Abstract
L-glutamate, the major excitatory neurotransmitter, also has a role in non-neuronal tissues and modulates immune responses. Whether NMDA receptor (NMDAR) signalling is involved in T-cell development is unknown. In this study, we show that mouse thymocytes expressed an array of glutamate receptors, including NMDARs subunits. Sustained calcium (Ca2+) signals and caspase-3 activation in thymocytes were induced by interaction with antigen-pulsed dendritic cells (DCs) and were inhibited by NMDAR antagonists MK801 and memantine. NMDARs were transiently activated, triggered the sustained Ca2+ signal and were corecruited with the PDZ-domain adaptor postsynaptic density (PSD)-95 to thymocyte-DC contact zones. Although T-cell receptor (TCR) activation was sufficient for relocalization of NMDAR and PSD-95 at the contact zone, NMDAR could be activated only in a synaptic context. In these T-DC contacts, thymocyte activation occurred in the absence of exogenous glutamate, indicating that DCs could be a physiological source of glutamate. DCs expressed glutamate, glutamate-specific vesicular glutamate transporters and were capable of fast glutamate release through a Ca2+-dependent mechanism. We suggest that glutamate released by DCs could elicit focal responses through NMDAR-signalling in T cells undergoing apoptosis. Thus, synapses between T and DCs could provide a functional platform for coupling TCR activation and NMDAR signalling, which might reflect on T-cell development and modulation of the immune response.
Keywords: calcium signalling, thymocyte, NMDAR, glutamate signalling, immunological synapse
-glutamate is the major excitatory neurotransmitter in the central nervous system (CNS). There is now evidence to suggest that glutamate acts as a signalling molecule in non-neuronal tissues,1, 2 with an emerging role as an immune modulator.3 Metabotropic G-protein-coupled glutamate receptors (mGluRs) are involved in T-cell activation,4 and in the inhibition of activation-induced cell death.5 Ionotropic glutamate receptors are involved in cell cycle progression, regulating activation and proliferation, chemotactic migration and integrin-mediated adhesion in T cells,6, 7 with some indications for a role of the N-methyl--aspartate receptor (NMDAR) in Ca2+ signalling.8, 9
Increases in [Ca2+]i are key signals in T-cell activation after T-cell receptor (TCR) engagement. T lymphocytes are believed to use store-operated calcium (SOC) channel entry as the main mode of Ca2+ influx.10 The SOC channels in lymphocytes are known as Ca2+ release-activated Ca2+ (CRAC) channels, whose molecular identity has been recently elucidated, with Orai1 as the pore-forming subunit and STIM1 as the sensor of stored Ca2+. Ca2+ signalling in T cells also involves purinergic receptors, voltage-gated Ca2+ channels, and activate other channels such as Ca2+-dependent voltage-activated K+ channels.11, 12 The relationships between all these Ca2+ routes, remain to be elucidated.
The immunological synapse (IS) is a structure that mediates information exchange, which forms in the contact zone between an antigen-presenting cell (APC) and a T-cell bearing a specific TCR. It was first suggested to facilitate the directed secretion of cytokines between T cells and APCs.13 Cytokines may be released into the synapse, having an immediate effect on clustered receptors in the contact zone.14 The synapse formed between T cells and dendritic cells (DCs) shows cell-to-cell adhesion, stability and close apposition of membranes,15 which may help to optimize soluble mediator concentration at the T cell-APC interface, thereby limiting bystander effects. Neuronal synapses and ISs show anatomical similarity, although ISs form rapidly and are transient.16 To what extent these synapses are functionally similar is not known.
Circulating T cells are thought to be exposed to glutamate in the plasma, glutamate-rich peripheral organs,1, 2 and in the brain, facilitating cross-talk with the CNS.17 It is therefore likely that GluRs are not permanently stimulated in T cells, and we postulated that their activation occurs as a result of IS formation. In analogy to neurons, glutamate should therefore be rapidly released, with faster kinetics than those reported through the cystine/glutamate antiporter (Xc− system).18 Indeed, the possibility that glutamate released by APCs at ISs mediates communication with T cells through GluR-mediated Ca2+ signalling has never been analyzed.
As glutamate mediates apoptosis of neuronal cells through NMDARs and Ca2+ signalling,19 we analyzed thymocyte development, a process in which thymocytes that recognize self-antigens with high affinity are eliminated by Ca2+-dependent apoptosis.20 Our findings indicate that DCs are capable of fast Ca2+-dependent glutamate release, while thymocytes express NMDARs, which modulate TCR-dependent Ca2+ signalling and apoptosis only in a synaptic context, revealing a novel aspect of IS functioning.
Results
NMDARs are expressed in thymocytes
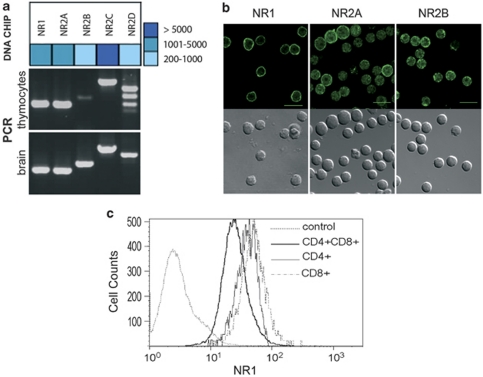
More than 30 different GluRs are expressed in the CNS.21 As only mGluRs have been detected in thymocytes,22 we used neurotransmission-dedicated oligoarrays to analyze their GluR repertoire. Thymocytes expressed most known GluRs (Supplementary Figure S1a), including NMDAR GluN1, GluN2A and GluN2B subunits (IUPHAR nomenclature of subunits), previously referred to as NR1, NR2A and NR2B subunits23 (Figure 1a). These findings were confirmed by PCR (Figure 1a), and confocal microscopy, using well-characterized antibodies (Figure 1b, Supplementary Figure S1c). To study NMDAR function, we focused on NR1, the obligate subunit of NMDARs, which was expressed in thymocytes at levels similar to what was observed in the brain (Figure 1a, Supplementary Table S1). FACS analysis, indicated that 97.3% of thymocytes expressed NR1, with higher expression in CD4+ or CD8+ cells as compared with CD4+CD8+ double-positive (DP) cells (Figure 1c).
Figure 1.
