Abstract
Elimination of autoreactive CD4+ T cells through the death receptor Fas/CD95 is an important mechanism of immunological self-tolerance. Fas deficiency results in systemic autoimmunity, yet does not affect the kinetics of T-cell responses to acute antigen exposure or infection. Here we show that Fas and TCR-induced apoptosis are largely restricted to CD4+ T cells with an effector memory phenotype (effector memory T cells (TEM)), whereas central memory and activated naïve CD4+ T cells are relatively resistant to both. Sensitivity of TEM to Fas-induced apoptosis depends on enrichment of Fas in lipid raft microdomains, and is linked to more efficient formation of the Fas death-inducing signaling complex. These results explain how Fas can cull T cells reactive against self-antigens without affecting acute immune responses. This work also identifies Fas-induced apoptosis as a possible immunotherapeutic strategy to eliminate TEM linked to the pathogenesis of a number of autoimmune diseases.
Keywords: Fas, death receptors, lipid raft microdomains, CD4 T cells, apoptosis
The TNF-family receptor Fas/CD95 can directly induce apoptotic programmed cell death and is essential for maintaining immunological self-tolerance. Humans with dominant-negative Fas mutations in the autoimmune lymphoproliferative syndrome (ALPS) and mice deficient for Fas or Fas ligand are highly predisposed to autoimmunity.1, 2, 3 Recent findings have shown that expression of Fas on T cells is important for the function of Fas in maintaining immunological self-tolerance.4 In vitro, apoptosis induced by the TCR, termed restimulation-induced cell death (RICD), is dependent on Fas in CD4+ T cells.5 However, it has been difficult to demonstrate defective deletion of Fas-deficient T cells in response to acute infections, immunization or superantigen stimulation in vivo.6 Rather, Fas appears to have a role mainly in deletion of T cells that are chronically or repeatedly exposed to TCR stimulation.6
T cells previously exposed to antigen acquire phenotypic and functional characteristics of memory T cells, including the ability to self-renew more efficiently and respond to antigen with minimal co-stimulation.7 These properties are important for maintaining immunity to pathogens but may also allow the perpetuation of pathological autoimmune responses. Memory T cells exist in functionally distinct subsets based on expression of CCR7 and CD62L, which allow lymph node homing,8 and the TNF-family member CD27. Memory T cells expressing CD62L, CD27 and CCR7 are termed ‘central memory' (TCM) because they efficiently home to lymph nodes and self-renew. By contrast, effector memory T cells (TEM) migrate to extralymphatic sites and have immediate potential to produce effector cytokines on TCR restimulation. Although the precise interplay between effector and TCM is not fully understood, TEM have been implicated in the pathogenesis of a number of T-cell-dependent autoimmune diseases and animal models of autoimmunity.9, 10
Here, we have found that TCR and Fas-mediated apoptosis in primary human and mouse CD4+T cells is largely restricted to cells with an effector memory phenotype, and have linked this sensitivity to Fas localization to lipid raft microdomains and more efficient assembly and activation of the Fas-associated death-inducing signaling complex (DISC).
Results
Fas-induced apoptosis is restricted to the effector memory subset of human CD4+ T cells
We observed a wide variation in sensitivity to Fas-induced apoptosis between donors in samples of total CD4+ T cells from healthy volunteers, and reasoned that this may correlate with the variation in the percentage of CD45RO+ memory T cells. To assay the apoptosis sensitivity of different T-cell subsets defined by surface markers, we developed a multi-parameter flow cytometric assay to determine the percentage of annexin V-positive early apoptotic cells simultaneously with surface markers for memory T cells (CD45RA−) and the chemokine receptor CCR7 and the TNF-receptor family member CD27, which distinguish effector and central memory CD4+ T-cell subsets.11, 12 As previously observed,12 naïve CD45RA+ T cells were predominantly CCR7+/CD27+, while CD45RA− memory T cells could be divided into TCM (CCR7+/CD27+) or TEM (CCR7−/CD27−) and a small subset of CCR7−CD27+ ‘transitional' memory cells, (Figure 1a, bottom left). Naive CD4+ T cells did not express detectable Fas as previously reported,13 while TCM and TEM CD4+ T cells expressed identical levels of surface Fas (Figure 1b). Correlating with their lack of surface Fas, naïve T cells were highly resistant to apoptosis induced by crosslinked anti-Fas antibodies (Figure 1c). Strikingly, TCM phenotype CCR7+CD27+ T cells were also almost completely resistant to apoptosis induced by Fas crosslinking, whereas TEM contained nearly all the Fas-sensitive T cells in the memory T-cell pool (Figure 1c). TEM CD4+ T cells were also the most sensitive to apoptosis induced by a stabilized form of FasL (FasL-LZ), the physiological ligand for Fas (Figure 1d). Taken together, these data show that within freshly isolated CD4+ T cells, TEM are virtually the only cells with a functional Fas receptor.
Figure 1.
