Abstract
Tunneling nanotubes (TNTs) can be induced in rat hippocampal astrocytes and neurons with H2O2 or serum depletion. Major cytoskeletal component of TNTs is F-actin. TNTs transfer endoplasmic reticulum, mitochondria, Golgi, endosome and intracellular as well as extracellular amyloid β. TNT development is a property of cells under stress. When two populations of cells are co-cultured, it is the stressed cells that always develop TNTs toward the unstressed cells. p53 is crucial for TNT development. When p53 function is deleted by either dominant negative construct or siRNAs, TNT development is inhibited. In addition, we find that among the genes activated by p53, epidermal growth factor receptor is also important to TNT development. Akt, phosphoinositide 3-kinase and mTOR are involved in TNT induction. Our data suggest that TNTs might be a mechanism for cells to respond to harmful signals and transfer cellular substances or energy to another cell under stress.
Keywords: tunneling-nanotube, p53, EGFR
Tunneling nanotubes (TNTs) were first described in 2004 as a thin membranous, hovering freely channels between cells with diameter of 50-200 nm mediating communication between cells.1 As a new principle of cell–cell communication, TNTs are found in rat pheochromocytoma PC12 cells,1 HEK293 cells,1, 2 EBV-transformed human B-cell line,3 murine macrophage J774 cells,3 DU 145 human prostate cancer cells,4 THP-1 monocytes,5 hepatic HepG2 cells,6 TRVb-1 cells,6 bovine mammary gland epithelial cells,6 human monocyte-derived macrophages,3, 7 primary cultures of rat astrocytes,8 myeloid-lineage dendritic cells5 and hematopoietic stem and progenitor cells.9 Similar cell–cell communication projections were described in drosophila imaginal discs in vivo in 1999.10 The cytoskeletal components of TNTs are reported to be F-actin, myosin Va and microtubules.11 TNTs are suggested to transfer endosome-related organelles, surface receptors, mitochondria, lysosomes, GPI-anchored GFP and calcium fluxes.12 Some particles like human immunodeficiency virus (HIV) and PrPSc use TNTs as infection highways to transfer from one cell to another.13, 14, 15, 16 However, the development mechanisms of TNTs are still unknown. Here, we address what is the initiator for TNT development, whether TNTs are induced by the target cells or they spout out by the initiating cells, and what is the downstream pathway of TNT development.
In our study, we induced TNTs in cultured rat hippocampal astrocytes with H2O2 or serum depletion. Major cytoskeletal component of TNTs is F-actin. TNTs transfer endoplasmic reticulum (ER), mitochondria, Golgi and endosome as described by others. In addition, TNTs transfer intracellular and extracellular amyloid β (Aβ) at the speed 2–8 times to ER, mitochondria, Golgi and endosome. We find that TNT development is a property of cells under stress. When co-culturing two populations of cells, it is the stressed cells that always develop TNTs toward the unstressed cells. p53 is critical for TNT development. When p53 function is deleted by either dominant negative or siRNAs, TNT development is abolished. Also TNTs can be induced in p53+/+ osteosarcoma HT1080 cells, which can be inhibited by p53 siRNAs and dominant negative mutant. In p53-deficient MG63 osteosarcoma cells, no TNTs are induced. This phenotype can be rescued by expressing p53 in MG63 cells. In addition, we find that among the genes activated by p53, epidermal growth factor receptor (EGFR) is also important to TNT development. The downstream pathways involve Akt, phosphoinositide 3-kinase (PI3K) and mTOR. Our data suggest that TNTs might be a mechanism for cells to transfer cellular substances or energy to another cell when they sense their lives are under threaten. This is the first time, to our knowledge, the mechanisms of TNT development and its hypothesized function are described.
Results
TNT induction in neurons/astrocytes and their properties
TNTs were induced by H2O2 (100 μM, 24 h) or serum depletion (7 days) in rat primary hippocampal astrocytes (Figures 1a, b, c and g) and neurons (Figures 1d, d1 and g). In HEK293 cells, TNTs could be induced by H2O2 or serum depletion as well (Figures 1e, f and h). The distribution of nanotube length were quantified and the majority of TNTs lasted for 21-30 μm long (Figure 1j). Carbenoxolone (CBX, 1 and 10 mM) did not block TNT induction in both astrocytes and neurons indicating that TNTs were not gap junctions (Figure 1k). TNTs hovered freely in culture medium, did not attach to culture surface as described by others1 (Supplementary Figure S1). The A–Z axis scanning revealed the relative density of a TNT, which did not attach to bottom surface between two cells (Figure 1i). Major cytoskeletal component of TNTs connecting astrocytes and astrocytes (Figure 1l) as well as astrocytes and neurons (Figure 1m) was F-actin demonstrated by fluorescent F-actin marker. This was further confirmed by the fact that adding F-actin-disrupting agent latrunculin A (1 μM) or cytochalasin D (2 μg/ml) blocked TNT induction in astrocytes and neurons (Figure 1n), whereas microtubule-disrupting agent nocodazole (10 μg/ml) or microtubule-stabilizing agent paclitaxel (10 μM) did not alter TNT induction (Figure 1n). Plasma membrane marker showed that TNTs were continuous extensions from the cell membrane (Figures 1o and o1). After microinjection of Alexa488 dye to astrocytes, the material transfer from one cell to another cell was observed in 189/400 cases, suggested that the substance transfer from one cell to another was uni-directional (Figures 1p and q).
Figure 1.
