Abstract
Acute lung injury (ALI) and its more severe form, acute respiratory distress syndrome, are complex illnesses involving the interplay of both environmental (such as mechanical ventilation) and genetic factors. To understand better the underlying mechanisms of pathogenesis associated with ALI, we recently identified several candidate genes by global expression profiling in preclinical models of ALI and relevant single-nucleotide polymorphisms. We summarize here several strategies successfully used to identify novel ALI candidate genes and detail the validation of variants in these genes as contributing factors to ALI pathobiology, conclusions based on functional analyses, and specific genetic association studies conducted in ALI cohorts. Continued insights into ALI pathogenesis and identification of genetic variants, which confer ALI risk and severity, promise to reveal novel molecular therapeutic targets that can be translated into personalized treatments to reduce the very high, unacceptable mortality of this disorder.
Keywords: acute lung injury, SNP, microarray, PBEF, inflammation
Acute lung injury (ALI), and its more severe form, acute respiratory distress syndrome (ARDS), are complex syndromes of critical illness that were first described by Thomas L. Petty and his colleagues in 1967. These physician scientists, apprised of the literature emanating from battlefield casualties associated with the Vietnam War, noted the similarities between critically ill patients in their hospitals with severe hypoxemia and diffuse alveolar infiltrates and battlefield trauma victims airlifted to nearby medical facilities with respiratory insufficiency. These physicians were the first to coin the term, ARDS, and their subsequent report in Lancet (1) remains one of the most widely cited papers in the “critical care” literature.
A great deal has been learned about ALI and ARDS since the initial Lancet report, with the definition of ALI and ARDS now standardized (2). Both ALI and ARDS are characterized by diffuse alveolar Infiltrates, and the presence of severe hypoxemia defined as a PaO2:FiO2 (p/f) less than 300 in ALI or a p/f less than 200 in ARDS in the absence of elevated left atrial pressure. A p/f ratio is the index to characterize ARDS, where FiO2 is simply the percentage of oxygen in a gas mixture. ALI exhibits profound inflammation, deranged vascular permeability, alveolar flooding with protein-rich exudate leading to atelectasis and an increase in the work of breathing. This is substantial due to the increase in dead space, a need for a higher minute ventilation, and reductions in pulmonary compliance. Inevitably, respiratory failure ensues and mechanical ventilation is required. The syndromes account for approximately 150,000 to 200,000 cases/yr, extended intensive care unit (ICU) hospitalization with major healthcare costs, and continue to suffer from a very high mortality rate of 30–40% (3).
In the last decade, several insights into ALI susceptibility and severity have evolved, including the potentially deleterious role of mechanical ventilation that is now recognized to potentially worsen pre-existing lung injury and, indeed, may incite de novo lung injury (4). Ventilator-associated lung injury or ventilator-induced lung injury (VILI) can be minimized by restricting the ventilator-delivered Vt and maintaining plateau airway pressures below 30 cm H2O, which reduces ARDS mortality and days spent on the ventilator (4). Lung injury caused by high-volume ventilation has also proven a very useful experimental model for investigators studying ALI.
Another important insight into ALI and ARDS has been the realization that this acute critical illness represents an important example of health care disparities. Moss and Mannino (5) evaluated the annual mortality rate in ARDS cases over a 2-decade period, and noted a marked disparity in ALI mortality, with African Americans exhibiting a substantially increased mortality rate, even when corrected for socioeconomic status and health care access; and similar data have been reported for sepsis, a major inciting stimulus for ALI/ARDS. More recently, a review of ARDSnet trial participants (6) demonstrated that, compared with whites (European descent) patients, African descent cases with ALI have a significantly higher risk of death due to increased severity of disease at presentation. A significantly higher risk of death was noted for Hispanics with ALI, which, unlike African American cases, was unexplained by the increased acuity at the time of presentation (6).
