Abstract
The plasma membrane constitutes a barrier that maintains the essential differences between the cytosol and the extracellular environment. Plasmalemmal injury is a common event during the life of many cells that often leads to their premature, necrotic death. Blebbing – a display of plasmalemmal protrusions – is a characteristic feature of injured cells. In this study, we disclose a previously unknown role for blebbing in furnishing resistance to plasmalemmal injury. Blebs serve as precursors for injury-induced intracellular compartments that trap damaged segments of the plasma membrane. Hence, loss of cytosol and the detrimental influx of extracellular constituents are confined to blebs that are sealed off from the cell body by plugs of annexin A1 – a Ca2+- and membrane-binding protein. Our findings shed light on a fundamental process that contributes to the survival of injured cells. By targeting annexin A1/blebbing, new therapeutic approaches could be developed to avert the necrotic loss of cells in a variety of human pathologies.
Keywords: plasma membrane, repair, annexin, calcium, blebbing
Physical disruption of the plasma membrane is common in cells that operate under conditions of mechanical stress.1, 2 The permeability barrier can also be breached by chemical means: pathogens gain access to host cells by secreting pore-forming toxins and phospholipases, and the host's own immune system uses pore-forming proteins to eliminate both pathogens and the pathogen-invaded cells.3, 4, 5 Nucleated cells survive the disruption of their plasma membrane by a process of resealing.1, 2, 6, 7, 8, 9 If resealing fails, the intracellular contents are lost. But irreversible damage can occur even before this event if the influx of extracellular Ca2+ is not restrained.9, 10, 11 An abrupt elevation in the intracellular concentration of Ca2+ ([Ca2+]i) activates intracellular proteinases and phospholipases, thereby causing generalized damage to the phospholipid bilayer and the cytoskeleton.2, 12 It is thus hardly surprising that an elevation in [Ca2+]i is sensed as an ‘immediate danger' signal by an injured cell.2, 13, 14 In injured cells the influx of extracellular Ca2+ triggers a process of plasma membrane blebbing, which is often considered to be a harbinger of cell death.15, 16, 17, 18, 19, 20, 21, 22 Blebs are sprouted by cells whose plasma membrane has become locally detached from the actin cortex.15, 16, 17, 18, 19 The detachment and surge of cytoplasm into the newly formed blebs are driven by a contraction of the cortical actomyosin, which generates hydrostatic pressure and thus drives herniations of the plasma membrane.19, 23 Consequently, blebbing can be triggered by a variety of Ca2+-dependent or Ca2+-independent mechanisms that are known to activate myosin or to induce a rearrangement of cytoskeletal and cortical structures.18, 24 Blebbing is manifested in a number of physiological processes, such as cytokinesis, cell spreading and locomotion.15, 16, 17, 18, 19 However, it is most commonly associated with cell injury and apoptosis.17, 18, 20, 21, 22 Albeit so, the specific role of blebbing in physiological and pathological processes is still controversial.17, 18 In this study, we present a hitherto unknown role of blebbing as a mechanism of resistance to plasmalemmal injury. In this capacity, blebs act as a trap for the damaged membrane segment and its adjoining cytosol, and are sealed off from the cell body by plugs of annexin A1 – a Ca2+- and membrane-binding protein.25 Following plasmalemmal injury, the loss of intracellular constituents is thus confined to the area of the blebs. Above all, the sealed-off blebs serve as a sink of potentially damaging Ca2+, thereby averting detrimental, Ca2+-triggered proteolysis and lipolysis within the cell proper.
Results
Plasmalemmal injury triggers blebbing
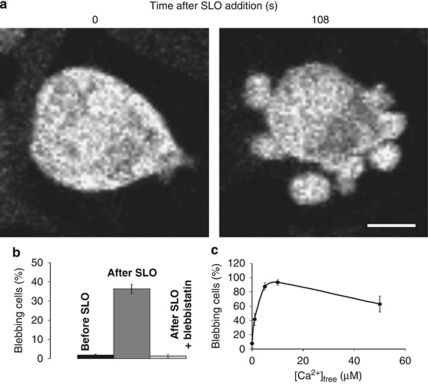
Human embryonic kidney (HEK 293) cells responded to permeabilization with streptolysin O (SLO) – a bacterial pore-forming toxin – by sprouting multiple transient blebs (Figure 1a; Supplementary Movie 1). Whereas only 1.8±0.6% of the cells blebbed before the addition of SLO (440 cells in 16 independent experiments), 36.4±2.4% of the same cell population blebbed after its application (Figure 1b; t-test; P<0.01).
Figure 1.
An injury-induced influx of Ca2+ triggers blebbing, which is mediated by the contraction of myosin II. (a) HEK 293 cells sprout numerous blebs after SLO-induced perforation. The images of the CFP-expressing cells are presented in the grey-scale mode. Magnification bar=10 μm. (b) Bar graph depicting the percentage of cells manifesting blebs before the addition of SLO, after the addition of SLO, and after pre-treatment with blebbistatin followed by the addition of SLO. (c) The graph shows the percentage of SLO-perforated cells manifesting blebs at the indicated [Ca2+]free. Mean values (±S.E.M.) are presented
Contraction of the cortical actomyosin was a driving force in bleb formation (Figure 1b); only 1.4±0.8% of the cells that had been pre-treated with blebbistatin – a selective inhibitor of myosin II ATPase26 – responded to SLO permeabilization by blebbing (125 cells in 4 experiments; t-test; P<0.01).