T cells express a large array of i- and m-GluRs. (a) Oligoarray (Neurotrans) analysis of NMDAR mRNA expression on thymocytes compared with brain (upper panel), and validated by RT-PCR compared with brain (lower panel). Oligoarray data are shown as a heat map. Data for all GluRs are shown in Supplementary Figure S1 using primers shown in Supplementary Table S1. (b) Immunofluorescence analysis of NMDAR subunit expression on thymocytes. For each indicated NMDAR subunit (NR1, NR2A, NR2B), a Nomarski image (bottom row) and a confocal fluorescence image is shown in an equatorial plane (top row). Images for other ionotropic glutamate receptors (iGluRs) are shown in Supplementary Figure S1c. Control staining consisting in IgG2b isotype control (for anti-NR1 antibody) or purified rabbit IgG (for anti-NR2A, NR2B, GluR2/3, KA2 antibodies) were negative using the same settings as the specific staining (Supplementary Figure S1c). (c) Flow cytometry analysis of NR1 expression in thymocytes. Antibodies that detect NR1 as a band at the expected molecular weight by western blotting in brain microsomal preparations were used. Relative fluorescence intensity is shown for CD4+, CD8+ and DP subpopulations. Data are representative of three independent experiments. IgG2b control isotype is shown as control for NR1 staining
Ca2+ signalling and apoptosis in thymocytes in contact with DCs, involves NMDAR
To examine NMDAR function in thymocytes, we focused on Ca2+ and apoptosis signalling. Indeed, both are associated with NMDAR activity in neurones. Glutamate and NMDA, at doses of 1–500 μM, did not induce Ca2+ signalling or apoptosis in thymocytes (data not shown). Thus, we analyzed whether a thymocyte-DC synaptic context could provide the environment required for coupling Ca2+ signalling to apoptosis.
To set up an in vitro system of T cell-DC synapses, we used HNT-TCR-transgenic mice in which most T cells express the same TCR directed to the HA 126–138 peptide. In vivo administration of HA 126–138 in transgenic mice induces massive apoptosis, mostly of DP thymocytes.24 Coculture of thymocytes from transgenic mice and HA-pulsed DCs, enhances the probability of antigen-dependent synaptic contacts. To assess DC capacity to induce thymocyte apoptosis, we monitored the expression of Nur77, CD69 and caspase-3. Nur77, an immediate early gene required for the induction of apoptosis in negative selection25 is a specific marker of clonal deletion in vivo.26 CD69, an early T-cell activation marker upregulated after TCR engagement and involved in negative selection,27 was thus used to discriminate thymocytes that have contacted DCs. Activated caspase-3 in living cells, allowed early detection of apoptosis, avoiding bystander effects occurring at later time points. Nur77, CD69 and activated caspase-3 that we measured in vivo, were also upregulated in vitro, indicating functional synapses in vitro (Supplementary Figure S2a). In contrast to thymocytes, peripheral CD4+ T cells in contact with DCs showed increased CD69 expression, without caspase-3 activation (Supplementary Figure S2b) and proliferated as expected in response to HA peptide (data not shown).
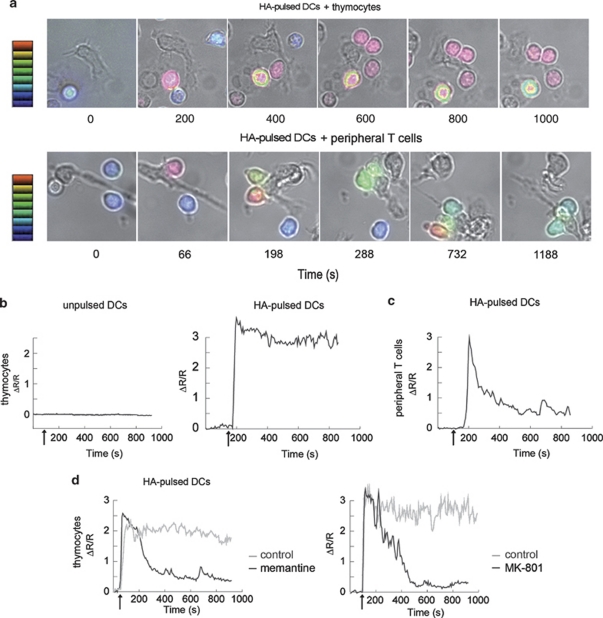
Then, we used this in vitro system to monitor the Ca2+ signal elicited in thymocytes contacting DCs. Antigen-specific contacts of thymocytes with DCs, in a glutamate-free medium, resulted in rapid and sustained increase in [Ca2+]i in T cells (Figures 2a and b). A majority of the thymocytes established long-lasting contacts, 90% of which resulted in a Ca2+ peak (ΔR/R=2.93±0.08, n=119), followed by a sustained high-level plateau lasting at least 10 min (Figure 2b and Supplementary Video S1). A few unstable contacts with no measurable Ca2+ signals were observed in the absence of HA-peptide (Figure 2b). Interestingly, contacts with peripheral splenic CD4+ T cells resulted in a Ca2+ signal with a peak similar to that for thymocytes (ΔR/R=3.72±0.19, n=32), but rapidly decreasing (50% decrease from the peak value in 91±13 s, n=30) to a plateau maintained at a basal level of approximately one-fifth of the peak value (Figures 2a and c, Supplementary Videos S1 and S2).
Figure 2.
NMDAR modulates Ca2+ signalling in thymocytes in contact with DCs, in the absence of exogenous glutamate. (a) Representative time-lapse microscopy images (DIC/Fura-2 overlay) of thymocytes (upper set) and peripheral T cells (lower set) making contact with HA-pulsed DCs. The scale indicates Ca2+ level, expressed as ΔR/R values, ranging from 0 (blue) to 3 (red). In thymocytes, steep [Ca2+]i increases were recorded 44±3 s (n=101) after initial contact with the DC, and was, in most synapses, rapidly followed by the establishment of a stable contact (50±2 s, n=93) associated with a sustained high-level plateau lasting at least 10 min. (b) Representative Ca2+ signal in Fura-2 loaded thymocytes in contact with unpulsed DCs (left panel) or with HA-pulsed DCs (right panel) Arrows: contact. (c) Representative Ca2+ signal in Fura-2 loaded peripheral T cells in contact with HA-pulsed DCs. Arrow: contact. (d) Representative traces of the Ca2+ response in thymocytes in contact with HA-pulsed DCs in the presence (black curves) or absence (grey curves) of the NMDAR antagonists memantine (100 μM) (left panel) and MK801 (100 μM) (right panel). Arrows: contact
Thus, thymocyte-DC contact, in the absence of exogenous glutamate, results in a sustained Ca2+ signal with a high plateau and apoptosis signalling. These results also point to a potential relationship between the sustained shape of the Ca2+ signal and T-cell fate.
