Abstract
Among lung pathologies, α1AT, chronic obstructive pulmonary disease (COPD), emphysema, and asthma are diseases triggered by local environmental stress in the airway that we refer to herein collectively as airway stress diseases (ASDs). A deficiency of α-1-antitrypsin (α1AT) is an inherited genetic disorder that is a consequence of the misfolding of α1AT during protein synthesis in liver hepatocytes, reducing secretion to the plasma and delivery to the lung. Deficiency of α1AT in the lung triggers a similar pathological phenotype to other ASDs. Moreover, the loss of α1AT in the lung is a well-known environmental risk factor for COPD/emphysema. To date there are no effective therapeutic approaches to address ASDs, which reflects a general lack of understanding of their cellular basis. Herein, we propose that ASDs are disorders of proteostasis. That is, they are initiated and propagated by a common theme—a challenge to protein folding capacity maintained by the proteostasis network (PN) (see Balch et al., Science 2008;319:916–919). The PN is a network of chaperones and degradative components that generates and manages protein folding pathways responsible for normal human physiology. In ASD, we suggest that the PN system fails to respond to the increased burden of unfolded proteins due to genetic and environmental stresses, thus triggering pulmonary pathophysiology. We introduce the enabling concept of proteostasis regulators (PRs), small molecules that regulate signaling pathways that control the composition and activity of PN components, as a new and general approach for therapeutic management of ASDs.
Keywords: proteostasis, inflammation, antitrypsin, COPD, emphysema
Cellular integrity relies on the correct folding of its protein constituents to maintain the specialized functions of cells and tissues that support normal human physiology and protect us from disease pathology. Generation and maintenance of the protein fold for function is the task of the protein homeostasis or proteostasis system (1–5). The proteostasis network or PN consists of more than 2,000 chaperone and degradative components along with their respective adaptive signaling pathways. Folding components include the ubiquitous Hsp40, Hsp/Hsc70, and Hsp90 chaperone-cochaperone systems. Protective degradative pathways include the ubiquitin-proteasome system (UPS) (6) and autophagic-lysosomal-endosomal pathways that remove defective and/or unwanted proteins (7–10). Signaling pathways that modulate the composition of the PN include the unfolded protein response (UPR) (11), the heat-shock response (HSR) (12, 13), ubiquitin-proteasome system (UPS), as well as Ca2+-sensing (14, 15) and inflammatory (16) pathways. The components and signaling pathways responsible for generating PN protective pathways vary considerably across the diversity of cell types comprising the human “cellome” (2, 13, 17, 18). These observations suggest that the PN is central for both the generation and the maintenance of the protein fold for specialized cell and tissue function during the human healthspan.
The PN can be significantly challenged by alterations in the genome (mutation and single nucleotide polymorphisms [SNPs]) such as occurs in the liver with α1AT deficiency (19–22), in response to pathogen challenge (parasites, bacteria, and viruses) (23–25), to environmental stressors including toxins, and in the case of lung airborne pollutants such as allergens (asthma) and smoking (chronic obstructive pulmonary disease/emphysema), which trigger inflammatory responses (26–28). Collectively, these events trigger protein misfolding through disruption of folding pathways and/or post-translational oxidative insult, accelerating the decline of proteostasis protection that occurs normally in response to aging (2, 29, 30). These events can trigger multiple alarm systems including inflammatory responses characteristic of chronic obstructive pulmonary disease (COPD), emphysema, and asthma (16).
Variation in the concentration and/or composition of the components of the PN occurs in response to both cell-autonomous (that is, within the cell) and cell-nonautonomous (that is, environmental or outside the cell) events that can overwhelm PN function, leading to a breakdown of the network and subsequently triggering human pathology characteristic of many inherited and sporadic protein folding diseases (1, 2, 5). Deficiency of α1AT is an inherited disease that challenges both liver and pulmonary function. In the liver, α1AT variants trigger cell autonomous proteostasis disease; in the lung, its reduced levels triggers an environmental stress response similar to that involved in COPD, emphysema, and asthma, a pathology that we now collectively refer to as airway stress disease (ASD).
Herein, we describe ASDs from the perspective of proteostasis (1, 2, 5) and propose a new paradigm for deconvolution of disease mechanism and therapeutic management. We posit that environment-triggered ASDs (31) exceed the capacity of the proteostasis program to maintain the protein fold in the lung, a hypothesis that is consistent with the impact of smoking and pollutants (airborn toxins and allergens) across a broad spectrum of pulmonary disorders (32, 33). In Part 1, we define proteostasis and the concept of the proteostasis boundary as a 3-dimensional surface that encompasses folding kinetics and protein stability (energetics) to explain how proteostasis operates. In Part 2, we briefly focus on the cell biological, biochemical, and biophysical properties of α1AT variant protein folds that trigger proteostasis disease in the liver as an example of the response of the proteostasis system to a cell-autonomous challenge. In Part 3, we use α1AT variants as a defined example of a cell-nonautonomous (environmental) challenge to proteostasis that triggers human pulmonary ASD pathology (21). We emphasize the role of the PN in maintenance of lung function in response to those environmental factors that can trigger progressive pathological failure of the proteostasis program. In Part 4, we will discuss emerging pharmacological strategies that may be used to enhance PN function to protect human biology from ASDs. We emphasize that an understanding of proteostasis in pulmonary disease presents an unanticipated opportunity to revise both our perception of lung dysfunction and the potential for therapeutic options that could have significant clinical benefit by reducing the onset of disease.
PART 1. THE PN IN HEALTH AND DISEASE
What is proteostasis? Proteins in the crowded proteome environment of a cell require folding assistance throughout their normal lifespan. PN players generate, maintain, and remove proteins (1, 2, 4, 29, 30) (Figure 1). We define the PN as a fully integrated, layered system (Figure 1) that contains chaperones (both general [Hsc/Hsp70, Hsp90] and their protein client specific co-chaperones such as Hsp40-, Hop-, p23-, and tetratricopeptide repeat [TRP]–containing proteins), folding enzymes such as protein disulfide isomerases, degradation components belonging to the UPS and lysosome-endosome-autophagy networks, and multiple signaling networks (e.g., HSR and UPR) that control both the composition and concentration of the PN components in response to the cellular genome and the local environment to maintain proteome health.
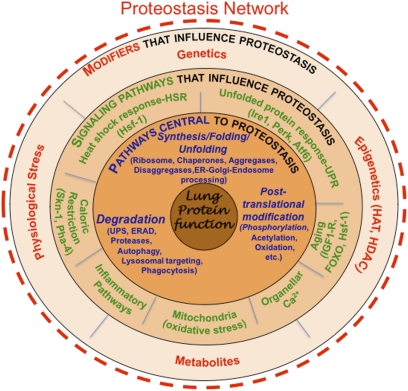
Figure 1.
