Abstract
Since the advent of the new proteomics era more than a decade ago, large-scale studies of protein profiling have been exploited to identify the distinctive molecular signatures in a wide array of biological systems spanning areas of basic biological research, various disease states, and biomarker discovery directed toward therapeutic applications. Recent advances in protein separation and identification techniques have significantly improved proteomics approaches, leading to enhancement of the depth and breadth of proteome coverage. Proteomic signatures specific for invasive lung cancer and preinvasive lesions have begun to emerge. In this review we provide a critical assessment of the state of recent advances in proteomic approaches and the biological lessons they have yielded, with specific emphasis on the discovery of biomarker signatures for the early detection of lung cancer.
Keywords: proteomics, biomarker, early detection, lung cancer
CHALLENGES IN LUNG CANCER RESEARCH
Lung cancer is the deadliest cancer in the United States and worldwide, accounting for 15% of all cancer incidence and 29% of all cancer deaths, with a 5-year survival rate of only 15% (1, 2). Lung cancer represents a spectrum of diseases with tremendous heterogeneity at the pathological and molecular levels (3–6) that is strongly associated with smoking as a risk factor. With about 20% of the United States adult population smoking and 1 billion smokers worldwide, it was estimated that in 2009 lung cancer claimed more lives than breast, prostate, colon, liver, kidney, and melanoma cancers combined (2, 7). Despite the recent improvements of bronchoscopic and surgical techniques as well as advances in chemotherapy and radiation therapy treatments, attempts to improve patient outcomes are faced with immense challenges. Several noninvasive detection technologies have been investigated. Imaging techniques, such as chest radiography, low-dose spiral computed tomography, sputum cytology, and molecular biomarkers in various biological samples, have been tested for their diagnostic value for early detection of lung cancer (8, 9). Although these tests vary in their sensitivity and specificity, only low-dose chest computed tomography was shown to reduce lung cancer–specific mortality (10–12). This encouraging finding calls for new molecular strategies to address the noninvasive diagnostic and risk assessment for lung cancer. These molecular strategies will have to demonstrate clinical utility and may complement currently tested strategies.
NATURAL HISTORY OF LUNG CANCER PROGRESSION AND A WINDOW FOR EARLY DETECTION
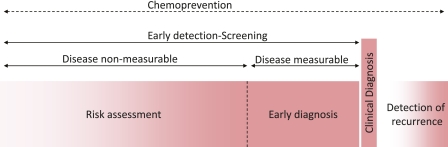
Lung cancer can be considered to result from a long history of repeated airway damage and repair cycles. Although clinically addressed at the time of diagnosis, the disease process develops for months and years before affecting patients' lives. This rather long disease process (Figure 1) represents a window of opportunity where the intervention should take place with the aim of preventing the development of disease (e.g., primary prevention, such as smoking cessation, or chemoprevention). Only about 20% of high-risk individuals are likely to develop lung cancer (13), but the questions of who will develop a malignancy and at what rate the disease will progress remain. The next significant hope in achieving a difference in the management of the disease, short of preventing its development, is to diagnose it early while it is measurable yet presymptomatic. To this aim, the search for lung cancer–specific biomarkers has been intensified; however, no biomarker has been proven clinically useful for the early diagnosis of lung cancer (14).
Figure 1.
Diagram illustrating the natural history of lung cancer development and positioning several windows of clinical relevance in the management of lung cancer, showing a long window of opportunity for “chemoprevention,” a window of “early detection” divided conceptually by periods of time based on whether the disease is measureable (a window of “risk assessment” covers the entire early detection period and may be called window of “early diagnosis” should the tumor be measurable) a window of “clinical diagnosis,” and a window of “detection of recurrence.” These windows correspond to the many clinical outcomes that may require the development of different biomarker profiles for optimal management of lung cancer.