NMDAR triggers the sustained Ca2+ response in thymocytes and is involved in thymocyte apoptosis
The primary mechanism of Ca2+ mobilization in T cells is the release of Ca2+ from intracellular stores followed by Ca2+ influx through CRAC channels.10 We hypothesized that NMDARs could participate in Ca2+ influx, thereby contributing to the sustained Ca2+ plateau observed during thymocyte apoptosis.
In the T-DC synapse model, we incubated T cells either with MK801 or memantine, two non-competitive open-channel NMDAR blockers, and then measured T-DC contact-induced Ca2+ signals and apoptosis in presence of these inhibitors. MK801 and memantine modulated Ca2+ signals in approximately 50% of thymocytes, resulting in a transient signal with a similar amplitude at the initial peak (3.15±0.14, n=42 and 2.81±0.12, n=33, for MK801 and memantine, respectively) to control cells, but with a plateau close to basal levels (50% decrease in the peak value after 148±16 s, n=18 and 275±37 s, n=14, respectively) (Figure 2d and Supplementary Video S3). To rule out the possibility that NMDAR blockers had non-specific effects on CRAC or other off-target effects on channels involved in T-cell activation, we monitored the Ca2+ signal by FACS on thymocytes activated by anti-CD3/anti-CD28 cross-linking, in the absence of glutamate. Approximately 60% of thymocytes responded to TCR stimulation by a Ca2+ signal that was unaffected by MK801 or memantine (Supplementary Figure S3), indicating that these drugs had no major effect on Ca2+ signalling resulting from TCR triggering.
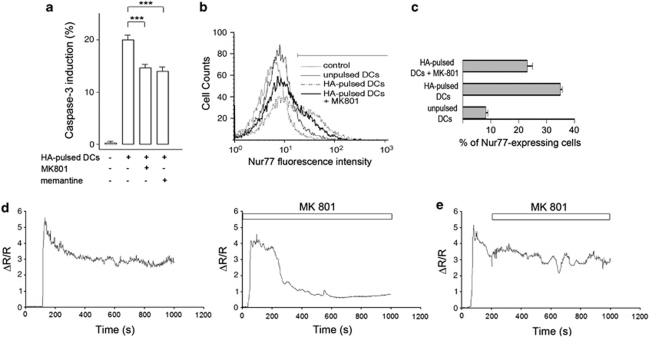
NMDAR blockers also significantly affected the percentages of activated caspase-3-expressing cells (27±0.7% inhibition with MK801, n=24 and 30±0.85% with memantine, n=15) (Figure 3a), indicating a role for NMDARs in apoptosis. It is noteworthy that the effect on caspase-3 expression might be underestimated because only 50% of thymocytes engaged in a synapse were sensitive to the drugs, as measured in Ca2+ signalling experiments. Activation of Nur77 expression, which is Ca2+ dependent, was affected by MK801 as measured by FACS analysis, 4 h after thymocyte-DC contact (Figures 3b and c), suggesting that Nur77 could be an early target of the Ca2+ signal mediated by NMDAR.
Figure 3.
NMDAR triggers a sustained Ca2+ signal, is transiently activated and is involved in caspase-3 activation and Nur77 induction, in thymocyte-DC contacts. (a) Inhibition of caspase-3 (t=6 h) expression in DP thymocytes co-cultured with unpulsed or HA-pulsed DCs with or without MK801 (100 μM) and memantine (100 μM). Data are means of six replicates for five independent cultures (*** P<0.001). The effect of memantine or MK801 on apoptosis was dose-dependent and was significant at 10 μM, with the maximal effect observed at 100 μM. A dose of 10 μM MK801 resulted in 11.4±1% inhibition of apoptosis (n=6) and a transient Ca2+ signal in 50% thymocytes (n=10) (data not shown). (b) Flow cytometry analysis of Nur77 expression in DP thymocytes, before and 4 h after contact with antigen-loaded DCs, in the presence or absence of MK801 (100 μM). The graphs are representative of three experiments. (c) Quantification of Nur77-expressing DP thymocytes before and 4 h after contact with HA-loaded DCs, in the presence or absence of MK801 (100 μM). (d) Representative traces of the Ca2+ response in thymocytes in contact with HA-pulsed DCs. Thymocytes were preincubated with MK801 (100 μM), NMDA (300 μM) and -serine (20 μM) to block any NMDARs that would be present at the membrane before synapse formation. Thymocytes were then washed and added to HA-pulsed DCs in the absence or presence of the NMDAR antagonists MK801 (100 μM). (e) Representative traces of the Ca2+ response in thymocytes in contact with HA-pulsed DCs MK801 (100 μM) was applied after the beginning of Ca2+ response (t=208 s)
As previously mentioned glutamate or NMDA did not induce any Ca2+ signalling, in freshly dissociated thymocytes. Patch-clamp recordings confirmed that no NMDA current was detectable, when NMDA and -serine were co-applied (data not shown), indicating NMDA insensitivity of isolated thymocytes. These data, combined to the observation that NMDAR blockers inhibit both Ca2+ and apoptosis signalling in DC-contacting thymocytes, suggest that NMDA channels are activated only during or after synapse formation. To further analyze the involvement of NMDA channels in this process, we asked if (1) NMDARs are activated only after synapse formation and (2) the sustained Ca2+ response requires a transient NMDAR activation. Using the T-DC contact model, we incubated freshly dissociated thymocytes with MK801, NMDA and -serine to block all activable NMDARs present at the membrane. We then washed the thymocytes and recorded the Ca2+ signals after synaptic T-DC contact. The sustained Ca2+ responses (n=47, 95% of cells with a sustained signal) (Figure 3d), were similar to the responses recorded in absence of thymocyte pretreatment, suggesting that NMDARs are active only after synaptic contact even if their agonists are present. In the same conditions of pretreatment, addition of MK801 in the bath solution before synapse formation revealed a sensitivity of the Ca2+ plateau to this NMDAR blocker (n=22, 55% of cells with a transient signal) (Figure 3d). Using the same protocol, 200 s after the beginning of Ca2+ signalling because of T-DC contacts, we applied MK801. MK801 application after synapse formation failed to inhibit Ca2+ response (n=20, 90% of cells with a sustained signal) (Figure 3e).