Human effector memory CD4+ T cells are selectively susceptible to Fas-induced apoptosis directly ex vivo. (a) Markers used to subset CD4+ T cells into naïve and memory subsets (CD45RA) and then into central (TCM) and effector (TEM) subsets (CCR7 and CD27) are shown. (b) Histogram overlay indicates surface Fas expression within the CD4+ sub-populations gated as above with shaded histograms representing isotype controls. Data are representative of four different donors. (c) Apoptosis was induced by 6–8 h treatment with the indicated concentrations of crosslinked anti-Fas mAb APO-1-3 in freshly isolated CD4+ T-cell subsets using CD4, CD45RA, CD27 and CCR7 surface staining and the apoptosis marker annexin V. Specific cell death was calculated for each T-cell subset as mentioned in the Materials and Methods section. (d) Apoptosis was induced in unseparated CD4+ T cells with the indicated concentration of FasL-LZ for 6–8 h and induced cell death was quantitated as in c. The data in c and d are an average of experiments from six separate donor samples, error bars indicate ±S.E.M. Values statistically different between naïve and memory or TCM versus TEM: *P≤0.03, **P≤0.009, ***P≤0.0008, paired Student's t-test
In order to test whether Fas-induced apoptosis is restricted to cells with a TEM phenotype also in activated T cells, we purified populations of naïve or memory CD4+ T cells from normal donors, activated them with anti CD3/CD28 followed by expansion in IL-2, conditions extensively used to define and study RICD.14, 15 We observed progressive conversion of memory cells to a TEM phenotype, downregulating CD27 and CCR7 and gaining CD70 expression (Figure 2a, data not shown). Naïve CD4+ T cells gained CD45RO and lost CD45RA expression, but maintained a CCR7+/CD27+ TCM phenotype for up to 2 weeks of culture in IL-2 (Figure 2a). After activation, naïve T cells also acquired nearly equivalent levels of Fas to that expressed on activated memory T cells (Figure 2b). When activated T cells derived from naïve T-cell precursors were treated with anti-Fas antibody or FasL, they remained relatively resistant to Fas-induced apoptosis compared with memory T cells (Figures 2c and d). Memory T cells retaining TCM-like phenotype were as resistant to Fas-induced apoptosis as naïve T cells. Apoptosis of TEM cells accounted for nearly all of the Fas sensitivity of activated T cells similar to cells analyzed directly ex vivo (Figures 2c and d). As cells with a TCM and TEM phenotype were cultured together in the experiments, these data indicate cell-autonomous differences. These results show that a TEM phenotype is the best predictor of sensitivity to Fas-induced apoptosis in CD4+ T cells, both directly ex vivo and after in vitro expansion.
Figure 2.
Activated human memory and effector memory CD4+ T cells have the highest susceptibility to Fas-induced apoptosis. (a) Surface staining of CD3/CD28 activated and IL-2 expanded naïve and memory CD4+ T cells for TCM and TEM markers and (b) surface Fas levels in each population are indicated. Activated naïve and memory T cells were stimulated with either anti-Fas mAb APO-1-3 (c) or FasL-LZ (d) and apoptosis quantitated in cells with the indicated phenotype using CCR7 and CD27 surface markers. Data are averages ±S.E.M. of a minimum of six donors, *P<0.01, **P<0.05, ***P<0.0001, paired Student's t-test
In activated CD4+ T cells, much of the apoptosis triggered by restimulation of the TCR (RICD) is mediated through Fas–FasL interactions.16 T cells sorted initially for a naïve and TCM phenotype and then activated and expanded in vitro were significantly more resistant to RICD than memory and TEM cells (Figure 3a). TCM had significant residual sensitivity to TCR-induced apoptosis, which may be due to conversion of these cells to a TEM phenotype during in vitro expansion. Indeed, when activated T cells were assayed for RICD simultaneously with surface markers, TCR-induced apoptosis in TCM with a CD27+CCR7+ phenotype at the time of restimulation was reduced (Figure 3d). To determine the extent to which TCR-induced cell death was dependent on Fas–FasL interactions, we used blocking antibodies against FasL (Figure 3b) and also studied CD4+ T cells purified from the blood of ALPS patients containing Fas mutations that dominantly interfere with Fas apoptosis (Figures 3c and d). Anti-FasL (Nok-2) antibodies efficiently blocked RICD in restimulated naïve and memory subsets, indicating that memory and TEM cells undergo increased apoptosis induced by Fas-specific RICD mechanisms (Figure 3b). ALPS patient T cells were significantly protected from Fas-induced apoptosis, with the greatest differences occurring in T cells from ALPS patients harboring Fas mutations in the death domain (DD), consistent with previous data showing that Fas DD mutations cause the most severe impairment of Fas-mediated apoptotic signaling.17 TEM contained the most Fas-sensitive cells, which were also dramatically resistant to Fas-induced apoptosis in ALPS patients (Figure 3c). Much of the TCR-induced apoptosis seen in the TEM cells was dependent on Fas–FasL interactions, as TEM from ALPS patients were significantly resistant to RICD compared with healthy donors (Figure 3d).
Figure 3.