PROTEOMIC APPROACHES FOR DISCOVERY AND VALIDATION OF CANCER BIOMARKERS
Considerable progress has been made in the past decade in identifying tumor characteristics through advances of molecular biology technologies. Much of this progress was driven by increasing knowledge of tumor-related aberrations that affect nucleic acids at genomic, transcriptional, and posttranscriptional levels. Proteins are the functional end product of genes that ultimately control vital biological processes via their expression level and posttranslational modifications. Moreover, the number of proteins produced by cells far exceeds the number of genes because proteins vary in their stability compared with mRNA and are subjected to many levels of posttranscriptional and posttranslational regulations, such as splicing variants, fusions, and posttranslational modifications. Therefore, to advance our understanding of the biology of lung cancer and to obtain a more integrated view of the disease biology, it is critical to capture the full spectrum of the variations in protein expression patterns, their posttranslational modifications, and their functions in cancer cells. Thus, the main objectives of applying proteomics research in lung cancer are (1) to use the molecular complexity of the proteome and provide the depth and breadth necessary for the discovery of the full spectrum of protein expression changes in clinically relevant specimens, (2) to derive lessons in pathogenesis, and (3) to build a comprehensive understanding of the disease after integration with other molecular approaches. Hence, proteomics presents an attractive alternative to comprehensive genomic analysis of tumors qualitatively and quantitatively.
Proteomic Discovery Platforms
Several analytical approaches have been adopted to identify novel proteins and to help us understand their structure, function, and interaction with proteins and other molecules. There have been attempts to bring this knowledge to the clinic by means of new diagnostic and predictive biomarkers as well as the identification of therapeutic targets. A list of the most common proteomic approaches is summarized in Table 1. These were reviewed in details elsewhere (15–17).
TABLE 1.
COMPARISON OF PROTEOMIC APPROACHES IN CANCER BIOMARKER DISCOVERY RESEARCH
| 2-D gel separation | MALDI MS | LC-MS/MS | LC-MRM MS | Protein/antibody arrays | |
|---|---|---|---|---|---|
| Purpose | Profiling, separation and identification | Profiling | Inventory and identification | Targeted protein quantitation | Targeted protein detection |
| Protein detection and identification | Protein pI and MW. Identification by peptide mapping and sequencing, | Detection of intact peptides and proteins | Identification via peptide sequences. | Peptide specific MS/MS transitions. | Detection using antibodies or ligands |
| Quantitation | Semi-quantitative | Semiquantitative | Semiquantitative | Quantitative, labeled reference peptides. Label free techniques | Not quantitative |
| PTM detection | Yes | No | Yes | Yes | Yes |
| Throughput | Low | High | High | Low | High |
| Reproducibility | High | High | High | High | High |
| Sensitivity | Moderate | low | High | High | High |
| Depth of analysis | 100–1,000 proteins | 100–300 peaks | 500–4,000 proteins | 1–100 proteins | 0–500 proteins |
| Major disadvantages | pI and MW range limitations. Contamination by polysaccharides and nucleic acids | Detection of abundant proteins. Limited MW range (2–30 kDa). No identification. | High false discovery rate | Cost of labeled peptides for absolute quantitation | Antibody specificity and availability. Only known proteins can be detected. |
Definition of abbreviations: 2-D = two-dimensional; LC-MRM MS = liquid chromatography coupled to multiple reaction monitoring mass spectrometry; LC-MS/MS = liquid chromatography coupled to tandem mass spectrometry; MALDI MS = matrix-assisted laser desorption ionization mass spectrometry; ; MW = molecular weight; pI = isoelectric point; PTM = posttranslational modification.
PROTEOMIC APPLICATIONS IN LUNG CANCER RESEARCH
Analysis of Preinvasive Lesions for Risk Assessment Biomarkers
Preinvasive lesions of the lung are attractive specimens when considering the investigation of how lung cancer develops and progresses from a normal epithelium to an invasive phenotype. Biopsy specimens are collected using autofluorescence bronchoscopy. Although working with these specimens remains challenging because of their scarcity and the difficulty in bringing a detailed histological classification to daily practice, these lesions are likely to harbor molecular alterations that are indicative of lung cancer risk, including tumors of different histological subtypes. A high-throughput proteomic approach combined with an in vitro carcinogenesis cell model and tissue immunoassay analysis identified changes in proteins associated with sequential pathogenesis of non–small cell lung cancer (NSCLC) (18). We reported proteomic patterns specific for normal alveolar and normal bronchial tissues, preinvasive lesions, and invasive lung cancer tissues (19, 20). The proteomic profiles obtained by matrix-assisted laser desorption ionization mass spectrometry (MALDI MS) of normal lung, preinvasive lesions, of the lung and invasive lung cancer tissues were able to cluster these three groups in a continuum based on 38 discriminatory features (molecular weight signatures expressed as mass-to-charge ratios, m/z) .