TNTs are induced by H2O2 or serum deprivation in rat astrocytes and neurons. (a) Rat hippocampal astrocytes were microinjected with Alexa488 dye and a TNT (arrow) developed at 24 h after H2O2 treatment. The arrow indicates a TNT. (b) Phase contrast picture of a. The arrow indicates a TNT. (c) In the cells microinjected with Alexa488 at 24 h after H2O2 treatment, TNTs could be induced between astrocytes as well as between neurons and astrocytes. The arrows indicate TNTs. Scale bar for a–c: 20 μm. (d) Neurons developed a TNT (arrow) after serum depletion for 7 days. Scale bar: 50 μm. (d1) High-magnification picture of d. Scale bar: 20 μm. (e) HEK293 cells under control condition. (f) HEK293 cells developed TNTs (arrows) after serum depletion for 7 days. Scale bar for e and f: 20 μm. (g) Quantification of TNT induction by H2O2 and serum depletion in astrocytes and neurons. Number of TNTs were counted in 100 cells in three independent preparations (n=3). Data represented mean±S.E., **P<0.01 compared with control groups. (h) Quantification of TNT induction by H2O2 and serum depletion in HEK293 cells (n=3). Data represented mean±S.E., **P<0.01 compared with control groups. (i) Top left panel: a TNT (arrow) was taken X–Z scanning (framed area). Top right panel: X–Z section showed that the TNT did not attach to the bottom culture surface. Bottom panel: the relative density was calculated at the scanning plate 1, 20, 40 for Z axis and 1, 8, 16 for X axis. Scale bar: 50 μm. (j) Distributions of TNT length (n=100). (k) Gap junction inhibitor CBX (1 and 10 mM) did not inhibit TNT development in astrocytes and neurons (n=3). Data represented mean±S.E., **P<0.01 compared with control groups. (l) Astrocytes infected with Organelle Light Intracellular Targeted Fluorescent Proteins actin-GFP developed TNTs (arrows). (m) Neurons and astrocytes infected with Organelle Light Intracellular Targeted Fluorescent Proteins actin-GFP developed TNTs between astrocytes (arrow) and between neurons and astrocytes (arrow head). Scale bar for l and m: 20 μm. (n) F-actin-disrupting agent latrunculin A or cytochalasin D blocked TNT induction in neurons (left panel) and astrocytes (right panel), whereas microtubule-disrupting agent nocodazole or microtubule-stabilizing agent paclitaxel did not alter TNT induction (n=3). Data represented mean±S.E., **P<0.01 compared with control groups. (o) Astrocytes infected with Organelle Light Intracellular Targeted Fluorescent Proteins plasma membrane (PM)-GFP developed TNTs. Scale bar: 20 μm. (o1) High-magnification picture of o. Scale bar: 5 μm. (p) A TNT (arrow) transferred materials from an astrocyte injected with Alexa488 to the target cell (arrow head) at time 0. (q) A TNT (arrow) transferred materials from an astrocyte injected with Alexa488 to the target cell (arrow head) at time 10 min. Scale bar for p and q: 20 μm
When astrocytes were microinjected with Alexa488 dye, the substance transfer speed was calculated as 185.47±26.84 nm/min (Figures 2a and b, a1, a2, b1 and b2). Astrocytes infected by specific cellular compartment markers Cellular Lights Intracellular Targeted Fluorescent Proteins showed that TNTs could transfer ER, Golgi, endosome and mitochondria (Figures 2c, c1, d, d1, e, e1, f and f1). The transfer velocities at different time points for ER, Golgi, endosome and mitochondria in TNTs were calculated arranging from around 2 to 15, 5 to 15, 5 to 12 and 1 to 5 μm/s, respectively (Figure 2g). The accordingly travel distances at different time points for ER, Golgi, endosome and mitochondria arranged from around 10 to 75, 25 to 75, 10 to 60 and 5 to 25 μm (Figure 2g).
Figure 2.
TNTs transfer ER, Golgi, endosome and mitochondria. (a) Astrocytes injected with Alexa488 developed TNTs at time 0. (b) The same cell in a at the time of 10 min. Scale bar for a and b: 20 μm. (a1) High-magnification picture of a TNT in a. (b1) The same spot in a1 (arrow) moved upward at the time of 10 min. (a2) High-magnification picture of a TNT in a. (b2) The same spot in a2 (arrow) moved upward at the time of 10 min. Scale bar for a1–b2: 2 μm. (c) Astrocytes infected with Organelle Light Intracellular Targeted Fluorescent Proteins ER-GFP developed TNTs. Scale bar: 50 μm. (c1) High-magnification picture of c. Arrows: TNTs. Scale bar: 25 μm. (d) Astrocytes infected with Organelle Light Intracellular Targeted Fluorescent Proteins Golgi-RFP developed TNTs. Scale bar: 50 μm. (d1) High-magnification picture of d Arrows: TNTs. Scale bar: 25 μm. (e) Astrocytes infected with Organelle Light Intracellular Targeted Fluorescent Proteins endosome-GFP developed TNTs. Scale bar: 50 μm. (e1) High-magnification picture of e. Arrows: TNTs. Scale bar: 25 μm. (f) Astrocytes infected with Organelle Light Intracellular Targeted Fluorescent Proteins mitochondria (mito)-RFP developed TNTs. Scale bar: 50 μm. (f1) High-magnification picture of f. Arrows: TNTs. Scale bar: 25 μm. (g) Top panels or left panel: particle movement velocities changed with time for ER, Golgi, endosome and mitochondria measured by movement of Organelle Light Intracellular Targeted Fluorescent Proteins markers in TNTs. Data represented mean±S.E. Bottom panels or right panel: particle movement distances changed with time for ER, Golgi, endosome and mitochondria measured by movement of Organelle Light Intracellular Targeted Fluorescent Proteins markers in TNTs. Data represented mean±S.E.
Aβ can be transferred by TNTs and induced cytotoxicity
As HIV and PrPSc were suggested to use TNTs as infection highways to transfer from one cell to another,13, 14, 15, 16 we suspected that in rat astrocytes and neurons, TNTs could also be hijacked by cytotoxic particles as a spreading tunnel. Intracellular Aβ induced cytotoxicity in various systems. Rat brains overexpressing human APP-inducing intracellular Aβ accumulation had significant neuronal loss in the cortical regions and inhibition of intracellular Aβ accumulation could promote cell survival.17 Overexpressing human APP751 in P19 cells caused an increase in intracellular Aβ level associating with a decrease in mitochondria membrane potential.18 A strong piece of direct evidence for intracellular Aβ cytotoxicity came from an experiment in which microinjection of Aβ1−42 induced significant cell death in human primary cultured neurons.19 When intracellularly expressed fusion protein Aβ–EGFP was transfected into rat astrocytes (Figure 3a) or microinjected into rat neurons (Figure 3c), the spreading of EGFP to another cells was observed 30 min after transfection or microinjection (Figures 3b and d), suggesting that intracellular Aβ could use TNTs to transfer to another cell. The calculations of spreading velocities and travel distances with time indicated that in both astrocytes and neurons, intracellular Aβ traveled much faster than ER, Golgi, endosome and mitochondria (Figure 3e).
Figure 3.