Fas-dependent RICD is restricted to human TEM (a) Sorted naïve, TCM, TEM and total memory CD4+ T cells were initially activated with CD3/CD28 followed by IL-2 for 8–10 days and subsequently restimulated with plate-bound anti-CD3 for 6–8 h and analyzed for cell death. Data shown are an average from six different donors, error bars are ± S.E.M, P-values indicate statistical significance between naïve, memory and TCM, TEM subsets, *P<0.01, **P<0.001, ***P<0.0003. (b) Fas-specific apoptosis in RICD is tested using anti-FasL (Nok2, 25 μg/ml) blocking antibodies in naïve and memory subsets. Data are an average of two experiments done in triplicates. ***P<0.0009, *P=0.028. (c) Fas-specific cell death induced by anti-Fas antibodies in 1 week activated CD4+ subsets derived from normal donors (n=3) or ALPS patients (n=3) with either DD or non-DD Fas mutations are indicated using multiparameter gating strategy. Error bars are ±S.E.M. ***P<0.0005. (d) Anti-CD3-induced cell death is shown for the same subsets of activated CD4+ T cells from normal donors (n=3) or ALPS patients with dominant-negative mutations in the Fas DD (n=3). ***P<0.0005, paired Student's t-test
TCR triggering also results in synthesis and secretion of FasL. To determine whether differences in FasL production could contribute to the differential apoptosis of T-cell subsets, we measured FasL after TCR restimulation. Naïve and memory T cells produced comparable amounts of FasL whereas TCM and TEM produced slightly less (Supplementary Figures S1a and b). Interestingly, despite the similar levels of FasL as measured by ELISA, FasL produced by restimulated naïve T cells was less cytotoxic for the Fas-sensitive cell line SKW6.4 (Supplementary Figure S1c). This suggests that differences in posttranslational processing of FasL may contribute to the reduced TCR-induced apoptosis of naïve restimulated T cells.
Previous studies have identified murine central and effector CD4+ memory T cells based on surface expression of -selectin (CD62L) and CD44.18 We tested naïve (CD62Lhi/CD44lo), central memory (CD62Lhi/CD44hi) and effector memory (CD62Llo/CD44hi) CD4+ T-cell subsets for apoptosis in response to TCR crosslinking in cells from wild-type C57Bl/6 mice as well as Fas-deficient lpr/lpr mice on the B6 background. Unlike human T cells, murine naïve T cells did express some surface Fas (Figure 4e). Nevertheless, TEM phenotype cells were the most susceptible to RICD and FasL-induced apoptosis (Figures 4a and c), and experiments Fas-deficient Lpr T cells confirmed that FasL and RICD were completely dependent on Fas (Figures 4b and d). After activation and expansion in vitro with IL-2, naïve and TCM T cells downmodulated CD62L and the majority acquired an effector memory (CD62Llo/CD44hi) phenotype (Supplementary Figure S2a). In accordance with these phenotypic changes, naïve T cells acquired sensitivity to FasL-induced apoptosis that was higher than the apoptosis observed in activated TEM or TCM, but lower than that of TEM in resting T cells (Supplementary Figure S2d). In response to RICD, in vitro expanded TEM remained the most sensitive to apoptosis (Supplementary Figure S2c). In Lpr Fas-deficient activated T cells, there was slight upregulation of surface Fas that is likely because of the known ‘leakiness' of the lpr mutation in the Fas gene19 (Supplementary Figure S2b). Despite this small amount of Fas expression, Lpr T cells remained totally resistant to FasL-induced cell death (Supplementary Figure S2d). These data indicate that Fas and RICD in mouse CD4+ T cells is largely confined to TEM phenotype CD4+ T cells directly after isolation, but that conversion to TEM takes place more freely in mouse T cells after activation through the TCR and expansion in IL-2.
Figure 4.
Fas-dependent apoptosis is largely restricted to murine TEM phenotype CD4+ T cells. Sorted naïve (CD62Lhi/CD44lo), central memory (CD62Lhi/CD44hi) and effector memory (CD62Llo/CD44hi) CD4+ T cells from WT B6 (a and c) or B6 LPR (b and d) mice were treated directly after sorting with plate-bound anti-CD3 (a and b) or exogenous FasL-LZ (c and d) for 8 h. Apoptosis was quantitated by annexin/PI staining. Fas staining on the ex vivo sorted CD4+ T-cell subsets are shown in (e). All experiments are from two repeats with three replicates, error bars are ±S.E.M., and *P<0.04, **P<0.004, ***P<0.0005, paired Student's t-test. In c, * above the line indicate significant differences between TEM and TCM and lower indicate TEM versus naïve
Fas function in CD4+ T-cell subsets is regulated at the level of the DISC rather than bcl-2 family proteins
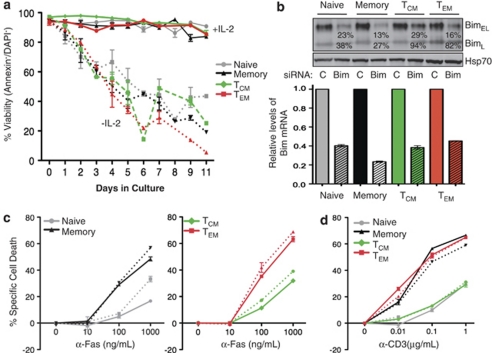
The differential sensitivity of CD4+ T-cell subsets to Fas-induced apoptosis may be regulated by cell-intrinsic survival factors, such as bcl-2 family members, or more proximally, at the Fas signaling complex. Flow cytometry revealed similar levels of bcl-2 and bcl-xL, two pro-survival members of the bcl-2 family,20 in activated human naïve, memory, TCM and TEM cells (Supplementary Figure S3a). The BH3 protein Bim triggers apoptosis of effector T cells after cytokine deprivation and is critical for the contraction phase of T cells after an acute immune response.21, 22 We tested the ability of naïve and memory T-cell subsets to undergo apoptosis after deprivation of IL-2 and found that all died with similar kinetics (Figure 5a). A previous study implicated reduced activity of AKT and higher levels of Bim in the increased sensitivity of TEM to apoptotic stimuli.23 However, we did not find reduced phosphorylated AKT or FOXO3a in TEM, and found slightly lower levels of Bim in TEM compared with other subsets (Supplementary Figure S3b). Additionally, apoptosis in response to anti-Fas (Figure 5c) or anti-CD3 (Figure 5d) was not dampened in the absence of Bim siRNA, which produced a 60–80% knockdown of Bim protein and mRNA (Figure 5b). The PI3-kinase inhibitor LY294002, also did not sensitize cells to Fas-mediated apoptosis (Supplementary Figure S4a) or spontaneous cell death (Supplementary Figure S4b) in the different CD4 subsets. Thus, the resistance of TCM and activated naïve T cells to apoptosis triggered by Fas or the TCR does not appear to depend on the activity of the PI3-kinase or AKT kinase pathways or the expression of Bim, which is regulated by these pathways.
