusually low sporulation within their two respective fruiting body groups (KF4.3.9 and MC3.5.9) grew as tan colonies rather than as the majority yellow phenotype (Table 1). However, group-mates among these seven clones varied significantly in both swarming rate (Fig. 1 C and D and Table S1) and spore production (Fig. 3 B and D and Table S3), indicating that even these variants do not represent simple dual-state phase variation.
We screened 48 colonies derived from each of four clones isolated from the most internally diverse fruiting body, MC3.5.9. Three of the four parental clones (c6, c16, and c24) were yellow, and one was tan (c29). None of the 48 colonies derived from the tan clone were yellow, as would be expected for ≈25% of colonies under DK1622-like phase variation. Among these four clones only one instance of apparent phase variation was observed. In that case, the biphasic diversity observed differed dramatically both from the patterns of diversity documented among our original fruiting body isolates and from DK1622 phase variation. Colonies derived from isolate MC3.5.9c6 showed two clearly distinguishable phenotypes of colony opacity during social swarming. Importantly, cultures derived from colonies of both opacity types form robust fruiting bodies and do not vary significantly in swarming rate. This limitation of variation among MC3.5.9c6 cells to colony opacity contrasts starkly with the variation observed among the original isolates from fruiting body MC3.5.9, which did not include similar variation in colony opacity but did include three distinct swarming-rate clusters and clones with severe developmental deficiencies. Colonies derived from the other three parental clones selected for the control experiments showed no variation for any visual phenotype. Thus, exhibition of phase variation is itself yet another phenotype that seems to vary among closely related group mates within natural fruiting bodies.
Endemic Variation.
Our results show that much of the detectable diversity within natural Myxococcus social groups derives from endemic mutation rather than intergroup migration. Here we consider migration to be the combination of dispersal to a new location and physical immigration into a new social group at that location. Five fruiting bodies, including the most phenotypically diverse one (MC3.5.9), contained only a single haplotype for all six loci sequenced here. Another four were polymorphic only at the pilA locus, with just two alleles present in each case. Only one fruiting body was polymorphic at more than one locus (MC3.3.5, polymorphic at pilA and Mxan_0533). In contrast, in almost all pairwise comparisons of fruiting body groups, the dominant six-locus haplotypes from the paired groups differed at most loci. The sole exceptional comparison is between MC3.3.5 and MC3.5.9, which share identical majority haplotypes. However, genetic variation seems to be structured even across these two fruiting bodies that were isolated at the centimeter scale because variants with similar phenotype profiles are represented by multiple clones within each fruiting body group but are absent from the other (Table 1).
In contrast to patterns revealing endemic variation, the occurrence of some alleles that are shared by clones from multiple fruiting bodies isolated from different locations is consistent with some degree of recombination across groups and populations, possibly mediated by phage transduction (54). Such patterns are not unexpected in light of previous evidence for horizontal gene transfer in Myxococcus populations (7, 8, 55).
Migration.
Our data suggest that the rate at which Myxococcus social variants arise by mutation and subsequently increase to detectable frequencies within their natal groups is high relative to the rate at which variants migrate into “foreign” groups and subsequently persist. The among-group migration rate will greatly affect the relative importance of within- vs. among-group selection (56) in determining the fate of new mutations in social genes. Low migration will promote spatiotemporal clustering of genetically similar lineages [i.e., high relatedness within groups (5, 37, 57)], high cotransmission of social diversity across generations (41), and the among-group component of selection in multilevel selection models of social evolution (56).
Biological traits that affect migration rate are thus likely to influence how cooperation is maintained in a metapopulation and the evolutionary forces causing socially proficient genotypes to diversify. In Myxococcus, most cells are highly cohesive owing to the production of cell-surface adhesins, which should hinder emigration away from natal kin groups. Moreover, kin discrimination mechanisms that hinder immigration (58) by members of neighboring groups appear to be pervasive in natural M. xanthus populations. This inference derives from experiments in which neighboring swarms of genetically very similar cm-scale isolates failed to merge on agar plates for most pairings (36) (Fig. 4). Cooperation benefits buttressed by low migration may thus contribute to selection for cell–cell adhesion and territorial kin discrimination.