Proteomic Analysis of Invasive Tumors
MALDI MS has been applied to fresh frozen lung tumors and has demonstrated the ability not only to distinguish lung cancers from other tissues but also to define proteomic profiles and histological classes predictive of tumor behavior (20). This technology has progressed to the high-throughput proteomic profiling of formalin-fixed paraffin-embedded (FFPE) tissue sections after on-tissue tryptic digestion and to the top-down identification of most abundant proteins. This method was applied to FFPE-archived specimens in different tissue types, including lung cancer (21, 22).
A major advance in the field has been to increase the depth of the proteomic analysis through a bottom-up approach called “shotgun proteomics.” In shotgun analyses, protein mixtures are digested to peptides, which are then analyzed most commonly by multidimensional liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) (23). MS/MS spectra encode the sequences of peptides as well as the masses and sequence positions of any posttranslational modifications. Matching of MS/MS spectra to database sequences enables the identification of the peptides and therefore the proteins from which they are derived. Shotgun analyses by LC-MS/MS can also provide for quantitative analysis of protein components (24). Shotgun proteomics has proven to be the most versatile and effective method for dissecting multiprotein complexes, signaling networks, and complex subcellular proteomes (25). Shotgun analyses have generated the most complete proteomic inventories of major eukaryotic subcellular organelles, whole cell and tissue proteomes, and proteomes of human biofluids, including plasma and serum. Using shotgun analysis of pooled tissue lysates from early stages of NSCLC tumors and normal adjacent tissues, we identified dozens of proteins that are differentially expressed in NSCLC compared with nonmalignant control tissues (26). Recently, a comparison of the proteome of FFPE tissues and fresh frozen tissues by shotgun analysis revealed an equivalent number of identified proteins with 92% overlap among the proteins identified (27), which opens the prospect of further applying high-dimensional proteomic analysis to archived materials. Using an immunoaffininty step in tandem with LC-MS/MS, Rikova and colleagues profiled the global changes in phosphoproteome in 41 NSCLC cell lines and 150 primary tumors (28). Several novel activated tyrosine kinases were identified in this study, including PDGFRα and DDR1, which had not been previously implicated in NSCLC. More recently, another group adapting similar approaches successfully identified several other signaling tyrosine kinases that are altered in mesothelioma cell lines, such as JAK1, STAT1, cortactin, FER, p130Cas (BCAR1), SRC, and FYN (29). In summary, shotgun proteomics has proven to be an invaluable tool for the discovery of novel molecular alterations associated with the pathogenesis of complex biological systems such as lung cancer. Future studies designed with a clearly defined biological question that targets certain fractions of the proteome or subproteome (e.g., membranal proteome or glycoproteome) should yield a more in-depth view of the molecular biology of lung cancer.
MALDI Profiling and Imaging Mass Spectrometry
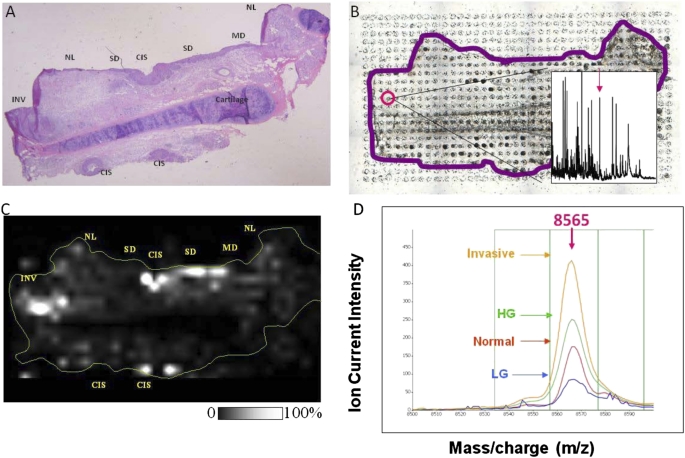
A novel strategy that combines tissue imaging technology and MALDI MS was used to identify lung cancer risk–associated biomarkers in which direct mapping and high-resolution imaging of biomolecules present in tissue sections were performed (30). Imaging MS allows simultaneous mapping of hundreds biomolecules (metabolites, lipids, peptides, and proteins) with an exact correlation to tissue architecture. Based on signal intensity (for a chosen m/z value), relative quantification can be obtained throughout the investigated section (Figure 2) (31). Quantification of the signals to provide a histology-independent lung cancer risk assessment based on proteomic data will take this effort to the next level toward early detection of lung cancer.
Figure 2.