Managing proteostasis in human lung disease. Illustrated are the layers of interactions that facilitate the function of the proteostasis network to generate and maintain functional proteins in the lung and counter the challenges to the protein fold as occurs in α-1-antitrypsin (α1AT) and airway stress disease (ASD). The proteostasis network is composed of the components outlined in the first layer (in blue font), including the ribosome, chaperones, aggregases, and disaggregases that direct folding, as well as pathways that select proteins for degradation (e.g., the ubiquitin-proteasome system [UPS], endoplasmic reticulum [ER]-associated degradation [ERAD] systems, proteases, autophagic pathways, lysosomal/endosomal targeting pathways, and phagocytic pathways; the latter being responsible for the recognition, uptake, and degradation of extracellular proteins). The second layer includes signaling pathways (in green font) that regulate the levels and activity of components found in the first layer to allow adjustment of composition in response to folding stress such as found in α1AT deficiency and ASDs. The third layer (in red font) includes genetic and epigenetic pathways, physiologic stressors, and intracellular metabolites that affect the activities defined by the second and first layers. These are the genetic and epigenetic and environmental influences that challenge the protein fold and lead to α1AT deficiency and ASD. The proteostasis network is under dynamic biological control at all times and can be therapeutically adjusted by proteostasis regulators (PRs), a new class of small molecules that can be used to rebalance the proteostasis program to benefit human health (3, 5). Reproduced with permission from Reference (2).
It is now evident that the composition and capacity of the PN is unique to each cell type (2), such as the alveolar cells found in the lung that are subject to ASD. This is because each cell type presents a unique and dynamic challenge to the PN based on the genomic and proteomic program required to generate and maintain their specialized physiologies and to protect the cell against pathophysiological insults. Most PN components have sisoforms differentially distributed in the cytosol and the subcellular compartments comprising the exocytic, endocytic, lysosomal, autophagic, and mitochondrial environments suggesting a high degree of compartment specialization in proteostasis function. Moreover, we now recognize that the PN includes neuronal signaling pathways (neuroendocrine pathways) that integrate PN function across cell and tissue boundaries (30, 34) (Figure 1).
Proteostasis is constantly stressed by SNPs, mutation(s) and the local extracellular environment: a task that compromises the PN in, for example, ASDs in response to airborne pollutants and allergens. Thus, folding stress can stretch the inherited capacity of the PN to deal with misfolded proteins. Regulatory pathways that respond to stress conditions can either resolve the folding protein or promote cell death when pushed beyond the limits of sustainable correction by the PN (16, 35).
It is important to appreciate that the PN is based on an energetic standard (2) and performs a highly adaptable quality control function. The PN per se has no knowledge of “function” necessary for providing an exact quality control decision. Rather, the proteostasis system sets in as yet unknown ways variable energetic standards in each cell type that supports the protein fold in a manner that is ultimately advantageous for cell and tissue function to promote organismal survival (2, 36, 37). This standard can be augmented in both a cell-autonomous and cell-nonautonomous fashion through UPR, HSR, and inflammatory responses to address folding problems. Thus, it is the local activity of the PN that ultimately dictates functionality rather than the protein sequence per se (2, 38). Indeed, what is an acceptable protein seqeuence/fold at at one timepoint in the cell, can be discarded in response to both genetic and environmental factors that affect proteostasis capacity. We refer to the balance of protein folding and degradative pathways maintained by the proteostasis system to promote healthspan as the yin and yang of the proteome balance (3).
The balance that drives the dynamic physiology of each cell type can become out-of-balance (imbalanced) in disease such as occurs in ASDs (1, 3, 29, 30). What do we mean by imbalance of PN function? To understand the role of proteostasis in human heath and the response that protects us from pathology, we need to quantify the general relationship between the energetics of protein folding pathways and PN components that are essential for the biological protein folding reaction. We have previously used mathematical modeling based on classic Michalis-Menten kinetics to address this conceptual challenge, which we referred to as FoldEx (38). In FoldEx, we described how the inherited energetics of the polypeptide chain sequence, such as found in α1AT, are read by the PN in the endoplasmic reticulum (ER), the first step in the exocytic pathway, to achieve a state of functional folding acceptable for export to post-ER compartments and secretion at the cell surface (38). We have recently generalized the FoldEx model to include all protein folding reactions in the cell that are managed by the PN (2). This model is referred to as FoldFx and expands the concept of folding for export from the ER to that of achieving and maintaining general protein function in the context of the local PN.
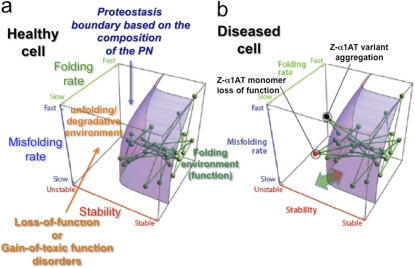
A critical feature of the FoldEx/Fx models is the concept of the “proteostasis boundary” or “PB,” that is, the integrated cellular proteostasis system that supports the protein fold (2, 39) (Figure 2a). The location and shape of the PB in 3-dimensional space is directly linked to the local composition and concentration of both folding and degradative PN components essential for generating and maintaining the protein fold (Figure 2a). The PB defines a surface that dictates the minimal energetics that a protein must have to achieve folding and function in the context of a given PN capacity of the cell. Thus, the position of a protein relative to the PB maintained by the PN is defined by each protein's folding thermodynamics (from unstable to stable, x axis), folding kinetics (from slow to fast, z axis) and misfolding kinetics (from slow to fast, y axis). The functional state of a protein in the cell (as indicated by the nodes [the proteins] and the edges [their links to other proteins within the network] [Figure 2a]) establishes the success or failure of biology in generating and managing the fold. If it is below the PB shown in Figure 2a, its fold and function is supported by the PN; if above the PB, the PN is not adequate to support the fold for function (e.g., its folding pathway is compromised, it is more sensitive to misfolding, and/or its thermodynamic stability is compromised) and the protein is either degraded or forms nonfunctional aggregates, which disrupts biology (Figure 2b). Diseases that increase the demand on the PN and that cannot be met by stress-responsive pathways that increase PN capacity (Figure 2b, green arrow), cause a collapse of the PB (Figure 2b, red arrow). This can result in the failure of folding of multiple proteins and a major disruption of biological networks required for cell integrity. For further description of the PB, we refer the reader to references (2, 4, 5).
Figure 2.
