Abstract
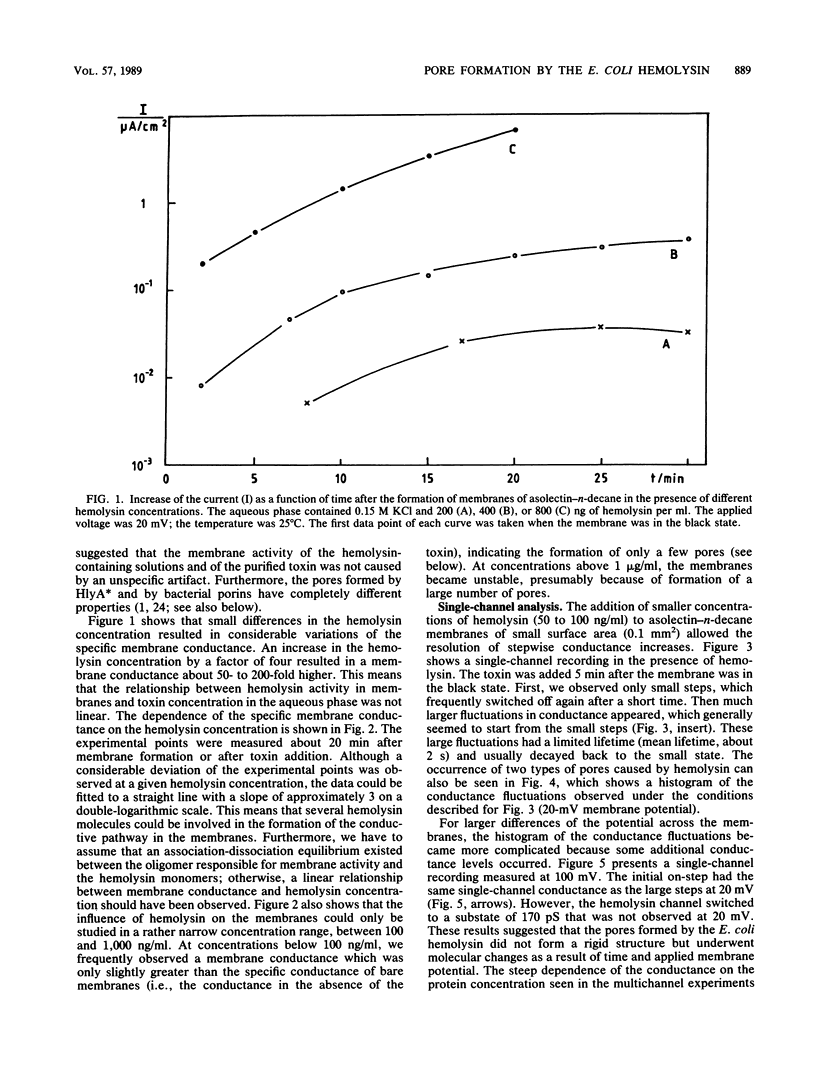
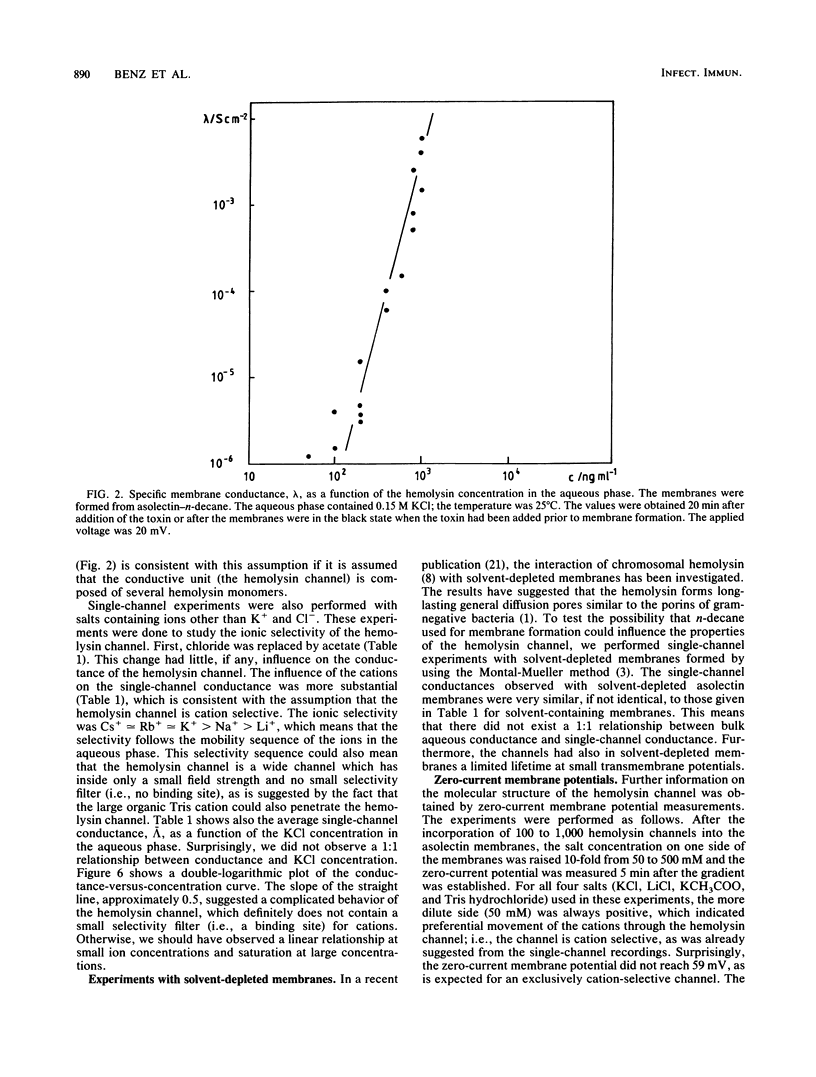
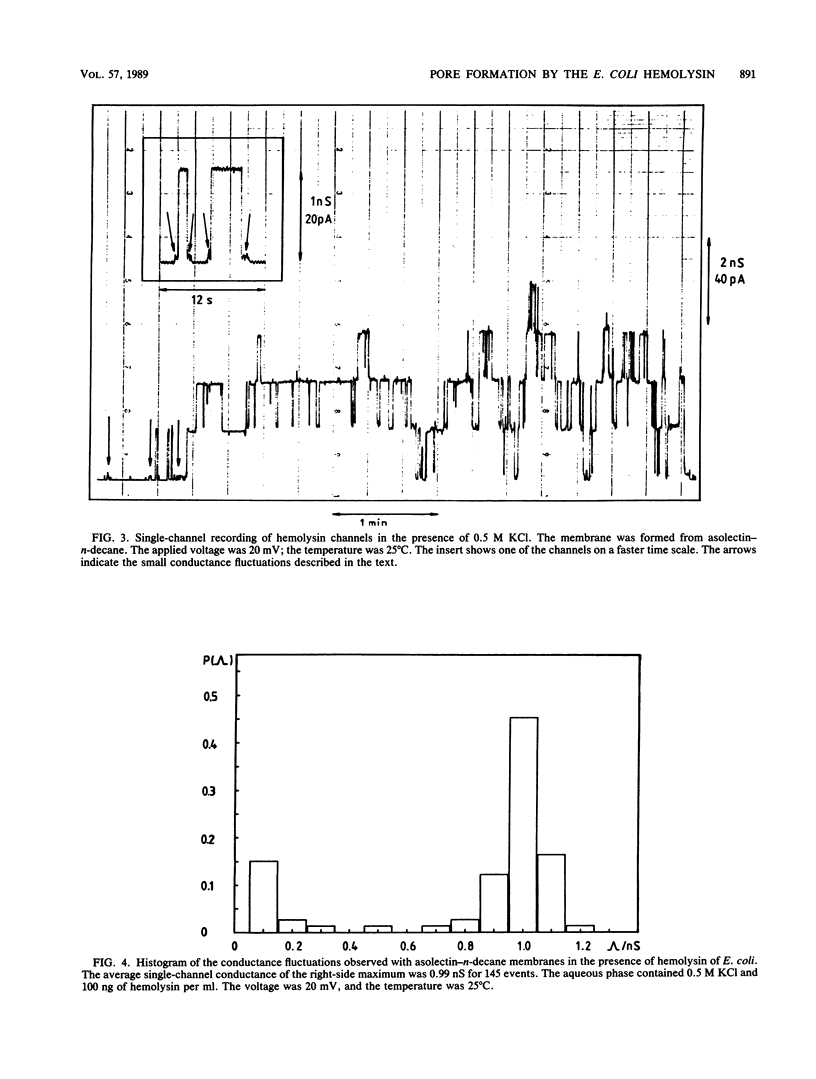
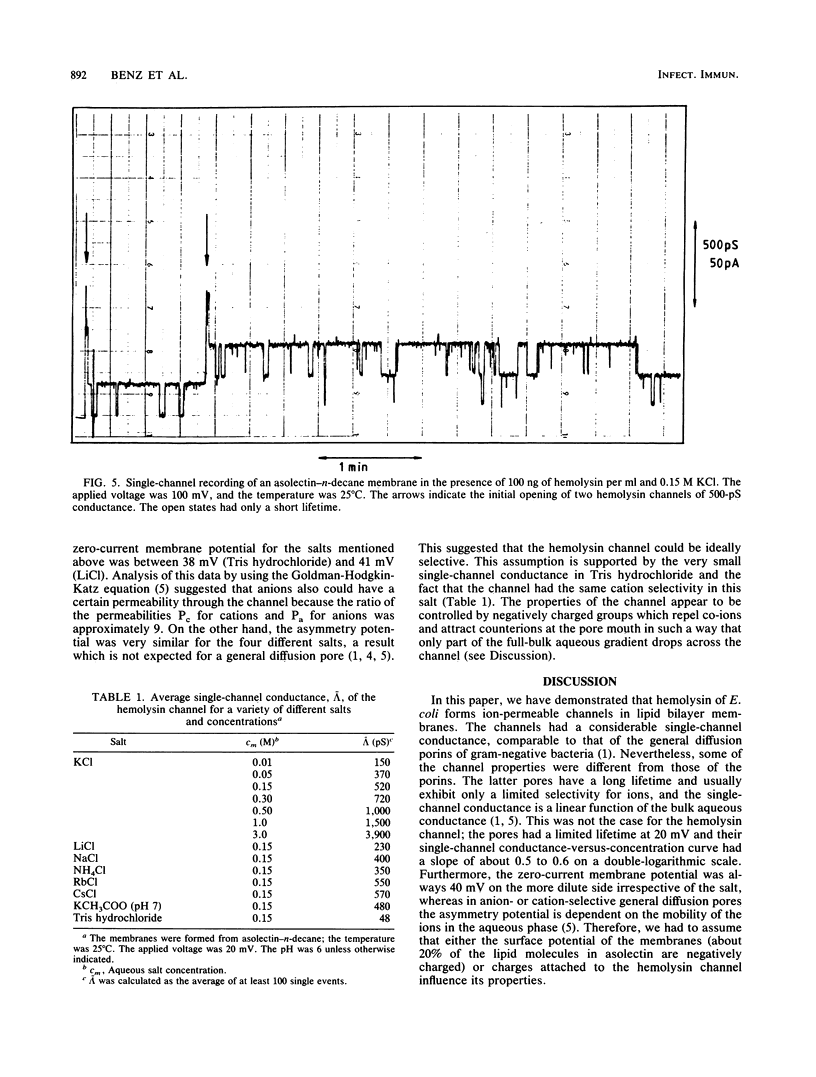
Lipid bilayer experiments were performed in the presence of hemolysin of Escherichia coli. The toxin had a rather low activity in membranes formed of pure lipids, such as phosphatidylcholine or phosphatidylserine. In membranes from asolectin, a crude lipid mixture from soybean, hemolysin was able to increase the conductance by many orders of magnitude in a steep concentration-dependent fashion, which suggested that several hemolysin molecules could be involved in the conductive unit. Furthermore, the much higher toxin activity in asolectin membranes would be consistent with the assumption that this lipid contains a receptor needed for membrane activity of the toxin. The results of single-channel records showed that the membrane activity of hemolysin is due to the formation of ion-permeable channels with a single-channel conductance of about 500 pS in 0.15 M KCl. The hemolysin channel seemed to be formed by a toxin oligomer which showed an association-dissociation reaction and had a mean lifetime of about 2 s at small transmembrane voltages. The conductance of the hemolysin channels was only moderately dependent on the salt concentration in the aqueous phase. Zero-current membrane potential experiments showed that the hemolysin channel is cation selective. The mobility sequence of the cations in the channel was