Preinvasive proteins biomarker candidate from a lung tumor biopsy detected by matrix-assisted laser desorption ionization imaging mass spectrometry (MALDI MS). (A) Hematoxylin and eosin image of the tissue section. (B) Array of matrix deposited on a serial section for MS analysis. (C) MALDI MS image of the protein at m/z 8565, demonstrating overexpression in high-grade preinvasive and invasive areas. Gray scale provided, white corresponding to the highest intensity. (D) MALDI MS peak of the candidate biomarker protein at m/z 8,565 demonstrating differential peak intensity variations in normal, preinvasive, and invasive tissues (LG, low grade; HG, high grade).
Proteomic Analysis of Blood
Proteomic analysis of blood represents an appealing choice to researchers addressing the discovery of biomarkers because it can be quickly and easily obtained noninvasively in large quantities over time. Given the low abundance of known cancer markers in serum or plasma, it is critical to select proteomic technologies that provide sufficient depth of analysis for biomarker discovery. Several recent studies have investigated the extent to which proteomic technologies can unravel the complexity of the plasma proteome. In this regard, the Human Proteome Organization completed a comprehensive collaborative study to characterize the human serum and plasma proteomes (32). The rapid proteomic profiling of blood, tissue, or urine with minimal sample preparation, using the peak pattern as a diagnostic tool, has generated great enthusiasm but has been minimally successful at providing robust signatures to translate to the clinic. In this approach, the focus is on the use of MS peak patterns of abundant proteins or peptide fragments that correlate with an early disease stage but are usually not part of the disease mechanism. Several studies have used MALDI MS to study proteins and peptides in serum (33, 34). For example, we previously identified a seven-signal proteomic signature diagnostic of stage I NSCLC using MALDI MS analysis of undepleted and unfractionated serum with an overall accuracy of 78% and a sensitivity of 67.4% (34). Patz and colleagues identified four differentially expressed serum proteins (transferrin, retinol-binding protein, antitrypsin, and haptoglobin) that discriminate between NSCLC and control subjects (35). Using the same MALD MS approaches, several other groups have reported serum protein expression profiles that distinguish patients with various cancers from control subjects (recently reviewed by Ocak and colleagues [15]). Despite the numerous advantages and novel biological insights brought by MALDI MS technology, several preanalytical and analytical limitations hinder wider applications and implementation of this approach in the clinical setting. The major preanalytical challenges are related to the lack of standardized sample collection and preparation techniques, leading to the introduction of analytical bias and the lack of reproducibility. The extreme complexity of biofluids, such as blood, serum, or plasma, and the low abundance of most of the specific protein markers are among other factors that reduce the sensitivity of detection by MS technologies. In fact, the sensitivity of MALDI MS and most other MS technologies is limited to the most abundant proteins, typically within the 1 μg/ml range, whereas most of the known serum biomarkers are 1,000-fold less concentrated. Finally, although implementation of MALDI MS techniques to fresh tissue or blood samples may provide a large number of discriminatory peaks, it does not allow direct identification of the corresponding proteins (15).
Validation of Diagnostic Serum Biomarkers
After the discovery of new biomarkers, the next critical steps are to validate and evaluate their performance in clinically relevant patient populations (36). Multiple levels of validation have to take place before confirming the clinical utility of the biomarker (37, 38). This includes confirmation of detected changes in protein level by different techniques and correlation with biological outcomes of lung cancer such as early detection, chemosensitivity, or survival. These phases of clinical validation will evaluate a biomarker's performance in relevant clinical context and how it may affect clinical management of risk or disease (39).
Biochemical methods for protein markers validation have been dominated by immuno-based assays. Although immuno-based detection assays have been the most trusted and reliable method for biomarker validation, they rely on the tight and specific binding of the antibodies against the targeted molecule but are limited by the quality of antibodies and are labor intensive and are relatively low throughput (40). Recently, multiple reaction monitoring, a high-throughput liquid chromatography tandem mass spectrometry–based method, has been developed that can allow biomarker validation (41, 42). In this approach, biological specimens are depleted of abundant proteins and minimally processed with the optional addition of standard labeled proteins. Kuhn and colleagues used this approach to identify a panel of serum biomarkers from patients with rheumatoid arthritis (43). Another novel technology that combines the specificity of immune assays with the sensitivity of MS, denoted stable isotope standards and capture by antipeptide antibodies (SISCAPA), was developed to quantify peptides in complex digests (44). In this method, antipeptide antibodies immobilized on nano-affinity columns are used to enrich specific peptides along with spiked stable-isotope–labeled internal standards of the same sequence. Upon elution from the antipeptide antibody supports, electrospray MS is used to quantify the peptides (natural and labeled). SISCAPA is thus limited to sequence-defined (predetermined) analytes but offers the possibility of significantly increased sensitivity by removing unwanted peptides from the set delivered to the MS. No blood-based biomarker for lung cancer has been validated using these techniques, although ongoing technical improvements of protein separation and detection may allow for applications of these approaches as validation platforms in the near future. Blood sample repositories were recently developed in the context of a joint NCI/SPORE/EDRN effort and are available for phase II validation of candidate biomarkers (http://edrn.nci.nih.gov/resources/sample-reference-sets).