The proteostasis boundary that controls lung function in health and disease. The location of each node (or green sphere) in the network represents a corresponding protein's folding energetics (stability, x axis; folding kinetics, z axis; and misfolding kinetics, y axis). Each connection (green line) represents a physical or functional interaction that maintains the biological operation of the cell. The purple surface defines the proteostasis boundary (PB), defined by the composition of the proteostasis network (PN) in the lung. The location of the PB impacts the function of proteins based on their unique folding/misfolding kinetics and thermodynamic stability. It is shown as being the same for all proteins reflecting the general features of proteostasis capacity inherent in the highly abundant chaperone and degradative PN components (5). Many specialized components of the PN may augment the folding/misfolding kinetics or stability of a protein in the context of the general proteostasis program to achieve function. (a) All of the nodes driving normal lung function are within the proteostasis boundary of a healthy cell, indicating that their function is fully supported by the PN. (b) Mutations such as found in α1AT deficiency or in response to environmental challenges (e.g., allergens, air pollutants, or smoke leading to ASDs) can alter the folding kinetics or energetics of a protein, making their corresponding nodes fall outside the proteostasis boundary. This can lead to either loss of function (red node, i.e., Z-variant α1AT monomer) or aggregation (black node, i.e., Z-variant α1AT ER associated aggregate). Both states occur in α1AT deficiency. They likely strongly influence onset and progress of all ASDs. The double-ended green-red arrow at the base of the PB indicates that PN capacity can be increased by stress signaling pathways (green tip) or collapsed (red tip) by stress challenges that exceed the capacity of the PN to correct the folding problem (see text). Reproduced with permission from Reference (2).
In summary, the proteostasis system provides a folding environment that generates and protects protein function in biology that can be severely challenged in both acute and chronic diseases such as ASDs.
PART 2. α1AT DEFICIENCY: A CELL-AUTONOMOUS PROTEOSTASIS CHALLENGE TO HEPATOCYTES
As a member of the serine protease inhibitor (serpin) family, α1AT is the most abundant antiprotease in the serum. α1AT is mainly produced by hepatocytes in the liver, but also in unknown amounts by other cells or tissues such as lung alveolar cells lining the interstitium and lung macrophages (21). Given that α1AT (patho)physiology has been extensively reviewed (19–22), it is presented below only briefly to inform the reader of the features of disease relevant to the proteostasis concept.
The α1AT locus is pleomorphic with approximately 75 alleles. The M variant has normal function (Figure 3a). Other variants have different impacts on the properties of the protein fold and, hence, the PN and presentation of disease. Among the mutations leading to α1AT deficiency (6), the most common and severe form of disease occurs in response to a substitution of glutamic acid with a lysine at position 342, referred to as the Z-variant. The presence of misfolded Z-variant α1AT in the hepatocyte provides an example of cell-autonomous disease triggering liver dysfunction and cancer (Figure 3b). Following translocation into the ER, wild-type (WT) α1AT only transiently interacts with the ER lumenal PN chaperones BiP and calnexin to generate the normal fold. In contrast, monomeric and misfolded soluble intermediates of the Z-variant of α1AT can be detected to form stable complexes containing the PN folding chaperone components calnexin-ERp57/BiP/GRP94 (19, 40). This soluble but misfolded pool challenges the PN folding capacity of the ER and is targeted for degradation (19, 40, 41). Degradation of the soluble misfolded α1AT variants involves fold sensing pathways, targeting α1AT through ER-associated degradation (ERAD) (42–45) and/or glycoprotein ERAD (GERAD) (46) pathways. ERAD involves the Sec61 translocon and cytosolic p97/valosin containing protein (VCP)-dependent complexes, SCF (Skp1, Cullins, F-box proteins)-ubiquitin ligase complexes, and the cytosolic proteasome (6, 41). The GERAD pathway involves a carbohydrate recognition/calnexin-linked cycle and the EDEM family of proteins that target misfolded cargo to the Sec61 translocon (47–50).
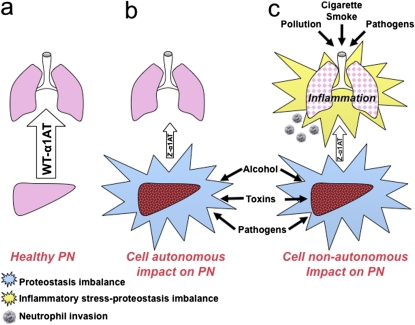
Figure 3.
Role of proteostasis in cell-nonautonomous ASD. (a) Wild-type (WT) α1AT is correctly folded and secreted from hepatocytes in the presence of a healthy PN (large arrow), populating the lung with a protective anti-protease environment. (b) In α1AT deficiency and, in particular, Z-variant disease, α1AT is misfolded and poorly secreted (small arrow). The presence of variant α1AT in liver leads to progressive hepatocyte pathology by a cell-autonomous mechanism by challenging the capacity of the PN to deal with the folding problem. Proteostasis imbalance in the liver is influenced by the presence of both the misfolded monomer and the accumulation of aggregated Z-variant in the ER of the hepatocyte. Liver disease can be exacerbated by environmental insults including alcohol, toxins, and pathogens. (c) α1AT deficiency affects the lung by a cell-nonautonomous mechanism. The loss of anti-protease protection normally afforded by WT α1AT is similar to environmental insults such as pollution and cigarette smoke, which taxes pulmonary proteostasis capacity through oxidative stress and triggers inflammatory correction pathways. Pathogens can further exacerbate inflammatory responses to amplify loss of proteostasis capacity.
In addition to generation of soluble misfolded monomers that are targeted to the proteasome, the Z-variant mutation also triggers polymer/aggregate formation in the ER, preventing export. Due to a conformational change in the “reactive loop” that is responsible for protease inhibition (19, 21), the E342K substitution opens up a β-sheet and allows the reactive loop of a second α1AT molecule to insert at this point to form a dimer that extends into polymers, the mechanism of which is under debate (19, 51–53). The abnormal aggregate accumulates in the ER of the hepatocyte. The formation of Z-α1AT aggregates can lead to a significant gain-of-toxic function in the liver. The hepatic cell partially protects itself from these ER-associated inclusion bodies using proteostasis-based ER-targeted autophagic pathways (10, 19, 20, 40, 54, 55).
Thus, in the FoldEx/Fx model of a healthy cell, such as a normal liver secreting wild-type α1AT, the position of the α1AT node and its edges lie beneath the PB, indicating that the PN of the ER is adequate to support folding for function and serum sufficiency (Figure 2a). In contrast, the energetics and kinetics of folding and misfolding of α1AT variant alleles largely fall outside the capacity of the PN and, hence, trigger liver disease in cell-autonomous fashion (Figure 3b). Declining PN function during aging (56, 57) is likely to significantly contribute to the advance of cell-autonomous α1AT deficiency and the progressive, age-related onset of liver disease (1, 29, 30).