similar to their mobility sequence in the aqueous phase, which was consistent with the assumption that the hemolysin channel is wide and that the interior field strength is not very high. From the single-channel conductance, a lower limit of about 1.0 nm for the effective channel diameter could be estimated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Boehler-Kohler B. A., Dieterle R., Boos W. Porin activity in the osmotic shock fluid of Escherichia coli. J Bacteriol. 1978 Sep;135(3):1080–1090. doi: 10.1128/jb.135.3.1080-1090.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Fröhlich O., Läuger P., Montal M. Electrical capacity of black lipid films and of lipid bilayers made from monolayers. Biochim Biophys Acta. 1975 Jul 3;394(3):323–334. doi: 10.1016/0005-2736(75)90287-4. [DOI] [PubMed] [Google Scholar]
- Benz R., Janko K., Boos W., Läuger P. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1978 Aug 17;511(3):305–319. doi: 10.1016/0005-2736(78)90269-9. [DOI] [PubMed] [Google Scholar]
- Benz R., Janko K., Läuger P. Ionic selectivity of pores formed by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1979 Mar 8;551(2):238–247. doi: 10.1016/0005-2736(89)90002-3. [DOI] [PubMed] [Google Scholar]
- Benz R., Läuger P. Transport kinetics of dipicrylamine through lipid bilayer membranes. Effects of membrane structure. Biochim Biophys Acta. 1977 Jul 14;468(2):245–258. doi: 10.1016/0005-2736(77)90118-3. [DOI] [PubMed] [Google Scholar]