The recognition of ALI health disparities, as well as the recognition that only a fraction of patients exposed to ALI-inciting events (sepsis, trauma, pneumonia, aspiration) actually progress to development of the syndrome, incited significant interest in the identification of genetic factors potentially contributing to ALI susceptibility and prognosis, an unanswered question in ALI pathogenesis and epidemiology. The identification of genes contributing to ALI would potentially provide a better understanding of ALI pathobiology, yield novel biomarkers, identify individuals or populations at risk, and prove useful for the development of highly efficacious and individualized therapies.
GENOMIC STRATEGIES TO IDENTIFY ALI CANDIDATE GENES
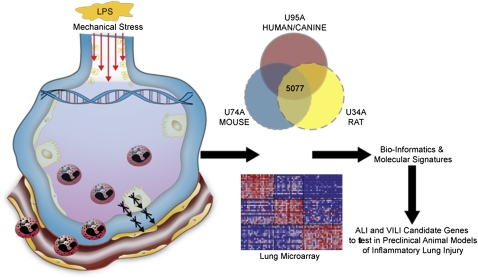
Many challenges exist in assessing genetic influences in ALI, such as the extensive comorbidities and phenotypic variance of critically ill patients, the diversity in lung injury–evoking stimuli, incomplete gene penetrance, complex gene–environment interactions, likelihood for locus heterogeneity, and the sporadic nature of the disease. Despite these significant challenges, we have successfully employed a systems biology approach, combining genomic and genetic strategies in preclinical models of ALI and VILI, to generate a list of putative ALI candidate genes (http://www.uic.edu/labs/iprm/diseasefocus_ali.html) that contribute to ALI susceptibility and severity. These approaches generally included combining extensive gene expression profiling on microarray samples of lung tissue RNA from preclinical ALI models after LPS exposure or high Vt ventilation (VILI) with stringent filtering modalities (Figure 1) (7). Additional useful complementary approaches included using novel bioinformatic methods, such as evaluation of evolutionarily conserved genes (ortholog genes) across multiple species (murine, rat, and canine and human cellular models of ALI) as a filter for ALI/VILI genes linked to a Eukaryote Gene Orthologs database (8). In some studies, we employed a consomic rat strategy using VILI-resistant and VILI-sensitive rat strains combined with chromosome transversion and expression profiling (9). These complementary strategies confirmed long-suspected ALI-associated candidate genes (IL-6, IL-1α, PAI1) (10), but, more importantly, identified novel genes not previously implicated in ALI (PBEF, GADD45α, CXCR4, COX2, MYLK, MIF) that may have a high likelihood of positively contributing toward an ALI phenotype (11–14). Table 1 highlights the utility of these approaches, with the number of PubMed citations as an index of novelty over the past decade of inquiry. Importantly, a key aspect of these successful approaches has been validation of the “candidate gene approach” by studies using genetically engineered mice and single-nucleotide polymorphism (SNP) assessment in human ALI populations. In these studies, the relevant ALI candidate genes were often sequenced to identify novel SNPs or insertion/deletions that were subsequently tested for differences in allelic frequency between ALI cases (ALI susceptibility and outcome) and control patients at risk for ALI (11–14). The ultimate goal was to ascertain functionality of these genetic variants via effects on promoter activity or structure–function changes in the protein itself, as detailed subsequently here, and the general strategy used is shown in Figure 1.
Figure 1.
Novel approaches to identify acute lung injury (ALI) genes: the candidate gene approach. Several species involved in preclinical models of ALI were subjected to either LPS (intratracheally administered) or mechanical stress (high Vt)–induced lung injury. Lung tissue RNA was isolated and subjected to microarray analysis. Dysregulated gene functional profiles were analyzed using Onto-Express. In addition, Ingenuity Pathway Analysis was also performed to identify potential candidate gene signatures, their signaling networks and overlaps.
TABLE 1.