PART 3. α1AT AS A MODEL FOR ENVIRONMENT-TRIGGERED CELL-NONAUTONOMOUS ASD
Serum α1AT is delivered to the lung by as-yet uncharacterized transepithelial transport pathways (Figure 3a). Unlike the cell-autonomous challenge α1AT variants poses to the hepatocyte during nascent synthesis, reduced levels of α1AT in the lung trigger cell-nonautonomous disease and pulmonary pathophysiology similar to that found in ASDs such as COPD and emphysema (Figure 3c). As should be evident, the clinical course of disease will reflect the biochemical and biophysical properties of a particular allele and its impact on the α1AT fold, some being more severe than others.
The principal role of α1AT in the lung is to inhibit, by irreversible binding, serine proteases, in particular those secreted by neutrophils for host defense purposes. Inhibition maintains lung integrity by preventing the degradation of the extracellular matrix in the alveolar interstitium (21, 22, 40, 58, 59). Thus, α1AT serves as an “environmental factor” in the lung that is necessary to prevent protein folding stress engendered by general protease attack on the lung epithelial layer. Its absence triggers inflammatory responses that signal to the proteostasis system to make every effort to repair the problem(s), at least until proteostasis capacity is reached. Beyond this point, pathology becomes the dominating feature of disease and therefore is a symptom, not a cause.
Consistent with its role as a cell-nonautonomous proteostasis agent in ASD, one of the most important genetic disorders predisposing the lung to COPD and emphysema is a deficiency in α1AT (19, 21, 22, 31, 52, 60). In other words, a folding challenge to proteostasis makes the lung more susceptible to environmental stress. Alleles identified to date that can be classified as potential deficiency-inducing and lung stress–inducing folds have been estimated to occur in 10% of cases of COPD (61), which suggests a substantial and undiagnosed impact of α1AT deficiency in the human population (48). These alleles induce panacinar emphysema, chronic bronchitis, bronchiectasis, and pulmonary vascular dilatation with severe hypoxaemia as early as the third to fifth decade of life (59, 62). Conversely, whereas α1AT is a risk factor for ASD, α1AT deficiency pathology is also strikingly enhanced by many environmental airway threats, particularly smoking, which triggers general oxidative stress and protein misfolding in lung cells, further exacerbating the already strained PN capacity. Thus, an inherited or environmental challenge to the lung proteostasis system predisposes pulmonary tissue to folding stress (63–67), suggesting that α1AT and ASDs have a common origin: inadequate protection by the endogenous cellular proteostasis program that becomes progressively weaker with age.
Although monomeric α1AT deficiency clearly contributes to variant α1AT ASD, Z-α1AT aggregates may also have a cell-nonautonomous impact on proteostasis. The Z-α1AT variant has been detected in lung lavages and in lung tissue sections from individuals with disease (19, 21, 22, 54, 68–70). One possibility is that these polymers may accelerate pulmonary symptoms through a gain-of-toxic-function mechanism not unlike the impact of toxic oligomers in triggering many neurodegenerative diseases (12, 29, 56, 71). It has been shown that wild-type α1AT can be internalized by endothelial cells and is proinflammatory (72). Z-α1AT has been reported to be localized with and chemotactic for neutrophils (70, 73, 74). We speculate that Z-variant α1AT aggregates may be taken up by endothelial cells as the UPR is activated in monocytes from individuals with α1AT deficiency (75). An excess of neutrophils and associated inflammatory response(s) found in COPD, emphysema and asthma are thought to accelerate the disease state. Indeed, the importance of proteostasis capacity is evident in hits recovered in statistically powered genome-wide association studies (GWAS) (76). These studies are beginning to define the architectural principles that could challenge the pulmonary proteostasis program. Genes involved in nicotine addiction leading to increased smoking may prematurely challenge the PN; genes involved in extracellular matrix stability may tax maintenance and repair proteostasis pathways responsible for fibrosis; and genes resulting in enhanced susceptibility to oxidative stress are all events that would have a common origin in exceeding PN capacity required to maintain the protein fold for normal lung cell function.
Thus, wild-type α1AT serves as a protective environmental factor in a cell-nonautonomous fashion in the pulmonary function. In contrast, variant-triggered α1AT deficiency alters the sustainability of alveolar epithelial integrity in response to protein misfolding, leading us to suggest that all ASDs have a common origin triggered by a challenge to the proteostasis system.
PART 4. THERAPEUTIC MANIPULATION OF THE PN: A NEW PARADIGM FOR ASD MANAGEMENT
In Parts 1–3 we have attempted to use α1AT deficiency to illustrate the operation of the proteostasis program in managing protein folding in a cell-autonomous fashion in the hepatocyte (Figure 3b), and we used the known role of α1AT as an environmental factor in the lung (Figure 3c) as an example of a cell-nonautonomous environmental threat to pulmonary physiology. In both instances, we have argued that disease is a consequence of proteostasis stress. From a therapeutic perspective, this new focus on the mechanism of pathogenesis (the proteostasis system) rather than symptoms (the inflammatory response) provides us with a rigorously defined pharmacological approach to address ASDs.
Currently, the limited treatments available for ASD pulmonary dysfunction (including those caused by α1AT deficiency) involve therapeutic inhalation of steroids like formoterol or salmeterol to reduce endpoint inflammatory/(immune) responses (77), lung transplantation, and/or intravenous augmentation (weekly or bimonthly) of pooled plasma-purified α1AT (62, 78, 79). The former create as many problems as they try to resolve, the latter are the most specific to α1AT disease etiology but are costly, and they currently lack proof of efficacy, if not negatively indicated, as a therapeutic alternative (80).
A new proteostasis-based therapeutic strategy for pulmonary disease would take advantage small molecules that regulate the composition and concentration of PN components through regulatory pathways including, among others, HSR (26, 81–86), UPR (87–93), UPS (94–96), anti-oxidant response elements (ARE), inhibition or activation histone deacetylase (HDAC)/histone acetylase (HAT) pathways that impact protein folding pathways (97–103), and mitochondrial UPR (oxidative stress pathways) (104). Each of these pathways protects the cell in response to the types of folding stress that would occur in ASDs. We refer to these compounds as proteostasis regulators (PRs) (1, 2, 5). The utility of PRs for the correction of misfolding disease is supported by recent observations that correction of cellular-folding defects can be achieved by adjusting the activity of the PN in cystic fibrosis (105), neurogenerative disease (85, 106–108), Friedreich's Ataxia (97, 98), and cancer (2, 91, 92, 96, 99, 100, 109, 110), all diseases that challenge proteostasis. By altering the composition of the PN (Figure 1) and hence the shape of the PB (Figure 2), these are anticipated to, for example, reduce and/or prevent misfolding in response to cell-autonomous inherited mutation/SNPs and/or misfolding in a cell-nonautonomous fashion in response to, for example, oxidative damage that can occur through environmental stressors such as smoking, air pollutants, or allergens (Figure 3). PRs could also operate at the tissue and organismal level in a cell-nonautonomous fashion through neural signaling pathways. As shown recently in C. elegans models of heat stress (30, 34), neuroendocrine-derived signaling pathways play a major role in regulation of PN stress protection in a cell-nonautonomous manner.