Together, these results suggest that NMDARs are necessary for the induction of the sustained Ca2+ response but are only transiently activated at the beginning of the synaptic response. Furthermore, these receptors are also involved in the induction of thymocyte apoptosis after contact with DCs.
Thymocyte–DC interaction induces NR1 and PSD-95 clustering at the contact zone
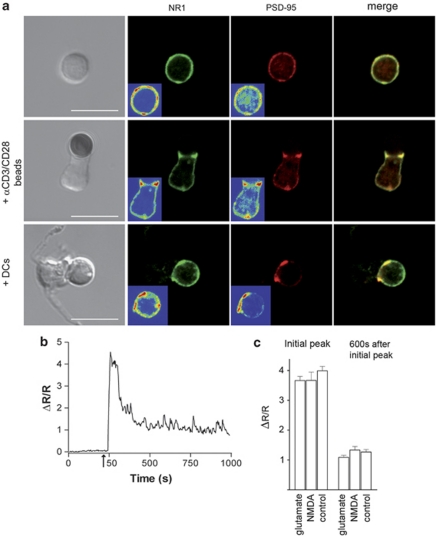
Transient activation of NMDA channels at the synapse suggest that an important trafficking of these receptors could occur in thymocytes. The TCR signalosome includes PDZ domain-containing adaptors, increasingly thought to be involved in ISs.28 Postsynaptic density (PSD)-95, accumulates in the active zones of neuronal synapses, forming so-called PSDs. It is the first-line signalling adaptor binding directly to the C-terminus of NMDAR subunits,29 involved in stabilization and functional recruitment of NMDARs at the excitatory synapse.30 Staining for NR1 and PSD-95 indicated that both were distributed over the entire resting thymocytes, but were colocalized in the contact zone in T-DC synapses (Figure 4a and Supplementary Figure S4). When thymocytes were stimulated with anti-CD3/CD28-coated beads in the absence of glutamate, PSD-95 was nonetheless colocalized with NR1 in the contact zone (Figure 4a), indicating that the relocalization of NMDAR and PSD-95 to the synapse depends on TCR stimulation. Interestingly, TCR stimulation using anti-CD3/CD28-coated beads, in the presence or absence of NMDA (or glutamate itself), did not recapitulate the effect of glutamate observed in a synaptic context. The Ca2+ signal was not sustained with a high-level plateau (Figures 4b and c), no significant apoptosis was induced (data not shown) and no NMDA currents were recorded (data not shown), indicating that in addition to glutamate, one or several other signals provided by the DC, are necessary to activate NMDARs.
Figure 4.
TCR triggering is sufficient to induce NMDAR relocalization at the thymocyte-DC contact zone, whereas NMDAR can be activated only in a synaptic context. (a) Double labelling of PSD-95 (red) and NR1 (green) on isolated thymocytes, thymocytes in contact with anti-CD3/CD28-coated beads and thymocytes in contact with a DC. Merged pictures are shown in each case (yellow), Scale bars, 10 μm. Profiles of distribution of fluorescence intensities and colocalization analysis of PSD-95 and NR1 are shown in Supplementary Figure S4. Controls consisted in mouse IgG2b isotype control for NR1 and purified rabbit IgG for PSD-95 (not shown). (b) Representative trace of Ca2+ response in thymocytes in contact with anti-CD3/CD28-coated beads. Arrow: contact. (c) Histogram of the mean Ca2+ amplitudes from 6 to 11 representative traces at the initial peak and at the plateau phase (600 s after the initial peak), in the absence or presence of glutamate (300 μM) and NMDA (100 μM)
We conclude that intense trafficking of NMDAR may occur in thymocytes on contact with DCs. TCR triggering is sufficient to induce NR1 and PSD-95 clustering at the synapse, while the NMDAR is activated only in a complete synaptic context.
DCs are capable of fast glutamate release
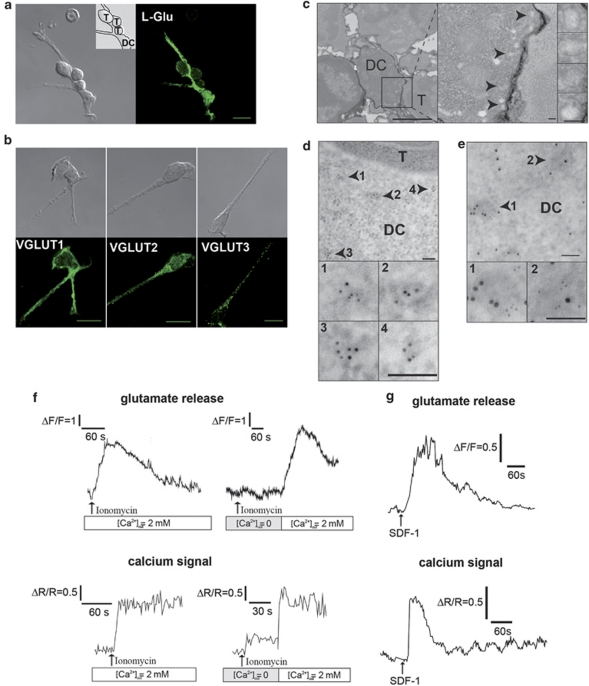
As all the Ca2+ signalling experiments in T-DC contacts were performed in the absence of exogenous glutamate, we wondered which cell was the physiological source of glutamate. Immunostaining with a specific antibody to glutamate showed immunoreactivity mainly in DCs indicating that DC is the source of glutamate in T-DC synapses (Figure 5a). Moreover, DCs express the glutamate-specific vesicular glutamate transporters (VGLUT)1 and VGLUT2, which confer a glutamatergic phenotype to neurons31 and VGLUT3 (Figure 5b and Supplementary Figure S5b). Vesicular structures of 100 nm size, with a characteristic electron-dense membrane were observed in DCs, opposite the contact zone and often located in a polarized cytoplasmic region-containing mitochondria (Figure 5c). Immunogold labelling of VGLUT2 showed clusters of particles delineating discrete vesicles, opposite the contact zone in DCs (Figure 5d and Supplementary Figure S5d). Purified DCs express mRNAs encoding components involved in neurotransmitter exocytosis through SNARE complex formation (synaptophysin, Snap-23 and VAMP-2) or in the regulation of vesicle docking (Munc-18), as well as synaptotagmin-I (Supplementary Figures S5c and e), a Ca2+ sensor for glutamate release. VGLUT2 and synaptotagmin-I were co-expressed in the same vesicular structures (Figure 5e), suggesting the presence in DCs of a compartment competent for both glutamate accumulation and exocytosis. The question of glutamate production by immune cells was previously addressed, by measuring glutamate accumulation over 24 h in culture.18 Instead, we focused on real-time monitoring of glutamate release by freshly isolated DCs, using the -glutamate dehydrogenase (GDH)-linked assay.32 In the presence of external Ca2+, ionomycin (Figure 5f) and SDF-1α (a physiological stimulus in astrocytes)33, 34 (Figure 5g) triggered both [Ca2+]i increases and glutamate release in DCs with a similar time course.
