Abstract
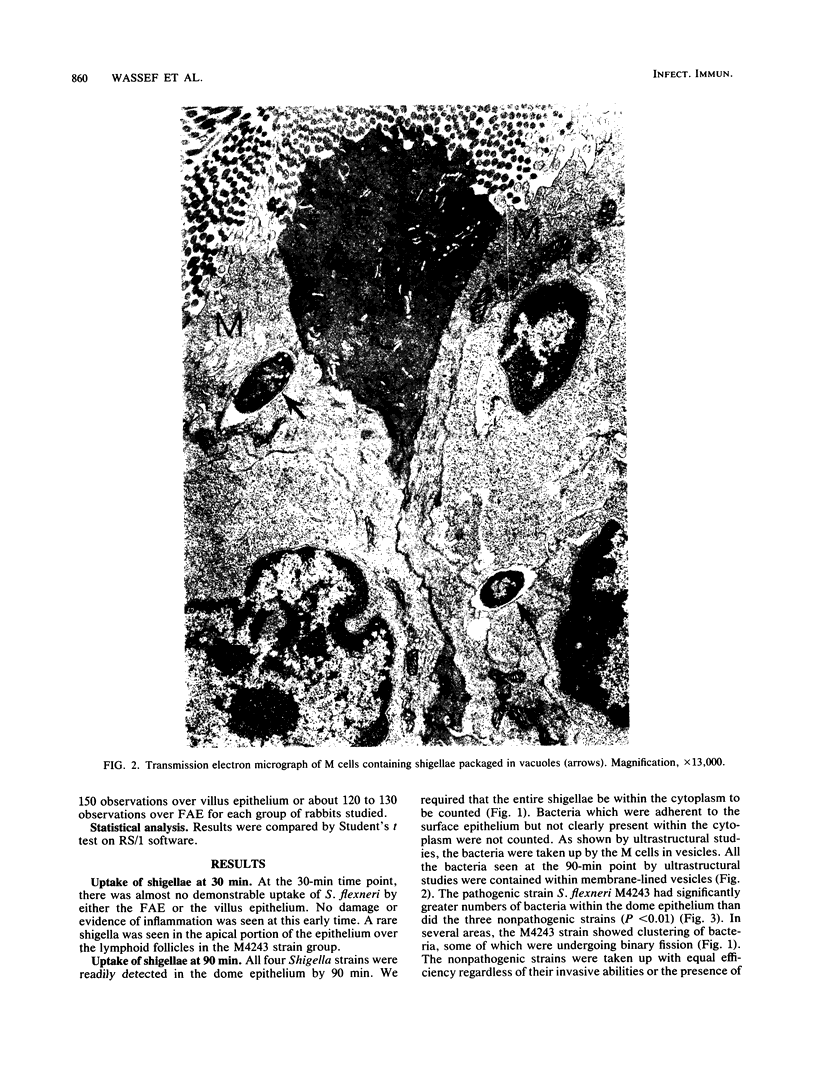
Strains of Shigella flexneri with different invasive and pathogenic potentials were inoculated into the intestinal lumen of acutely ligated loops in nonimmune rabbits. After 90 min, tissues processed for ultrastructural as well as light microscopy showed that the bacilli were phagocytosed by M cells over lymphoid follicles of Peyer's patches and carried in vacuoles into the epithelium. Nonpathogenic as well as pathogenic strains were readily taken up regardless of the presence of the 140-megadalton virulence plasmid. More virulent than avirulent shigellae were found in M cells at 90 min, reflecting replication or preferential uptake of the virulent strains. Heat-killed shigellae of the virulent strain were taken up by M cells to the same degree as the avirulent strains. Incubation of the bacteria for 18 h resulted in surface ulceration which was limited to epithelium overlying lymphoid follicles (M cell areas) in acute loops exposed to the virulent shigellae. Villus epithelium adjacent to the ulcerated follicular domes was intact, although there was mucus depletion. In the present study, we found that pathogenic shigellae appear to replicate in the M cells, escape from the phagocytic vesicles, and thereby initiate the ulcerations in this experimental model of dysentery. While initial antigen processing in the gut for a mucosal immune response may require uptake of luminal microorganisms by M cells, this may pose a threat under some circumstances.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bockman D. E., Cooper M. D. Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix, and Peyer's patches. An electron microscopic study. Am J Anat. 1973 Apr;136(4):455–477. doi: 10.1002/aja.1001360406. [DOI] [PubMed] [Google Scholar]
- FORMAL S. B., LABREC E. H., KENT T. H., FALKOW S. ABORTIVE INTESTINAL INFECTION WITH AN ESCHERICHIA COLI-SHIGELLA FLEXNERI HYBRID STRAIN. J Bacteriol. 1965 May;89:1374–1382. doi: 10.1128/jb.89.5.1374-1382.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Kent T. H., Austin S., Labrec E. H. Fluorescent-antibody and histological study of vaccinated and control monkeys challenged with Shigella flexneri. J Bacteriol. 1966 Jun;91(6):2368–2376. doi: 10.1128/jb.91.6.2368-2376.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Labrec E. H., Palmer A., Falkow S. Protection of Monkeys Against Experimental Shigellosis with Attenuated Vaccines. J Bacteriol. 1965 Jul;90(1):63–68. doi: 10.1128/jb.90.1.63-68.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura Y. Functional morphology of microfold cells (M cells) in Peyer's patches--phagocytosis and transport of BCG by M cells into rabbit Peyer's patches. Gastroenterol Jpn. 1986 Aug;21(4):325–335. [PubMed] [Google Scholar]
- Inman L. R., Cantey J. R. Specific adherence of Escherichia coli (strain RDEC-1) to membranous (M) cells of the Peyer's patch in Escherichia coli diarrhea in the rabbit. J Clin Invest. 1983 Jan;71(1):1–8. doi: 10.1172/JCI110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan K. P., Sharpnack D. D., Collins H., Formal S. B., O'Brien A. D. Morphologic evaluation of the effects of Shiga toxin and E coli Shiga-like toxin on the rabbit intestine. Am J Pathol. 1986 Oct;125(1):69–80. [PMC free article] [PubMed] [Google Scholar]