PART 5. OVERVIEW: PROTEOSTASIS AND THE MANAGEMENT OF HUMAN LUNG DISEASE
In Parts 1–4 we have attempted to provide a fresh perspective on ASD based on the concept that challenges to the protein fold are the common initiating event. The slow onset of pulmonary disease is defined by repetitive challenges to the PN capacity, which ultimately fails in response to aging. With this view in mind, we envision that PRs would fulfill an unmet need in pulmonary therapeutics by strengthening the biology of the proteostasis program that routinely protects us from environmental folding insults (1, 17, 18, 111). The lung proteostasis program, being one of the major frontline defenses to human pathology, is likely to benefit significantly if managed more effectively. Pharmacological management through PRs may provide considerable benefit in the clinic by giving lung tissue, through its own emergent biological protective proteostasis programs, a chance to protect itself against folding stress, apoptosis, and fibrosis. If applied in a timely fashion, given the role of proteostasis in development and longevity (5, 29, 30, 56), PRs may provide a favorable environment for regeneration of pulmonary function through management of the stem cell environment.
Funding for this research supported by the National Institutes of Health grants GM42336, GM33301, NS067643, AG031097, HL079442, HL095524, DK051870, AG036634, AG18917 and by the generous support of the AlphaOne Foundation and the Cystic Fibrosis Foundation.
Author Disclosure: M.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. W.E.B. was a consultant for FoldRX and Proteostasis Therapuetics Inc. (PTI). He is a co-founder of PTI.
References
- 1.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science 2008;319:916–919. [DOI] [PubMed] [Google Scholar]
- 2.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem 2009;78:959–991. [DOI] [PubMed] [Google Scholar]
- 3.Hutt DM, Balch WE. The proteome in balance. Science 2010; (In press). [DOI] [PMC free article] [PubMed]
- 4.Hutt DM, Powers ET, Balch WE. The proteostasis boundary in misfolding diseases of membrane traffic. FEBS Lett 2009;583:2639–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth DM, Balch WE. Modeling general proteostasis: proteome balance in health and disease. Curr Opin Cell Biol (2011), doi:10.1016/j.ceb.2010.11.001. [DOI] [PMC free article] [PubMed]
- 6.Finley D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu Rev Biochem 2009;78:477–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broadley SA, Hartl FU. The role of molecular chaperones in human misfolding diseases. FEBS Lett 2009;583:2647–2653. [DOI] [PubMed] [Google Scholar]
- 8.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 2009;43:67–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroeger H, Miranda E, MacLeod I, Perez J, Crowther DC, Marciniak SJ, Lomas DA. Endoplasmic reticulum-associated degradation (ERAD) and autophagy cooperate to degrade polymerogenic mutant serpins. J Biol Chem 2009;284:22793–22802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hidvegi T, Ewing M, Hale P, Dippold C, Beckett C, Kemp C, Maurice N, Mukherjee A, Goldbach C, Watkins S, et al. An autophagy-enhancing drug promotes degradation of mutant alpha1-antitrypsin Z and reduces hepatic fibrosis. Science 2010;329:229–232. [DOI] [PubMed] [Google Scholar]
- 11.Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol 2008;3:399–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev 2008;22:1427–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gidalevitz T, Kikis EA, Morimoto RI. A cellular perspective on conformational disease: the role of genetic background and proteostasis networks. Curr Opin Struct Biol 2010;20:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burdakov D, Petersen OH, Verkhratsky A. Intraluminal calcium as a primary regulator of endoplasmic reticulum function. Cell Calcium 2005;38:303–310. [DOI] [PubMed] [Google Scholar]
- 15.Mu TW, Ong DS, Wang YJ, Balch WE, Yates JR III, Segatori L, Kelly JW. Chemical and biological approaches synergize to ameliorate protein-folding diseases. Cell 2008;134:769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature 2008;454:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panowski SH, Dillin A. Signals of youth: endocrine regulation of aging in caenorhabditis elegans. Trends Endocrinol Metab 2009;20:259–264. [DOI] [PubMed] [Google Scholar]
- 18.Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu Rev Biochem 2008;77:727–754. [DOI] [PubMed] [Google Scholar]
- 19.Perlmutter DH. Autophagic disposal of the aggregation-prone protein that causes liver inflammation and carcinogenesis in alpha-1-antitrypsin deficiency. Cell Death Differ 2009;16:39–45. [DOI] [PubMed] [Google Scholar]
- 20.Gooptu B, Lomas DA. Conformational pathology of the serpins: themes, variations, and therapeutic strategies. Annu Rev Biochem 2009;78:147–176. [DOI] [PubMed] [Google Scholar]
- 21.Gooptu B, Ekeowa UI, Lomas DA. Mechanisms of emphysema in alpha1-antitrypsin deficiency: molecular and cellular insights. Eur Respir J 2009;34:475–488. [DOI] [PubMed] [Google Scholar]
- 22.Ekeowa UI, Gooptu B, Belorgey D, Hagglof P, Karlsson-Li S, Miranda E, Perez J, MacLeod I, Kroger H, Marciniak SJ, et al. Alpha1-antitrypsin deficiency, chronic obstructive pulmonary disease and the serpinopathies. Clin Sci (Lond) 2009;116:837–850. [DOI] [PubMed] [Google Scholar]
- 23.Geller R, Vignuzzi M, Andino R, Frydman J. Evolutionary constraints on chaperone-mediated folding provide an antiviral approach refractory to development of drug resistance. Genes Dev 2007;21:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shonhai A, Maier AG, Przyborski JM, Blatch GL. Intracellular protozoan parasites of humans: the role of molecular chaperones in development and pathogenesis. Protein Pept Lett 2011;18:143–157. [DOI] [PubMed] [Google Scholar]
- 25.Anelli T, Sitia R. Physiology and pathology of proteostasis in the early secretory compartment. Semin Cell Dev Biol 2010;21:520–525. [DOI] [PubMed] [Google Scholar]