SLO-induced blebbing was the direct consequence of an injury-induced influx of Ca2+. Even after permeabilization, relatively few cells blebbed if [Ca2+]free in the extracellular milieu was below 100 nM (Figure 1c; experimental point: [Ca2+]free=0). When [Ca2+]free was increased to 1 μM, the percentage of blebbing cells increased significantly, attaining a maximum at 5–10 μM (Figure 1c; for each experimental condition, a minimal number of 65 cells was recorded in four independent experiments; P<0.01 for any experimental condition compared with the experimental point [Ca2+]free=0). A further increase in extracellular [Ca2+]free inhibited blebbing (Figure 1c; see also Figure 1b; the bar ‘after SLO' represents data obtained at extracellular [Ca2+]=2.5 mM). This inhibition is most likely the consequence of a sharp elevation in [Ca2+]i in permanently permeabilized cells9 that causes generalized damage to the phospholipid bilayer and cytoskeleton and leads to a depletion of intracellular ATP levels.2
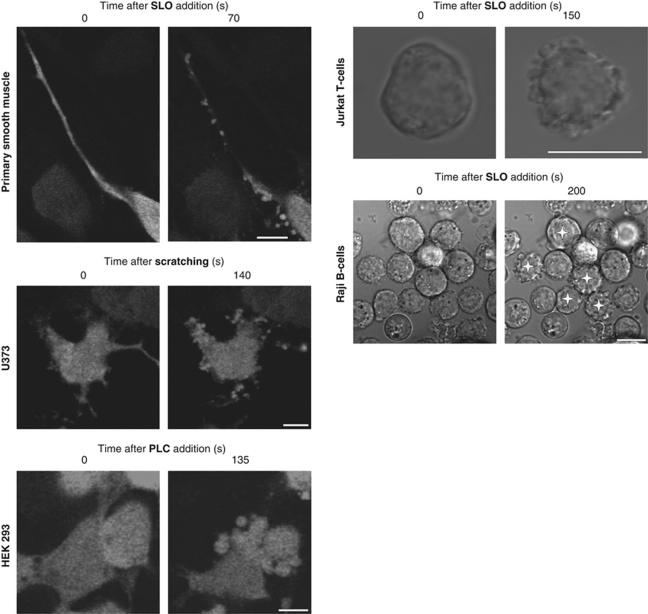
Blebbing was a universal cellular response to plasmalemmal injury: all cell types we have tested so far blebbed in response to injury irrespective of the nature of the damaging agent (Figure 2; Supplementary Movies 1 and 2).
Figure 2.
Blebbing is a universal cellular response to plasmalemmal injury. Blebbing was recorded in SLO-perforated primary cultures of human myometrial smooth muscle cells, Jurkat T cells and Raji B cells, in mechanically injured human astrocytoma cells (U 373 cells), and in HEK 293 cells that had been treated with bacterial phospholipase C. Raji cells are shown at low magnification: blebbing cells are marked by asterisks. The images of non-transfected (Raji and Jurkat) or the CFP-expressing cells (smooth muscle, U 373, HEK 293) are presented in grey-scale mode. Magnification bar=10 μm
Blebbing protects injured cells
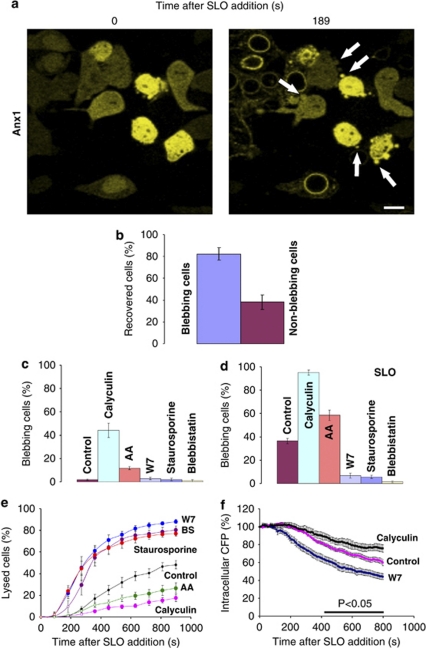
To evaluate the role of blebbing during plasmalemmal injury, we simultaneously monitored bleb dynamics and the outcome of the damage in individual living cells. The fate of the perforated cells was visualized by monitoring the intracellular dynamics of annexin A1 (Supplementary Figure S1; see also Babiychuk et al., 2009). The number of cells manifesting an irreversible translocation of annexin A1 (lysed cells) was scored in blebbing cells as well as in those that failed to bleb after exposure to SLO (Figure 3a). Blebbing cells were more prone to recovery after an SLO attack than were non-blebbing ones, thereby indicating that blebbing contributed to cell survival in the wake of plasmalemmal injury (Figure 3b).
Figure 3.