Figure 5.
Comparable apoptosis after cytokine deprivation and lack of a requirement for Bim in TCR and Fas-induced apoptosis in human CD4+ T cells. (a) Activated T-cell subsets were expanded in growth medium with or without IL2. Viability was determined by FACS using annexin/DAPI staining on the days indicated. (b) Lysates of T-cell subsets transfected for 72 h with control (c) or Bim siRNA (Bim) were immunoblotted for Bim protein. Quantitative RT-PCR for Bim mRNA from the same cells is indicated below. (c and d) The apoptotic response induced by anti-Fas antibodies (c) and anti-TCR (d) in cells transfected with control siRNA (straight lines) or Bim siRNA (dashed lines) 72 h after transfection is indicated. Experiments are averages of two or five repeats done in triplicates and error bars are ±S.E.M.
To determine whether efficient Fas-induced apoptosis induction in TEM is coupled to early events in signal transduction, we studied the recruitment and processing of FADD and caspase-8, components of the Fas DISC,24 in activated T cells derived from memory or naïve CD4+ T cells. Recruitment of FADD to the DISC was severely reduced in naïve compared with memory T cells (Figure 6a). Recruitment of the p51/53 caspase-8 proenzyme was also significantly reduced in naïve T-cell preparations, and cleavage of caspase-8 into the p41/43 forms was impaired in the DISC of naïve T cells. Importantly, even weak bivalent uncrosslinked anti-Fas mAb (APO1-1) treatment results in FADD recruitment and significant caspase-8 cleavage (Supplementary Figure S5a). The lack of caspase-8 cleavage in the APO1-1-treated cells can be seen more clearly in the overexposure (Supplementary Figure S5a, lower inset). In cells derived from purified TCM and TEM, FADD recruitment and caspase-8 cleavage was reduced in TCM, especially at later time points (Supplementary Figure S6).
Figure 6.
Human memory CD4+ T cells form a more efficient Fas DISC. (a) Immunoblot analysis of DISC components in naïve and memory human CD4+ T cells after stimulating (30 min) with anti-Fas antibody and immunoprecipitating with protein A/G beads as indicated. Lysates from both anti-Fas-treated and control cells contained low levels of p41/43 processed caspase-8, likely because of autoprocessing after lysis, T-cell activation or spontaneous cell death. Lines represent lanes not shown and the data are a representative of eight independent experiments. (b) Densitometry analysis of recruited DISC components normalized to Fas in DISC is shown. Right panel compares caspase-8 cleavage as a ratio of unprocessed versus cleaved caspase-8 in the DISC
c-FLIP, a protein containing tandem DED domains and a pseudocaspase domain, can compete with caspase-8 for recruitment to the Fas DISC and inhibit activation of caspase-8 in the signaling complex when present at high levels relative to caspase-8.25, 26 Analysis of the Fas signaling complex for the presence of c-FLIP revealed increased levels of processed c-FLIPL (long isoform) as well as the short isoform, c-FLIPS in the DISC of memory versus naïve and TEM versus TCM subsets. Total levels of c-FLIPL and c-FLIPS were also higher in these Fas-sensitive subsets (Supplementary Figure S7). Therefore, c-FLIP is unlikely to regulate the assembly of the DISC in these experiments.
Measurement of caspase-8 p18 (cytosolic active caspase-8) and poly-ADP ribose polymerase (PARP) cleavage, which depends on mitochondrial permeability transition,27 revealed that Fas-resistant naïve T cells failed to produce the p18 subunit or cleaved PARP after Fas stimulation. Additionally, there was delayed production of these cleavage products after treatment with crosslinked anti-Fas mAb (Supplementary Figure S8). Thus, the reduced DISC activity seen in naïve T cells after Fas stimulation is likely the limiting factor in production of active caspase-8 and subsequent activation of effector caspases and resultant cell death.