Aβ can be transferred by TNTs and induced cytotoxicity. (a) Intracellularly expressed fusion protein Aβ-EGFP was transfected into astrocytes at time 0. (b) EGFP transferred from one astrocytes to another ones at the time of 30 min. Arrows: TNTs. (c) Intracellularly expressed fusion protein Aβ-EGFP was microinjected into neurons at time 0. (d) EGFP transferred from one neurons to another ones at the time of 30 min. Arrows: TNTs. Scale bar for a–d: 20 μm. (e) Intracellular Aβ movement velocities and distance changed with time in TNTs in astrocytes and neurons. Data represented mean±S.E. (f) Aβ monomers were transferred by TNTs (arrow). (g) Protofibrils of Aβ were transferred by TNTs (arrow). Scale bar for f and g: 20 μm. (h) Top panel: Aβ transferred by TNTs induced significant cell death. Bottom panel: TNTs were induced by H2O2 in both EGFP and Aβ infected groups. Data represented mean±S.E. **P<0.01 compared with control groups
As Aβ was normally secreted outside of cells, we also investigated whether extracellular Aβ could be uptaken by astrocytes and transferred by TNTs. After 24 h of incubation with monomers or protofibrils of tetracysteine-labeled Aβ1–42 (TC-Aβ, 1 μm) in the culture medium, TNTs were induced by H2O2 in astrocytes. Both monomers and protofibrils of Aβ were detected in the TNTs, suggesting that TNTs could transfer extracellular Aβ uptaken by astrocytes. To further investigate whether Aβ transferred by TNTs was toxic to cells, we first infected astrocytes with adeno-virus packaged with intracellularly expressed Aβ. These astrocytes were then trypsinized and added onto neuronal culture. TNTs were induced by H2O2, and neuronal cell death ratios were indicated by terminal deoxynucleotidyl transferase-biotin dUTP nick-end labeling (TUNEL) at 24 h after induction. After the induction of TNTs, Aβ induced significantly greater neuronal cell death compared with the EGFP controls and Aβ alone without TNT induction (Figure 3h). As neurons were not transduced with Aβ, the remarkable neuronal cell death can be attributed to Aβ transferred by TNTs from astrocytes. With astrocytes infected with EGFP controls, although with the induction of TNTs, neurons showed no significant difference on cell death than EGFP without TNT induction, suggesting that high cell death ratio in Aβ+H2O2 group could not be attributed to H2O2 toxicity (Figure 3h).
Induction of TNTs depended on p53, EGFR, Akt, PI3K and mTOR activations
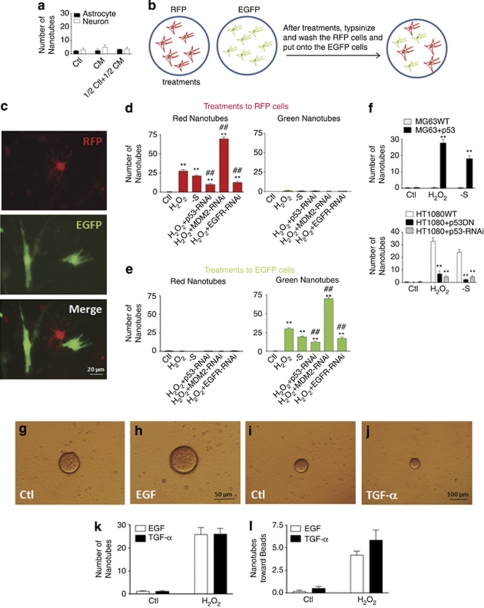
To determine the initiator for TNT induction, we first tested the effects of nerve growth factor (NGF) and scratching. Heparin-coated acrylic beads were soaked in a solution containing NGF (100 μg/ml) and placed onto cell cultures. Our data showed that both NGF (Figures 4a–c and f) and scratching (Figures 4d, e and g) did not induce TNTs in either astrocytes or neurons. As H2O2 and serum depletion, two stresses inducing TNTs, triggered apoptosis in both neurons and astrocytes,19, 20 we then examined whether p53, Bax and caspases, key players in apoptosis, were involved in TNT development. The results suggested that p53 overexpression increased TNT induction with or without H2O2 treatment. p53 dominant negative mutant blocked development of TNTs (Figures 4h, h1, i, i1, j, j1, k and k1). However, Bax-neutralizing antibodies (6A7 and 2D2)19 or pan caspase inhibitor Z-VAD-fmk did not alter TNT induction in astrocytes (Figure 4n) and neurons (Figure 4o). To confirm the determining role of p53 in TNT induction, siRNAs to p53 (p53-1 and p53-2) significantly reduced TNT induction in astrocytes (Figure 4p). When the function of MDM2, a negative regulator of p53, was blocked by its siRNAs (MDM2-1 and MDM2-2), the induction of TNTs was enhanced (Figures 4l, l1 and p). It was reported that among numerous genes upregulated by p53 activation, EGFR was related to cell growth and proliferation.21 The involvement of EGFR was also investigated by its siRNAs (EGFR-1 and EGFR-2). Our data showed that EGFR was required for TNT induction (Figures 4m, m1 and p).
Figure 4.
TNT development is p53 dependent. (a) Neurons in culture under the control conditions. (b) Heparin-coated acrylic beads were added to cell culture. (c) Heparin-coated acrylic beads soaked with NGF were added to cell culture. Scale bar for a–c: 100 μm. (d) Neurons in culture under the control conditions. (e) Neurons in culture after scratching. Scale bar for d and e: 50 μm. (f) Quantification of TNT induction with NGF-containing beads added in neurons and astrocytes (n=3). Data represented mean±S.E. (g) Quantification of TNT induction with scratching in neurons and astrocytes (n=3). Data represented mean±S.E. (h) Astrocytes transfected with EGFP were treated in control medium. (i) Astrocytes transfected with EGFP were treated in H2O2 for 24 h. (j) Astrocytes transfected with EGFP and wild-type p53 were treated in H2O2 for 24 h. (k) Astrocytes transfected with EGFP and dominant negative mutant p53 were treated in H2O2 for 24 h. (l) Astrocytes transfected with EGFP and siRNA to MDM2 were treated in H2O2 for 24 h. (m) Astrocytes transfected with EGFP and siRNA to EGFR were treated in H2O2 for 24 h. Scale bar for h–m: 20 μm. (h1) High-magnification picture of h. (i1) High-magnification picture of i. (j1) High-magnification picture of j. (k1) High-magnification picture of k. (l1) High-magnification picture of l. (m1) High-magnification picture of m. Scale bar for h1–m1: 5 μm. (n) p53 dominant negative mutant blocked TNT induction in astrocytes (n=3). Data represented mean±S.E., **P<0.01 compared with control groups. (o) p53 dominant negative mutant blocked TNT induction in neurons (n=3). Data represented mean±S.E., **P<0.01 compared with control groups. (p) Effects of siRNAs to p53, MDM2 and EGFR on TNT induction (n=3). Data represented mean±S.E., **P<0.01 compared with control groups