Blebbing protects cells from plasmalemmal damage. (a) Lysed cells are characterized by membrane-translocated annexin A1-YFP (Anx1). Cells recovering from an SLO attack (characterized by a cytoplasmic localization of annexin A1-YFP) protrude numerous blebs (arrows). Magnification bar=10 μm. (b) Augmented blebbing is associated with cells that are recovering from an SLO attack: 82±6% of the blebbing cells (n=85) recovered from an SLO attack, as opposed to 38±6% of the non-blebbing ones (n=140; t-test, P<0.01). Mean values (±S.E.M.) for nine independent experiments are presented. (c) Non-permeabilized HEK 293 cells (double-transfected with annexin A1-YFP and CFP) that had been treated with either acrylamide (AA; 241 cells; 9 independent experiments) or calyculin (203 cells; 9 experiments) were more prone to blebbing than untreated cells (control: 440 cells; 15 experiments; P<0.01 for both experimental conditions compared with the control). In non-permeabilized cells that had been treated with staurosporine (226 cells; 9 experiments), W7 (231 cells; 9 experiments) or blebbistatin (BS; 125 cells; 4 experiments), the inhibition was obscured by a low level of background blebbing. Mean values (±S.E.M.) are presented. (d) After pharmacological treatment the cells supplying the data in (c) were permeabilized with SLO. SLO-permeabilized cells that had been pre-treated with either acrylamide or calyculin were more prone to blebbing than untreated SLO-permeabilized cells. In contrast, pre-treatment with staurosporine, W7 or blebbistatin inhibited SLO-induced blebbing. Mean values (±S.E.M.) are presented (P<0.01 for any experimental condition compared to the control). (e, f) The cells supplying the data in (d) were analysed for their annexin A1 (e) or CFP (f) status. Mean values (±S.E.M.) are presented. In (e), the filled symbols denote the times after SLO exposure at which the differences between any experimental condition and the control reached statistical significance (t-test; P<0.01). In (f), the horizontal bar denotes the times after SLO exposure at which the differences between any experimental condition reached statistical significance (t-test; P<0.05)
To afford causal evidence of the protective function of blebbing, we monitored the resistance to an SLO attack by cells whose blebbing was either inhibited or activated pharmacologically. Acrylamide and calyculin – a broad-spectrum phosphatase inhibitor – are known to activate blebbing.16 Indeed, blebbing was significantly amplified in non-permeabilized HEK 293 cells that had been treated with either acrilamide or calyculin (Figure 3c). Staurosporine, a broad-spectrum kinase inhibitor, W7, an inhibitor of myosin light chain kinase, and blebbistatin are known to inhibit blebbing.16, 26 In non-permeabilized HEK 293 cells, an inhibition induced by either agent was obscured by a low level of background blebbing in control cells (Figure 3c). SLO permeabilization resulted in significant amplification of the background blebbing (Figures 1a and 3c, d, control), which revealed the inhibitory effects of staurosporine, W7 or blebbistatin (Figure 3d). In SLO-permeabilized cells that had been pre-treated with either acrylamide or calyculin, blebbing was further amplified (Figure 3c and d). The analysis of the intracellular dynamics of annexin A1 revealed acrylamide- and calyculin-pre-treated cells to be more likely to withstand an SLO attack than control cells (Figure 3e; Supplementary Figure S2). On the other hand, cells that had been pre-treated with staurosporine, W7 or blebbistatin were more likely to be lysed than control cells (Figure 3e; Supplementary Figure S2). Accordingly, the cytoplasmic components (tracked with the cytoplasmic cyan-fluorescent protein, CFP) were lost more rapidly from W7-pre-treated cells than from either the control cells or the calyculin-pre-treated ones (Figure 3f). Hence, cells that had been pharmacologically manipulated to bleb extensively were more likely to withstand a toxin attack than were cells in which blebbing had been inhibited.
Damaged regions are confined to blebs
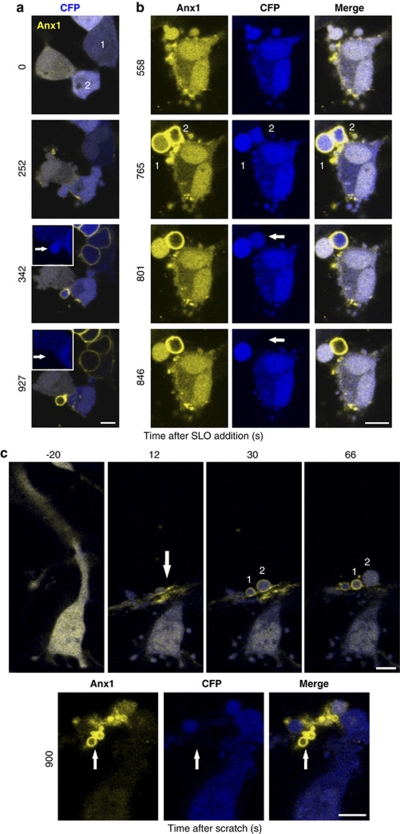
The most compelling evidence for a protective role of blebbing during plasmalemmal injury is afforded by a direct observation of blebs in action. We simultaneously monitored blebbing, an elevation in [Ca2+]i (membrane translocation of annexin A1) and the loss of cytoplasm (CFP-leakage) in SLO-treated HEK 293 cells (Figure 4). The cell marked ‘1' in Figure 4a did not respond to the SLO attack by blebbing (Supplementary Movie 3). After permeabilization this cell was confronted with a massive influx of Ca2+, which led to a global translocation of annexin A1 (Figure 4a: time after SLO addition=342 and 972 s) and a complete loss of cytoplasm (Figure 4a: time after SLO addition=972 s). In contrast, cell ‘2' in Figure 4a responded to an SLO attack by extensive blebbing (Figure 4a: time after SLO addition=252 s; Supplementary Movie 3). As for cell ‘1', the membrane permeability barrier in cell ‘2' was effectively breached by SLO. However, the elevation in [Ca2+] did not implicate the entire cytoplasm, but was confined to a single bleb (Figure 4a: time after SLO addition=342 and 927 s). Similarly, although cytoplasmic CFP leaked out of the bleb, it was retained by the rest of the cell (Figure 4a: time after SLO addition=927 s; arrows in the insets).
Figure 4.
Blebbing protects injured cells by isolating the damaged regions. Blebbing, an elevation in [Ca2+]i (membrane translocation of annexin A1) and the loss of cytoplasm (CFP-leakage) were simultaneously monitored in SLO-permeabilized HEK 293 cells (a, b) and in mechanically injured U 373 cells (c). Numbers and arrows highlight the regions of interest that are discussed in the text. Magnification bar=10 μm
In Figure 4b (Supplementary Movie 4), the SLO-induced elevation in Ca2+ and leakage of CFP are documented for two blebs. An efficient permeabilization of the cell was accompanied by a Ca2+ elevation in both of the blebs but not in the rest of the cytoplasm (Figure 4b: time after SLO addition=765 s). After permeabilization, the damaged plasma membrane of bleb ‘1' was efficiently repaired and excess of Ca2+ was pumped out, thereby resulting in a back translocation of annexin A1 (Figure 4b: times after SLO addition=801 and 846 s). In contrast, the repair mechanism in bleb ‘2' was inefficient: the intrableb [Ca2+] remained elevated and annexin A1 remained associated with the plasma membrane (Figure 4b). The loss of cytoplasm was exclusively confined to the irreversibly perforated bleb (Figure 4b, arrows): no leakage of cytoplasmic CFP occurred either from the repaired bleb or from the cell body.