Circulating Autoantibodies
Tumor-associated antigens (TAAs) are proteins that are altered in a variety of ways in cancer cells that render them immunogenic. These include overexpression, mutations, misfolding, truncation, and degradation (45). A large number of TAA targets have been identified from patient sera in several immunological diseases and malignancies using various high-throughput screening platforms, such as cDNA expression phage display libraries and protein microarrays (46). In lung cancer, autoantibodies against the protein gene product 9.5 have been identified as a potential lung cancer TAA using immunoreactivity of patients' sera against tumor proteins isolated by two-dimensional proteomics (47). Using phage display libraries, TAAs have been detected in the blood of patients who developed lung cancer up to 5 years before tumors were detected with spiral computed tomography using screening (48). Therefore, monitoring these autoantibodies in serum from individuals at high risk for lung cancer represents an attractive option for developing a screening test. Using these approaches, several groups reported the identification of large numbers of immunogenic peptides that are potential targets for autoantibodies. For example, two separate groups identified several potential immunoreactive peptides for autoantibodies using the T-7 cDNA-based phage library as a screen from sera of patients with NSCLC (48–50). Using similar techniques, Chen and colleagues identified and validated ubiquitin 1 among several other peptides as a potential autoantibody target in lung adenocarcinoma from sera of patients with early-stage disease (51). A recent study by Wu and colleagues reported the identification of six peptide clones discriminatory of NSCLC using phage display techniques, but only one protein identity has been confirmed (52). However, most of the identified antigens were found to elicit antibodies in a relatively small proportion of patients. One other common challenge to these phage display techniques has been the inability to detect posttranslational modifications. Recently this obstacle was overcome by the development of a multidimensional fractionation technique using liquid chromatography to isolate a mixture of native proteins extracted from cancer cell lines. Using this method, antibodies directed against C-terminal hydrolase L3 ubiquitin were identified in the sera of patients with colon cancer and, more recently, in the blood of patients with lung adenocarcinoma (53). The validation of these novel lung cancer autoantibodies mandated the development of robust detection assays that are sensitive, reproducible, and high throughput. To test the utility of autoantibodies as a diagnostic tool for lung cancer, indirect ELISA tests were developed and validated for a panel of six known lung cancer TAAs (p53, NY-ESO-1, cancer-associated antigen, GBU4-5, Annexin 1, and SOX2) (54, 55). These efforts yielded an assay with high reproducibility, precision, and linearity that was able to identify nearly 40% of primary lung cancers via a peripheral blood test. This approach promises to address the need for early diagnosis (Figure 1), particularly for presymptomatic, curable disease. These assays need further clinical validation in large cohorts of high-risk patients, retrospectively and prospectively, before moving to the clinical practice.
SUMMARY AND FUTURE CLINICAL IMPLICATIONS
The importance of clinical proteomics derives from its future fundamental impact on our understanding of complex disease processes such as lung cancer and from the new opportunities in the early detection, prognosis, and therapeutic management of the disease. In this review we attempt to provide an up-to-date overview of the recent progress in proteomics technologies and their wide range of applications in lung cancer, with a main emphasis on early detection. The rapid development of proteomic technologies has led to the assembly of large protein inventories and to a better understanding of how they interact, insight into the role of specific posttranslational modifications, and addressing some of their biological functions. Although proteomic profiling of lung cancer and related biological specimens has yet to demonstrate clinical utility in early detection, it has the potential to highlight differences between lung cancer and nonmalignant lesions and between different levels of risks as well as stages and histology subtypes. Molecular profiling may assist with identifying high-risk populations and offers a unique opportunity to study early carcinogenesis. Integrating the findings from different scales of biological organization from gene to protein to cell using systems biology approaches will provide a global view of the key molecular changes associated with tumor progression. Therefore, systems biology can potentially expedite the translation of “omics” to personalized molecular medicine in the foreseeable future.