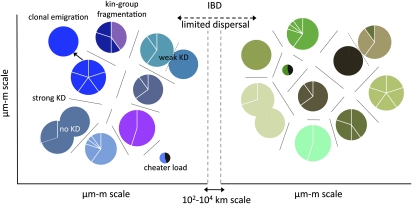
Fig. 4.
Simple hypothetical model of natural Myxococcus population biology. Circles represent social groups within which individuals directly interact. Sectors represent genetically distinct within-group variants. Distinctly colored circles represent among-group genetic differentiation, with lines separating kin discrimination (KD) units (36). Overlapping circles represent lack of KD between highly similar, but nonetheless genetically distinct, social groups. Multicolored circles represent kin-group fragmentation (see text). Small circles with a black sector represent cheater-infected groups burdened by cheater load (17, 29, 32). Distinct color ranges across left vs. right panels represent population differentiation across large spatial scales due to isolation by distance (IBD) (8). The arrow at Left represents establishment of a new clonal group by clonal emigration.
Limitation of Socially Defective Cheaters.
Cheater strains with social defects in clonal groups that can exploit cooperative genotypes in mixed groups during Myxococcus development readily appear by mutation in laboratory populations (32). When rare, these cheaters have a within-group advantage over cooperators but lose that advantage and impose cheating load (i.e., reduce group productivity) (12) when they reach high frequencies (29, 32). Many isolates described here exhibit low spore production (e.g., MC3.5.9c29; Fig. 3D) and/or slow swarming (e.g., KF4.3.9c1; Fig. 1C and Table S3). These clones may represent cheaters of natural origin that defect from “fair” production levels for a social compound required for development or social motility but exploit others who produce more of that compound. Alternatively, socially deficient strains may be present owing to genetic drift or selection on some trait other than the socially defective one.
Only mutations creating socially defective cheaters that have an advantage within a group across an organism's entire life cycle will increase substantially within groups. The mutation rate to cheaters that have such a net within-group advantage (at least when rare) remains unknown. Pleiotropy may limit this mutation rate (58, 59). Cheater mutations that are net beneficial within groups may nonetheless be net deleterious across groups in a larger metapopulation owing to among-group selection mediated by cheater load (17, 29, 32, 57) and promoted by limited migration (Fig. 4).
The mutation rate to socially defective cheating alleles that are net beneficial within groups might be lower than the rate at which such alleles are lost from a metapopulation owing to their net deleterious effect at the among-group level. In this scenario, the overall frequency of socially defective cheaters in a metapopulation should be determined by the relative magnitude of that mutation rate and the strength of among-group selection against the spectrum of cheaters that arise by mutation (60, 61). Alternatively, if the combination of mutation rate to socially defective cheaters and intergroup cheater migration is sufficiently high relative to the rate of cheater loss via among-group selection, all social groups in a metapopulation could become infected by cheaters. Four of the 10 Myxococcus fruiting body groups examined here did not harbor variation in spore production or social swarming at frequencies above our detection limits, suggesting that these groups did not harbor cheaters at the time they were sampled. This result is consistent with (but not demonstrative of) the possibility that socially defective cheater frequencies in natural Myxococcus populations are largely determined by “kin selection–mutation balance” (61), with kin selection in this scenario being mediated by selection among spatially structured kin groups.
Coevolution.
High cotransmission of within-group diversity across generations aligns the evolutionary interests of clustered lineages (40, 41). Under low migration, diverse lineages that repeatedly and preferentially interact may coevolve to reduce conflict (62–64) and chimeric load (i.e., reduced group productivity caused by within-group diversity). Cheating load is one form of chimeric load. If coevolution within cheater-infected groups proceeds rapidly relative to the rate of cheater loss by among-group selection, immunity to socially defective cheaters and policing behaviors that suppress them may evolve, as has occurred in experimental populations (65–67). Indeed, cheaters themselves may reevolve proficiency at cooperation by novel genetic routes (66) and thereby perhaps reach new cooperation fitness peaks (68) not accessible to noncheaters.