In some cells, only a transient intra-bleb translocation of annexin A1 occurred, and was followed by bleb retraction (Supplementary Movie 5).
Not all blebbing cells (see Figure 1a: 36.4±2.4% cells blebbing after SLO addition) showed a localized intra-bleb translocation of annexin A1 (17.5±2.1% 369 cells tested in 12 independent experiments): presumably, the most robust cells resealed their plasmalemmal lesions even before intra-bleb [Ca2+] elevated to the extent required for annexin A1 membrane translocation (10 μM, see Babiychuk et al., 2009).
The protective role of blebbing during plasmalemmal injury was not restricted to the toxin attack. Supplementary Movie 6 documents a membrane injury followed by a plasmalemmal repair (transient translocation of annexin A1-YFP) localized within a bleb in a HEK 293 cell that had been treated with bacterial phospholipase C. Figure 4c details the protective action of blebbing in a mechanically damaged cell. A layer of U 373 cells, which form long cytoplasmic protrusions in culture, was scratched with the needle of a microsyringe above the area shown in the images. The mechanical force bent the cytoplasmic protrusion and locally injured the plasma membrane (arrow). The damage was accompanied by a simultaneous elevation in local [Ca2+]i, which manifested itself by a local translocation of annexin A1 (Figure 4c: time after scratch=12 s; Supplementary Movie 7). Within a few seconds, numerous blebs had sprouted around the cell body. However, the translocation of annexin A1, which followed a substantial elevation in intrableb [Ca2+], was observed only in the blebs of the damaged region. Bleb ‘1' remained permeabilized, whereas bleb ‘2' was efficiently repaired: the intrableb [Ca2+] was lowered and annexin A1 back-translocated (Figure 4c: time after scratching=66 s). Fifteen minutes after scratching, annexin A1 had accumulated within the damaged region and was associated with the membranes of numerous small blebs (Figure 4c: time after scratching=900 s). These blebs were directly connected to the extracellular milieu: the intrableb [Ca2+] remained high and the intrableb cytoplasm leaked out (Figure 4c: time after scratching=900 s, small arrows). However, neither a global elevation in [Ca2+]i nor a global loss of cytoplasm was observed.
In the experiments described above, a selective loss of the cytoplasmic components from the damaged blebs was directly demonstrated by monitoring the leakage of CFP. The intrableb Ca2+ elevation was indirectly assessed by monitoring the Ca2+-dependent translocation of annexin A1.9 To demonstrate the localized intra-bleb elevation in Ca2+ directly, we used Fluo 5F – a Ca2+-sensitive fluorescent dye – which permits a monitoring of Ca2+ fluxes in the high micromolar range. The elevation, observed in non-transfected HEK 293 cells, is documented in Figure 5 and in Supplementary Movie 8. These results also confirmed that the selective intra-bleb elevation in Ca2+ was not a transfection artefact. It should be noted that Ca2+ measurements with cytoplasmic fluorescent dyes reflect fairly accurately the events that occur during the initial stages of permeabilization; however, they cannot be applied for the evaluation of relatively long-term processes such as plasmalemmal repair. After an initial elevation, the Ca2+-induced fluorescent signal declines in repaired (active Ca2+ extrusion) and in permanently permeabilized cells (loss of fluorescent dye) alike.9 The rapid loss of fluorescent dye from perforated blebs was presumably responsible for a decreased incidence of Fluo 5F-positive blebs (8.8±1.7% 356 cells analysed in seven independent experiments) compared with the annexin A1-positive blebs (17.5±2.1%).
Figure 5.
Intrableb elevation in Ca2+. SLO was added (time=0) to non-transfected HEK 293 cells that had been pre-loaded with Fluo 5F. At time points=90, 108 and 117 s, [Ca2+] was higher in the bleb (arrow) than in the cytoplasm. Fluorescence is shown in the rainbow mode: low [Ca2+] – blue; high [Ca2+] – red. Magnification bar=10 μm
Taken together, these experiments demonstrate that blebbing protects damaged cells by isolating the damaged regions, thereby preventing a global loss of cytoplasm and an uncontrolled influx of Ca2+. Our observations also indicate that the damaged blebs are sealed off from the rest of the cytoplasm.