The development of specific and sensitive diagnostic biomarkers from biological fluids, such as sputum, blood, or exhaled breath, should improve early detection strategies, monitoring of disease progression, treatment response, and surveillance for recurrence. There is a need for extensive validation using novel proteomics research platforms and demonstration of clinical utility.
Acknowledgments
We thank Dr. Clay Callison and Mr. William Alborn for their insightful comments and suggestions.
Support by grant CA102353 awarded (P.P.M.).
Author Disclosure: M.H. and J.S.M.R. do not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.C. owns a patent through Vanderbilt University. P.P.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharjee A, Richards WG, Staunton J, Li C, Monti S, Vasa P, Ladd C, Beheshti J, Bueno R, Gillette M, et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc Natl Acad Sci USA 2001;98:13790–13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. Eur Respir J 2001;18:1059–1068. [DOI] [PubMed] [Google Scholar]
- 5.Beer DG, Kardia SL, Huang CC, Giordano TJ, Levin AM, Misek DE, Lin L, Chen G, Gharib TG, Thomas DG, et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat Med 2002;8:816–824. [DOI] [PubMed] [Google Scholar]
- 6.Garber ME, Troyanskaya OG, Schluens K, Petersen S, Thaesler Z, Pacyna-Gengelbach M, van de Rijn M, Rosen GD, Perou CM, Whyte RI, et al. Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci USA 2001;98:13784–13789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010;19:1893–1907. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet 2000;355:479–485. [DOI] [PubMed] [Google Scholar]
- 9.Patz EF Jr, Caporaso NE, Dubinett SM, Massion PP, Hirsch FR, Minna JD, Gatsonis C, Duan F, Adams A, Apgar C, et al. National Lung Cancer Screening Trial American College of Radiology Imaging Network Specimen Biorepository originating from the Contemporary Screening for the Detection of Lung Cancer Trial (NLST, ACRIN 6654): design, intent, and availability of specimens for validation of lung cancer biomarkers. J Thorac Oncol 2010;5:1502–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manser RL, Irving LB, Byrnes G, Abramson MJ, Stone CA, Campbell DA. Screening for lung cancer: a systematic review and meta-analysis of controlled trials. Thorax 2003;58:784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manser R. Screening for lung cancer: a review. Curr Opin Pulm Med 2004;10:266–271. [DOI] [PubMed] [Google Scholar]
- 12.Pastorino U. Lung cancer screening. Br J Cancer 2010;102:1681–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institute of Health NCIN. NCI Office of Media Relations. Twenty percent fewer lung cancer deaths seen among those who were screened with low-dose spiral CT than with chest X-ray. US NIH News 2010.
- 14.Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer 2005;5:845–856. [DOI] [PubMed] [Google Scholar]
- 15.Ocak S, Chaurand P, Massion PP. Mass spectrometry-based proteomic profiling of lung cancer. Proc Am Thorac Soc 2009;6:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Addona TA, Abbatiello SE, Schilling B, Skates SJ, Mani DR, Bunk DM, Spiegelman CH, Zimmerman LJ, Ham AJ, Keshishian H, et al. Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nat Biotechnol 2009;27:633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Angulo AM, Hennessy BT, Mills GB. Future of personalized medicine in oncology: a systems biology approach. J Clin Oncol 2010;28:2777–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen J, Behrens C, Wistuba II, Feng L, Lee JJ, Hong WK, Lotan R. Identification and validation of differences in protein levels in normal, premalignant, and malignant lung cells and tissues using high-throughput Western Array and immunohistochemistry. Cancer Res 2006;66:11194–11206. [DOI] [PubMed] [Google Scholar]