To address whether TNTs were induced by the target cells or they spouted out by the initiating cells, we examined if conditioned medium from TNT containing cells could induce TNTs in another batch of cells. Conditioned medium from cells treated with serum deprivation for 7 days was collected and 5% serum was added to conditioned medium. Such medium was used to treat the second batch of cells to observe if TNTs were induced. Our data showed that there was no TNT induction in complete conditioned medium treated or 1/2 control medium with 1/2 conditioned medium treated groups (Figure 5a), suggesting that TNT growth might be a property of initiating cells, not the target cells. To further confirm this hypothesis, astrocytes were distinguished into two groups by transfecting EGFP or RFP respectively. One group of colored cells was treated with TNT-inducing insults, and then trypsinized and washed, and then co-cultured with another color group of cells. By doing this, we obtained co-cultures containing both green (EGFP expressing) and red (RFP expressing) cells and could observe TNT development between two colored cell groups (Figure 5b). After induction by H2O2 or serum depletion, TNTs were developed between two groups of cells (Figure 5c). When the red cells were treated with insults, there were significant number of the red TNTs developed from the red cells to the green cells, but not from the green cells to the red one (Figure 5d). Blockage of the functions of p53 and EGFR inhibited TNT induction as described above, while siRNA to MDM2 increased TNT induction (Figure 5d, left panel). Similarly, when the green cells were treated with TNT-inducing insults, only green TNTs were observed to grow from the green cells to the red cells, but not from the red ones to the green one (Figure 5e). p53, EGFR and MDM2 had the similar roles in this scenario (Figure 5e, right panel). TNT induction was abolished in p53-deficient osteosarcoma MG63 cells, and recovered by adding p53 back to cells (Figure 5f, top panel). In p53+/+ osteosarcoma HT1080 cells, TNTs could be induced. However, p53 dominant negative mutant and siRNA blocked the development of TNTs in HT1080 cells (Figure 5f, bottom panel). Heparin-coated acrylic beads were soaked in a solution containing two major ligands for EGFR, epidermal growth factor (EGF, 100 μg/ml, Figures 5g and h)or transforming growth factor-α (TGF-α, 100 μg/ml, Figures 5i and j), and then added to astrocytes with or without insults. Both number of TNTs or the TNTs directing to the beads did not alter under either EGF or TGF-α treatments (Figures 5k and l) suggesting that EGF or TGF-α were not involved in TNT induction or guidance.
Figure 5.
TNT development is a property of stressed cells. (a) Conditioned medium from serum depletion (7 days) cells was supplemented by serum and added to second batch of cells (n=3). Data represented mean±S.E. (b) Experiment procedure for co-culture of RFP and EGFP-expressing astrocytes. (c) Co-cultured cells were pictured at red (top panel), green (middle panel) and merged (bottom panel) filters. Scale bar: 20 μm. (d) When stress was applied to red cells, TNTs developed from red cells toward green cells (left panel), not from green cells to red cells (right panel) (n=3). Data represented mean±S.E, **P<0.01 compared with control groups, ##P<0.01 compared with H2O2 group. (e) When stress was applied to green cells, TNTs developed from green cells toward red cells (right panel), not from red cells to green cells (left panel) (n=3). Data represented mean±S.E., **P<0.01 compared with control groups, ##P<0.01 compared with H2O2 group. (f) TNT induction in MG63 (top panel) and HT1080 (bottom panel) cells (n=3). Data represented mean±S.E., **P<0.01 compared with control groups. (g) Astrocytes in culture under the control conditions. (h) Heparin-coated acrylic beads soaked with EGF were added to cell culture. Scale bar for g and h 50 μm. (i) Astrocytes in culture under the control conditions. (j) Heparin-coated acrylic beads soaked with TGF-α were added to cell culture. Scale bar for i and j: 100 μm. (k) Quantification of TNT induction with EGF-containing beads added in astrocytes (n=3). Data represented mean±S.E. (l) Quantification of TNT induction with TGF-α-containing beads added in astrocytes (n=3). Data represented mean±S.E.
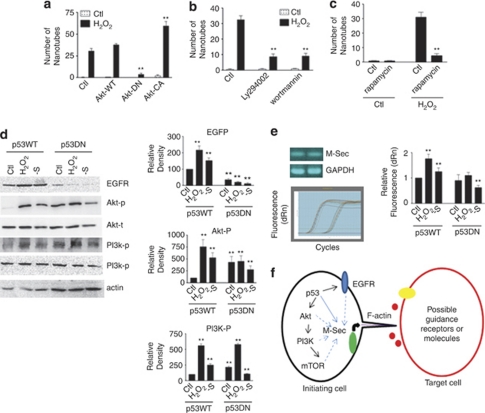
EGFR could activate Akt, PI3K and mTOR.22 To explore the downstream pathways of TNT induction, we examined the involvements of Akt, PI3K and mTOR. Akt dominant negative mutant blocked TNT induction, whereas Akt constitutively active mutant enhanced TNT development (Figure 6a) suggesting that Akt was involved in TNT induction. Applying PI3K inhibitors ly294002 (50 μM) or wortmannin (10 nM) showed that PI3K was important to TNT induction (Figure 6b). TNT development was inhibited by mTOR inhibitor rapamycin (0.1 nM) suggesting that mTOR was also involved in TNT development (Figure 6c). Western blots showed that in astrocytes, the levels of EGFR, phosphorylated Akt and phosphorylated PI3K increased with H2O2 and serum deprivation treatments (Figure 6d). In the astrocytes transfected with p53 dominant negative mutant, with H2O2 and serum deprivation treatments, the enhancement of EGFR and phosphorylated Akt was suppressed (Figure 6d) The increase in phosphorylated PI3K was inhibited in serum deprivation, but not in H2O2 treatment with p53 dominant negative mutant transfection (Figure 6d). It was reported that M-Sec promoted TNT formation.23 The expression of M-Sec in rat hippocampal astrocytes was first confirmed by RT-PCR (Figure 6e). Real-time PCR showed that activation of p53 with H2O2 or serum deprivation treatments effectively induced overexpression of M-Sec (Figure 6e, right panel). In the astrocytes transfected with p53 dominant negative mutant, there was no significant increase in M-Sec, whereas the level of M-Sec decreased in serum deprivation treatment group (Figure 6e, right panel).
Figure 6.