Clusters of socially cotransmitted lineages may also coevolve to reduce chimeric load generated by behavioral incongruities among interacting strains that are each socially proficient in clonal groups (34, 36, 69). Cotransmitted lineages might even coevolve to perform distinct mutually beneficial functions that raise cooperative group productivity beyond that achievable by clonal groups. Unique trajectories of coevolution among clusters of cotransmitted lineages may promote diversification across kin groups.
Regeneration of Clonality.
New clonal groups can be established by clonal emigration from an internally diverse group (Fig. 4) (58) or by local selective sweeps of adaptive mutants that purge variation from an existing kin group (70). Alternatively, new kin discrimination alleles might arise by mutations that generate biological barriers to migration across clonal cell patches (23) and thereby fragment a preexisting group into multiple kin discrimination units (Fig. 4). The rate at which clonal groups of cooperative genotypes are freshly established (or reestablished) is unknown but is an important parameter for understanding cheater–cooperator population dynamics.
What Maintains Diversity Within and Between Groups?
Natural variation in M. xanthus social traits documented here and previously (42–45) may be nonadaptive and may have reached detectable frequencies by genetic drift or hitchhiking (27) or might reflect pleiotropic byproducts of evolutionary adaptation at some alternative trait. For example, even variation that causes large fitness differences during laboratory developmental competition experiments (34–36, 71) need not have been shaped by selection for within-group competitiveness during development. Indeed, the strongest developmental cheaters yet identified in M. xanthus originated in a selective regime in which evolving populations never underwent development (32, 72). Alternatively, selective sweeps may be driving some observed variants to fixation. Finally, Myxococcus social diversity may be maintained by various forms of balancing selection.
Within kin groups, frequency-dependent selection might maintain both cooperators and socially defective cheaters (32) or multiple genotypes that mutually benefit one another owing to differential expression of cooperative traits (66). Within-group diversity might also be promoted by specialized performance among genotypes across variable environmental conditions [e.g., surface conditions (45, 47, 73), prey composition (44), etc.]. Across kin groups, balancing selection might take the form of kin-group specialization to different microhabitats or nontransitive fitness relationships (74) during competitive interactions, such as the production of anticompetitor compounds (75) by adjacent kin groups.
The extensive diversity within natural Myxococcus social groups documented here suggests that within-group conflict is likely to play a major role in myxobacterial social evolution. Migration among kin groups seems to be low relative to the rate at which persisting variants arise by mutation and coevolution among socially cotransmitted Myxococcus lineages is likely to occur. The relative roles that the fundamental forces of evolution—mutation, distinct forms of selection, migration, genetic drift, and recombination—play in shaping natural social variation in the myxobacteria remain to be quantified. Doing so will require estimation of mutation rates, identification of loci and alleles responsible for observed social variation, screening for population genetic signatures of distinct evolutionary forces, and characterization of fitness relationships among social interactants under conditions relevant to natural habitats.
Methods
Greater detail is provided in SI Methods. Fruiting bodies were isolated from soil samples collected at forested locations near Bloomington, Indiana in a hierarchical sample design (five 2-cm scale sample sites at each of five 10-m scale sites at each of three 10-km scale sites). M. xanthus cultures initiated with whole fruiting bodies were stored frozen, as were cultures derived from 48 individual clones isolated from each fruiting body. Phenotype and genetic analyses focused on clones isolated from 10 fruiting bodies representing all three 10-km scale sites. Swarming rate on soft agar medium was measured for all isolated clones, and spore productivity was measured for five or more clones from each fruiting body. Sequences from six genetic loci known to be highly polymorphic from previous M. xanthus genome comparisons were used to classify strains by their multilocus genotype (Table 1) and construct maximum-likelihood and Baysian inference phylograms of all distinct genotypes (Fig. S1).
Acknowledgments
We thank H. Peitz for sharing genomic data; T. Platt for helpful suggestions regarding the control experiments; and E. Hanschen and M. Toups for helpful suggestions regarding statistical and phylogenetic analyses. This study was supported by National Institutes of Health Grant GM079690 (to G.J.V.).
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution V: Cooperation and Conflict,” held January 7–8, 2011, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/SACKLER_cooperation.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper are deposited in the GenBank database (accession nos. JF819182–JF819591 and JF741968–JF742049).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100307108/-/DCSupplemental.