- 26.Salminen A, Lehtonen M, Paimela T, Kaarniranta K. Celastrol: molecular targets of thunder god vine. Biochem Biophys Res Commun 2010;394:439–442. [DOI] [PubMed] [Google Scholar]
- 27.Bodas M, Min T, Vij N. Early-age-related changes in proteostasis augment immunopathogenesis of sepsis and acute lung injury. PLoS ONE 2010;5:e15480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sartori C, Scherrer U. Turning up the heat in the lungs. A key mechanism to preserve their function. Adv Exp Med Biol 2003;543:263–275. [PubMed] [Google Scholar]
- 29.Morimoto RI, Cuervo AM. Protein homeostasis and aging: taking care of proteins from the cradle to the grave. J Gerontol A Biol Sci Med Sci 2009;64:167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prahlad V, Morimoto RI. Integrating the stress response: lessons for neurodegenerative diseases from C. elegans. Trends Cell Biol 2009;19:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan ES, Silverman EK. Genetics of COPD and emphysema. Chest 2009;136:859–866. [DOI] [PubMed] [Google Scholar]
- 32.Castaldi PJ, Cho MH, Cohn M, Langerman F, Moran S, Tarragona N, Moukhachen H, Venugopal R, Hasimja D, Kao E, et al. The COPD genetic association compendium: a comprehensive online database of COPD genetic associations. Hum Mol Genet 2010;19:526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stockley RA, Mannino D, Barnes PJ. Burden and pathogenesis of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2009;6:524–526. [DOI] [PubMed] [Google Scholar]
- 34.Prahlad V, Cornelius T, Morimoto RI. Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science 2008;320:811–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci USA 2008;105:18525–18530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol 2010;11:515–528. [DOI] [PubMed] [Google Scholar]
- 37.Lindquist S. Protein folding sculpting evolutionary change. Cold Spring Harb Symp Quant Biol 2009;74:103–108. [DOI] [PubMed] [Google Scholar]
- 38.Wiseman RL, Powers ET, Buxbaum JN, Kelly JW, Balch WE. An adaptable standard for protein export from the endoplasmic reticulum. Cell 2007;131:809–821. [DOI] [PubMed] [Google Scholar]
- 39.Wiseman RL, Koulov A, Powers E, Kelly JW, Balch WE. Protein energetics in maturation of the early secretory pathway. Curr Opin Cell Biol 2007;19:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perlmutter DH, Brodsky JL, Balistreri WF, Trapnell BC. Molecular pathogenesis of alpha-1-antitrypsin deficiency-associated liver disease: a meeting review. Hepatology 2007;45:1313–1323. [DOI] [PubMed] [Google Scholar]
- 41.McCracken AA, Brodsky JL. Recognition and delivery of ERAD substrates to the proteasome and alternative paths for cell survival. Curr Top Microbiol Immunol 2005;300:17–40. [DOI] [PubMed] [Google Scholar]
- 42.Brodsky JL, Scott CM. Tipping the delicate balance: defining how proteasome maturation affects the degradation of a substrate for autophagy and endoplasmic reticulum associated degradation (ERAD). Autophagy 2007;3:623–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Termine DJ, Moremen KW, Sifers RN. The mammalian UPR boosts glycoprotein ERAD by suppressing the proteolytic downregulation of ER mannosidase I. J Cell Sci 2009;122:976–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sifers RN. Defining the ERAD connection: assembly required. Cell Cycle 2009;8:4026–4027. [PubMed] [Google Scholar]
- 45.Scott CM, Kruse KB, Schmidt BZ, Perlmutter DH, McCracken AA, Brodsky JL. ADD66, a gene involved in the endoplasmic reticulum-associated degradation of alpha-1-antitrypsin-Z in yeast, facilitates proteasome activity and assembly. Mol Biol Cell 2007;18:3776–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cabral CM, Liu Y, Moremen KW, Sifers RN. Organizational diversity among distinct glycoprotein endoplasmic reticulum-associated degradation programs. Mol Biol Cell 2002;13:2639–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Y, Termine DJ, Swulius MT, Moremen KW, Sifers RN. Human endoplasmic reticulum mannosidase I is subject to regulated proteolysis. J Biol Chem 2007;282:4841–4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan S, Huang L, McPherson J, Muzny D, Rouhani F, Brantly M, Gibbs R, Sifers RN. Single nucleotide polymorphism-mediated translational suppression of endoplasmic reticulum mannosidase I modifies the onset of end-stage liver disease in alpha1-antitrypsin deficiency. Hepatology 2009;50:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maattanen P, Gehring K, Bergeron JJ, Thomas DY. Protein quality control in the ER: the recognition of misfolded proteins. Semin Cell Dev Biol 2010;21:500–511. [DOI] [PubMed] [Google Scholar]
- 50.Kanehara K, Kawaguchi S, Ng DT. The EDEM and Yos9p families of lectin-like ERAD factors. Semin Cell Dev Biol 2007;18:743–750. [DOI] [PubMed] [Google Scholar]
- 51.Miranda E, Perez J, Ekeowa UI, Hadzic N, Kalsheker N, Gooptu B, Portmann B, Belorgey D, Hill M, Chambers S, et al. A novel monoclonal antibody to characterize pathogenic polymers in liver disease associated with alpha(1)-antitrypsin deficiency. Hepatology 2010. [DOI] [PubMed]
- 52.Knaupp AS, Bottomley SP. Serpin polymerization and its role in disease–the molecular basis of alpha1-antitrypsin deficiency. IUBMB Life 2009;61:1–5. [DOI] [PubMed] [Google Scholar]
- 53.Gooptu B, Miranda E, Nobeli I, Mallya M, Purkiss A, Brown SC, Summers C, Phillips RL, Lomas DA, Barrett TE. Crystallographic and cellular characterisation of two mechanisms stabilising the native fold of alpha1-antitrypsin: implications for disease and drug design. J Mol Biol 2009;387:857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaushal S, Annamali M, Blomenkamp K, Rudnick D, Halloran D, Brunt EM, Teckman JH. Rapamycin reduces intrahepatic alpha-1-antitrypsin mutant Z protein polymers and liver injury in a mouse model. Exp Biol Med (Maywood) 2010;235:700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gosai SJ, Kwak JH, Luke CJ, Long OS, King DE, Kovatch KJ, Johnston PA, Shun TY, Lazo JS, Perlmutter DH, et al. Automated high-content live animal drug screening using C. elegans expressing the aggregation prone serpin alpha1-antitrypsin Z. PLoS ONE 2010;5:e15460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Douglas PM, Dillin A. Protein homeostasis and aging in neurodegeneration. J Cell Biol 2010;190:719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ben-Zvi A, Miller EA, Morimoto RI. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc Natl Acad Sci USA 2009;106:14914–14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salahuddin P. Genetic variants of alpha1-antitrypsin. Curr Protein Pept Sci 2010;11:101–117. [DOI] [PubMed] [Google Scholar]
