Abstract
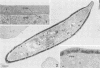
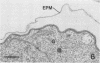
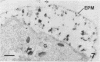
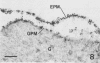
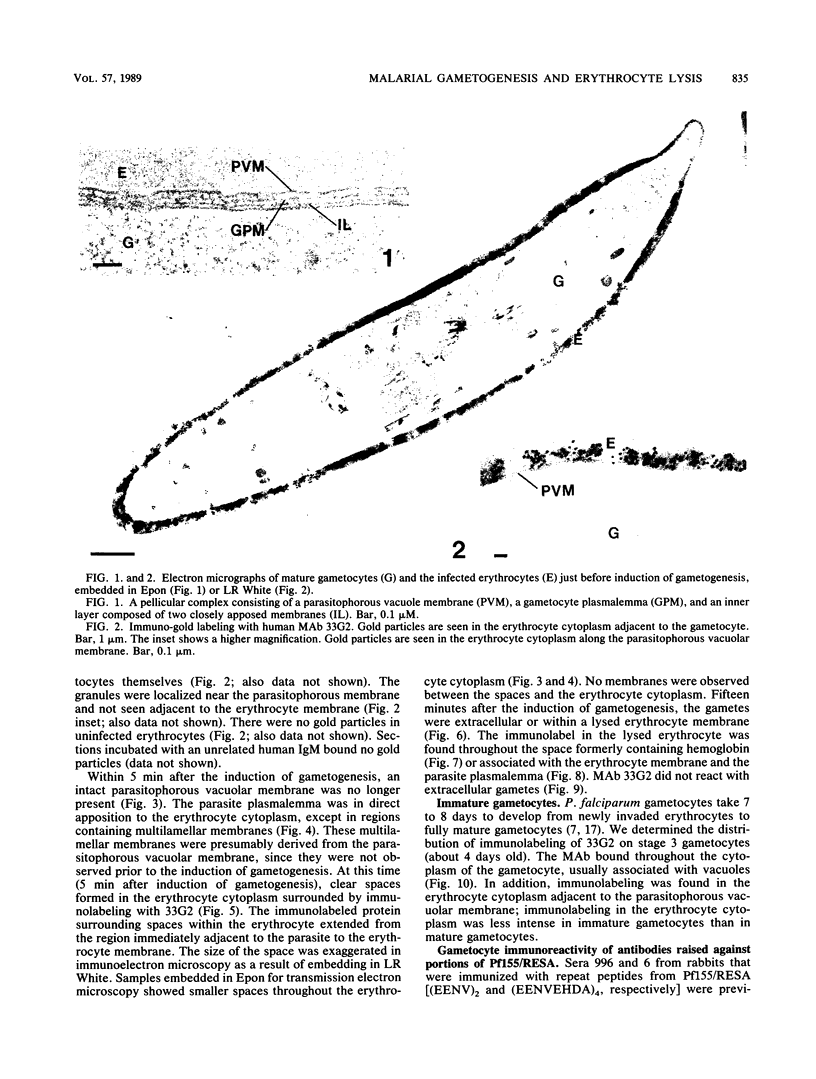
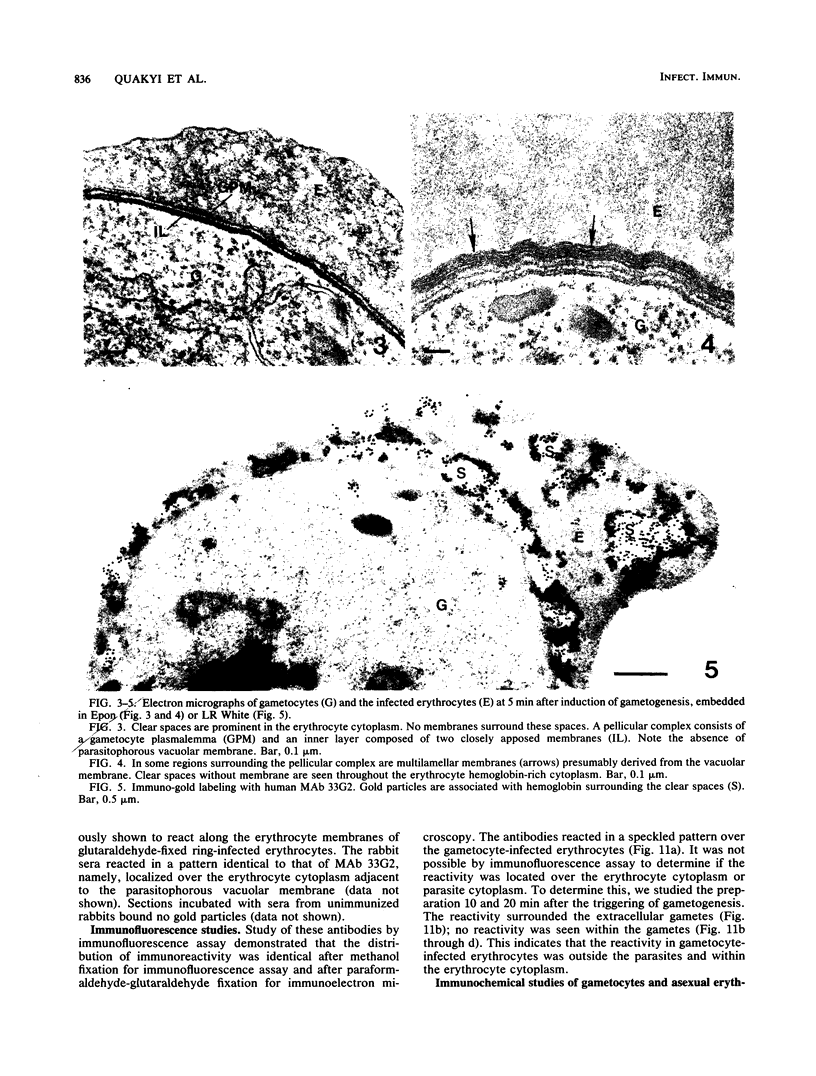
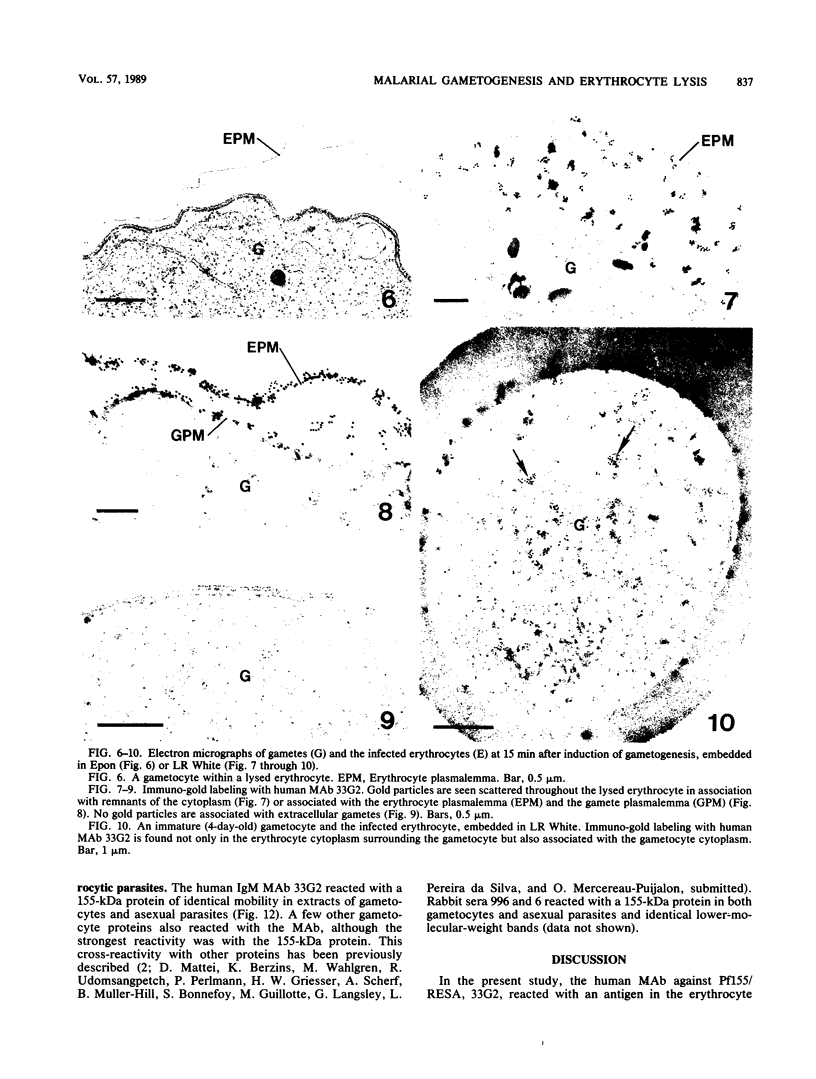
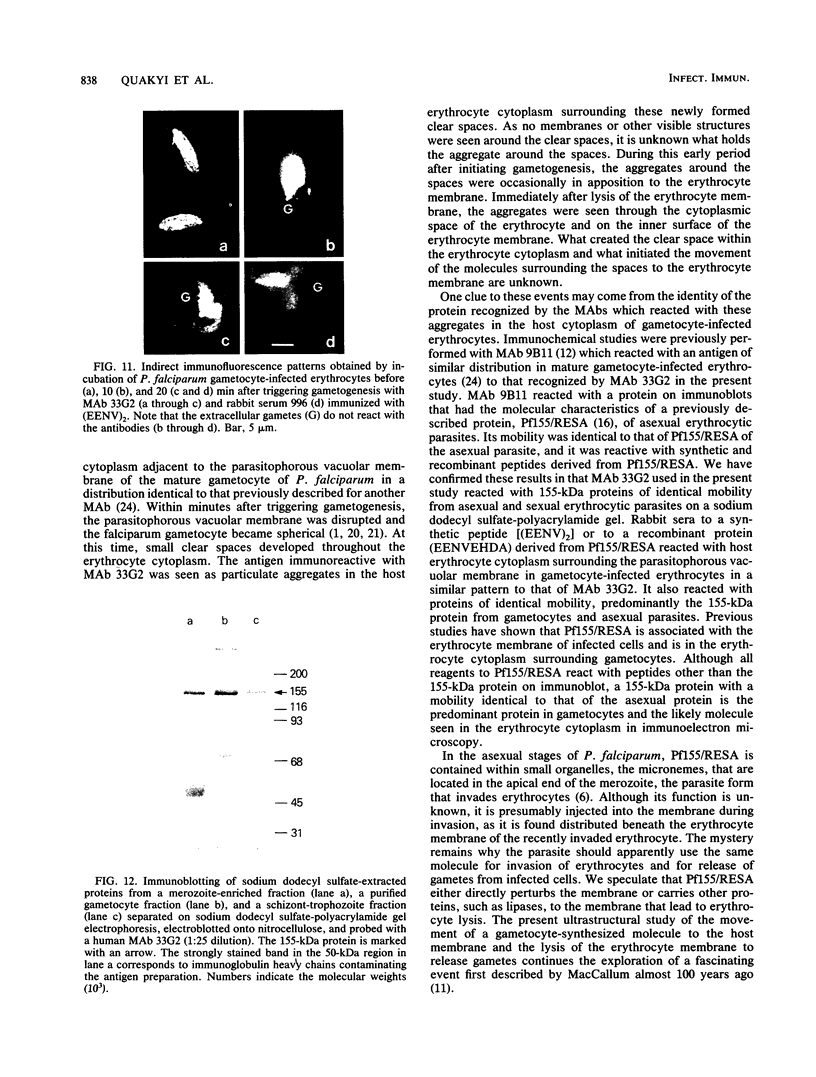
Erythrocytes containing mature gametocytes of Plasmodium falciparum circulate in the blood until they are ingested by a mosquito, an event that triggers gametogenesis and lysis of the infected erythrocyte. It was previously shown that a parasite protein (Pf155/RESA) accumulates in the erythrocyte cytoplasm next to the parasitophorous vacuolar membrane (S. Uni, A. Masuda, M. J. Stewart, R. Nussenzweig, and M. Aikawa, Am. J. Trop. Med. Hyg., 36:481-488, 1987). Using a monoclonal antibody to Pf155/RESA and rabbit sera to two different repeat peptides of Pf155/RESA, we have studied the location of Pf155/RESA after induction of gametogenesis. Five minutes after triggering gametogenesis, the parasitophorous membrane no longer surrounded the parasite, bringing the parasite membrane in contact with the erythrocyte cytoplasm. Clear spaces appeared throughout the hemoglobin-rich host cytoplasm; Pf155/RESA was now localized in the cytoplasm directly surrounding the spaces. No membrane existed between the spaces and the erythrocyte cytoplasm. The spaces with surrounding Pf155/RESA protein extended to the erythrocyte membrane. After lysis of the erythrocyte membrane (15 min after triggering gametogenesis), the protein was distributed along the erythrocyte membrane and throughout the