Akt, PI3K and mTOR are involved in TNT development. (a) TNT induction in astrocytes transfected with wild-type Akt, dominant negative Akt and constitutively active Akt (n=3). Data represented mean±S.E., **P<0.01 compared with control groups. (b) TNT induction with treatments of PI3K inhibitors ly294002 and wortmannin (n=3). Data represented mean±S.E., **P<0.01 compared with control groups. (c) TNT induction with treatment of mTOR inhibitor rapamycin (n=3). Data represented mean±S.E., **P<0.01 compared with control groups. (d) Western blots showing the levels of EGFR, phosphorylated Akt, total Akt, phosphorylated PI3K, total PI3K and actin (n=3). Data represented mean±S.E., **P<0.01 compared with control groups. (e) Top left panel: the expression of M-Sec was confirmed by RT-PCR in rat hippocampal astrocytes. Bottom left panel: Fluorescence recorded from real-time PCR (45 cycles). Right panel: quantification of real-time RT-PCR suggested that M-Sec increased with p53 activation (n=3). Data represented mean±S.E., **P<0.01 compared with control groups. (f) Schematic drawing of TNT induction pathways
Discussion
TNTs are newly discovered cell–cell connections.1 It is hypothesized that TNTs are kind of a ‘highway' for substance transportation between cells.24 Cellular compartments, such as ER, endosome, mitochondria, can be transferred through TNTs.12 Our results indicate that TNTs are a general cellular machinery for cell–cell communication. Such communication may occur in a relatively long distance. Membrane continuity is a unique way of cell–cell communication. Our finding supports that, as a form of membrane continuity, TNTs may be efficient communication tunnels facilitating information and material exchange. Consistent with the previous reports,1, 12 our data show TNTs transport cellular substances uni-directionally suggesting that TNTs mediate selective and targeted interaction between cells. Here, we hypothesize, from our data that TNTs are important structures to transfer cellular contents or energy from the insulted cells to other cells when cells are under stress. Our data from the co-culture experiments show that only the stressed cells develop TNTs toward the uninsulted cells, not vice versa. Furthermore, our results also indicate that induction of TNTs is initiated from the insulted cells, not by the target cells, suggesting that the ability to develop TNTs is a potential defense response to stress. Cells may use TNTs to transfer undamaged cellular organelles, useful substance or energy to other cells when undergo apoptosis. Some pathogens, including PrPSc and HIV-1, are suggested to ‘hijack' TNTs for their cell to cell spreading.13, 14, 15, 16 Our data also show that TNTs can transfer intracellular and extracellular Aβ peptides, which are proven cytotoxic to neurons and astrocytes.19, 25
As a transcription factor, p53 responds to various stress signals and transcribes a series of genes.21 Although p53 has numerous functions regulating cellular events, many of these functions prevent duplication errors when cells are under stress. In this sense, p53 pathway enhances cell division fidelity and prevents cancer formation.21 Here, we find that p53 is also critical for TNT development. Therefore, the dependence of p53 of TNT formation suggests that TNT induction may be a manner for cells to deal with cellular stress and reduce the possibility to develop into cancer cells. Recently, it has been hypothesized that oncogenesis and neurodegeneration may share common mechanistic foundations.26 p53 is an upstream key player in apoptosis and neurodegeneration. It is possible that by activating p53, cells undergo apoptosis keep harmful materials locally inside the membrane structures. In the other hand, through developing TNTs triggered by p53 activation, cells may send out useful or recyclable materials to other healthy cells. Noticing that transfer velocity of cytotoxic intracellular Aβ is much faster than cellular orgnelles, it is possible that cytotoxic or transmissible particles, such as PrPSc and HIV-1, can hijack TNTs as their spreading highways. Contradictory to our results, in mouse embryonic fibroblasts, p53 is reported to inhibit the formation of Cdc42-induced filopodia, but not Rac1-induced lamellipodia or RhoA-induced stress fiber formation.27 We think TNTs may or may not be a kind of filopodia as TNTs do not attach to culture surface as most of filopodia. Therefore, the induction mechanism for TNTs can be totally different than filopodia. Furthermore, the stress used in this study to induce TNTs are oxidative stress and serum deprivation, which may trigger different pathways than Cdc42-induced filopodia formation. Interestingly, we also find that the major cytoskeletal component of TNTs is F-actin. As actin genes are transcriptional targets for p5328, 29 and p53 interacts directly with F-actin,30 it is likely that p53 regulates and interacts with actin during TNT development.
Among the genes upregulated by p53, we find that EGFR is required for TNT induction. EGFR has important roles in cell proliferation.22 Data show that overexpression of EGFR and its ligands occurs in various epithelial cancers, most notably gliomas and breast, pancreas and liver carcinoma.22 Dominant negative construct to EGFR blocks ductal branching and outgrowth in virgin mice.31 EGFR ligand TGF-α expression driven by keratin promoter in skin results in hypertrophy and hyperkeratosis with alopecia or stunted hair growth.32, 33 TGF-α expression commonly leads to enhancement of cellular proliferation, increased mitotic index, reduction of apoptosis, hyperplasia of skin, mammary glands and liver.22 Deletion of TGF-α in TGF-α knockout mice induces a significant reduction in the number of dopaminergic neurons in the substantia nigra.34 It is also reported that EGFR ligands have tissue specificity during particular development stage.22 This could explain, at least partially, why we do not find EGF or TGF-α can induce TNT development or guide TNT directions in this study. EGFR is suggested to activate Akt, PI3K and mTOR, which have important roles in protein synthesis and metabolism, ribosome biogenesis, transcription, cell proliferation and migration.22, 35, 36 Akt, PI3K and mTOR are also associated with both oncogenesis and neurodegenerative diseases.26 Our data show that Akt, PI3K and mTOR are involved in TNT development, suggesting that TNT development may have an important role in preventing cancer formation and neurodegeneration. Furthermore, TNT-inducing insults H2O2 and serum deprivation can also induce overexpression of M-Sec in wild-type astrocytes, but not in p53-deficient cells, suggesting that M-Sec might be regulated by p53 activation.
Therefore, we propose that harmful insults induce p53 activation, which then in turn, upregulates EGFR expression and activates Akt/PI3K/mTOR pathway. p53 activation or EGFR or Akt/PI3K/mTOR induces M-Sec overexpression, which together with RalA and the exocyst complex can trigger F-actin polymerization23 and contributes to TNT development from the cell membrane (Figure 6f). The development of TNTs is a property of the initiating cells, however, at the same time, there may be some guidance receptors on the target cell surface or guidance molecules released by the target cells. These receptors or molecules, although are not responsible for the initiation of TNTs, may contribute to guide the directions of TNTs to find the target cells (Figure 6f). These findings have important implications to discover TNT function and its roles in cell–cell communication.