- 59.Kaplan A, Cosentino L. Alpha1-antitrypsin deficiency: forgotten etiology. Can Fam Physician 2010;56:19–24. [PMC free article] [PubMed] [Google Scholar]
- 60.Dahl M, Nordestgaard BG. Markers of early disease and prognosis in copd. Int J Chron Obstruct Pulmon Dis 2009;4:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Serres FJ, Blanco I, Fernandez-Bustillo E. Estimating the risk for alpha-1 antitrypsin deficiency among copd patients: Evidence supporting targeted screening. COPD 2006;3:133–139. [DOI] [PubMed] [Google Scholar]
- 62.Fromer L. Improving diagnosis and management of alpha-1 antitrypsin deficiency in primary care: translating knowledge into action. COPD 2010;7:192–198. [DOI] [PubMed] [Google Scholar]
- 63.Yang P, Bamlet WR, Sun Z, Ebbert JO, Aubry MC, Krowka MJ, Taylor WR, Marks RS, Deschamps C, Swensen SJ, et al. Alpha1-antitrypsin and neutrophil elastase imbalance and lung cancer risk. Chest 2005;128:445–452. [DOI] [PubMed] [Google Scholar]
- 64.Yang P, Wentzlaff KA, Katzmann JA, Marks RS, Allen MS, Lesnick TG, Lindor NM, Myers JL, Wiegert E, Midthun DE, et al. Alpha1-antitrypsin deficiency allele carriers among lung cancer patients. Cancer Epidemiol Biomarkers Prev 1999;8:461–465. [PubMed] [Google Scholar]
- 65.Sun Z, Yang P. Role of imbalance between neutrophil elastase and alpha 1-antitrypsin in cancer development and progression. Lancet Oncol 2004;5:182–190. [DOI] [PubMed] [Google Scholar]
- 66.Doi K, Horiuchi T, Uchinami M, Tabo T, Kimura N, Yokomachi J, Yoshida M, Tanaka K. Neutrophil elastase inhibitor reduces hepatic metastases induced by ischaemia-reperfusion in rats. Eur J Surg 2002;168:507–510. [DOI] [PubMed] [Google Scholar]
- 67.Yamaguchi K, Shimada S, Tashima S, Ogawa M. A potentially novel peptidase, resembling but distinct from neutrophil elastase, produced by carcinoma cells. Oncol Rep 2000;7:1017–1021. [DOI] [PubMed] [Google Scholar]
- 68.Davies MJ, Lomas DA. The molecular aetiology of the serpinopathies. Int J Biochem Cell Biol 2008;40:1273–1286. [DOI] [PubMed] [Google Scholar]
- 69.Morrison HM, Kramps JA, Burnett D, Stockley RA. Lung lavage fluid from patients with alpha 1-proteinase inhibitor deficiency or chronic obstructive bronchitis: anti-elastase function and cell profile. Clin Sci (Lond) 1987;72:373–381. [DOI] [PubMed] [Google Scholar]
- 70.Elliott PR, Bilton D, Lomas DA. Lung polymers in Z alpha1-antitrypsin deficiency-related emphysema. Am J Respir Cell Mol Biol 1998;18:670–674. [DOI] [PubMed] [Google Scholar]
- 71.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res 2008;192:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sohrab S, Petrusca DN, Lockett AD, Schweitzer KS, Rush NI, Gu Y, Kamocki K, Garrison J, Petrache I. Mechanism of alpha-1 antitrypsin endocytosis by lung endothelium. FASEB J 2009;23:3149–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mulgrew AT, Taggart CC, Lawless MW, Greene CM, Brantly ML, O'Neill SJ, McElvaney NG. Z alpha1-antitrypsin polymerizes in the lung and acts as a neutrophil chemoattractant. Chest 2004;125:1952–1957. [DOI] [PubMed] [Google Scholar]
- 74.Mahadeva R, Atkinson C, Li Z, Stewart S, Janciauskiene S, Kelley DG, Parmar J, Pitman R, Shapiro SD, Lomas DA. Polymers of Z alpha1-antitrypsin co-localize with neutrophils in emphysematous alveoli and are chemotactic in vivo. Am J Pathol 2005;166:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carroll TP, Greene CM, O'Connor CA, Nolan AM, O'Neill SJ, McElvaney NG. Evidence for unfolded protein response activation in monocytes from individuals with alpha-1 antitrypsin deficiency. J Immunol 2010;184:4538–4546. [DOI] [PubMed] [Google Scholar]
- 76.Hall IP, Lomas DA. The genetics of obstructive lung disease: big is beautiful. Thorax 2010;65:760–761. [DOI] [PubMed] [Google Scholar]
- 77.Welte T. Optimising treatment for COPD–new strategies for combination therapy. Int J Clin Pract 2009;63:1136–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kohnlein T, Welte T. Alpha-1 antitrypsin deficiency: pathogenesis, clinical presentation, diagnosis, and treatment. Am J Med 2008;121:3–9. [DOI] [PubMed] [Google Scholar]
- 79.Sinn PL, Burnight ER, McCray PB Jr. Progress and prospects: prospects of repeated pulmonary administration of viral vectors. Gene Ther 2009;16:1059–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gotzsche PC, Johansen HK. Intravenous alpha-1 antitrypsin augmentation therapy for treating patients with alpha-1 antitrypsin deficiency and lung disease. Cochrane Database Syst Rev 2010;7:CD007851. [DOI] [PubMed] [Google Scholar]
- 81.Kannaiyan R, Shanmugam MK, Sethi G. Molecular targets of celastrol derived from thunder of god vine: Potential role in the treatment of inflammatory disorders and cancer. Cancer Lett 2011;303:9–20. [DOI] [PubMed] [Google Scholar]
- 82.Brown IR. Heat shock proteins and protection of the nervous system. Ann N Y Acad Sci 2007;1113:147–158. [DOI] [PubMed] [Google Scholar]