- 19.Rahman SM, Shyr Y, Yildiz PB, Gonzalez AL, Li H, Zhang X, Chaurand P, Yanagisawa K, Slovis BS, Miller RF, et al. Proteomic patterns of preinvasive bronchial lesions. Am J Respir Crit Care Med 2005;172:1556–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yanagisawa K, Shyr Y, Xu BJ, Massion PP, Larsen PH, White BC, Roberts JR, Edgerton M, Gonzalez A, Nadaf S, et al. Proteomic patterns of tumour subsets in non-small-cell lung cancer. Lancet 2003;362:433–439. [DOI] [PubMed] [Google Scholar]
- 21.Groseclose MR, Massion PP, Chaurand P, Caprioli RM. High-throughput proteomic analysis of formalin-fixed paraffin-embedded tissue microarrays using MALDI imaging mass spectrometry. Proteomics 2008;8:3715–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groseclose MR, Andersson M, Hardesty WM, Caprioli RM. Identification of proteins directly from tissue: in situ tryptic digestions coupled with imaging mass spectrometry. J Mass Spectrom 2007;42:254–262. [DOI] [PubMed] [Google Scholar]
- 23.Liebler DC. Shotgun mass spec goes independent. Nat Methods 2004;1:16–17. [DOI] [PubMed] [Google Scholar]
- 24.Ong SE, Foster LJ, Mann M. Mass spectrometric-based approaches in quantitative proteomics. Methods 2003;29:124–130. [DOI] [PubMed] [Google Scholar]
- 25.Yates JR III, Gilchrist A, Howell KE, Bergeron JJ. Proteomics of organelles and large cellular structures. Nat Rev Mol Cell Biol 2005;6:702–714. [DOI] [PubMed] [Google Scholar]
- 26.Takefumi Kikuchi QL. Robbert Slebos, Bing Zhang, Ming Li, Jamshedur Rahman, Lisa Zimmerman, David Tabb, Adriana Gonzalez, Daniel Liebler, David Carbone, and Pierre Massion: Shotgun proteomic analysis of non-small cell lung cancer. Proceedings of the 99th annual meeting of the American Association for Cancer Research 2008.
- 27.Sprung RW Jr, Brock JW, Tanksley JP, Li M, Washington MK, Slebos RJ, Liebler DC. Equivalence of protein inventories obtained from formalin-fixed paraffin-embedded and frozen tissue in multidimensional liquid chromatography-tandem mass spectrometry shotgun proteomic analysis. Mol Cell Proteomics 2009;8:1988–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007;131:1190–1203. [DOI] [PubMed] [Google Scholar]
- 29.Menges CW, Chen Y, Mossman BT, Chernoff J, Yeung AT, Testa JR. A Phosphotyrosine Proteomic Screen Identifies Multiple Tyrosine Kinase Signaling Pathways Aberrantly Activated in Malignant Mesothelioma. Genes Cancer 2010;1:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaurand P, Sanders ME, Jensen RA, Caprioli RM. Proteomics in diagnostic pathology: profiling and imaging proteins directly in tissue sections. Am J Pathol 2004;165:1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaurand P, Norris JL, Cornett DS, Mobley JA, Caprioli RM. New developments in profiling and imaging of proteins from tissue sections by MALDI mass spectrometry. J Proteome Res 2006;5:2889–2900. [DOI] [PubMed] [Google Scholar]
- 32.Anderson L, Hunter CL. Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol Cell Proteomics 2006;5:573–588. [DOI] [PubMed] [Google Scholar]
- 33.Sidransky D, Irizarry R, Califano JA, Li X, Ren H, Benoit N, Mao L. Serum protein MALDI profiling to distinguish upper aerodigestive tract cancer patients from control subjects. J Natl Cancer Inst 2003;95:1711–1717. [DOI] [PubMed] [Google Scholar]
- 34.Yildiz PB, Shyr Y, Rahman JS, Wardwell NR, Zimmerman LJ, Shakhtour B, Gray WH, Chen S, Li M, Roder H, et al. Diagnostic accuracy of MALDI mass spectrometric analysis of unfractionated serum in lung cancer. J Thorac Oncol 2007;2:893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patz EF Jr, Campa MJ, Gottlin EB, Kusmartseva I, Guan XR, Herndon JE II. Panel of serum biomarkers for the diagnosis of lung cancer. J Clin Oncol 2007;25:5578–5583. [DOI] [PubMed] [Google Scholar]
- 36.George SL. Statistical issues in translational cancer research. Clin Cancer Res 2008;14:5954–5958. [DOI] [PubMed] [Google Scholar]
- 37.Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, Winget M, Yasui Y. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst 2001;93:1054–1061. [DOI] [PubMed] [Google Scholar]