References
- 1.Krebs J, Davies N. Ecology: An Evolutionary Approach. Oxford: Blackwell Science; 1997. [Google Scholar]
- 2.Oxley PR, Spivak M, Oldroyd BP. Six quantitative trait loci influence task thresholds for hygienic behaviour in honeybees (Apis mellifera) Mol Ecol. 2010;19:1452–1461. doi: 10.1111/j.1365-294X.2010.04569.x. [DOI] [PubMed] [Google Scholar]
- 3.Waddington SJ, et al. Genetic polyethism in leaf-cutting ants. Behav Ecol. 2010;21:1165–1169. [Google Scholar]
- 4.Fortunato A, Strassmann JE, Santorelli L, Queller DC. Co-occurrence in nature of different clones of the social amoeba, Dictyostelium discoideum. Mol Ecol. 2003;12:1031–1038. doi: 10.1046/j.1365-294x.2003.01792.x. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert OM, Queller DC, Strassmann JE. Discovery of a large clonal patch of a social amoeba: Implications for social evolution. Mol Ecol. 2009;18:1273–1281. doi: 10.1111/j.1365-294X.2009.04108.x. [DOI] [PubMed] [Google Scholar]
- 6.Köhler T, Buckling A, van Delden C. Cooperation and virulence of clinical Pseudomonas aeruginosa populations. Proc Natl Acad Sci USA. 2009;106:6339–6344. doi: 10.1073/pnas.0811741106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vos M, Velicer GJ. Genetic population structure of the soil bacterium Myxococcus xanthus at the centimeter scale. Appl Environ Microbiol. 2006;72:3615–3625. doi: 10.1128/AEM.72.5.3615-3625.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vos M, Velicer GJ. Isolation by distance in the spore-forming soil bacterium Myxococcus xanthus. Curr Biol. 2008;18:386–391. doi: 10.1016/j.cub.2008.02.050. [DOI] [PubMed] [Google Scholar]
- 9.Wilder CN, Allada G, Schuster M. Instantaneous within-patient diversity of Pseudomonas aeruginosa quorum-sensing populations from cystic fibrosis lung infections. Infect Immun. 2009;77:5631–5639. doi: 10.1128/IAI.00755-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wollenberg MS, Ruby EG. Population structure of Vibrio fischeri within the light organs of Euprymna scolopes squid from Two Oahu (Hawaii) populations. Appl Environ Microbiol. 2009;75:193–202. doi: 10.1128/AEM.01792-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadell CD, Xavier JB, Foster KR. The sociobiology of biofilms. FEMS Microbiol Rev. 2009;33:206–224. doi: 10.1111/j.1574-6976.2008.00150.x. [DOI] [PubMed] [Google Scholar]
- 12.Velicer GJ. Social strife in the microbial world. Trends Microbiol. 2003;11:330–337. doi: 10.1016/s0966-842x(03)00152-5. [DOI] [PubMed] [Google Scholar]
- 13.West SA, et al. The social lives of microbes. Annu Rev Ecol Evol Syst. 2007;38:53–77. [Google Scholar]
- 14.Shimkets LJ, Dworkin M, Reichenbach H. In: The Myxobacteria. The Prokaryotes. Dworkin M, et al., editors. New York: Springer; 2006. [Google Scholar]
- 15.Wu SS, Kaiser D. Genetic and functional evidence that Type IV pili are required for social gliding motility in Myxococcus xanthus. Mol Microbiol. 1995;18:547–558. doi: 10.1111/j.1365-2958.1995.mmi_18030547.x. [DOI] [PubMed] [Google Scholar]
- 16.Berleman JE, Kirby JR. Deciphering the hunting strategy of a bacterial wolfpack. FEMS Microbiol Rev. 2009;33:942–957. doi: 10.1111/j.1574-6976.2009.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velicer GJ, Vos M. Sociobiology of the myxobacteria. Annu Rev Microbiol. 2009;63:599–623. doi: 10.1146/annurev.micro.091208.073158. [DOI] [PubMed] [Google Scholar]
- 18.Hodgkin J, Kaiser D. Cell-to-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc Natl Acad Sci USA. 1977;74:2938–2942. doi: 10.1073/pnas.74.7.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodgkin JDK. Genetics of gliding motility in Myxococcus xanthus. Mol Gen Genet. 1979;171:177–191. [Google Scholar]