Figure 5.
DCs may be the physiological source of glutamate in thymocyte-DC synapses. (a) Confocal immunofluorescence image of glutamate labelling in a DC in contact with three thymocytes (right). Corresponding Nomarski image (left), with an inset indicating the cell contours. Scale bar, 10 μm. (b) Immunofluorescence analysis of VGLUT1, VGLUT2 and VGLUT3 in DCs (bottom row), with the corresponding Nomarski images (top row). Scale bar, 10 μm. Typical punctate fluorescent staining of VGLUT2 and VGLUT3, was observed in DCs (Supplementary Figure S5b). (c) Morphological electron micrograph of thymocyte-DC conjugates showing a group of vesicle structures at the cell–cell interface. Original image (left, scale bar, 2 μm). Magnification of the synapse (middle, scale bar, 100 nm) and of the vesicles (right, scale bar, 100 nm). Arrows: individual vesicles in the DC. (d) Immunogold staining of VGLUT2 in the T cell-DC contact zone. Top: low magnification with vesicles in the DC indicated by arrows. Bottom: higher magnification. Scale bar, 100 nm. (e) Immunogold colocalization of VGLUT2 (5 nm) and synaptotagmin I (10 nm) on the same vesicular structures (arrows). Top: individual vesicles indicated by arrows (scale bar, 2 μm). Bottom: magnifications (scale bars, 100 nm). Control staining is shown in Supplementary Figure S5d (f) Typical traces of glutamate release (upper panels) from DCs stimulated with ionomycin (1 μM), monitored in continuous culture, by recording NADH fluorescence emission. Horizontal scale: time in seconds. Vertical scale: arbitrary units. The amounts of glutamate released represented 40% of the fluorescence increase induced by 10 μM glutamate. Lower panels: Ca2+ signals induced by ionomycin in the presence or absence of extracellular Ca2+ in Fura-2-loaded DCs. (g) Typical traces of glutamate release (uper panel) from DCs stimulated with SDF-1α (100 nM), monitored as in (f). Vertical scale: arbitrary units. Lower panel: Ca2+ signal induced by SDF-1α in Fura-2-loaded DCs, in the presence of extracellular Ca2+
We conclude that DCs show features required for regulated glutamate exocytosis and are capable of fast glutamate release in a Ca2+-dependent manner.
Discussion
In previous studies, GluRs were potentially involved in immune regulation,3 T cells were thought to be exposed to glutamate in the CNS, in the bloodstream or in certain peripheral organs7 and monocyte-derived DCs have been pointed out as a source of slow glutamate release accumulating in T-DC long-term cocultures.18 As T cells communicate through ISs, which are structurally similar to neuronal synapses,16 several questions are left unanswered. Does the IS use glutamate signalling? Is that signalling linked to Ca2+ signals governing T-cell fate? How important is synapse structure in glutamatergic communication between T cells and DCs? To what extent are immunological and neuronal synapses functionally similar?
The NMDAR, a source of Ca2+ entry in T-cell signalling at the IS
Until recently, most of the studies examining Ca2+ entries in T cells were not carried out on T cells contacting APCs. Thymocytes responded to DC contact with an immediate Ca2+ signal, with a sustained plateau sensitive to NMDAR blockers. Our data suggest that this sustained Ca2+ signal is probably not due to a sustained activity of the NMDAR. Similarly to the mechanisms involved in synaptic plasticity (LTD and LTP)35 there might be a transient activation of NMDARs, which could act as a trigger of a sustained Ca2+ response carried by other effectors. However, no detectable NMDAR signal was observed in resting thymocytes and in thymocytes stimulated with anti-CD3/CD28-coated beads (i.e., not engaged in a full synapse with DCs). It is physiologically relevant that the contact with DC may be necessary to activate NMDARs. Our observation that sustained Ca2+ signalling and apoptosis occurred only in the context of IS and not in solitary thymocytes is in line with the need for a tight control of T-cell activation by antigen-specific contact with DC, to ensure proper thymic negative selection. Indeed, the IS is the physiological structure dedicated to communication in the immune system and represents a logical strategy to provide protection against permanent exposure of thymocytes to glutamate. Such a strategy could also make sense in activation of peripheral T cells, which are exposed to substantial glutamate concentration in serum. The sustained, high-level Ca2+ plateau observed in this study was characteristic of thymocytes and correlated with antigen-dependent induction of apoptosis. It was not observed in naïve peripheral CD4+ splenic T cells that showed glutamate signalling-dependent proliferation (PA and SC-K unpublished data) but no apoptosis. The correlation between T-cell fate and the shape of the Ca2+ signal suggests that the elevated plateau phase is intimately linked to thymocyte apoptosis. This opens new challenges to identify the switches controlling pro-survival versus pro-apoptosis outcome of NMDAR stimulation.