space between the gamete and the erythrocyte membrane. The mechanism by which Pf155/RESA remained aggregated around the spaces and its role in erythrocyte lysis are unknown. It is of interest that the parasite appeared to use the same molecule during invasion of erythrocytes and during release of gametes from infected erythrocytes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M., Carter R., Ito Y., Nijhout M. M. New observations on gametogenesis, fertilization, and zygote transformation in Plasmodium gallinaceum. J Protozool. 1984 Aug;31(3):403–413. doi: 10.1111/j.1550-7408.1984.tb02987.x. [DOI] [PubMed] [Google Scholar]
- Anders R. F. Multiple cross-reactivities amongst antigens of Plasmodium falciparum impair the development of protective immunity against malaria. Parasite Immunol. 1986 Nov;8(6):529–539. doi: 10.1111/j.1365-3024.1986.tb00867.x. [DOI] [PubMed] [Google Scholar]
- Aslund L., Sjölander A., Wahlgren M., Wåhlin B., Ruangjirachuporn W., Berzins K., Wigzell H., Perlmann P., Pettersson U. Synthetic gene construct expressing a repeated and highly immunogenic epitope of the Plasmodium falciparum antigen Pf155. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1399–1403. doi: 10.1073/pnas.84.5.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzins K., Perlmann H., Wåhlin B., Carlsson J., Wahlgren M., Udomsangpetch R., Björkman A., Patarroyo M. E., Perlmann P. Rabbit and human antibodies to a repeated amino acid sequence of a Plasmodium falciparum antigen, Pf 155, react with the native protein and inhibit merozoite invasion. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1065–1069. doi: 10.1073/pnas.83.