Annexin A1 plugs damaged blebs
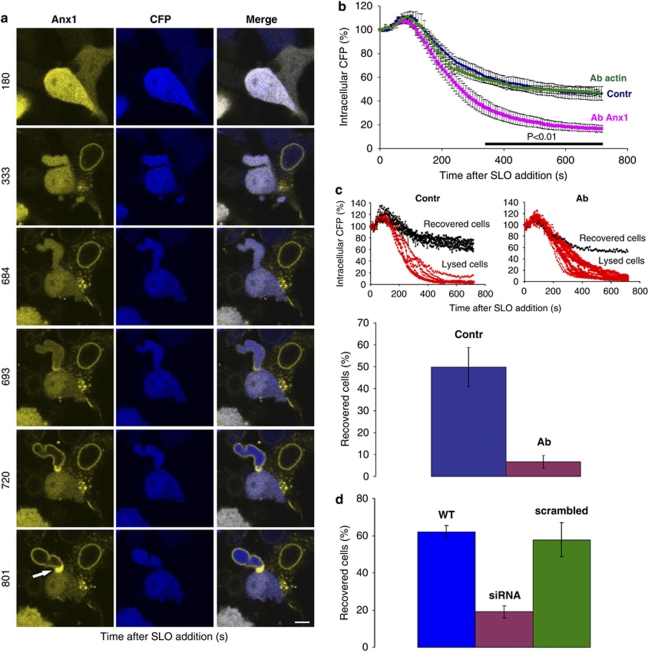
As it is inconceivable that even the narrow neck of a bleb can alone restrict for long the process of diffusion between the bleb and the cell body, we explored possible diffusion-blocking mechanisms. A close inspection of the blebs in recovering cells revealed a spectacular accumulation of annexin A1 at the base of the neck region (Figure 6a: arrow; Supplementary Movies 9 and 10). This accumulation of annexin A1 was not a consequence of cell folding because it was not mirrored by a corresponding accumulation of cytoplasmic CFP (Figure 6a). Among 64 cells that showed a localized intra-bleb translocation of annexin A1, 18 cells (31.6±7% 12 independent experiments) showed a marked accretion of annexin A1 at the region of the bleb-to-cell contact (7.9±1.3-fold increase in fluorescence compared with the fluorescence within a cytoplasmic region of interest) similar to that shown in Figure 6a. It should be noted that, owing to their relatively small size, some of these structures inevitably escape detection in our experimental settings, being localized outside the confocal plane even if the corresponding, much larger bleb is in focus.
Figure 6.
Annexin A1 blocks the communication between the damaged bleb and the rest of the cytoplasm. (a) A massive accumulation of annexin A1 is seen at the base of the SLO-perforated bleb (arrow). The cell in the upper right-hand corner is irreversibly perforated. Magnification bar=10 μm. (b) The SLO-induced loss of CFP recorded from a whole field of HEK 293 cells (containing minimally 15 cells) was significantly faster in the presence of the anti-annexin A1 antibody (Ab Anx1) than that either in the presence of the anti-β-actin antibody of the same isotype (Ab actin) or in the absence of antibody (Contr). Mean values (±S.E.M.) of three independent experiments. The horizontal bar indicates the times after SLO exposure at which the differences between experimental conditions (Ab Anx1 compared with either Ab actin or control) reached statistical significance (t-test; P<0.01). (c) Upper panels: assessed individually, SLO-permeabilized cells either completely lost their cytoplasm (lysed cells) or, after a partial loss of CFP, were able to repair the damaged plasma membrane and thus averted a further loss of cytoplasm (recovered cells). Representative experiments are shown. When perforated in the presence of the anti-annexin A1 antibody (Ab), only 6.6±2.9% of the cells (lower panel; 99 individual cells, n=5) recovered from the SLO attack, whereas 49.8±8.9% (lower panel; 73 individual cells, n=5) recovered from the perforation in the absence of the antibody (Contr; t-test P<0.01). Mean values (±S.E.M.) are represented. (d) SLO-induced lysis was significantly accelerated in HEK 293 cells stably expressing annexin A1-specific siRNA (siRNA) compared with either wild-type HEK 293 or HEK 293 cells transfected with scrambled siRNA (WT: 292 individual cells, n=9; siRNA: 348 individual cells, n=12; scrambled siRNA: 125 individual cells, n=5; t-test P<0.01). Mean values (±S.E.M.) are presented
In the presence of Ca2+, annexin A1 aggregates and fuses biological membranes.25, 27, 28, 29 It is, moreover, instrumental in the inward vesiculation in multivesicular endosomes – a process that is driven by a budding and fusion of the organelle-limiting membrane.30 Hence, massive, Ca2+-induced local accumulation of annexin A1 would cross-link the neck membranes of a bleb and efficiently block any communication between it and the rest of the cell. Annexin A1 might also trigger a sealing of the lipid bilayer within the bleb-to-cell contact region by recruiting intracellular vesicles according to the ‘classical' resealing pathway.1, 2
We tested these postulates either by inactivating annexin A1 with a blocking antibody13 or by downregulating annexin A1 expression level with siRNA. In the first set of experiments, native HEK 293 cells expressing endogenous annexin A1 were permeabilized in the presence of the antibody, which penetrated the cells via the perforated plasma membrane.6 In these cells, the SLO-induced loss of cytoplasmic CFP, collectively recorded from the whole observation field, was significantly faster than in those that had been perforated in the absence of the antibody (Figure 6b). A substitution of anti-annexin A1 antibody by anti-β-actin antibody (clone AC-15) of the same isotype (IgG1) failed to accelerate cell lysis in SLO-perforated cells (Figure 6b). When assessed individually, it was found that cells had either completely lost their cytoplasm (lysed cells) or, after a partial loss of CFP, were able to repair the damaged plasma membrane and thus avert a further loss of cytoplasm (Figure 6c). The percentage of lysed cells was significantly higher when the perforation occurred in the presence of the anti-annexin A1 antibody (Figure 6c). In the second set of experiments, annexin A1-specific siRNA was stably expressed in HEK 293 cells, resulting in a sevenfold reduction of annexin A1 protein levels (Supplementary Figure S3). In these cells, SLO-induced lysis was significantly accelerated (Figure 6d). Scrambled siRNA failed to decrease annexin A1 levels (Supplementary Figure S3) and had no effect on cell lysis (Figure 6d). These findings demonstrate that annexin A1 is directly involved in protecting cells against plasmalemmal injury.
Discussion
Formerly, the blebbing of injured cells was deemed to be a harbinger of death.31 Indeed, we have ourselves observed that blebbing of some injured cells forebodes their lysis. However, here we show that injured cells that failed to bleb were prone to dying, whereas those that manifested blebbing were more likely to recover. Moreover, a pharmacologically induced increase in blebbing led to a significant increase in the cell-survival rate. Hence, in injured cells, blebbing represents a cell's attempt to escape death rather than a mechanism promoting it.