DC, a site of glutamate mobilization for exocytosis signalling
A recent study in T cells cocultured with monocyte-derived DCs pulsed with a superantigen, showed glutamate production in the milieu, detectable from day 1 (in the 5 μM range) and accumulating in the coculture with time. This glutamate production involved a slow release mechanism, mediated by the cystine/glutamate antiporter.18 In this study, we showed a rapid and substantial release of glutamate by DCs, consistent with previous reports of synaptic polarization and Ca2+-regulated release of pre-assembled vesicular cytokine stores for DCs.36 We used several approaches to suggest that DCs are competent for both glutamate accumulation and release. (1) Immunofluorescence showed strong punctate staining for glutamate and VGLUT vesicular transporters, and staining for the Ca2+ sensor synaptotagmin-I and for VAMP-2, a SNARE component of the vesicular machinery for exocytosis. (2) Electron microscopy showed vesicle-like structures in the T-DC contact zones. (3) Double immunogold staining suggested that DCs are competent for both glutamate accumulation mediated by VGLUT and glutamate release through a Ca2+- and SNARE-dependent mechanism. (4) An enzymatic-linked assay showed ionomycin and SDF-1α-induced -glutamate release by DCs. It remains to define the precise SNARE-dependent mechanism of exocytosis, using specific drugs or toxins, and to determine the type of Ca2+ entry involved in exocytosis signalling; mechanisms similar to those reported for astrocytes might be expected.33 In our hands, riluzole and bafilomycin inhibited the Ca2+ signal and caspase-3 induction in thymocytes contacting DCs (PA and SC-K unpuplished data). However, this approach is not entirely appropriate as exocytosis of chemokines might be also affected. Indeed, we have shown that SDF-1α, a physiological stimulus-mobilizing intracellular Ca2+,34 triggers the release of glutamate from DCs. How SDF-1 operates in the minimal system of T-DC synapses for glutamate release remains to be determined. Ca2+ transients in DCs interacting with T cells may be involved in triggering glutamate exocytosis, but they have rarely been observed here and in human DCs,34 suggesting that local Ca2+ fluxes in DCs might be involved in glutamate release. As thymic epithelial cells, did not release measurable glutamate in real-time (PA, TC and SC-K, unpublished data), DCs may be specialized for rapid glutamate delivery to T cells. Glutamate should be considered an ‘immunotransmitter' as suggested for cytokines and chemokines.37
PSD-95, a signalling adaptor indicative of the glutamatergic phenotype of the IS
In neuronal synapses, PSDs, which contain an intricate network of scaffolding PDZ-domain proteins, are thought to participate in synapse genesis and to organize Ca2+ sources, sensors and effectors, and a number of signalling proteins.30 Several studies showed a role for PDZ-domain proteins such as CARMA138 and hDlg/Dlgh1/SAP9728 in T-cell activation and TCR activation-induced synapse assembly, respectively. Our findings add to the current knowledge the possibility of PSD-95-mediated connections between NMDARs and TCRs. Although the role of GluRs in T-DC communication was not entirely unexpected, we were surprised to find that TCR triggering alone was sufficient to promote the clustering of both NMDAR and PSD-95 in the contact zone. We suggest that TCR signalling triggers the modelling of the nascent IS into a functionally competent synapse for glutamate signalling. Hence, our data indicated that NMDAR can be activated only in a synaptic context. An additional signal from the DC may be needed to activate NMDARs. We suggest that the IS may provide a functional platform for coupling detection of TCR ligation and NMDAR activation with subsequent focal NMDAR signalling. PSD-95 may well be a primary adaptor, connecting TCR and NMDAR signalling. A challenge will be to understand the precise molecular mechanism of the interconnection between TCR and NMDAR signalling. Furthermore, a transient activation of NMDARs at the synapse would suggest an important trafficking of these receptors in thymocytes. Although our data indicate that PSD95 might be involved, the mechanism of NMDAR exocytosis at the synapse as showed in neurones35 remains to be solved.
In conclusion, for years, glutamate signalling was thought to take place primarily in the CNS; it is now known to control key peripheral physiological functions, such as insulin secretion, keratinocyte differentiation, and remodelling of bone mass. Accordingly, therapeutic applications are being developed for diabetes, psoriasis, and osteoporosis.2 Our findings suggest an additional site of peripheral glutamate signalling and possible involvement of NMDARs in a major physiolological function of the thymus: the negative selection. There is still a long road to solve the complexity of the cooperation of different GluRs in T-DC synapses. Understanding the precise role of glutamate signalling in determining T-cell fate by modulating the proliferation/differentiation balance, the effector/regulatory and effector/memory transitions, might reflect on the physiology of the adaptive immune response. Our findings together with previous data3 raise potential applications for drug-mediated immunomodulation through interactions with -glutamate signalling.
Materials and Methods
Mice
The generation and genotyping of HNT-TCR transgenic mice specific for the HA 126–138 (HNTNGVTAACSHE) peptide presented by MHC class II I-Ad have been described elsewhere.24 All animals had a B10.D2 (H2d) genetic background and were used at the age of 6–10 weeks. All animal experiments conformed to European Community guidelines of animal protection and local legal and ethical requirements. All experiments were approved by local authorities and direction des Services Veterinaires des Hauts-De-Seine (authorization for animal experimentation to SC-K, No. 92–131). T cells and DCs were isolated, purified and cocultured using standard methods as described in Supplementary online Materials and Methods.
Antibodies and reagents
All antibodies were proved to be specific and are widely used in the neuronal system. Antibodies against NR2A and NR2B were purchased from Upstate Biotechnology (Millipore SAS, Molsheim, France). Antibodies against NR1 (M68), VGLUT1, VGLUT2 and VGLUT3 used in flow cytometry or confocal microscopy were from Synaptic Systems (Goettingen, Germany). Anti-NR1 antibody M68, widely used in neurones, recognized a band at the expected molecular weight (116 KD) in western blotting experiments of rat brain microsomal preparations and of thymocytes extracts immunoprecipitated by anti-PSD-95 (data not shown). Biotinylated anti-CD3 (145–2C11) and anti-CD28 (37.51) were from Pharmingen (BD Biosciences, Le Pont de Claix, France). Antibodies against PSD-95 and glutamate were from Zymed (San Francisco, CA, USA) and Sigma-Aldrich (Lyon, France), respectively. All inhibitors and antagonists specific for GluRs were from Tocris (Bristol, UK).
DNA chip analysis of the GluR repertoire
DNA chip analysis was performed on Neurotrans NT280M oligochips from GeneScore (www.genescore.fr), registered on GEO (Gene Expression Omnibus) (No GPL4746), already used for studies in the CNS and containing oligonucleotides for 280 genes involved in neurotransmission (two oligonucleotides for each gene).39 Modifications are available in Supplementary online Materials and Methods.