NOVEL ACUTE LUNG INJURY CANDIDATE GENES IDENTIFIED BY AN EXPANDED CANDIDATE GENE APPROACH: NUMBER OF PUBMATRIX CITATIONS
| Encoded Protein | Gene Symbol | ALI | VILI | ARDS | Vascular Permeability | Inflammation |
|---|---|---|---|---|---|---|
| IL-6 | IL6 | 380 | 79 | 98 | 215 | 12,207 |
| Tissue factor | TF | 696 | 57 | 144 | 1,154 | 12,167 |
| Vascular endothelial growth factor | VEGF | 102 | 46 | 35 | 2,494 | 2,543 |
| IL-1 receptor antagonist | IL1RA | 19 | 4 | 11 | 9 | 1,098 |
| Vascular endothelial growth factor receptor | VEGFR | 30 | 19 | 7 | 992 | 964 |
| Plasminogen activator inhibitor–1 | PAI1 | 66 | 16 | 22 | 32 | 887 |
| IL-13 | IL13 | 12 | 2 | 3 | 11 | 834 |
| Akt, protein kinase B | AKT | 32 | 9 | 3 | 61 | 589 |
| Chemokine receptor–4 | CXCR4 | 13 | 3 | 1 | 2 | 352 |
| Cyclo-oxygenase–2 | COX2 | 5 | 0 | 0 | 3 | 216 |
| Plasminogen activator, urokinase receptor | PAUR | 13 | 1 | 1 | 9 | 196 |
| IL-1b | IL1B | 3 | 0 | 1 | 2 | 183 |
| Pre–B-cell colony enhancing factor | PBEF1 | 17 | 1 | 1 | 5 | 114 |
| Myosin light chain kinase | MYLK | 28 | 6 | 1 | 155 | 85 |
| c-Met | MET | 8 | 1 | 0 | 5 | 58 |
| Aquaporin-1 | AQP1 | 16 | 0 | 6 | 48 | 27 |
| Sphingosine 1-phosphate receptor–1 | EDG1 | 0 | 0 | 0 | 8 | 19 |
| Growth arrest DNA damage–inducible, alpha | GADD45A | 0 | 0 | 0 | 0 | 4 |
Definition of abbreviations: ALI = acute lung injury; ARDS = acute respiratory distress syndrome; VILI = ventilator-induced lung injury.
PubMatrix, a tool for multiplex literature mining (http://pubmatrix.grc.nia.nih.gov/), was used in January 2011.
REPRESENTATIVE NOVEL ALI CANDIDATE GENES IDENTIFIED BY GENOMIC STRATEGIES
Pre–B-Cell Colony–Enhancing Factor: a Novel ALI Candidate Gene
An early success for the candidate gene approach was the identification of pre–B-cell colony–enhancing factor (PBEF), an obscure gene (fewer than 10 PubMed citations) first identified in 1994 as a protein secreted by activated lymphocytes in bone marrow stromal cells that stimulates early stage-B cell formation (listed in Table 1). Both gene and protein expressions of PBEF were consistently elevated (>fivefold) in saline lavage-, sepsis-, and high-ventilation–induced injury models of murine, canine ALI, and in bronchoalveolar lavage (BAL) fluid and serum samples from critically ill ICU patients with ALI and sepsis (7, 11) (two- to fivefold increase over controls). Studies are currently underway to extend the validation of PBEF as a useful ALI biomarker.
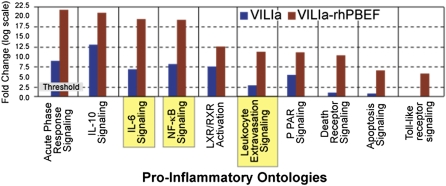
We subsequently highlighted the capacity for PBEF gene to influence ALI beyond any B-cell regulatory function, with a key role in regulating vascular permeability, as well as inhibiting endothelial and neutrophil apoptosis. Immunohistochemical staining in ALI lung tissues localized PBEF expression to vascular endothelial cells (ECs) and infiltrating neutrophils (11). We further demonstrated recombinant human PBEF (rhPBEF) as a direct rat neutrophil chemotactic factor, and rhPBEF and VILI were synergistic in producing neutrophilic alveolitis accompanied by increased BAL levels of polymorphonuclear chemoattractants and increases in lung vascular and alveolar permeability (15). Gene expression profiling identified induction of VILI-associated gene modules, such as nuclear factor (NF)–κB, leukocyte extravasation, apoptosis, and Toll-like receptor pathways. Likewise, these gene modules are synergistically augmented in mice that were subjected to both VILI and rhPBEF challenge, as summarized in Figure 2. Because we hypothesize that endogenous PBEF released by stretch-induced injury exacerbates the permeability response, we next examined the effect of reduced endogenous PBEF on VILI. Heterozygous PBEF+/− mice were significantly protected (reduced BAL protein, BAL IL-6 levels, peak inspiratory pressures) when exposed to severe VILI, and exhibited significantly reduced gene expression of VILI-associated modules (15).