- 83.Miyata Y, Chang L, Bainor A, McQuade TJ, Walczak CP, Zhang Y, Larsen MJ, Kirchhoff P, Gestwicki JE. High-throughput screen for Escherichia coli heat shock protein 70 (Hsp70/DnaK): ATPase assay in low volume by exploiting energy transfer. J Biomol Screen 2010;15:1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wisen S, Bertelsen EB, Thompson AD, Patury S, Ung P, Chang L, Evans CG, Walter GM, Wipf P, Carlson HA, et al. Binding of a small molecule at a protein-protein interface regulates the chaperone activity of Hsp70-Hsp40. ACS Chem Biol 2010;5:611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Evans CG, Chang L, Gestwicki JE. Heat shock protein 70 (Hsp70) as an emerging drug target. J Med Chem 2010;53:4585–4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chang L, Bertelsen EB, Wisen S, Larsen EM, Zuiderweg ER, Gestwicki JE. High-throughput screen for small molecules that modulate the ATPase activity of the molecular chaperone DnaK. Anal Biochem 2008;372:167–176. [DOI] [PubMed] [Google Scholar]
- 87.Wiseman RL, Zhang Y, Lee KP, Harding HP, Haynes CM, Price J, Sicheri F, Ron D. Flavonol activation defines an unanticipated ligand-binding site in the kinase-RNase domain of IRE1. Mol Cell 2010;38:291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Volchuk A, Ron D. The endoplasmic reticulum stress response in the pancreatic beta-cell. Diabetes Obes Metab 2010;12:48–57. [DOI] [PubMed] [Google Scholar]
- 89.Wiseman RL, Balch WE. A new pharmacology–drugging stressed folding pathways. Trends Mol Med 2005;11:347–350. [DOI] [PubMed] [Google Scholar]
- 90.Wright CM, Chovatiya RJ, Jameson NE, Turner DM, Zhu G, Werner S, Huryn DM, Pipas JM, Day BW, Wipf P, et al. Pyrimidinone-peptoid hybrid molecules with distinct effects on molecular chaperone function and cell proliferation. Bioorg Med Chem 2008;16:3291–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Porter JR, Fritz CC, Depew KM. Discovery and development of Hsp90 inhibitors: a promising pathway for cancer therapy. Curr Opin Chem Biol 2010;14:412–420. [DOI] [PubMed] [Google Scholar]
- 92.Kim YS, Alarcon SV, Lee S, Lee MJ, Giaccone G, Neckers L, Trepel JB. Update on Hsp90 inhibitors in clinical trial. Curr Top Med Chem 2009;9:1479–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Luo W, Sun W, Taldone T, Rodina A, Chiosis G. Heat shock protein 90 in neurodegenerative diseases. Mol Neurodegener 2010;5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee BH, Lee MJ, Park S, Oh DC, Elsasser S, Chen PC, Gartner C, Dimova N, Hanna J, Gygi SP, et al. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature 2010;467:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roelofs J, Park S, Haas W, Tian G, McAllister FE, Huo Y, Lee BH, Zhang F, Shi Y, Gygi SP, et al. Chaperone-mediated pathway of proteasome regulatory particle assembly. Nature 2009;459:861–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Genin E, Reboud-Ravaux M, Vidal J. Proteasome inhibitors: recent advances and new perspectives in medicinal chemistry. Curr Top Med Chem 2010;10:232–256. [DOI] [PubMed] [Google Scholar]
- 97.Rai M, Soragni E, Chou CJ, Barnes G, Jones S, Rusche JR, Gottesfeld JM, Pandolfo M. Two new pimelic diphenylamide HDAC inhibitors induce sustained frataxin upregulation in cells from Friedreich's ataxia patients and in a mouse model. PLoS ONE 2010;5:e8825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thomas EA, Coppola G, Desplats PA, Tang B, Soragni E, Burnett R, Gao F, Fitzgerald KM, Borok JF, Herman D, et al. The HDAC inhibitor 4b ameliorates the disease phenotype and transcriptional abnormalities in Huntington's disease transgenic mice. Proc Natl Acad Sci USA 2008;105:15564–15569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wiech NL, Fisher JF, Helquist P, Wiest O. Inhibition of histone deacetylases: a pharmacological approach to the treatment of non-cancer disorders. Curr Top Med Chem 2009;9:257–271. [DOI] [PubMed] [Google Scholar]
- 100.Glauben R, Sonnenberg E, Zeitz M, Siegmund B. HDAC inhibitors in models of inflammation-related tumorigenesis. Cancer Lett 2009;280:154–159. [DOI] [PubMed] [Google Scholar]
- 101.Camins A, Sureda FX, Junyent F, Verdaguer E, Folch J, Pelegri C, Vilaplana J, Beas-Zarate C, Pallas M. Sirtuin activators: designing molecules to extend life span. Biochim Biophys Acta 2010;1799:740–749. [DOI] [PubMed] [Google Scholar]
- 102.Sun AY, Wang Q, Simonyi A, Sun GY. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol 2010;41:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sadruddin S, Arora R. Resveratrol: biologic and therapeutic implications. J Cardiometab Syndr 2009;4:102–106. [DOI] [PubMed] [Google Scholar]
- 104.Haynes CM, Ron D. The mitochondrial UPR-protecting organelle protein homeostasis. J Cell Sci 2010;123:3849–3855. [DOI] [PubMed] [Google Scholar]
- 105.Hutt DM, Herman D, Rodrigues AP, Noel S, Pilewski JM, Matteson J, Hoch B, Kellner W, Kelly JW, Schmidt A, et al. Reduced histone deacetylase 7 activity restores function to misfolded CFTR in cystic fibrosis. Nat Chem Biol 2010;6:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jinwal UK, O'Leary JC III, Borysov SI, Jones JR, Li Q, Koren J III, Abisambra JF, Vestal GD, Lawson LY, Johnson AG, et al. Hsc70 rapidly engages tau after microtubule destabilization. J Biol Chem 2010;285:16798–16805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Patury S, Miyata Y, Gestwicki JE. Pharmacological targeting of the Hsp70 chaperone. Curr Top Med Chem 2009;9:1337–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jinwal UK, Miyata Y, Koren J III, Jones JR, Trotter JH, Chang L, O'Leary J, Morgan D, Lee DC, Shults CL, et al. Chemical manipulation of Hsp70 ATPase activity regulates tau stability. J Neurosci 2009;29:12079–12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beumer JH, Tawbi H. Role of histone deacetylases and their inhibitors in cancer biology and treatment. Curr Clin Pharmacol 2010;5:196–208. [DOI] [PubMed] [Google Scholar]
- 110.Mitsiades CS, Hideshima T, Chauhan D, McMillin DW, Klippel S, Laubach JP, Munshi NC, Anderson KC, Richardson PG. Emerging treatments for multiple myeloma: beyond immunomodulatory drugs and bortezomib. Semin Hematol 2009;46:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cohen E, Dillin A. The insulin paradox: aging, proteotoxicity and neurodegeneration. Nat Rev Neurosci 2008;9:759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]