Single-cell Ca2+ video imaging
Single-cell Ca2+ video imaging on T-DC conjugates was carried out using a modified version of a previously described method.40 Purified DCs were plated on glass delta T culture dishes (Bioptechs, Butler, PA, USA) coated with polylysine and maintained at 37 °C in recording solution (mammalian saline: 116 mM NaCl, 5.6 mM KCl, 1.2 mM MgCl2, 2 mM CaCl2, 5 mM NaHCO3, 1 mM NaH2PO4, 20 mM HEPES, pH 7.3, supplemented with 2 g/l glucose). Thymocytes or CD4+ T cells were loaded for 30 min with 4 μM Fura-2/AM (Molecular Probes ,Invitrogen, Cergy Pontoise, France) by incubation for 30 min at 37 °C in culture medium. When indicated T cells were preincubated for 10 min with the NMDAR antagonists and then dispensed on top of the DCs maintained at 37 °C using a temperature-controlled dish, which was fixed above a warmed epifluorescence 40 × oil objective (Bioptechs, Farmingdale, NY, USA) and a PTR 200 perfusion temperature regulator (ALA Scientific Instruments, Farmingdale, NY, USA). Successive fluorescence and DIC images were recorded for 20 min. Light for excitation was supplied by a high pressure 100 W xenon arc lamp and the 340 and 380 nm wavelengths were selected with a monochromator (Cairn Research Ltd. Faversham Kent, UK). Fluorescence images were collected with a Sensicam QE CCD camera (PCO Computer Optics GmbH, Kelheim, Germany), digitized, and integrated in real time by an image processor (Metafluor, Molecular Devices, Downingtown, PA, USA). The light transmission images were taken every 6 s, alternating with the fluorescent images. The fluorescent signals were analyzed offline, using an image processor (Metamorph, Molecular Devices) to account for cell movements during the 20-min recordings. Background fluorescence was subtracted from the corresponding fluorescent images. To allow comparison between different labs, results are expressed as (ΔR/R), where R is the ratio (R) between fluorescence signals at 340 and 380 nm obtained before the addition of any agent, and ΔR the difference between the ratios measured during a response and R. For single cell Ca2+ video imaging in isolated thymocytes and DCs, a modification of this protocol was used and is provided in Supplementary online Materials and Methods.
Extracellular glutamate imaging
We used the -GDH-linked assay, a fluorescence-based assay allowing dynamic quantification of glutamate release in non-excitable cells, to monitor rapid glutamate release from DCs.32 Purified DCs (5 × 105) were incubated for 20 min on glass coverslips treated with polylysine, to allow adhesion. DCs were washed and bathed in the recording solution (mammalian saline as above, supplemented with NAD (1 mM), and -GDH at 50 IU/ml (Sigma-Aldrich). Glutamate released from the cells was immediately oxidized to α-ketoglutarate by GDH, with the formation of NADH and fluorescence emission at 450 nm. Controls without -GDH indicated that detectable levels of NADH are not produced by DCs, either spontaneously or after induction by ionomycin. Agents were added directly to the culture dish.
Flow cytometry and confocal microscopy
Flow cytometry (four colour flow cytometry, activated caspase-3 detection and Ca2+ flux studies) and confocal microscopy on T-DC conjugates were performed using standard procedures as described in Supplementary online Materials and Methods. The controls consisted of mouse isotype controls (as control for the monoclonal M68 anti-NR1, anti-Nur77 and anti-synaptotagmine antibodies), and purified rabbit IgG as control for anti-NR2A, NR2B, GluR2/3, PSD-95, KA2, glutamate, VGLUT antibodies. The same settings and exposure time were used for image acquisition by confocal microscopy of the specific staining and the controls.
Electron microscopy
Morphological analysis and post-embedding immunocytochemistry and immunogold labelling of T-DC conjugates was performed using classical techniques as described in Supplementary online Materials and Methods. Mouse isotype controls and purified rabbit IgG were used as controls for immunogold staining, with the same exposure time and magnification as specific staining.
Statistical analysis
Data are expressed as means ± S.E.M. Statistical significance was assessed by the non-parametric Mann–Whitney test, using Prism, version 5.0, software (GraphPad Software, San Diego, CA, USA). The significance level was set at P=0.05. The Kolmogorov–Smirnov test (Cellquest Software, BD Biosciences) was used to compare FACS profiles.
Acknowledgments
We thank A Trautmann and B Malissen for helpful discussions and critical reading of the paper. We thank JF Renaud and A Lombet for continuous support and N Kerlero de Rosbo for reading the paper. We thank B Lucas for helpful discussions. We thank C Rücker-Martin and V Nicolas for advice and assistance on confocal microscopy, L Dauphinot for expertize in PCR, D Jaillard for excellent assistance with electron microscopy, JP Mauger and S Wick for access to the animal facility. This work was supported by grants from Association de la Recherche contre le Cancer (to SC-K and to TC), Fondation de France (to SC-K), Bonus Qualité Recherche de l'Université Paris-Sud (to OM and SC-K), Centre National de la Recherche Scientifique, Conseil Régional Ile de France (Sesame) and Association Marie Lannelongue. PA was supported as a PhD candidate by a studentship from the Ministère de l'Education nationale, de la Recherche et de la Technologie. GLC and SP are supported by the Medical Research Council. GLC is a Royal Society-Wolfson Merit Award Holder.
Glossary
- APC
antigen-presenting cell
- Ca2+
calcium
- CRAC
Ca2+ release-activated Ca2+
- CNS
central nervous system
- DC
dendritic cell
- DP
CD4+CD8+ double-positive
- GluR
glutamate receptor
- IS
immunological synapse
- mGluR
metabotropic glutamate receptor
- NMDA
N-methyl--aspartate
- NMDAR
NMDA receptor
- PSD
postsynaptic density
- SOC
store-operated calcium channel
- TCR
T-cell receptor
- VGLUT
vesicular glutamate transporter
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by A Verkhratsky
Supplementary Material
References
- Nedergaard M, Takano T, Hansen AJ. Beyond the role of glutamate as a neurotransmitter. Nat Rev Neurosci. 2002;3:748–755. doi: 10.1038/nrn916. [DOI] [PubMed] [Google Scholar]
- Skerry TM, Genever PG. Glutamate signalling in non-neuronal tissues. Trends Pharmacol Sci. 2001;22:174–181. doi: 10.1016/s0165-6147(00)01642-4. [DOI] [PubMed] [Google Scholar]
- Pacheco R, Gallart T, Lluis C, Franco R. Role of glutamate on T-cell mediated immunity. J Neuroimmunol. 2007;185:9–19. doi: 10.1016/j.jneuroim.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Pacheco R, Ciruela F, Casado V, Mallol J, Gallart T, Lluis C, et al. Group I metabotropic glutamate receptors mediate a dual role of glutamate in T cell activation. J Biol Chem. 2004;279:33352–33358. doi: 10.1074/jbc.M401761200. [DOI] [PubMed] [Google Scholar]