Figure 2.
Mechanisms of pre–B-cell colony–enhancing factor (PBEF)–mediated ventilator-induced lung injury (VILI) pathobiology: results of expression profiling in ALI preclinical models. Dysregulated genes grouped into their respective canonical pathways related to inflammation are summarized based on studies performed in ventilator-injured mice in comparison with mice subjected to VILI and injected of recombinant human PBEF. Adapted with permission from Reference 15.
To understand the molecular machinery underlying PBEF involvement in ALI and VILI, we generated a PBEF promoter–luciferase reporter, and demonstrated that mechanical stress, TNF, LPS, and other inflammatory stimuli drive promoter activity. A series of nested deletions determined that PBEF is a GC-rich TATA-less promoter, with functional response elements influencing transcription when exposed to mechanical and oxidative stress. Preliminary studies suggest a major role for members of the STAT family of transcription factors. Finally, to complete the highly translational research, we have generated neutralizing antibodies directed to reducing PBEF availability and have identified that both intratracheal and intravenous administration each resulted in significant protection from VILI (15).
Direct sequencing of the PBEF gene in 36 subjects (12 ALI, 12, sepsis, and 12 healthy control patients) identified 11 SNPs in PBEF gene with two promoter SNPs, T-1001G and C-1543T, associated with ALI and sepsis. The strongest association was found with the −1,543C/−1,001G haplotype, an independent risk factor for ALI susceptibility identified by multiple logistic regression analysis controlling for clinical factors (11). These results were subsequently confirmed by Bajwa and colleagues (16) in a comparable, but distinct, replicate ALI population. Their study demonstrated further that the −1,543G/−1,001C haplotype was associated with increased ICU mortality, whereas the −1,543T/−1,001T haplotype was associated with fewer ventilator days and decreased ICU mortality. Importantly, the functionality of these SNPs was defined, and each haplotype served to modulate stretch and oxidant response elements within the PBEF promoter.
In summary, we demonstrated PBEF to be a biomarker in sepsis and sepsis-induced ALI, with genetic variants conferring ALI susceptibility. We noted the mechanism of PBEF-induced pathobiology via NF-kB signaling, and have identified a putative therapeutic strategy targeting PBEF in VILI. These studies implicate PBEF as a key inflammatory mediator intimately involved in both the development and severity of ventilator-induced ALI.