- 20.Sun M, Wartel M, Cascales E, Shaevitz JW, Mignot T. Motor-driven intracellular transport powers bacterial gliding motility. Proc Natl Acad Sci USA. 2011;108:7559–7564. doi: 10.1073/pnas.1101101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg E, Varon M. Antibiotics and lytic enzymes. In: Rosenberg E, editor. Myxobacteria: Development and Cell Interactions. New York: Springer; 1984. pp. 109–125. [Google Scholar]
- 22.Nariya H, Inouye M. MazF, an mRNA interferase, mediates programmed cell death during multicellular Myxococcus development. Cell. 2008;132:55–66. doi: 10.1016/j.cell.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 23.Nadell CD, Foster KR, Xavier JB. Emergence of spatial structure in cell groups and the evolution of cooperation. PLOS Comput Biol. 2010;6:e1000716. doi: 10.1371/journal.pcbi.1000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldman BS, et al. Evolution of sensory complexity recorded in a myxobacterial genome. Proc Natl Acad Sci USA. 2006;103:15200–15205. doi: 10.1073/pnas.0607335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drake JW, Charlesworth B, Charlesworth D, Crow JF. Rates of spontaneous mutation. Genetics. 1998;148:1667–1686. doi: 10.1093/genetics/148.4.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eyre-Walker A, Keightley PD. The distribution of fitness effects of new mutations. Nat Rev Genet. 2007;8:610–618. doi: 10.1038/nrg2146. [DOI] [PubMed] [Google Scholar]
- 27.Maynard Smith J. The population genetics of bacteria. Proc R Soc Lond B Biol Sci. 1991;245:37–41. [Google Scholar]
- 28.Wright S. Evolution in Mendelian populations. Genetics. 1931;16:97–159. doi: 10.1093/genetics/16.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiegna F, Velicer GJ. Competitive fates of bacterial social parasites: persistence and self-induced extinction of Myxococcus xanthus cheaters. Proc Biol Sci. 2003;270:1527–1534. doi: 10.1098/rspb.2003.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross-Gillespie A, Gardner A, West SA, Griffin AS. Frequency dependence and cooperation: Theory and a test with bacteria. Am Nat. 2007;170:331–342. doi: 10.1086/519860. [DOI] [PubMed] [Google Scholar]
- 31.Sandoz KM, Mitzimberg SM, Schuster M. Social cheating in Pseudomonas aeruginosa quorum sensing. Proc Natl Acad Sci USA. 2007;104:15876–15881. doi: 10.1073/pnas.0705653104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Velicer GJ, Kroos L, Lenski RE. Developmental cheating in the social bacterium Myxococcus xanthus. Nature. 2000;404:598–601. doi: 10.1038/35007066. [DOI] [PubMed] [Google Scholar]
- 33.Buttery NJ, Rozen DE, Wolf JB, Thompson CR. Quantification of social behavior in D. discoideum reveals complex fixed and facultative strategies. Curr Biol. 2009;19:1373–1377. doi: 10.1016/j.cub.2009.06.058. [DOI] [PubMed] [Google Scholar]
- 34.Fiegna F, Velicer GJ. Exploitative and hierarchical antagonism in a cooperative bacterium. PLoS Biol. 2005;3:e370. doi: 10.1371/journal.pbio.0030370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strassmann JE, Zhu Y, Queller DC. Altruism and social cheating in the social amoeba Dictyostelium discoideum. Nature. 2000;408:965–967. doi: 10.1038/35050087. [DOI] [PubMed] [Google Scholar]
- 36.Vos M, Velicer GJ. Social conflict in centimeter-and global-scale populations of the bacterium Myxococcus xanthus. Curr Biol. 2009;19:1763–1767. doi: 10.1016/j.cub.2009.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foster KR, Wenseleers T, Ratnieks FLW. Kin selection is the key to altruism. Trends Ecol Evol. 2006;21:57–60. doi: 10.1016/j.tree.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 38.Hamilton WD. The genetical evolution of social behaviour. I. J Theor Biol. 1964;7:1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 39.Sachs JL, Mueller UG, Wilcox TP, Bull JJ. The evolution of cooperation. Q Rev Biol. 2004;79:135–160. doi: 10.1086/383541. [DOI] [PubMed] [Google Scholar]