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzins K., Wahlgren M., Perlmann P. Studies on the specificity of anti-erythrocyte antibodies in the serum of patients with malaria. Clin Exp Immunol. 1983 Nov;54(2):313–318. [PMC free article] [PubMed] [Google Scholar]
- Brown G. V., Culvenor J. G., Crewther P. E., Bianco A. E., Coppel R. L., Saint R. B., Stahl H. D., Kemp D. J., Anders R. F. Localization of the ring-infected erythrocyte surface antigen (RESA) of Plasmodium falciparum in merozoites and ring-infected erythrocytes. J Exp Med. 1985 Aug 1;162(2):774–779. doi: 10.1084/jem.162.2.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R., Miller L. H. Evidence for environmental modulation of gametocytogenesis in Plasmodium falciparum in continuous culture. Bull World Health Organ. 1979;57 (Suppl 1):37–52. [PMC free article] [PubMed] [Google Scholar]
- Coppel R. L., Cowman A. F., Anders R. F., Bianco A. E., Saint R. B., Lingelbach K. R., Kemp D. J., Brown G. V. Immune sera recognize on erythrocytes Plasmodium falciparum antigen composed of repeated amino acid sequences. 1984 Aug 30-Sep 5Nature. 310(5980):789–792. doi: 10.1038/310789a0. [DOI] [PubMed] [Google Scholar]
- Hadley T. J. Invasion of erythrocytes by malaria parasites: a cellular and molecular overview. Annu Rev Microbiol. 1986;40:451–477. doi: 10.1146/annurev.mi.40.100186.002315. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Masuda A., Zavala F., Nussenzweig V., Nussenzweig R. S. Monoclonal anti-gametocyte antibodies identify an antigen present in all blood stages of Plasmodium falciparum. Mol Biochem Parasitol. 1986 Jun;19(3):213–222. doi: 10.1016/0166-6851(86)90003-4. [DOI] [PubMed] [Google Scholar]