Growth Arrest and DNA Damage–Inducible 45α
Growth arrest and DNA damage–inducible 45α (GADD45α), a 21-kD, predominantly nuclear protein and a member of an evolutionarily conserved gene family, is implicated as a stress sensor that modulates the response of mammalian cells to genotoxic or physiological stress. GADD45α induces cell cycle arrest and apoptosis in most of the cells. Interestingly, it also promotes DNA repair functions and survival and maintains genomic stability in a p53-responsive manner. Despite its multiple known and often opposing functions, the role of GADD45α in ALI, in endothelial/epithelial barrier dysfunction, or in repair of the injured lung is unknown. GADD45α exhibited differential expression in orthologous global gene expression profiling studies, in multi-species preclinical ALI models, in region-specific lung tissue expression profiling and was markedly upregulated in response to VILI (8, 12) (Figure 2). We explored the mechanistic involvement of GADD45α in endotoxin (LPS)- and ventilator-induced inflammatory lung injury (VILI) by comparing multiple biochemical and genomic parameters of inflammatory lung injury in wild-type C57BL/6 and GADD45α−/− mice exposed to high Vt ventilation (VILI) or intratracheal LPS. GADD45α−/− mice were modestly susceptible to LPS-induced injury, but profoundly susceptible to VILI, demonstrating increased inflammation and increased microvascular permeability, with striking neutrophilic alveolitis, with increased vascular permeability and significantly elevated levels of inflammatory cytokines in BAL (12). Expression profiling of lung homogenates revealed strong dysregulation in the B-cell receptor signaling pathway in GADD45α−/− mice, suggesting the involvement of phosphatidylinositol 3-kinase/Akt signaling components, which were confirmed with a threefold reduction in Akt protein and phospho-Akt levels in GADD45α−/− lungs. Transfection of constitutively active Akt1 transgene reversed EC permeability induced by LPS (12) as well as VILI-induced vascular permeability in GADD45α−/− mice. Thus, both AKT and GADD45A are extremely attractive ALI candidate genes. The human GADD45A gene contains 25 validated SNPs (National Center for Biotechnology Information SNP database), the role of which in the ALI pathogenesis is unknown. We are further characterizing the role of GADD45α and the association of GADD45α genetic variants with sepsis and ALI.
ALI CANDIDATE GENES IDENTIFIED BY VASCULAR BARRIER–REGULATORY PATHWAY ANALYSIS
Myosin Light Chain Kinase as a Candidate Gene (MYLK )
Complementing the global gene expression profiling approach in humans or animal model ALI, we have also employed an alternate strategy to identify ALI candidate genes via the interrogation of genes involved in known ALI-regulated biologic pathways. As alveolar–vascular barrier dysfunction and increases in vascular permeability are essential features of inflammatory lung disease, such as ALI and VILI, we evaluated gene products in this pathway as potential ALI candidate genes, and initially focused on the non–muscle myosin light chain (MLC) kinase isoform (nmMLCK) (originally cloned in the Garcia laboratory) with our structure–function studies identifying a substantial role for nmMLCK in cytoskeleton rearrangement of ECs regulating vascular barrier function, angiogenesis, and leukocyte diapedesis. Thus, MYLK gene is an essential element of the inflammatory response (17, 18), and a strongly viable candidate to affect ALI susceptibility and outcome. nmMLCK is an enzyme that phosphorylates MLCs and regulates cellular actomyosin contraction. In two murine models of inflammatory lung injury induced by LPS or VILI, nmMLCK silencing or inhibition of kinase activity produced a dose-dependent attenuation of both LPS-induced lung inflammation and VILI (19). In addition, compared with wild-type mice, nmMLCK−/− knockout mice were significantly protected from VILI, with significant reductions in VILI-induced gene expression in biological pathways, such as nrf2-mediated oxidative stress, coagulation, p53-signaling, leukocyte extravasation, and IL-6 signaling. These studies validate nmMLCK as an attractive target for ameliorating the adverse effects of dysregulated lung inflammation in ALI and VILI.
The human MYLK gene resides on the short arm of chromosome 3 (3q21) and encodes three proteins, including nonmuscle and smooth muscle MLCK isoforms. MYLK gene sequencing identified 51 SNPs, including 5 coding MYLK SNPs conferring an amino acid change (Pro21His, Pro147Ser, Val261Ala, Ser1341Pro, and Arg1450Gln). Subsequently, association analysis of both single SNPs and haplotypes demonstrated very strong associations in both European descent and African descent ethnic groups with a fivefold increase in the risk of developing sepsis-induced ALI and severe sepsis (13, 19). We noted similar findings in association studies involving a cohort of trauma-induced ALI (20). We have also evaluated the association of 17 MYLK genetic variants with severe asthma in both European American and African American populations, and identified an SNP highly associated with severe asthma in African Americans consistent with data linking this chromosomal locus (MYLK, 3q21.1) to asthma and asthma-related phenotypes (21). Taken together, these data strongly implicate MYLK genetic variants as risk factors in inflammatory lung disorders, such as ALI and asthma.