- Benz R. Porin from bacterial and mitochondrial outer membranes. CRC Crit Rev Biochem. 1985;19(2):145–190. doi: 10.3109/10409238509082542. [DOI] [PubMed] [Google Scholar]
- Benz R., Schmid A., Nakae T., Vos-Scheperkeuter G. H. Pore formation by LamB of Escherichia coli in lipid bilayer membranes. J Bacteriol. 1986 Mar;165(3):978–986. doi: 10.1128/jb.165.3.978-986.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Mackman N., Nicaud J. M., Holland I. B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986 Apr;52(1):63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Damage to mammalian cells by proteins that form transmembrane pores. Rev Physiol Biochem Pharmacol. 1987;107:147–223. doi: 10.1007/BFb0027646. [DOI] [PubMed] [Google Scholar]
- Cavalieri S. J., Bohach G. A., Snyder I. S. Escherichia coli alpha-hemolysin: characteristics and probable role in pathogenicity. Microbiol Rev. 1984 Dec;48(4):326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorset D. L., Engel A., Massalski A., Rosenbusch J. P. Three Dimensional Structure of a Membrane Pore: Electron Microscopical Analysis of Escherichia coli Outer Membrane Matrix Porin. Biophys J. 1984 Jan;45(1):128–129. doi: 10.1016/S0006-3495(84)84135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Welch R. A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985 Jul;163(1):94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J., Hughes C. Genetics of Escherichia coli hemolysin. Curr Top Microbiol Immunol. 1985;118:139–162. doi: 10.1007/978-3-642-70586-1_8. [DOI] [PubMed] [Google Scholar]