- Chiocchetti A, Miglio G, Mesturini R, Varsaldi F, Mocellin M, Orilieri E, et al. Group I mGlu receptor stimulation inhibits activation-induced cell death of human T lymphocytes. Br J Pharmacol. 2006;148:760–768. doi: 10.1038/sj.bjp.0706746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldyrev AA, Carpenter DO, Johnson P. Emerging evidence for a similar role of glutamate receptors in the nervous and immune systems. J Neurochem. 2005;95:913–918. doi: 10.1111/j.1471-4159.2005.03456.x. [DOI] [PubMed] [Google Scholar]
- Ganor Y, Besser M, Ben-Zakay N, Unger T, Levite M. Human T cells express a functional ionotropic glutamate receptor GluR3, and glutamate by itself triggers integrin-mediated adhesion to laminin and fibronectin and chemotactic migration. J Immunol. 2003;170:4362–4372. doi: 10.4049/jimmunol.170.8.4362. [DOI] [PubMed] [Google Scholar]
- Lombardi G, Dianzani C, Miglio G, Canonico PL, Fantozzi R. Characterization of ionotropic glutamate receptors in human lymphocytes. Br J Pharmacol. 2001;133:936–944. doi: 10.1038/sj.bjp.0704134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglio G, Varsaldi F, Lombardi G. Human T lymphocytes express N-methyl-D-aspartate receptors functionally active in controlling T cell activation. Biochem Biophys Res Commun. 2005;338:1875–1883. doi: 10.1016/j.bbrc.2005.10.164. [DOI] [PubMed] [Google Scholar]
- Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotturi MF, Hunt SV, Jefferies WA. Roles of CRAC and Cav-like channels in T cells: more than one gatekeeper. Trends Pharmacol Sci. 2006;27:360–367. doi: 10.1016/j.tips.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Norcross MA. A synaptic basis for T-lymphocyte activation. Ann Immunol (Paris) 1984;135D:113–134. doi: 10.1016/s0769-2625(84)81105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006;7:247–255. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- Brossard C, Feuillet V, Schmitt A, Randriamampita C, Romao M, Raposo G, et al. Multifocal structure of the T cell – dendritic cell synapse. Eur J Immunol. 2005;35:1741–1753. doi: 10.1002/eji.200425857. [DOI] [PubMed] [Google Scholar]
- Dustin ML, Colman DR. Neural and immunological synaptic relations. Science. 2002;298:785–789. doi: 10.1126/science.1076386. [DOI] [PubMed] [Google Scholar]
- Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol. 2004;5:575–581. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- Pacheco R, Oliva H, Martinez-Navio JM, Climent N, Ciruela F, Gatell JM, et al. Glutamate released by dendritic cells as a novel modulator of T cell activation. J Immunol. 2006;177:6695–6704. doi: 10.4049/jimmunol.177.10.6695. [DOI] [PubMed] [Google Scholar]
- Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–337. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- Palmer E. Negative selection--clearing out the bad apples from the T-cell repertoire. Nat Rev Immunol. 2003;3:383–391. doi: 10.1038/nri1085. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Armstrong N. Structure and function of glutamate receptor ion channels. Annu Rev Physiol. 2004;66:161–181. doi: 10.1146/annurev.physiol.66.050802.084104. [DOI] [PubMed] [Google Scholar]
- Storto M, de Grazia U, Battaglia G, Felli MP, Maroder M, Gulino A, et al. Expression of metabotropic glutamate receptors in murine thymocytes and thymic stromal cells. J Neuroimmunol. 2000;109:112–120. doi: 10.1016/s0165-5728(00)00269-1. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56:2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liblau RS, Tisch R, Shokat K, Yang X, Dumont N, Goodnow CC, et al. Intravenous injection of soluble antigen induces thymic and peripheral T-cells apoptosis. Proc Natl Acad Sci U S A. 1996;93:3031–3036. doi: 10.1073/pnas.93.7.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woronicz JD, Calnan B, Ngo V, Winoto A. Requirement for the orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature. 1994;367:277–281. doi: 10.1038/367277a0. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Edmondson SG, Miller AD, Sellars M, Alexander ST, Somersan S, et al. Cutting edge: identification of the targets of clonal deletion in an unmanipulated thymus. J Immunol. 2003;170:10–13. doi: 10.4049/jimmunol.170.1.10. [DOI] [PubMed] [Google Scholar]
- Merkenschlager M, Graf D, Lovatt M, Bommhardt U, Zamoyska R, Fisher AG. How many thymocytes audition for selection. J Exp Med. 1997;186:1149–1158. doi: 10.1084/jem.186.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Tomassian T, Zhang M, Patel V, Schoenberger SP, Miceli MC. Dlgh1 coordinates actin polymerization, synaptic T cell receptor and lipid raft aggregation, and effector function in T cells. J Exp Med. 2005;201:419–430. doi: 10.1084/jem.20041428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Innocenti B, Parpura V, Haydon PG. Imaging extracellular waves of glutamate during calcium signaling in cultured astrocytes. J Neurosci. 2000;20:1800–1808. doi: 10.1523/JNEUROSCI.20-05-01800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, et al. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Montes M, McIlroy D, Hosmalin A, Trautmann A. Calcium responses elicited in human T cells and dendritic cells by cell-cell interaction and soluble ligands. Int Immunol. 1999;11:561–568. doi: 10.1093/intimm/11.4.561. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Borg C, Jalil A, Laderach D, Maruyama K, Wakasugi H, Charrier S, et al. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood. 2004;104:3267–3275. doi: 10.1182/blood-2004-01-0380. [DOI] [PubMed] [Google Scholar]
- Trautmann A. Chemokines as immunotransmitters. Nat Immunol. 2005;6:427–428. doi: 10.1038/ni0505-427. [DOI] [PubMed] [Google Scholar]
- Jun JE, Wilson LE, Vinuesa CG, Lesage S, Blery M, Miosge LA, et al. Identifying the MAGUK protein Carma-1 as a central regulator of humoral immune responses and atopy by genome-wide mouse mutagenesis. Immunity. 2003;18:751–762. doi: 10.1016/s1074-7613(03)00141-9. [DOI] [PubMed] [Google Scholar]
- Potier MC, Gibelin N, Cauli B, Le Bourdelles B, Lambolez B, Golfier G, et al. Developement of microarrays to study gene expression in tissue and single cells: analysis of neural transmissionIn: Geschwind DH, Gregg JP, (Eds)Microarrays for the Neurosciences: An Essential Guide MIT Press: Cambridge; 2002237–254. [Google Scholar]
- Delon J, Bercovici N, Raposo G, Liblau R, Trautmann A. Antigen-dependent and -independent Ca2+ responses triggered in T cells by dendritic cells compared with B cells. J Exp Med. 1998;188:1473–1484. doi: 10.1084/jem.188.8.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.