- 40.Sachs JL, Bull JJ. Experimental evolution of conflict mediation between genomes. Proc Natl Acad Sci USA. 2005;102:390–395. doi: 10.1073/pnas.0405738102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wade MJ. The co-evolutionary genetics of ecological communities. Nat Rev Genet. 2007;8:185–195. doi: 10.1038/nrg2031. [DOI] [PubMed] [Google Scholar]
- 42.Kraemer SA, Toups MA, Velicer GJ. Natural variation in developmental life-history traits of the bacterium Myxococcus xanthus. FEMS Microbiol Ecol. 2010;73:226–233. doi: 10.1111/j.1574-6941.2010.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krug D, et al. Discovering the hidden secondary metabolome of Myxococcus xanthus: A study of intraspecific diversity. Appl Environ Microbiol. 2008;74:3058–3068. doi: 10.1128/AEM.02863-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgan AD, MacLean RC, Hillesland KL, Velicer GJ. Comparative analysis of myxococcus predation on soil bacteria. Appl Environ Microbiol. 2010;76:6920–6927. doi: 10.1128/AEM.00414-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vos M, Velicer GJ. Natural variation of gliding motility in a centimetre-scale population of Myxococcus xanthus. FEMS Microbiol Ecol. 2008;64:343–350. doi: 10.1111/j.1574-6941.2008.00484.x. [DOI] [PubMed] [Google Scholar]
- 46.Chang BY, Dworkin M. Isolated fibrils rescue cohesion and development in the Dsp mutant of Myxococcus xanthus. J Bacteriol. 1994;176:7190–7196. doi: 10.1128/jb.176.23.7190-7196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi WY, Zusman DR. The two motility systems of Myxococcus xanthus show different selective advantages on various surfaces. Proc Natl Acad Sci USA. 1993;90:3378–3382. doi: 10.1073/pnas.90.8.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kadam SV, Velicer GJ. Variable patterns of density-dependent survival in social bacteria. Behav Ecol. 2006;17:833–838. [Google Scholar]
- 49.Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci USA. 1979;76:5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Velicer GJ, Lenski RE. Evolutionary trade-offs under conditions of resource abundance and scarcity: Experiments with bacteria. Ecology. 1999;80:1168–1179. [Google Scholar]
- 51.Beaumont HJE, Gallie J, Kost C, Ferguson GC, Rainey PB. Experimental evolution of bet hedging. Nature. 2009;462:90–93. doi: 10.1038/nature08504. [DOI] [PubMed] [Google Scholar]
- 52.Laue BE, Gill RE. Use of a phase variation-specific promoter of Myxococcus xanthus in a strategy for isolating a phase-locked mutant. J Bacteriol. 1994;176:5341–5349. doi: 10.1128/jb.176.17.5341-5349.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laue BE, Gill RE. Using a phase-locked mutant of Myxococcus xanthus to study the role of phase variation in development. J Bacteriol. 1995;177:4089–4096. doi: 10.1128/jb.177.14.4089-4096.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin S, Sodergren E, Masuda T, Kaiser D. Systematic isolation of transducing phages for Myxococcus xanthus. Virology. 1978;88:44–53. doi: 10.1016/0042-6822(78)90108-3. [DOI] [PubMed] [Google Scholar]
- 55.Vos M, Didelot X. A comparison of homologous recombination rates in bacteria and archaea. ISME J. 2009;3:199–208. doi: 10.1038/ismej.2008.93. [DOI] [PubMed] [Google Scholar]
- 56.Wade MJ. Soft selection, hard selection, kin selection, and group selection. Am Nat. 1985;125:61–73. [Google Scholar]