Materials and Methods
Chemicals, peptides, cDNA constructs, siRNAs and antibodies
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), CBX (Sigma, St. Louis, MO, USA), rapamycin (Sigma), ly294002 (Sigma), wortmannin (Sigma), latrunculin A (Sigma), cytochalasin D (Sigma), nocodazole (Sigma), paxlitaxel (Sigma), H2O2 (Beijing Chemicals Co., Beijing, China), NGF (Sigma), EGF, TGF-α and Z-VAD-fmk (CalBiochem, Gibbstown, NJ, USA) were used in the experiments. Organelle Light Intracellular Targeted Fluorescent Proteins for ER, endosome, Golgi, mitochondria, plasma membrane and actin were applied to label various cellular compartments as described by the manufacturer (Invitrogen). Antibodies to activated-Bax 6A7 and 2D2 (R&D System, Minneapolis, MN, USA) and IgG rabbit serum (Sigma) were injected at 25 μg/ml to neurons. The cDNA constructs of intracellularly expressed Aβ-EGFP, wild-type p53,19, 37 p53 dominant-negative mutant,19, 37 wild-type Akt,38, 39 dominant-negative Akt38, 39 and constitutively active Akt38, 39 were used. All siRNAs and controls (Qiagen, Hilden, Germany) were diluted into 5 nM before injection or transfection by HiPerFect (Qiagen) described by the manufacturer. The silencing efficiency and off-target effects of all siRNAs were verified by Qiagen. Antibodies to actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA), EGFR, phosphorylated Akt, total Akt, phosphorylated PI3K and total PI3K were used for western blots. For NGF, EGF, TGF-α applications, heparin-coated acrylic beads (Sigma) were soaked with 100 μg/ml growth factors for overnight at 4 °C.
TC-Aβ1–42 peptides were dissolved in 100% DMSO and adjusted to 1 mg/ml by adding distilled H2O. The solutions were adjusted to pH 7.4 with 2 M Tris base. After centrifugation at 6000 × g at 4 °C for 5 min, the supernatant was injected into a size exclusion chromatography column Superdex 75 HR 10/30 (GE Healthcare, Uppsala, Sweden) equilibrated with 10 mM Tris-HCl, pH 7.4. Peptides were fractionated at a flow rate of 0.5 ml/min and eluted in 1.5-column volumes. TC-Aβ1−42 elution was monitored by UV absorbance at 210, 254 and 280 nm. Under these conditions, TC-Aβ1−42 eluted as two well separated peaks; one corresponding to the void volume of Superdex 75 containing TC-Aβ protofibrils and the second peak corresponding to monomeric TC-Aβ. The protein concentrations of the TC-Aβ fractions were estimated by using the Micro bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL). The detection of TC-Aβ1−42 was performed with the TC-FlAsH In-Cell Tetracysteine Tag Detection Kits as described by the manufacturer (Invitrogen).
Cell cultures
Rat primary neurons and astrocytes were cultured from new born Sprague–Dawley rat hippocampus, following the regulations of Peking University Animal Care and Use Committee. In brief, fresh rat hippocampal tissues were washed with phosphate-buffered saline (PBS, 0.14 M NaCl, 0.003 M KCl, 0.01 M Na2HPO4, 0.002 M KH2PO4, pH 7.2) dissociated with 0.25% trypsin (Invitrogen), which was then inactivated by 10% decomplemented fetal bovine serum (FBS, HyClone, Logan, UT, USA). The mixture was triturated through pipette to make a homogenous mixture. After filtering the mixture through 70 μm sterilized filters, the flow-through was centrifuged. The pellet was then washed once by PBS and once by DMEM in Earle's balanced salt solution containing 0.225% sodium bicarbonate, 1 mM sodium pyruvate, 2 mM -glutamine, 0.1% dextrose, 1 × antibiotic Pen-Strep (all from Invitrogen) with 5% FBS. Cells were then plated on poly--lysine (Sigma) coated plates at the density of 3 × 106 cells/ml. Neurons and astrocytes were incubated at 37 °C in DMEM with 5% FBS and with 5% circulating CO2. Cytarabine was added to culture media 24 h after plating at 10 μM to inhibit cell growth in neuornal cultures, but not in astrocytic cultures. Medium was changed every 48 h. To obtain astrocyte culture, when cells were confluent on the surface of culture flask, the cells were rocked in a covered culture flask at 250 g for 18 h. The cells were washed twice with DMEM, and digested with 0.25% trypsin until the cells get rounded. The cells were transferred to another culture dish with DMEM and 5% FBS. Cells were treated for experiments at 7 days in culture. MG63 and HT1080 cells were maintained in DMEM and MEM (Invitrogen), respectively.
Confocal imaging
Cells were pictured using a Zeiss LSM-510 inverted confocal microscope (Carl Zeiss, Oberkochen, Germany). All image data were taken in the line-scanning mode of the cells excited at 488 nm for EFGP, Alexa488 or TC-FlAsH and 563 nm for RFP. X–Y and X–Z sections were taken 1 μm for every scanning step.
Microinjection
Thin-walled Borosilicate glass capillaries (outer diameter=1.0 mm, inner diameter=0.5 mm) with microfilament (MTW100F-4, World Precision Instrument, Sarasota, FL, USA) were pulled with a Flaming/Brown Micropipette Puller (P-97, Sutter, Novato, CA, USA) to obtain injection needles with a tip diameter of about 0.5 μm. Microinjections were performed in the cytosol of each cell using the Eppendorf Microinjector FemtoJet and Eppendorf Micromanipulator (Eppendorf, Hamburg, Germany). Neurons were injected with 25 fl/shot at an injection pressure of 100 hPa, a compensation pressure of 50 hPa and an injection time of 0.1 s. The solutions were injected at the indicated concentrations with 100 μg/ml Alexa488 (Molecular Probes, Eugene, OR, USA) as a fluorescent marker to recognize the injected cells.
Adeno-virus infection
Intracellular Aβ1−42 cDNA was subcloned from pEGFP-N3 into pAdTrack with BglII and XhoI digestions. Adeno-virus was packaged in HEK293 cells and the infectious particle was measured as 2 × 106/ml (MOI=1.33). The purified virus supernatant was added to cell culture medium at the dilution of 1 : 500 for 24 h.
Measurement of neuronal cell death
Cells were fixed in fresh 4% paraformaldehyde, 4% sucrose in PBS for 20 min at room temperature and permeablized in 0.1% Triton X-100, 0.1% sodium citrate in PBS for 2 min on ice. TUNEL staining was performed using the in situ cell death detection kit I as described by the manufacturer (Roche, Quebec, Canada). The percentage of cell death was determined by the ratio of the number of TUNEL-positive cells (red) over 100 EGFP-positive cells. The average of five counts was calculated as the percentage of neuronal cell death in a certain treatment.