Fas localization to lipid raft microdomains regulates efficiency of Fas signaling in memory CD4+ T-cell subsets
We have previously shown that efficient Fas-induced apoptosis depends on the localization of Fas to glycosphingolipid and cholesterol enriched, low-density lipoprotein fractions of the plasma membrane, termed ‘lipid rafts'.28 Cells in which Fas is prelocalized to lipid rafts, such as ‘type I' cell lines and T cells after stimulation through the TCR, are sensitive to apoptosis induced by bivalent anti-Fas, APO-1-1. Type II T-cell lines have little or no Fas present in lipid rafts and require stimulation with at least a dimer of Fas trimers or membrane-bound FasL to undergo apoptosis.28, 29 To test this paradigm, we stimulated activated naïve and memory CD4+ T cells with anti-Fas APO-1-1 or crosslinked anti-Fas (APO-1-3 plus anti-IgG3), and determined Fas-induced apoptosis. Naïve or TCM cells were consistently resistant to crosslinked and bivalent anti-Fas as well as FasL-LZ, although naïve T cells began to be sensitive to higher doses of crosslinked anti-Fas (Figure 7a). Density gradient fractionation of detergent lysates revealed a prominent amount of approximately 20% of the total Fas localized to fractions 3 and 4 containing lipid rafts in activated T cells derived from memory, but not in naïve T cells (Figure 7b). Enrichment of Fas in lipid rafts was specific, because the kinase lck, which localizes constitutively to lipid rafts, exhibited a similar partitioning into lipid rafts in T cells derived from purified naïve and memory precursors (Figure 7b). Additionally, use of filipin, a polyene antibiotic known to bind cholesterol,30 revealed no differences between naïve and memory T cells (Supplementary Figure S9a). Therefore, Fas appears to preferentially traffic to lipid raft microdomains in memory T cells and in TEM, and this correlates with higher sensitivity to Fas-induced apoptosis.
Figure 7.
Fas localization to lipid raft microdomains in human memory T cells promotes susceptibility to Fas-induced apoptosis. (a) Cell death from activated human CD4+ T-cell subsets was calculated from annexin/PI staining, after treatment for 8 h either with anti-Fas IgG1 (bivalent) or anti-Fas APO-1-3 crosslinked with anti-IgG3 (crosslinked). Data are an average of nine different donors and error bars are ± S.E.M. (b) Immunoblot analysis of the distribution of Fas in sucrose gradient fractions of memory and naïve CD4+ T cells. Lck is shown as a positive control for raft localization (bottom immunoblot panels). Densitometry analysis of the percentage of Fas and Lck in each fraction is shown in the lower graphs. Data are an average ± S.E.M. of five independent experiments. (c) Effect of the palmitoylation inhibitor 2-bromopalmitate on susceptibility of naïve and memory CD4+ T cells to apoptosis triggered by either bivalent or crosslinked anti-Fas mAb for 8 h is indicated. Data are an average of two experiments done in triplicates ± S.E.M., *P<0.02, **P<0.009, ***P<0.0001, paired Student's t-test
Localization of Fas to lipid raft microdomains requires palmitoylation of cysteine 199 in the juxtamembrane region of Fas, and treatment with the palmitate analog 2-bromopalmitate (BrPA) reduces the fraction of Fas in lipid raft microdomains and the efficiency of Fas-induced apoptosis in cell lines.31, 32 To determine if Fas localization to lipid rafts is required for efficient Fas signaling, we treated T cells with BrPA followed by stimulation with bivalent or crosslinked anti-Fas mAb. Naïve activated T cells, which were relatively resistant to apoptosis induced by either bivalent or crosslinked anti-Fas, were not further protected from Fas-induced apoptosis by BrPA, consistent with the low amounts of Fas localizing to lipid raft microdomains in these cells. Interestingly, in naïve cells, BrPA treatment increased sensitivity to apoptosis induced by anti-Fas mAb (Figure 7c) but not FasL (Supplementary Figure S9b). This may indicate that in naïve T cells, under certain conditions, Fas outside of lipid rafts may preferentially activate an apoptotic pathway, as occurs with TNFR1.33
BrPA was able to significantly protect memory T cells from apoptosis induced by bivalent, but not crosslinked anti-Fas (Figure 7c). This difference may be due to extrinsic crosslinking overcoming the reduced efficiency of Fas apoptosis signaling outside of lipid rafts. Importantly, BrPA protected T cells from FasL-induced apoptosis, with the greatest protection occurring in the highly Fas-sensitive TEM (Supplementary Figure S9b), indicating that lipid raft localization may be important for apoptosis induced by the physiological ligand for Fas. Taken together, these data show that localization of Fas to lipid raft microdomains accounts for at least part of the enhanced sensitivity of memory T cells and TEM to Fas-induced apoptosis.
Discussion
These results show that Fas-dependent apoptosis is highly restricted to cells with an effector memory phenotype in both mouse and human CD4+ T cells. Restriction of Fas-induced apoptosis to TEM could be demonstrated both with anti-Fas antibodies and a preparation of FasL stabilized with an isoleucine zipper (FasL-LZ). Apoptosis triggered through the TCR was also restricted to TEM and was dependent on Fas, as TEM from ALPS patients and Lpr mice were protected from RICD. Remarkably, TCM were as resistant to Fas and RICD as cells harboring Fas mutations from patients with ALPS. Once activated, the sensitivity of these T-cell subsets to Fas-mediated apoptosis correlated with their surface phenotype. Human T cells with a central memory (CCR7+/CD27+) phenotype remained relatively resistant to TCR and Fas-mediated apoptosis, while murine T cells largely converted to a TEM phenotype and became sensitive to Fas. The relative resistance of activated naïve and TCM cells to TCR and Fas-induced apoptosis may allow expansion of these cells, which is critical for efficient T-cell immune responses. Restraining expansion of TEM through Fas-mediated apoptosis may be important in preventing immunopathology and expansion of T cells that can provide help for autoreactive B cells.