- Härtlein M., Schiessl S., Wagner W., Rdest U., Kreft J., Goebel W. Transport of hemolysin by Escherichia coli. J Cell Biochem. 1983;22(2):87–97. doi: 10.1002/jcb.240220203. [DOI] [PubMed] [Google Scholar]
- Ikigai H., Nakae T. Conformational alteration in alpha-toxin from Staphylococcus aureus concomitant with the transformation of the water-soluble monomer to the membrane oligomer. Biochem Biophys Res Commun. 1985 Jul 16;130(1):175–181. doi: 10.1016/0006-291x(85)90398-5. [DOI] [PubMed] [Google Scholar]
- Ludwig A., Jarchau T., Benz R., Goebel W. The repeat domain of Escherichia coli haemolysin (HlyA) is responsible for its Ca2+-dependent binding to erythrocytes. Mol Gen Genet. 1988 Nov;214(3):553–561. doi: 10.1007/BF00330494. [DOI] [PubMed] [Google Scholar]
- Ludwig A., Vogel M., Goebel W. Mutations affecting activity and transport of haemolysin in Escherichia coli. Mol Gen Genet. 1987 Feb;206(2):238–245. doi: 10.1007/BF00333579. [DOI] [PubMed] [Google Scholar]
- Menestrina G., Mackman N., Holland I. B., Bhakdi S. Escherichia coli haemolysin forms voltage-dependent ion channels in lipid membranes. Biochim Biophys Acta. 1987 Nov 27;905(1):109–117. doi: 10.1016/0005-2736(87)90014-9. [DOI] [PubMed] [Google Scholar]
- Nicaud J. M., Mackman N., Gray L., Holland I. B. Regulation of haemolysin synthesis in E. coli determined by HLY genes of human origin. Mol Gen Genet. 1985;199(1):111–116. doi: 10.1007/BF00327519. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Porin channels in Escherichia coli: studies with liposomes reconstituted from purified proteins. J Bacteriol. 1983 Jan;153(1):241–252. doi: 10.1128/jb.153.1.241-252.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer J., König W., Hacker J., Goebel W. Bacterial adherence and hemolysin production from Escherichia coli induces histamine and leukotriene release from various cells. Infect Immun. 1985 Oct;50(1):271–278. doi: 10.1128/iai.50.1.271-278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer J., Vosbeck K., König W. Induction of inflammatory mediators from human polymorphonuclear granulocytes and rat mast cells by haemolysin-positive and -negative E. coli strains with different adhesins. Immunology. 1986 Dec;59(4):541–548. [PMC free article] [PubMed] [Google Scholar]
- Vogel M., Hess J., Then I., Juarez A., Goebel W. Characterization of a sequence (hlyR) which enhances synthesis and secretion of hemolysin in Escherichia coli. Mol Gen Genet. 1988 Apr;212(1):76–84. doi: 10.1007/BF00322447. [DOI] [PubMed] [Google Scholar]
- Wagner W., Kuhn M., Goebel W. Active and inactive forms of hemolysin (HlyA) from Escherichia coli. Biol Chem Hoppe Seyler. 1988 Jan;369(1):39–46. doi: 10.1515/bchm3.1988.369.1.39. [DOI] [PubMed] [Google Scholar]
- Wagner W., Vogel M., Goebel W. Transport of hemolysin across the outer membrane of Escherichia coli requires two functions. J Bacteriol. 1983 Apr;154(1):200–210. doi: 10.1128/jb.154.1.200-210.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