Real-time PCR
Cells were harvested and total RNA was isolated with TRIGene reagent (GenStar BioSolutions Co., Ltd., Beijing, China). Total RNA (2 μg) were reversely transcribed using TransScript II First-Strand cDNA Synthesis SuperMix (Beijing TransGen Biotech Co., Ltd., Beijing, China). Real-time PCRs were done by using TransStart Green q PCR SuperMix UDG (Beijing TransGen Biotech Co., Ltd.). Sequences of primers for the M-Sec were as following: forward primer: (5′-GTGCAGAACCTCTACCCCAATG-3′); and reverse primer: (5′-TGGAGAATGTCGATGGCCA-3′).23 Real-time PCR quantifications were run in triplicate for each sample and the average were determined. In order to use the comparative Ct method for relative quantification, the amplification efficiency of target and housekeeping gene must be approximately equal. Quantification was done using the comparative Ct method, expression levels for the target gene was normalized to the 18S rDNA (forward primer: (5′-CTTAGAGGGACAAGTGGCG-3′); reverse primer: (5′- GGACATCTAAGGGCATCACA-3′)) of each sample (2−ΔCt=2−(Ct(target gene)−Ct(GAPDH))). Amplification was performed for 45 cycles at 95 °C for 30 s, 58 °C for 30 s, 72 °C for 30 s, 95 °C for 1 min, 58 °C for 30 s and 95 °C for 30 s.
Western blots
Neuronal proteins were extracted in the cell lysis buffer (50 mM Tris pH 8.0, 150 mM NaCl, 1% NP-40, 0.1% SDS) and protein concentrations were measured by BCA assay (Pierce). Protein extracts were denatured at 100 °C for 5 min and separated on 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis at 70 V for about 2 h. Proteins were transferred to Immobilon-P polyvynilidene fluoride membrane (Millipore, Billerica, MA, USA) at 100 mA for 2 h. The membrane was blocked with 5% non-fat milk in Tris-buffered saline (TBS) with 0.1% tween20 (TBS-T) at room temperature for 1 h. Anti-EGFR, anti-Akt-p, anti-Akt-t, anti-PI3K-p, anti-PI3K-t and anti-actin antibodies were diluted at 1 : 1000 for western blots as primary antibodies. After three washes of 10 min each with TBS-T, the secondary antibody conjugated with horseradish peroxidase (HRP) was added in a dilution of 1 : 2500 as the secondary antibody. The secondary HRP was detected by enhanced chemiluminescence. Optical density was analyzed by Bio-Rad ChemiDox (Bio-Rad, Hercules, CA, USA). The relative density was calculated by the total absolute density of (EGFP/actin)100, (Akt-p/Akt-t/actin)*100 or (PI3K-p/PI3K-t/actin)*100.
Statistical evaluation
Statistical significance was assessed by one-way analysis of variances (ANOVAs). The Sheffé's test was applied as a post hoc for the significant difference shown by ANOVAs. A P-value of <0.05 was used as an indicative of statistical significance.
Acknowledgments
We thank Dr. A Levine (Rockefeller University) for providing wild-type and dominant negative mutant p53 constructs, Dr. J Woodgett (Ontario Cancer Institute) for providing wild-type, dominate negative and constitutively active mutant Akt constructs and Dr. H Lashuel (EPFL) for providing TC-Aβ. We also thank the assistance of confocal imaging from Dr. Shiqiang Wang and Mr. Rongchang Li (Peking University). This work was supported by the National Program of Basic Research sponsored by the Ministry of Science and Technology of China (2009CB941301), Peking University President Research Grant and Ministry of Education Recruiting Research Grant.
Glossary
- Aβ
amyloid β
- ANOVA
analysis of variance
- BCA
bicinchoninic acid
- CBX
carbenoxolone
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- ER
endoplasmic reticulum
- FBS
fetal bovine serum
- HIV
human immunodeficiency virus
- HRP
horseradish peroxidase
- NGF
nerve growth factor
- PBS
phosphate-buffered saline
- PI3K
phosphoinositide 3-kinase
- TBS
Tris-buffered saline
- TBS-T
Tris-buffered saline with tween20
- TC-Aβ
tetracysteine-amyloid β
- TGF-α
transforming growth factor-α
- TNT
tunneling nanotube
- TUNEL
terminal deoxynucleotidyl transferase-biotin dUTP nick-end labeling
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by N Bazan
Supplementary Material
References
- Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- Onfelt B, Davis DM. Can membrane nanotubes facilitate communication between immune cells. Biochem Soc Trans. 2004;32 (Part 5:676–678. doi: 10.1042/BST0320676. [DOI] [PubMed] [Google Scholar]
- Onfelt B, Nedvetzki S, Yanagi K, Davis DM. Cutting edge: membrane nanotubes connect immune cells. J Immunol. 2004;173:1511–1513. doi: 10.4049/jimmunol.173.3.1511. [DOI] [PubMed] [Google Scholar]
- Vidulescu C, Clejan S, O'Connor KC. Vesicle traffic through intercellular bridges in DU 145 human prostate cancer cells. J Cell Mol Med. 2004;8:388–396. doi: 10.1111/j.1582-4934.2004.tb00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–318. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Wustner D. Plasma membrane sterol distribution resembles the surface topography of living cells. Mol Biol Cell. 2007;18:211–228. doi: 10.1091/mbc.E06-05-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onfelt B, Nedvetzki S, Benninger RK, Purbhoo MA, Sowinski S, Hume AN, et al. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol. 2006;177:8476–8483. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]
- Zhu D, Tan KS, Zhang X, Sun AY, Sun GY, Lee JC. Hydrogen peroxide alters membrane and cytoskeleton properties and increases intercellular connections in astrocytes. J Cell Sci. 2005;118 (Part 16:3695–3703. doi: 10.1242/jcs.02507. [DOI] [PubMed] [Google Scholar]
- Freund D, Bauer N, Boxberger S, Feldmann S, Streller U, Ehninger G, et al. Polarization of human hematopoietic progenitors during contact with multipotent mesenchymal stromal cells: effects on proliferation and clonogenicity. Stem Cells Dev. 2006;15:815–829. doi: 10.1089/scd.2006.15.815. [DOI] [PubMed] [Google Scholar]
- Ramirez-Weber FA, Kornberg TB. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97:599–607. doi: 10.1016/s0092-8674(00)80771-0. [DOI] [PubMed] [Google Scholar]