We have found a similar hierarchy in sensitivity to anti-Fas-induced cell death as Riou et al.23 However, under these conditions, neither Bim levels nor phosphorylation of AKT and FOXO3a contribute to apoptosis sensitivity induced by Fas, TCR or cytokine deprivation in activated T cells. Other studies have found Bim making little or no contribution to Fas-mediated apoptosis in CD4+ T cells.6, 34, 35 Instead, we have found that sensitivity to TCR and Fas-induced apoptosis is regulated by the efficiency of Fas signal transduction, namely the assembly and activation of the Fas-associated DISC. FADD and caspase-8 recruitment was reduced in naïve versus memory and TCM versus TEM and the recruited caspase-8 was also processed inefficiently in Fas-resistant T cells. Despite the known role of c-FLIP in regulating caspase-8 recruitment and activation in the DISC, we did not find increased total or DISC-associated c-FLIP in Fas-resistant T cells. Recent work has described a novel mechanism of caspase-8 activation via polyubiquitination in the TRAIL DISC.36 Whether a similar mechanism regulates Fas DISC efficiency in TEM is not known. Overall, susceptibility of TEM to the extrinsic cell death pathway may help regulate these potentially dangerous cells in a number of environments.
A number of findings reported here support the notion that the presence of Fas in lipid raft microdomains enhances Fas apoptosis signaling in primary T-cell subsets. A significantly higher fraction of Fas occurs in lipid rafts of memory compared with naïve T cells. TEM resemble T cells that have been restimulated through the TCR in their sensitivity to apoptosis even with low levels of Fas oligomerization.28 However, the fact that naïve T cells, unlike type II tumor cells such as Jurkat, were resistant to apoptosis induced even by highly crosslinked anti-Fas mAb indicates that Fas resistance in these T-cell subsets involves mechanisms beyond simply localization to lipid rafts.
These results also have a number of diagnostic and therapeutic clinical implications. Multi-parameter gating, used here, allows measurement of cell death in samples directly ex vivo, dispensing with T-cell expansion in culture. In samples with complex mixtures of cell types, this method should enable more accurate diagnostic tests for cell death defects especially in ALPS or other diseases linked to defective T-cell apoptosis. Elimination of antigen-specific T cells through administration of antigen or antigen-bearing cells in a non-immunogenic manner has been proposed as a therapeutic strategy for a number of autoimmune diseases and allograft rejection.37, 38 In spontaneous autoimmune diseases, particularly systemic lupus erythematosus, TEM have been reported to be abnormally expanded,10 perhaps because TCR signaling that normally triggers RICD is altered.39 The hierarchy of apoptosis sensitivity we have described here might be disrupted in these patients, allowing pathological expansion of TEM. Understanding the mechanisms that underlie the more efficient assembly of the Fas DISC in TEM may allow the development of therapeutic strategies that sensitize T cells to antigen and TCR-mediated apoptosis in diseases mediated by autoreactive T cells. This work also predicts that Fas ligand might be used therapeutically to specifically eliminate TEM in diseases in which these cells cause pathology.
Materials and Methods
Antibodies and reagents
Antibodies were obtained from Kamiya (Seattle, WA, USA) (Apo1-1), Alexis Biochemicals, Enzo Life Sciences (Plymouth Meeting, PA, USA) (Apo1-3, cFLIP NF6), eBiosciences (San Diego, CA, USA) (anti-CD3 OKT3), Santa Cruz (Santa Cruz, CA, USA) (FasC20, Lck) BD Pharmingen (San Diego, CA, USA) (FADD), Cell Signaling (Danvers, MA, USA) (p-FoxO3a, FoxO3a, AKT, p-AKT, PARP, C-8 1C12) and Stressgen, Enzo Life Sciences (Plymouth Meeting, PA, USA) (Bim). C-8 (C15 clone) was from Alexis and anti-FasL (Nok2) was from BD. FasL-LZ is derived from overexpression of the extracellular region of FasL tagged with isoleucine zipper and Flag tag construct in 293T cells and purification of the protein on a Flag column. Stealth select siRNA (medium GC control and Bim) was obtained from Invitrogen (Carlsbad, CA, USA) and targets all isoforms of human Bim.