- Nijhout M. M., Carter R. Gamete development in malaria parasites: bicarbonate-dependent stimulation by pH in vitro. Parasitology. 1978 Feb;76(1):39–53. doi: 10.1017/s0031182000047375. [DOI] [PubMed] [Google Scholar]
- Nijhout M. M. Plasmodium gallinaceum: exflagellation stimulated by a mosquito factor. Exp Parasitol. 1979 Aug;48(1):75–80. doi: 10.1016/0014-4894(79)90056-0. [DOI] [PubMed] [Google Scholar]
- Perlmann H., Berzins K., Wahlgren M., Carlsson J., Björkman A., Patarroyo M. E., Perlmann P. Antibodies in malarial sera to parasite antigens in the membrane of erythrocytes infected with early asexual stages of Plasmodium falciparum. J Exp Med. 1984 Jun 1;159(6):1686–1704. doi: 10.1084/jem.159.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnudurai T., Lensen A. H., Meis J. F., Meuwissen J. H. Synchronization of Plasmodium falciparum gametocytes using an automated suspension culture system. Parasitology. 1986 Oct;93(Pt 2):263–274. doi: 10.1017/s003118200005143x. [DOI] [PubMed] [Google Scholar]
- Prieur A. M., Kaufmann M. T., Griscelli C., Dayer J. M. Specific interleukin-1 inhibitor in serum and urine of children with systemic juvenile chronic arthritis. Lancet. 1987 Nov 28;2(8570):1240–1242. doi: 10.1016/s0140-6736(87)91854-x. [DOI] [PubMed] [Google Scholar]
- Quakyi I. A., Carter R., Rener J., Kumar N., Good M. F., Miller L. H. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J Immunol. 1987 Dec 15;139(12):4213–4217. [PubMed] [Google Scholar]