- Gurke S, Barroso JF, Gerdes HH. The art of cellular communication: tunneling nanotubes bridge the divide. Histochem Cell Biol. 2008;129:539–550. doi: 10.1007/s00418-008-0412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes HH, Bukoreshtliev NV, Barroso JF. Tunneling nanotubes: a new route for the exchange of components between animal cells. FEBS Lett. 2007;581:2194–2201. doi: 10.1016/j.febslet.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT, et al. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol. 2009;11:328–336. doi: 10.1038/ncb1841. [DOI] [PubMed] [Google Scholar]
- Gousset K, Zurzolo C. Tunnelling nanotubes: a highway for prion spreading. Prion. 2009;3:94–98. doi: 10.4161/pri.3.2.8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DM, Sowinski S. Membrane nanotubes: dynamic long-distance connections between animal cells. Nat Rev Mol Cell Biol. 2008;9:431–436. doi: 10.1038/nrm2399. [DOI] [PubMed] [Google Scholar]
- Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- Kienlen-Campard P, Miolet S, Tasiaux B, Octave JN. Intracellular amyloid-beta 1-42, but not extracellular soluble amyloid-beta peptides, induces neuronal apoptosis. J Biol Chem. 2002;277:15666–15670. doi: 10.1074/jbc.M200887200. [DOI] [PubMed] [Google Scholar]
- Grant SM, Shankar SL, Chalmers-Redman RM, Tatton WG, Szyf M, Cuello AC. Mitochondrial abnormalities in neuroectodermal cells stably expressing human amyloid precursor protein (hAPP751) Neuroreport. 1999;10:41. doi: 10.1097/00001756-199901180-00008. [DOI] [PubMed] [Google Scholar]
- Zhang Y, McLaughlin R, Goodyer C, LeBlanc A. Selective cytotoxicity of intracellular amyloid beta peptide1-42 through p53 and Bax in cultured primary human neurons. J Cell Biol. 2002;156:519–529. doi: 10.1083/jcb.200110119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Goodyer C, LeBlanc A. Selective and protracted apoptosis in human primary neurons microinjected with active caspase-3, -6, -7, and -8. J Neurosci. 2000;20:8384–8389. doi: 10.1523/JNEUROSCI.20-22-08384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9:402–412. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284:31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- Hase K, Kimura S, Takatsu H, Ohmae M, Kawano S, Kitamura H, et al. M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat Cell Biol. 2009;11:1427–1432. doi: 10.1038/ncb1990. [DOI] [PubMed] [Google Scholar]
- Gurke S, Barroso JF, Hodneland E, Bukoreshtliev NV, Schlicker O, Gerdes HH. Tunneling nanotube (TNT)-like structures facilitate a constitutive, actomyosin-dependent exchange of endocytic organelles between normal rat kidney cells. Exp Cell Res. 2008;314:3669–3683. doi: 10.1016/j.yexcr.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Cui J, Chen Q, Yue X, Jiang X, Gao GF, Yu LC, et al. Galanin protects against intracellular amyloid toxicity in human primary neurons. J Alzheimers Dis. 2010;19:529–544. doi: 10.3233/JAD-2010-1246. [DOI] [PubMed] [Google Scholar]
- Morris LG, Veeriah S, Chan TA. Genetic determinants at the interface of cancer and neurodegenerative disease. Oncogene. 2010;29:3453–3464. doi: 10.1038/onc.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadea G, Lapasset L, Gauthier-Rouviere C, Roux P. Regulation of Cdc42-mediated morphological effects: a novel function for p53. EMBO J. 2002;21:2373–2382. doi: 10.1093/emboj/21.10.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenal I, Risler Y, Mignotte B. Down-regulation of actin genes precedes microfilament network disruption and actin cleavage during p53-mediated apoptosis. J Cell Sci. 1997;110 (Part 4:489–495. doi: 10.1242/jcs.110.4.489. [DOI] [PubMed] [Google Scholar]
- Comer KA, Dennis PA, Armstrong L, Catino JJ, Kastan MB, Kumar CC. Human smooth muscle alpha-actin gene is a transcriptional target of the p53 tumor suppressor protein. Oncogene. 1998;16:1299–1308. doi: 10.1038/sj.onc.1201645. [DOI] [PubMed] [Google Scholar]
- Metcalfe S, Weeds A, Okorokov AL, Milner J, Cockman M, Pope B. Wild-type p53 protein shows calcium-dependent binding to F-actin. Oncogene. 1999;18:2351–2355. doi: 10.1038/sj.onc.1202559. [DOI] [PubMed] [Google Scholar]
- Xie W, Paterson AJ, Chin E, Nabell LM, Kudlow JE. Targeted expression of a dominant negative epidermal growth factor receptor in the mammary gland of transgenic mice inhibits pubertal mammary duct development. Mol Endocrinol. 1997;11:1766–1781. doi: 10.1210/mend.11.12.0019. [DOI] [PubMed] [Google Scholar]
- Vassar R, Fuchs E. Transgenic mice provide new insights into the role of TGF-alpha during epidermal development and differentiation. Genes Dev. 1991;5:714–727. doi: 10.1101/gad.5.5.714. [DOI] [PubMed] [Google Scholar]
- Dominey AM, Wang XJ, King LE, Jr, Nanney LB, Gagne TA, Sellheyer K, et al. Targeted overexpression of transforming growth factor alpha in the epidermis of transgenic mice elicits hyperplasia, hyperkeratosis, and spontaneous, squamous papillomas. Cell Growth Differ. 1993;4:1071–1082. [PubMed] [Google Scholar]
- Blum M. A null mutation in TGF-alpha leads to a reduction in midbrain dopaminergic neurons in the substantia nigra. Nat Neurosci. 1998;1:374–377. doi: 10.1038/1584. [DOI] [PubMed] [Google Scholar]
- Averous J, Proud CG. When translation meets transformation: the mTOR story. Oncogene. 2006;25:6423–6435. doi: 10.1038/sj.onc.1209887. [DOI] [PubMed] [Google Scholar]
- Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 2006;21:362–369. doi: 10.1152/physiol.00024.2006. [DOI] [PubMed] [Google Scholar]
- Hinds PW, Finlay CA, Quartin RS, Baker SJ, Fearon ER, Vogelstein B, et al. Mutant p53 DNA clones from human colon carcinomas cooperate with ras in transforming primary rat cells: a comparison of the ‘hot spot' mutant phenotypes. Cell Growth Differ. 1990;1:571–580. [PubMed] [Google Scholar]
- Coffer PJ, Woodgett JR. Molecular cloning and characterisation of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur J Biochem. 1991;201:475–481. doi: 10.1111/j.1432-1033.1991.tb16305.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hong Y, Bounhar Y, Blacker M, Roucou X, Tounekti O, et al. p75 neurotrophin receptor protects primary cultures of human neurons against extracellular amyloid beta peptide cytotoxicity. J Neurosci. 2003;23:7385–7394. doi: 10.1523/JNEUROSCI.23-19-07385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.