CD4+ T-cell isolations and apoptosis assays
Peripheral blood leukocytes (PBLs) were obtained from normal donors in the Department of Transfusion Medicine, Clinical Center, NIH, under the Institutional Review Board (IRB) approved protocol. Peripheral blood from patients with ALPS Type Ia germline Fas mutations were obtained from NIAID clinical center under the IRB approved protocol number: 93-I-0063. For direct ex vivo apoptosis assays, CD8+ T cells were depleted by MACS separation and the PBL were incubated with Fas agonists, anti-Fas antibodies or FasL-LZ for 6–8 h. A multi-parameter FACS analysis was used to delineate the levels of Fas-induced apoptosis within various CD4+ T-cell subsets. Cells were stained with CD4 and CD45RA to differentiate between naïve (CD4+CD45RA+) and memory (CD4+CD45RA−) CD4+ T cells (Figure 1a). Surface receptors CCR7 and CD27 on memory T cells demarcate central (TCM, CD4+CD45RA− CCR7+/CD27+) or effector memory (TEM, CD4+CD45RA− CCR7−/CD27−) cells (Figure 1a). Co-staining was done with annexin for 20 min and analyzed by FACS. Cell death was calculated using the formula ((1–% live-treated cells)/(% live-untreated cells)), wherein cells that remain annexin negative are considered as live cells. For assays on activated T cells derived from ALPS patients, PBMCs were activated with concanavalin A (2.0 μg/ml, SIGMA, St Louis, MO, USA) for 48 h and expanded in RPMI medium containing IL-2 (100 IU/ml) for 3–5 days. Apoptosis assays were done as above. Mouse CD4+ T cells were isolated from 6- to 8-week-old wild-type (C57BL/6) or lpr mice using a CD4+ T-cell isolation kit (Miltenyi Biotec, Auburn, CA, USA) and sorted for naïve (CD62Lhi/CD44lo), central memory (CD62Lhi/CD44hi) and effector memory (CD62Llo/CD44hi) cells. For the ex vivo assays, cells were directly incubated on anti-CD3-coated plates or treated with FasL-LZ for 6–8 h and analyzed for apoptosis using annexin/PI staining. Sorted cells were activated on plate-bound CD3/28 (5 μg/ml) for 3 days and expanded in RPMI medium containing IL-2 (50 IU/ml) for another 5 days for the in vitro activated cell assays. All cell death assays were done in triplicates for each sample.
Lipid raft assays and Fas signaling complex analysis
For lipid raft separation Optiprep density centrifugation preparations were used as described previously.28 For depletion of palmitoylated Fas, activated naïve and memory cells were cultured for 18 h in the presence or absence of 100 μM 2-bromopalmitate, then treated with anti-Fas antibody and multi-parameter flow cytometry was used to detect apoptosis as described above. For analysis of the DISC, 107 activated naïve, memory, TCM or TEM cells were stimulated with either uncrosslinked (1 μg/ml) or crosslinked (0.2 μg/ml) anti-Fas antibodies and lysed in 1 ml of DISC lysis buffer. Immunoprecipitation was done with protein G beads (Pierce, Rockford, IL, USA) O/N at 4 °C. The beads were lysed with SDS buffer and immunoblotted against Fas, FADD, C-8 and cFLIP.
Acknowledgments
We would like to thank Jagan Muppidi for construction of the FasL-LZ vector, Mike Lenardo, John Ashwell and Josh Farber for critical reading of the paper, Andy Snow for providing Bim siRNA, Jim Simone and Jeff Lay for assistance in cell sorting. This research was supported (in part) by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health.
Glossary
- ALPS
autoimmune lymphoproliferative syndrome
- RICD
restimulation-induced cell death
- DISC
death-inducing signaling complex
- TCM
central memory T cells
- TEM
effector memory T cells
- DD
death domain
- PARP
poly-ADP ribose polymerase
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by H-U Simon
Supplementary Material
References
- Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- Straus SE, Sneller M, Lenardo MJ, Puck JM, Strober W. An inherited disorder of lymphocyte apoptosis: the autoimmune lymphoproliferative syndrome. Ann Intern Med. 1999;130:591–601. doi: 10.7326/0003-4819-130-7-199904060-00020. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Stranges PB, Watson J, Cooper CJ, Choisy-Rossi CM, Stonebraker AC, Beighton RA, et al. Elimination of antigen-presenting cells and autoreactive T cells by fas contributes to prevention of autoimmunity. Immunity. 2007;26:629–641. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RM, Chan FK, Chun HJ, Lenardo MJ. The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nat Immunol. 2000;1:469–474. doi: 10.1038/82712. [DOI] [PubMed] [Google Scholar]
- Hughes PD, Belz GT, Fortner KA, Budd RC, Strasser A, Bouillet P. Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity. 2008;28:197–205. doi: 10.1016/j.immuni.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S, Wherry E, Ahmed R. Vaccines: effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- Fritsch RD, Shen X, Illei GG, Yarboro CH, Prussin C, Hathcock KS, et al. Abnormal differentiation of memory T cells in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2184–2197. doi: 10.1002/art.21943. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Fritsch RD, Shen X, Sims GP, Hathcock KS, Hodes RJ, Lipsky PE. Stepwise differentiation of CD4 memory T cells defined by expression of CCR7 and CD27. J Immunol. 2005;175:6489–6497. doi: 10.4049/jimmunol.175.10.6489. [DOI] [PubMed] [Google Scholar]
- Miyawaki T, Uehara T, Nibu R, Tsuji T, Yachie A, Yonehara S, et al. Differential expression of apoptosis-related Fas antigen on lymphocyte subpopulations in human peripheral blood. J Immunol. 1992;149:3753–3758. [PubMed] [Google Scholar]