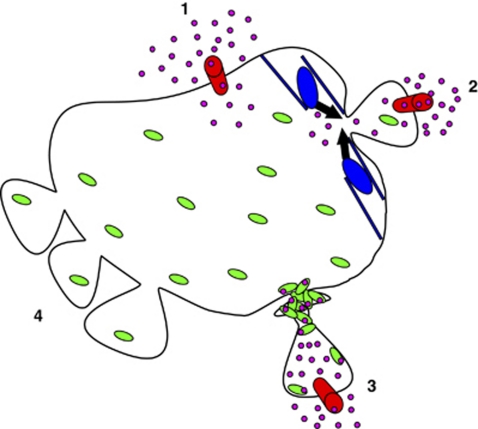
Our findings have disclosed blebbing to play a hitherto unknown role in protecting the plasma membrane during injury (Figure 7): (1) An injury to the plasma membrane (mechanical disruption, pores formed by cytotoxic proteins, lysis of the lipid bilayer) disrupts the permeability barrier, thereby permitting an influx of Ca2+ and an efflux of the intracellular constituents (not shown). (2) An injury-induced influx of Ca2+ triggers the phosphorylation of myosin II via Ca2+/calmodulin-dependent myosin light chain kinase. It is conceivable that myosin-driven contraction of the cortical cytoskeleton instigates a localized increase in hydrostatic pressure within the surrounding cytoplasm: the plasma membrane around the lesion herniates to form a bleb containing the damaged membrane. If the cell is able to repair the membrane lesion, the elevated intra-bleb [Ca2+] is pumped out and eventually the bleb retracts (not shown). If resealing fails, intra-bleb [Ca2+] continues to rise. (3) Once [Ca2+] within the perforated bleb reaches 10 μM (see Babiychuk et al.9), intra-bleb annexin A1 binds to the plasma membrane, thereby creating an ‘annexin A1 vacuum' within the bleb. Following the concentration gradient, cytosolic annexin A1 streams from the cell body into the bleb. From the cytoplasmic side, an aperture of a slender neck of the bleb is seen as a hot-spot of inflowing Ca2+: outside the opening the local Ca2+ concentration decreases rapidly due to diffusion into the cytoplasm and due to a Ca2+ sequestration by the endoplasmic reticulum and mitochondria. The locally elevated Ca2+ drives annexin A1 binding to the plasma membrane in the vicinity or within of the bleb aperture. A Ca2+-induced formation of ceramide within the plasma membrane exposed to the Ca2+ hot-spot might further increase the selectivity of annexin A1 binding.32 Thus concentrated, annexin A1 cross-links adjacent membranes of a slender neck of the bleb, thereby forming a plug. An efflux of cytosol is thus prevented and extracellular ‘invaders' remain trapped within the injured bleb.
Figure 7.
Blebbing confers resistance against cell lysis. (1) Plasmalemmal injury: injury – red cylinder; Ca2+ influx – pink circles. (2) Injury-induced bleb formation: myosin-driven contraction – black arrows; cortical cytoskeleton – blue; cytoplasmic annexin A1 – green ovals. (3) Plugged bleb: membrane-bound annexin A1 complexed with Ca2+ (green ovals+pink circles). (4) Spontaneously protruding blebs
The proposed mechanism accords with the observation that blebbing is not directly involved in the repair of the plasma membrane, that is, in the direct elimination of plasmalemmal lesions.8 Blebbing can be regarded as a damage-control mechanism, which is triggered after initial attempts at plasmalemmal resealing have failed. Above all, an uncontrolled intracellular Ca2+ overload is responsible for the death of perforated cells.9, 10, 11, 12 Repaired blebs are able to trap and evacuate elevated levels of Ca2+. However, if the membrane damage is too extensive to be repaired, the damaged regions are sacrificed to spare the rest of the cell: the plugged, irreversibly permeabilized blebs might be discarded by the cell.5, 31
We show that an injury to the plasma membrane causes bleb formation by triggering an influx of extracellular Ca2+. However, in non-perforated cells blebbing can also be induced by Ca2+-independent mechanisms via an activation of ROCK-dependent pathways.24 This phenomenon is routinely observed in cells exposed to toxic substances or at the onset of apoptosis.17, 18, 20 Apoptotic cells, in which the Ca2+ homeostasis is usually compromised,33 succumb more readily to plasmalemmal injury than do healthy cells.9, 10, 11 The ability of environmentally stressed or apoptotic cells to undergo spontaneous blebbing suggests that they might be able to anticipate their potential repair deficiency. The spontaneous protrusion of blebs, which often cover the whole cellular surface17, 18, 19 (see also Supplementary Figure S2, calyculin; Supplementary Movie 11), shields the cell body (Figure 7, step 4): an approaching toxin would first encounter the protruding membranes of the pre-existing blebs. A toxin-induced perforation of the blebs would result in an immediate confinement of the pores within the pre-existing traps. Hence, by forestalling the time-consuming step of targeted bleb formation (Figure 7, step 2), random blebbing before membrane damage would facilitate a speedy recovery of the perforated cells, and thus stave off an attack by invading pathogens or blood complement.
Cytotoxic T-cells and natural killer cells utilize a pore-forming protein, perforin, to eliminate virus-infected cells and tumours.34, 35 During perforin-induced cell death, the integrity of the plasma membrane is restored by repair responses that protect the cells from necrosis and permit granzymes to trigger apoptosis.5 We assume that blebbing is intimately involved in protecting perforin-permeabilized cells from necrosis. In support of this hypothesis, Keefe et al.5 have observed a selective accumulation of small extracellular dyes within the blebs of perforin-permeabilized cells that were undergoing apoptosis, whereas in irreversibly permeabilized, necrotic cells, the dyes were distributed throughout the entire cytoplasm.