- Keren D. F., Elliott H. L., Brown G. D., Yardley J. H. Atrophy of villi with hypertrophy and hyperplasia of Paneth cells in isolated (thiry-Vella) ileal loops in rabbits. Light-microscopic studies. Gastroenterology. 1975 Jan;68(1):83–93. [PubMed] [Google Scholar]
- Keren D. F., McDonald R. A., Formal S. B. Secretory immunoglobulin A response following peroral priming and challenge with Shigella flexneri lacking the 140-megadalton virulence plasmid. Infect Immun. 1986 Dec;54(3):920–923. doi: 10.1128/iai.54.3.920-923.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren D. F., Scott P. J., McDonald R. A., Kern S. E. Local IgA-memory response to bacterial antigens. Ann N Y Acad Sci. 1983 Jun 30;409:734–744. doi: 10.1111/j.1749-6632.1983.tb26912.x. [DOI] [PubMed] [Google Scholar]
- O'Brien A. D., Gentry M. K., Thompson M. R., Doctor B. P., Gemski P., Formal S. B. Shigellosis and Escherichia coli diarrhea: relative importance of invasive and toxigenic mechanisms. Am J Clin Nutr. 1979 Jan;32(1):229–233. doi: 10.1093/ajcn/32.1.229. [DOI] [PubMed] [Google Scholar]
- Oaks E. V., Wingfield M. E., Formal S. B. Plaque formation by virulent Shigella flexneri. Infect Immun. 1985 Apr;48(1):124–129. doi: 10.1128/iai.48.1.124-129.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. L. And now pathophysiology of M cells--good news and bad news from Peyer's patches. Gastroenterology. 1983 Aug;85(2):468–470. [PubMed] [Google Scholar]
- Owen R. L., Jones A. L. Epithelial cell specialization within human Peyer's patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974 Feb;66(2):189–203. [PubMed] [Google Scholar]
- Owen R. L., Pierce N. F., Apple R. T., Cray W. C., Jr M cell transport of Vibrio cholerae from the intestinal lumen into Peyer's patches: a mechanism for antigen sampling and for microbial transepithelial migration. J Infect Dis. 1986 Jun;153(6):1108–1118. doi: 10.1093/infdis/153.6.1108. [DOI] [PubMed] [Google Scholar]
- Owen R. L. Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer's patches in the normal unobstructed mouse intestine: an ultrastructural study. Gastroenterology. 1977 Mar;72(3):440–451. [PubMed] [Google Scholar]
- Rosner A. J., Keren D. F. Demonstration of M cells in the specialized follicle-associated epithelium overlying isolated lymphoid follicles in the gut. J Leukoc Biol. 1984 Apr;35(4):397–404. doi: 10.1002/jlb.35.4.397. [DOI] [PubMed] [Google Scholar]
- Rout W. R., Formal S. B., Giannella R. A., Dammin G. J. Pathophysiology of Shigella diarrhea in the rhesus monkey: intestinal transport, morphological, and bacteriological studies. Gastroenterology. 1975 Feb;68(2):270–278. [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneller M. C., Strober W. M cells and host defense. J Infect Dis. 1986 Nov;154(5):737–741. doi: 10.1093/infdis/154.5.737. [DOI] [PubMed] [Google Scholar]
- Wolf J. L., Dambrauskas R., Sharpe A. H., Trier J. S. Adherence to and penetration of the intestinal epithelium by reovirus type 1 in neonatal mice. Gastroenterology. 1987 Jan;92(1):82–91. doi: 10.1016/0016-5085(87)90842-0. [DOI] [PubMed] [Google Scholar]
- Wolf J. L., Kauffman R. S., Finberg R., Dambrauskas R., Fields B. N., Trier J. S. Determinants of reovirus interaction with the intestinal M cells and absorptive cells of murine intestine. Gastroenterology. 1983 Aug;85(2):291–300. [PubMed] [Google Scholar]