Sphingosine 1-Phosphate Receptor 1
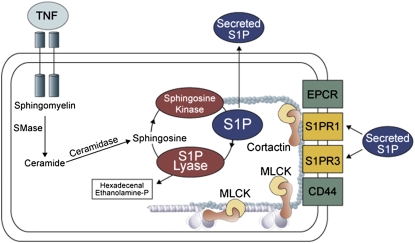
The bioactive sphingolipid metabolite, sphingosine 1-phosphate (S1P) is an important lipid mediator that enhances EC barrier function in vivo and in vitro by ligating the S1P receptor (S1PR) 1 (22–25), which is encoded by endothelial differentiation gene (EDG1 or S1PR1). S1PR1 is a pertussis toxin-sensitive, Gi-coupled receptor, which induces substantial increases in cortical actin polymerization that is critical to EC barrier enhancement in a Rac GTPase-dependent manner. Understanding the role of S1P in enhancing EC barrier function underscores its importance as a therapeutic target in reversing loss of EC barrier integrity. In vivo administration of selective competitive S1PR1 antagonists induces a dose-dependent disruption of barrier integrity in pulmonary endothelium, whereas S1PR1 agonists, SEW2871 and FTY720, promote vascular endothelial barrier function (22–25). A compelling argument for S1PR1 as an attractive ALI candidate gene is not only its ability to transduce signals that restore barrier integrity, but that S1PR1 is the target for transactivation by receptors for other potent barrier-protective agonists. These include endothelial protein C receptor (receptor for activated protein C) (26), c-Met (receptor for hepatocyte growth factor), and CD44 (receptor for high molecular weight hyaluronan) (27). We recently sequenced S1PR1 (EDG1) gene (14 African Americans and 13 European Americans), and identified 39 SNPs with several promoter SNPs associated with asthma, an inflammatory lung syndrome like ALI (28). Figure 3 highlights the S1P metabolic and signaling pathway, with SNPs now identified in each pathway component that is associated with ALI.
Figure 3.
Sphingosine 1-phosphate (S1P) generation and ALI-associated single-nucleotide polymorphism (SNPs) in the S1P biosynthetic pathway. S1P is a lipid mediator that essentially promotes lung endothelial barrier function. It is generated by the action sphingosine kinase and metabolized by sphingosine lyase. S1P-mediated ligation of its receptors results in increased tyrosine phosphorylation of cortactin, its rapid mobilization to the periphery, and interaction with myosin light chain (MLC) kinase (MLCK), finally culminating in enhanced endothelial cell barrier function. The SNPs associated with MLCK correlate to ALI susceptibility. The other modulators of this pathway are endothelial protein C receptor (EPCR) (26) and CD44 (27) via S1PR1 transactivation. Despite the fact that TNF, a cytokine that is elevated in lung injury, is known to promote S1P synthesis, endothelial cell (EC) barrier may still remain compromised, due to insufficient S1P buildup, or due to the negative regulatory SNPs in MYLK.
CONCLUSION
ALI is a major cause of morbidity and mortality in critically ill patients. Given the unacceptably high mortality rate observed in ALI, and the paucity of novel therapies and biomarkers, it is essential to recognize molecular targets associated with ALI to identify individuals at risk and to develop novel therapeutic targets and biomarkers. We have highlighted how global gene expression profiling in multispecies ALI models serves to broaden our net knowledge of ALI-implicated genes (29). Whereas the ALI association studies described here used mid-throughput genotyping strategies (such as Sequenome technology), high-throughput, whole-genome scanning technology has recently emerged as a powerful tool, particularly in detecting disease susceptibility genes with modest effects using very large samples of cases and controls. The Haplotype Mapping Project, which identified blocks of SNPs associated with each other, has allowed selection of the most informative SNPs for further disease association studies, with high-throughput SNP platforms now assessing over a million SNPs spanning the genome (i.e., genome-wide association studies [GWAS]). GWAS platforms have been successfully used in diverse disorders, such as age-related macular degeneration, inflammatory bowel disease, type 2 diabetes, and stroke. Although this approach has yet to be employed in either sepsis or ALI, the application of GWAS to the disease is clearly imminent, and, currently, there are several efforts to assess large European- and African-descent ALI cohorts. These studies may yield new mechanistic targets in ALI, and increase our understanding of the genetic basis for the significant health care disparities that exist in individuals of African and of Hispanic descent. We speculate that these GWAS studies will identify novel markers of risk for ALI that can be tested in future studies for utility in risk stratification and identification of subjects at high risk for poor outcomes, who are most likely to benefit from personalized treatment strategies. Continued challenges will be the gene–gene and gene–environment interactions, which add complexity to our understanding of the genome. Furthermore, sample size, genetic heterogeneity, and genome scan technology, based on “common disease, common variant,” will need to be considered, which may limit the power of GWAS (30). With these caveats in mind, these novel genetic approaches may prove exceptionally useful in ushering in the era of personalized medicine for critically ill individuals.