In conclusion, our study identifies a novel defence strategy that is adopted by cells as a response to plasmalemmal injury. Deficiencies in the intrinsic membrane-protection mechanisms may contribute to the pathogenesis of degenerative, infectious and immune diseases. Therefore, further experiments are required to establish whether blebbing is involved in protection against plasmalemmal injury in vivo.
Materials and Methods
Reagents
Monoclonal anti-annexin A1 antibody was from BD Biosciences (San Jose, CA, USA). Fluo 5F-AM was from Invitrogen AG (Basel, Switzerland). Restriction endonucleases, Taq polymerase and T4 DNA ligase were from New England Biolabs (Ipswich, MA, USA). Living Colours Fluorescent protein vectors peCFP-N1 and peYFP-N1 were from Clontech (Mountain View, CA, USA). SureSilencing shRNA plasmids were from SA Biosciences. Other reagents were from Sigma.
Cell culture and transfections
HEK cells, human astrocytoma cells (U 373 cells), primary smooth muscle cells (human myometrium), Jurkat T-cells and Raji B-cells were maintained and transfected as previously described.9, 36 The coding sequence of annexin A1 was cloned into the Living Colours Fluorescent protein vectors following PCR amplification from human bladder smooth muscle cDNA.36 Annexin A1-yellow-fluorescent protein and CFP were transiently expressed in target cells.36
RNAi knockdown of annexin A1 expression
Annexin A1 knockdown experiments were performed with shRNA targeting human annexin A1 (clone 4, Pos. 630–650): 5′-GGTGACCGATCTGAGGACTTT-3′ cloned into SureSilencing shRNA plasmids. Cells were transfected with shRNA using electroporation and a stable cell line established using neomycin resistance followed by clonal selection.37 Levels of annexin A1 were assessed by western blotting.
Imaging and intracellular calcium measurements
The cells seeded on glass coverslips were mounted in a perfusion chamber at 25°C in Tyrode's buffer (140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM glucose, 10 mM HEPES; pH=7.4) containing 2.5 mM CaCl2. At time point 0, the cells were challenged with 100 ng/ml SLO from Streptococcus pyogenes, 0.05 U/ml phospholipase C from Clostridium perfringens, or were mechanically injured by scraping with the needle of a microsyringe close to the field of observation. When indicated, the cells were pre-treated with acrylamide (4 mM, 18 h), calyculin (10 μM, 40 min), staurosporine (1 μM, 30 min), W7 (20 μM, 30 min) or blebbistatin (50 μM, 20 min) before the addition of SLO. For the calcium-imaging recordings, the cells were loaded for 30 min at 22°C with 3 μM Fluo 5F-AM in Tyrode's buffer. The fluorescence was recorded in an Axiovert 200M microscope with a laser scanning module LSM 510 META (Carl Zeiss AG, Feldbach, Switzerland) using a × 63 oil immersion lens.9 The images were analysed using the ‘Physiology evaluation' software package (Zeiss).
Acknowledgments
We gratefully acknowledge the financial support of the Swiss National Science Foundation (SNF Grants 320000-111778 to KM, 3100A0-121980/1 to EBB and 320030_128064/1 to AD) and the National Research Programme NRP 53 ‘Musculoskeletal Health-Chronic Pain' 405340-104679/1 (to AD).
Glossary
- SLO
streptolysin O
- CFP
cyan-fluorescent protein
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by SJ Martin
Supplementary Material
References
- McNeil PL, Steinhardt RA. Plasma membrane disruption: repair, prevention, adaptation. Annu Rev Cell Dev Biol. 2003;19:697–731. doi: 10.1146/annurev.cellbio.19.111301.140101. [DOI] [PubMed] [Google Scholar]
- McNeil PL, Kirchhausen T. An emergency responce team for membrane repair. Nat Rev Mol Cell Biol. 2005;6:499–505. doi: 10.1038/nrm1665. [DOI] [PubMed] [Google Scholar]
- Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- Parker MW, Feil SC. Pore-forming protein toxins: from structure to function. Prog Biophys Mol Biol. 2005;88:91–142. doi: 10.1016/j.pbiomolbio.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Keefe D, Shi L, Feske S, Massol R, Navarro F, Kirchhausen T, et al. Perforin triggers a plasma membrane-repair response that facilitates CTL induction of apoptosis. Immunity. 2005;23:249–262. doi: 10.1016/j.immuni.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Walev I, Bhakdi SC, Hofmann F, Djonder N, Valeva A, Aktories K, et al. Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O. Proc Natl Acad Sci USA. 2001;98:3185–3190. doi: 10.1073/pnas.051429498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilzer D, Gasser O, Moskovich O, Schifferli JA, Fishelson Z. Emission of membrane vesicles: roles in complement resistance, immunity and cancer. Springer Semin Immunopathol. 2005;27:375–387. doi: 10.1007/s00281-005-0004-1. [DOI] [PubMed] [Google Scholar]
- Idone V, Tam C, Goss JW, Toomre D, Pypaert M, Andrews NW. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J Cell Biol. 2008;180:905–914. doi: 10.1083/jcb.200708010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiychuk EB, Monastyrskaya K, Potez S, Draeger A. Intracellular Ca2+ operates a switch between repair and lysis of streptolysin O-perforated cells. Cell Death Differ. 2009;16:1126–1134. doi: 10.1038/cdd.2009.30. [DOI] [PubMed] [Google Scholar]
- Morgan BP, Luzio JP, Campbell AK. Intracellular Ca2+ and cell injury: a paradoxical role of Ca2+ in complement membrane attack. Cell Calcium. 1986;7:399–411. doi: 10.1016/0143-4160(86)90042-4. [DOI] [PubMed] [Google Scholar]