- Lenardo MJ. Interleukin-2 programs mouse αβ T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- Zheng L, Fisher G, Combadiere B, Hornung F, Martin D, Pelfrey C, et al. Mature T lymphocyte apoptosis in the healthy and diseased immune system. Adv Exp Med Biol. 1996;406:229–239. doi: 10.1007/978-1-4899-0274-0_24. [DOI] [PubMed] [Google Scholar]
- Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, et al. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- Bleesing JJ, Brown MR, Straus SE, Dale JK, Siegel RM, Johnson M, et al. Immunophenotypic profiles in families with autoimmune lymphoproliferative syndrome. Blood. 2001;98:2466–2473. doi: 10.1182/blood.v98.8.2466. [DOI] [PubMed] [Google Scholar]
- Ahmadzadeh M, Hussain S, Farber D. Heterogeneity of the memory CD4 T cell response: persisting effectors and resting memory T cells. J Immunol. 2001;166:926–935. doi: 10.4049/jimmunol.166.2.926. [DOI] [PubMed] [Google Scholar]
- Adachi M, Watanabe-Fukunaga R, Nagata S. Aberrant transcription caused by the insertion of an early transposable element in an intron of the Fas antigen gene of lpr mice. Proc Natl Acad Sci USA. 1993;90:1756–1760. doi: 10.1073/pnas.90.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petschner F, Zimmerman C, Strasser A, Grillot D, Nunez G, Pircher H. Constitutive expression of Bcl-xL or Bcl-2 prevents peptide antigen-induced T cell deletion but does not influence T cell homeostasis after a viral infection. Eur J Immunol. 1998;28:560–569. doi: 10.1002/(SICI)1521-4141(199802)28:02<560::AID-IMMU560>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Köntgen F, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, et al. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 2002;16:759–767. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- Riou C, Yassine-Diab B, Van grevenynghe J, Somogyi R, Greller LD, Gagnon D, et al. Convergence of TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells. J Exp Med. 2007;204:79–91. doi: 10.1084/jem.20061681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- Thome M, Tschopp J. Regulation of lymphocyte proliferation and death by FLIP. Nat Rev Immunol. 2001;1:50–58. doi: 10.1038/35095508. [DOI] [PubMed] [Google Scholar]
- Budd R, Yeh W, Tschopp J. cFLIP regulation of lymphocyte activation and development. Nat Rev Immunol. 2006;6:196–204. doi: 10.1038/nri1787. [DOI] [PubMed] [Google Scholar]
- Tewari M, Quan LT, O'Rourke K, Desnoyers S, Zeng Z, Beidler DR, et al. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- Muppidi JR, Siegel RM. Ligand-independent redistribution of Fas (CD95) into lipid rafts mediates clonotypic T cell death. Nat Immunol. 2004;5:182–189. doi: 10.1038/ni1024. [DOI] [PubMed] [Google Scholar]
- Holler N, Tardivel A, Kovacsovics-Bankowski M, Hertig S, Gaide O, Martinon F, et al. Two adjacent trimeric Fas ligands are required for Fas signaling and formation of a death-inducing signaling complex. Mol Cell Biol. 2003;23:1428–1440. doi: 10.1128/MCB.23.4.1428-1440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JM, Karnovsky MJ. Evaluation of the polyene antibiotic filipin as a cytochemical probe for membrane cholesterol. J Histochem Cytochem. 1980;28:161–168. doi: 10.1177/28.2.6766487. [DOI] [PubMed] [Google Scholar]
- Chakrabandhu K, Hérincs Z, Huault S, Dost B, Peng L, Conchonaud F, et al. Palmitoylation is required for efficient Fas cell death signaling. EMBO J. 2007;26:209–220. doi: 10.1038/sj.emboj.7601456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig C, Tchikov V, Schutze S, Peter ME. Palmitoylation of CD95 facilitates formation of SDS-stable receptor aggregates that initiate apoptosis signaling. EMBO J. 2007;26:221–231. doi: 10.1038/sj.emboj.7601460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legler DF, Micheau O, Doucey MA, Tschopp J, Bron C. Recruitment of TNF receptor 1 to lipid rafts is essential for TNFalpha-mediated NF-kappaB activation. Immunity. 2003;18:655–664. doi: 10.1016/s1074-7613(03)00092-x. [DOI] [PubMed] [Google Scholar]
- Snow AL, Oliveira JB, Zheng L, Dale JK, Fleisher TA, Lenardo MJ. Critical role for BIM in T cell receptor restimulation-induced death. Biol Direct. 2008;3:34. doi: 10.1186/1745-6150-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson J, Scatizzi JC, Siddiqui AM, Haines GK, III, Wu T, Li QZ, et al. Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity. 2008;28:206–217. doi: 10.1016/j.immuni.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Jin Z, Li Y, Pitti R, Lawrence D, Pham VC, Lill JR, et al. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell. 2009;137:721–735. doi: 10.1016/j.cell.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Askenasy N, Yolcu ES, Yaniv I, Shirwan H. Induction of tolerance using Fas ligand: a double-edged immunomodulator. Blood. 2005;105:1396–1404. doi: 10.1182/blood-2004-06-2364. [DOI] [PubMed] [Google Scholar]
- Zhou T, Song L, Yang P, Wang Z, Lui D, Jope RS. Bisindolylmaleimide VIII facilitates Fas-mediated apoptosis and inhibits T cell-mediated autoimmune diseases. Nat Med. 1999;5:42–48. doi: 10.1038/4723. [DOI] [PubMed] [Google Scholar]
- Kovacs B, Vassilopoulos D, Vogelgesang SA, Tsokos GC. Defective CD3-mediated cell death in activated T cells from patients with systemic lupus erythematosus: role of decreased intracellular TNF-alpha. Clin Immunol Immunopathol. 1996;81:293–302. doi: 10.1006/clin.1996.0192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.