Supported by National Heart, Lung, and Blood Institute/National Institutes of Health grants HL094394 and HL058064.
Author Disclosure: J.G.N.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet 1967;2:319–323. [DOI] [PubMed] [Google Scholar]
- 2.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American–European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818–824. [DOI] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–1693. [DOI] [PubMed] [Google Scholar]
- 4.ARDSnet Working Group. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301–1308. [DOI] [PubMed] [Google Scholar]
- 5.Moss M, Mannino DM. Race and gender differences in acute respiratory distress syndrome deaths in the United States: an analysis of multiple-cause mortality data (1979–1996). Crit Care Med 2002;30:1679–1685. [DOI] [PubMed] [Google Scholar]
- 6.Erickson SE, Shlipak MG, Martin GS, Wheeler AP, Ancukiewicz M, Matthay MA, Eisner MD. National Institutes of Health National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network. Racial and ethnic disparities in mortality from acute lung injury. Crit Care Med 2009;37:1–6.19050621 [Google Scholar]
- 7.Simon BA, Easley RB, Grigoryev DN, Ma SF, Ye SQ, Lavoie T, Tuder RM, Garcia JGN. Microarray analysis of regional cellular responses to local mechanical stress in acute lung injury. Am J Physiol Lung Cell Mol Physiol 2006;291:L851–L861. [DOI] [PubMed] [Google Scholar]
- 8.Grigoryev DN, Ma SF, Irizarry RA, Ye SQ, Quackenbush J, Garcia JGN. Orthologous gene-expression profiling in multi-species models: search for candidate genes. Genome Biol 2004;5:R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nonas SA, Moreno-Vinasco L, Ma SF, Jacobson JR, Desai AA, Dudek SM, Flores C, Hassoun PM, Sam L, Ye SQ, et al. Use of consomic rats for genomic insights into ventilator-associated lung injury. Am J Physiol Lung Cell Mol Physiol 2007;293:L292–L302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flores C, Ma SF, Maresso K, Wade MS, Villar J, Garcia JGN. IL6 gene-wide haplotype is associated with susceptibility to acute lung injury. Transl Res 2008;152:11–17. [DOI] [PubMed] [Google Scholar]
- 11.Ye SQ, Simon BA, Maloney JP, Zambelli-Weiner A, Gao L, Grant A, Easley RB, McVerry BJ, Tuder RM, Standiford T, et al. Pre–B-cell colony–enhancing factor as a potential novel biomarker in acute lung injury. Am J Respir Crit Care Med 2005;171:361–370. [DOI] [PubMed] [Google Scholar]
- 12.Meyer NJ, Huang Y, Singleton PA, Sammani S, Moitra J, Evenoski CL, Husain AN, Mitra S, Moreno-Vinasco L, Jacobson JR, et al. GADD45a is a novel candidate gene in inflammatory lung injury via influences on Akt signaling. FASEB J 2009;23:1325–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao L, Grant A, Halder I, Brower R, Sevransky J, Maloney JP, Moss M, Shanholtz C, Yates CR, Meduri GU, et al. Novel polymorphisms in the myosin light chain kinase gene confer risk for acute lung injury. Am J Respir Cell Mol Biol 2006;34:487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao L, Flores C, Fan-Ma S, Miller EJ, Moitra J, Moreno L, Wadgaonkar R, Simon B, Brower R, Sevransky J, et al. Macrophage migration inhibitory factor in acute lung injury: expression, biomarker, and associations. Transl Res 2007;150:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong SB, Sammani S, Moreno L, Mirzapoiazova T, Lang G, Barnard J, Lussier Y, Ye SQ, Garcia JGN. Pre–B-cell colony enhancing factor augments murine ventilator-mediated acute lung injury. Am J Respir Crit Care Med 2008;178:605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bajwa EK, Yu CJ, Gong MN, Thompson BT, Christiani DC. Pre–B-cell colony–enhancing factor gene polymorphisms and risk of acute respiratory distress syndrome. Crit Care Med 2007;35:1424–1425. [DOI] [PubMed] [Google Scholar]