- Rener J., Graves P. M., Carter R., Williams J. L., Burkot T. R. Target antigens of transmission-blocking immunity on gametes of plasmodium falciparum. J Exp Med. 1983 Sep 1;158(3):976–981. doi: 10.1084/jem.158.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden R. E., Canning E. U., Bray R. S., Smalley M. E. Gametocyte and gamete development in Plasmodium falciparum. Proc R Soc Lond B Biol Sci. 1978 Jun 5;201(1145):375–399. doi: 10.1098/rspb.1978.0051. [DOI] [PubMed] [Google Scholar]
- Sinden R. E., Canning E. U., Spain B. Gametogenesis and fertilization in Plasmodium yoelii nigeriensis: a transmission electron microscope study. Proc R Soc Lond B Biol Sci. 1976 Mar 30;193(1110):55–76. doi: 10.1098/rspb.1976.0031. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Udomsangpetch R., Lundgren K., Berzins K., Wåhlin B., Perlmann H., Troye-Blomberg M., Carlsson J., Wahlgren M., Perlmann P., Björkman A. Human monoclonal antibodies to Pf 155, a major antigen of malaria parasite Plasmodium falciparum. Science. 1986 Jan 3;231(4733):57–59. doi: 10.1126/science.3510452. [DOI] [PubMed] [Google Scholar]
- Uni S., Masuda A., Stewart M. J., Igarashi I., Nussenzweig R., Aikawa M. Ultrastructural localization of the 150/130 Kd antigens in sexual and asexual blood stages of Plasmodium falciparum-infected human erythrocytes. Am J Trop Med Hyg. 1987 May;36(3):481–488. doi: 10.4269/ajtmh.1987.36.481. [DOI] [PubMed] [Google Scholar]
- Walliker D., Quakyi I. A., Wellems T. E., McCutchan T. F., Szarfman A., London W. T., Corcoran L. M., Burkot T. R., Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987 Jun 26;236(4809):1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]