- 38.Srivastava S, Gopal-Srivastava R. Biomarkers in cancer screening: a public health perspective. J Nutr 2002; 132(8, Suppl)2471S–2475S. [DOI] [PubMed] [Google Scholar]
- 39.Moons KG. Criteria for scientific evaluation of novel markers: a perspective. Clin Chem 2010;56:537–541. [DOI] [PubMed] [Google Scholar]
- 40.Huang SN, Minassian H, More JD. Application of immunofluorescent staining on paraffin sections improved by trypsin digestion. Lab Invest 1976;35:383–390. [PubMed] [Google Scholar]
- 41.Gergov M, Ojanpera I, Vuori E. Simultaneous screening for 238 drugs in blood by liquid chromatography-ion spray tandem mass spectrometry with multiple-reaction monitoring. J Chromatogr B Analyt Technol Biomed Life Sci 2003;795:41–53. [DOI] [PubMed] [Google Scholar]
- 42.Kuzyk MA, Smith D, Yang J, Cross TJ, Jackson AM, Hardie DB, Anderson NL, Borchers CH. Multiple reaction monitoring-based, multiplexed, absolute quantitation of 45 proteins in human plasma. Mol Cell Proteomics 2009;8:1860–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuhn E, Wu J, Karl J, Liao H, Zolg W, Guild B. Quantification of C-reactive protein in the serum of patients with rheumatoid arthritis using multiple reaction monitoring mass spectrometry and C-13-labeled peptide standards. Proteomics 2004;4:1175–1186. [DOI] [PubMed] [Google Scholar]
- 44.Anderson NL, Polanski M, Pieper R, Gatlin T, Tirumalai RS, Conrads TP, Veenstra TD, Adkins JN, Pounds JG, Fagan R, et al. The human plasma proteome: a nonredundant list developed by combination of four separate sources. Mol Cell Proteomics 2004;3:311–326. [DOI] [PubMed] [Google Scholar]
- 45.Caron M, Choquet-Kastylevsky G, Joubert-Caron R. Cancer immunomics using autoantibody signatures for biomarker discovery. Mol Cell Proteomics 2007;6:1115–1122. [DOI] [PubMed] [Google Scholar]
- 46.Feng Z, Prentice R, Srivastava S. Research issues and strategies for genomic and proteomic biomarker discovery and validation: a statistical perspective. Pharmacogenomics 2004;5:709–719. [DOI] [PubMed] [Google Scholar]
- 47.Brichory F, Beer D, Le Naour F, Giordano T, Hanash S. Proteomics-based identification of protein gene product 9.5 as a tumor antigen that induces a humoral immune response in lung cancer. Cancer Res 2001;61:7908–7912. [PubMed] [Google Scholar]
- 48.Zhong L, Coe SP, Stromberg AJ, Khattar NH, Jett JR, Hirschowitz EA. Profiling tumor-associated antibodies for early detection of non-small cell lung cancer. J Thorac Oncol 2006;1:513–519. [PubMed] [Google Scholar]
- 49.Zhong L, Hidalgo GE, Stromberg AJ, Khattar NH, Jett JR, Hirschowitz EA. Using protein microarray as a diagnostic assay for non-small cell lung cancer. Am J Respir Crit Care Med 2005;172:1308–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khattar NH, Coe-Atkinson SP, Stromberg AJ, Jett JR, Hirschowitz EA. Lung cancer-associated auto-antibodies measured using seven amino acid peptides in a diagnostic blood test for lung cancer. Cancer Biol Ther 2010;10:267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen EI, Cociorva D, Norris JL, Yates JR III. Optimization of mass spectrometry-compatible surfactants for shotgun proteomics. J Proteome Res 2007;6:2529–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu D, Gao Y, Chen L, Qi Y, Kang Q, Wang H, Zhu L, Ye Y, Zhai M. Anti-tumor effects of a novel chimeric peptide on S180 and H22 xenografts bearing nude mice. Peptides 2010;31:850–864. [DOI] [PubMed] [Google Scholar]
- 53.Hanash S. Harnessing immunity for cancer marker discovery. Nat Biotechnol 2003;21:37–38. [DOI] [PubMed] [Google Scholar]
- 54.Chapman CJ, Murray A, McElveen JE, Sahin U, Luxemburger U, Tureci O, Wiewrodt R, Barnes AC, Robertson JF. Autoantibodies in lung cancer: possibilities for early detection and subsequent cure. Thorax 2008;63:228–233. [DOI] [PubMed] [Google Scholar]
- 55.Murray A, Chapman CJ, Healey G, Peek LJ, Parsons G, Baldwin D, Barnes A, Sewell HF, Fritsche HA, Robertson JF. Technical validation of an autoantibody test for lung cancer. Ann Oncol 2010;21:1687–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]