- 17.Garcia JG, Lazar V, Gilbert-McClain LI, Gallagher PJ, Verin AD. Myosin light chain kinase in endothelium: molecular cloning and regulation. Am J Respir Cell Mol Biol 1997;16:489–494. [DOI] [PubMed] [Google Scholar]
- 18.Petrache I, Verin AD, Crow MT, Birukova A, Liu F, Garcia JG. Differential effect of MLC kinase in TNF-alpha–induced endothelial cell apoptosis and barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 2001;280:L1168–L1178. [DOI] [PubMed] [Google Scholar]
- 19.Garcia JG, Moreno Vinasco L. Genomic insights into acute inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol 2006;291:L1113–L1117. [DOI] [PubMed] [Google Scholar]
- 20.Christie JD, Ma SF, Aplenc R, Li M, Lanken PN, Shah CV, Fuchs B, Albelda SM, Flores C, Garcia JGN. Variation in the MYLK gene is associated with development of acute lung injury after major trauma. Crit Care Med 2008;36:2794–2800. [DOI] [PubMed] [Google Scholar]
- 21.Flores C, Ma SF, Maresso K, Ober C, Garcia JG. A variant of the myosin light chain kinase gene is associated with severe asthma in African Americans. Genet Epidemiol 2007;31:296–305. [DOI] [PubMed] [Google Scholar]
- 22.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamburg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 2001;108:689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McVerry BJ, Peng X, Hassoun PM, Sammani S, Simon BA, Garcia JG. Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am J Respir Crit Care Med 2004;170:987–993. [DOI] [PubMed] [Google Scholar]
- 24.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin–induced inflammatory lung injury. Am J Respir Crit Care Med 2004;169:1245–1251. [DOI] [PubMed] [Google Scholar]
- 25.Sammani S, Camp SM, Natarajan V, Dudek SM, Garcia JGN. Differential effects of S1P receptors on airway and vascular barrier function in the murine lung. Am J Respir Cell Mol Biol 2010;43:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, Ye SQ, Garcia JG. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem 2005;280:17286–17293. [DOI] [PubMed] [Google Scholar]
- 27.Singleton PA, Dudek SM, Ma SF, Garcia JG. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation: novel role for hyaluronan and CD44 receptor family. J Biol Chem 2006;281:34381–34393. [DOI] [PubMed] [Google Scholar]
- 28.Sun X, Ma SF, Wade M, Flores C, Pino Yanes L, Moitra J, Ober C, Kittles R, Hussain A, Ford J, and Garcia JGN. Functional variants of the sphingosine 1-phosphate receptor 1 gene associate with susceptibility to asthma. J Allergy Clin Immunol 2010;126:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grigoryev DN, Finigan JH, Hassoun P, Garcia JG. Science review: searching for gene candidates in acute lung injury. Crit Care 2004;8:440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitsios GD, Zintzaras E. Genome-wide association studies: hypothesis—“free” or “engaged”? Transl Res 2009;154:161–164. [DOI] [PMC free article] [PubMed] [Google Scholar]