- 57.Gilbert OM, Foster KR, Mehdiabadi NJ, Strassmann JE, Queller DC. High relatedness maintains multicellular cooperation in a social amoeba by controlling cheater mutants. Proc Natl Acad Sci USA. 2007;104:8913–8917. doi: 10.1073/pnas.0702723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Travisano M, Velicer GJ. Strategies of microbial cheater control. Trends Microbiol. 2004;12:72–78. doi: 10.1016/j.tim.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 59.Foster KR, Shaulsky G, Strassmann JE, Queller DC, Thompson CR. Pleiotropy as a mechanism to stabilize cooperation. Nature. 2004;431:693–696. doi: 10.1038/nature02894. [DOI] [PubMed] [Google Scholar]
- 60.Crow JF, Kimura M. An Introduction to Population Genetics Theory. New York: Harper and Row; 1970. [Google Scholar]
- 61.Van Dyken JD, Linksvayer TA, Wade MJ. Kin selection-mutation balance: A model for the origin, maintenance, and consequences of social cheating. Am Nat. 2011;177:288–300. doi: 10.1086/658365. [DOI] [PubMed] [Google Scholar]
- 62.Bouma JE, Lenski RE. Evolution of a bacteria/plasmid association. Nature. 1988;335:351–352. doi: 10.1038/335351a0. [DOI] [PubMed] [Google Scholar]
- 63.Stewart AD, Logsdon JM, Jr, Kelley SE. An empirical study of the evolution of virulence under both horizontal and vertical transmission. Evolution. 2005;59:730–739. [PubMed] [Google Scholar]
- 64.Weeks AR, Turelli M, Harcombe WR, Reynolds KT, Hoffmann AA. From parasite to mutualist: Rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biol. 2007;5:e114. doi: 10.1371/journal.pbio.0050114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fiegna F, Yu YT, Kadam SV, Velicer GJ. Evolution of an obligate social cheater to a superior cooperator. Nature. 2006;441:310–314. doi: 10.1038/nature04677. [DOI] [PubMed] [Google Scholar]
- 66.Manhes P, Velicer GJ. Experimental evolution of selfish policing in social bacteria. Proc Natl Acad Sci USA. 2011;108:8357–8362. doi: 10.1073/pnas.1014695108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang QG, Buckling A, Ellis RJ, Godfray HC. Coevolution between cooperators and cheats in a microbial system. Evolution. 2009;63:2248–2256. doi: 10.1111/j.1558-5646.2009.00708.x. [DOI] [PubMed] [Google Scholar]
- 68.Wright S. The roles of mutation, inbreeding, crossbreeding and selection in evolution. In: Jones DF, editor. Proceedings of the Sixth International Congress in Genetics. New York: Brooklyn Botanic Garden; 1932. pp. 356–366. [Google Scholar]
- 69.Castillo DI, et al. A cost to chimerism in Dictyostelium discoideum on natural substrates. Evol Ecol Res. 2005;7:263–271. [Google Scholar]
- 70.Cohan FM. Bacterial species and speciation. Syst Biol. 2001;50:513–524. doi: 10.1080/10635150118398. [DOI] [PubMed] [Google Scholar]
- 71.Saxer G, et al. Cheating does not explain selective differences at high and low relatedness in a social amoeba. BMC Evol Biol. 2010;10:76. doi: 10.1186/1471-2148-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Velicer GJ, Kroos L, Lenski RE. Loss of social behaviors by Myxococcus xanthus during evolution in an unstructured habitat. Proc Natl Acad Sci USA. 1998;95:12376–12380. doi: 10.1073/pnas.95.21.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hillesland KL, Velicer GJ. Resource level affects relative performance of the two motility systems of Myxococcus xanthus. Microb Ecol. 2005;49:558–566. doi: 10.1007/s00248-004-0069-8. [DOI] [PubMed] [Google Scholar]
- 74.Kerr B, Riley MA, Feldman MW, Bohannan BJ. Local dispersal promotes biodiversity in a real-life game of rock-paper-scissors. Nature. 2002;418:171–174. doi: 10.1038/nature00823. [DOI] [PubMed] [Google Scholar]
- 75.Riley MA, Gordon DM. The ecological role of bacteriocins in bacterial competition. Trends Microbiol. 1999;7:129–133. doi: 10.1016/s0966-842x(99)01459-6. [DOI] [PubMed] [Google Scholar]