- Morgan BP. Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem J. 1989;264:1–14. doi: 10.1042/bj2640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeraerts MD, Ronveaux-Dupal MF, Lemasters JJ, Herman B. Cytosolic free Ca2+ and proteolysis in lethal oxidative injury in endothelial cells. Am J Physiol. 1991;261:C889–C896. doi: 10.1152/ajpcell.1991.261.5.C889. [DOI] [PubMed] [Google Scholar]
- McNeil AK, Rescher U, Gerke V, McNeil PL. Requirement for annexin A1 in plasma membrane repair. J Biol Chem. 2006;281:35202–35207. doi: 10.1074/jbc.M606406200. [DOI] [PubMed] [Google Scholar]
- Mirnikjoo B, Balasubramanian K, Schroit AJ. Suicidal membrane repair regulates phosphatidylserine externalization during apoptosis. J Biol Chem. 2009;284:22512–22516. doi: 10.1074/jbc.C109.022913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller H, Eggli P. Protrusive activity, cytoplasmic compartmentalization, and restriction rings in locomoting blebbing Walker carcinosarcoma cells are related to detachment of cortical actin from the plasma membrane. Cell Motil Cytoskeleton. 1998;41:181–193. doi: 10.1002/(SICI)1097-0169(1998)41:2<181::AID-CM8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Hagmann J, Burger MM, Dagan D. Regulation of plasma membrane blebbing by the cytoskeleton. J Cell Biochem. 1999;73:488–499. [PubMed] [Google Scholar]
- Charras GT. A short history of blebbing. J Microsc. 2008;231:466–478. doi: 10.1111/j.1365-2818.2008.02059.x. [DOI] [PubMed] [Google Scholar]
- Charras GT, Paluch E. Blebs lead the way: how to migrate without lamellipodia. Nat Rev Mol Cell Biol. 2008;9:730–736. doi: 10.1038/nrm2453. [DOI] [PubMed] [Google Scholar]
- Charras GT, Coughlin M, Mitchison TJ, Mahadevan L. Life and times of a cellular bleb. Biophys J. 2008;94:1836–1853. doi: 10.1529/biophysj.107.113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera P, Hartzell P, Davis G, Orrenius S. The formation of plasma membrane blebs in hepatocytes exposed to agents that increase cytosolic Ca2+ is mediated by the activation of a non-lysosomal proteolytic system. FEBS Lett. 1986;209:139–144. doi: 10.1016/0014-5793(86)81099-7. [DOI] [PubMed] [Google Scholar]
- Angus AA, Lee AA, Augustin DK, Lee EJ, Evans DJ, Fleiszig SM. Pseudomonas aeruginosa induces membrane blebs in epithelial cells, which are utilized as a niche for intracellular replication and motility. Infect Immun. 2008;76:1992–2001. doi: 10.1128/IAI.01221-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- Tinevez J-Y, Schulze U, Salbreux G, Roensch J, Joanny JF, Paluch E. Role of cortical tension in bleb growth. Proc Natl Acad Sci USA. 2009;106:18581–18586. doi: 10.1073/pnas.0903353106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001;3:339–345. doi: 10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- Gerke V, Moss SE. Annexins and membrane dynamics. Biochim Biophys Acta. 1997;1357:129–154. doi: 10.1016/s0167-4889(97)00038-4. [DOI] [PubMed] [Google Scholar]
- Limouze J, Straight AF, Mitchison T, Sellers JR. Specificity of blebbistatin, an inhibitor of myosin II. J Muscle Res Cell Motil. 2004;25:337–341. doi: 10.1007/s10974-004-6060-7. [DOI] [PubMed] [Google Scholar]
- Raynal P, Pollard HB. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994;1197:63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Rosengarth A, Luecke H. A calcium-driven conformational switch of the N-terminal and core domains of annexin A1. J Mol Biol. 2003;326:1317–1325. doi: 10.1016/s0022-2836(03)00027-5. [DOI] [PubMed] [Google Scholar]
- Monastyrskaya K, Babiychuk EB, Draeger A. The annexins: spatial and temporal coordination of signaling events during cellular stress. Cell Mol Life Sci. 2009;66:2623–2642. doi: 10.1007/s00018-009-0027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IJ, Bailey LM, Aghakhani MR, Moss SE, Futter CE. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J. 2006;25:1–12. doi: 10.1038/sj.emboj.7600759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segundo C, Medina F, Rodríguez C, Martínez-Palencia R, Leyva-Cobián F, Brieva JA. Surface molecule loss and bleb formation by human germinal center B cells undergoing apoptosis: role of apoptotic blebs in monocyte chemotaxis. Blood. 1999;94:1012–1020. [PubMed] [Google Scholar]
- Babiychuk EB, Monastyrskaya K, Draeger A. Fluorescent annexin A1 reveals dynamics of ceramide platforms in living cells. Traffic. 2008;9:1757–1775. doi: 10.1111/j.1600-0854.2008.00800.x. [DOI] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- Kagi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen KJ, et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Froelich CJ, Orth K, Turbov J, Seth P, Gottlieb R, Babior B, et al. New paradigm for lymphocyte granule-mediated cytotoxicity. J Biol Chem. 1996;271:29073–29079. doi: 10.1074/jbc.271.46.29073. [DOI] [PubMed] [Google Scholar]
- Monastyrskaya K, Babiychuk EB, Hostettler A, Rescher U, Draeger A. Annexins as intracellular calcium sensors. Cell Calcium. 2007;41:207–219. doi: 10.1016/j.ceca.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Monastyrskaya K, Babiychuk EB, Hostettler A, Wood P, Grewal T, Draeger A. Plasma membrane-associated annexin A6 reduces Ca2+ entry by stabilizing the cortical actin cytoskeleton. J Biol Chem. 2009;284:17227–17242. doi: 10.1074/jbc.M109.004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.