Abstract
Granzymes (gzms) are key components of T-killer (Tc) cells believed to mediate pro-apoptotic activities. Recent evidence suggests that gzms also possess non-cytotoxic activities that contribute to host defense. In this study, we show that Tc cells from lymphocytic choriomeningitis virus (LCMV)-infected wild-type (wt) and gzm A/B-deficient mice express similar levels of gzmK protein, with both mouse strains efficiently controlling infection. GzmK, in recombinant form or secreted by ex vivo-derived LCMV-immune gzmAxB−/− Tc cells, lacks pro-apoptotic activity. Instead, gzmK induces primary mouse macrophages to process and secrete interleukin-1β, independent of the ATP receptor P2X7. Together with the finding that IL-1Ra (Anakinra) treatment inhibits virus elimination but not generation of cytotoxic Tc cells in wt mice, the data suggest that Tc cells control LCMV through non-cytotoxic processes that involve gzmK.
Keywords: T-killer cell, virus control, orphan granzymes, inflammation
The cytotoxic cell granule secretory pathway is considered indispensable for eliminating tumor and virally infected cells. The underlying process is mediated by the pore-forming protein, perforin (perf), which delivers a family of serine proteases, namely the granzymes (gzms), to cells, presumably targeted for destruction. The gzms have been characterized by DNA sequencing of counterparts in the mouse, rat and humans and by the availability of recombinant proteins for functional and structural analyses.1, 2 On the basis of their transfer into target cells and secretion extracellularly, gzms were originally anticipated to act both intracellularly and mediate extracellular proteolytic events.3 However, the discovery that isolated gzms induced apoptosis in the presence of perf4, 5 encouraged the notion that proteases acted primarily as death effectors.
Human (Hu)gzmA was reported to induce pro-inflammatory cytokines in human monocytes and mouse macrophages more than 10 years ago,6 which may be due to processing of interleukin (IL)-1.7 The significance of this observation was not appreciated until recently when (Hu) and mouse (Mo)gzmAs were observed to lack cytotoxic activity in vitro,8 except under very restricted conditions.8, 9 In the absence of the apparent cytotoxicity mediated by (Hu) and (Mo)gzmAs, the possibility that the protease might act in vivo to induce pro-inflammatory cytokines was re-evaluated. In this regard, isolated (Hu) and (Mo)gzmAs, as well as human natural killer (NK) and mouse T-killer (Tc) cells secreting the protease were shown to induce human monocytic cells or pre-sensitized mouse peritoneal macrophages (PEMØs), respectively, to express and secrete pro-inflammatory cytokines IL-1β, tumor necrosis factor (TNF)-α and/or IL-6.8 In addition, caspase-1 inhibition was found to reduce (Hu)gzmA-induced IL-1β and TNF-α secretion by stimulated human monocytes, suggesting that gzmA may be another activator of the inflammasome platform systems.10 The physiological importance of this phenomenon was then validated by showing that gzmA knockout (ko) mice (gzmA−/−) resist the lethal effects of LPS.8 Together with the recent finding that (Mo)gzmM augments TLR4-driven inflammation and endotoxicosis,11 these observations set a biological precedent indicating that (Hu/Mo)gzms may have additional functions besides acting as pro-apoptotic mediators.
The highly cationic gzmK from humans and mouse has tryptase-like substrate preference similar to (Hu)gzmA, but the fine specificity is undoubtedly unique.12 Similar to (Hu)gzmA, the original report indicated that isolated Rat and (Hu)gzmKs are cytotoxic in vitro.13 A more recent study has suggested that isolated (Hu)gzmK induces biochemical events similar to (Hu)gzmA in target cells. In this study, (Hu)gzmK processes the nucleosome assembly protein (SET) releasing the DNAse NM23H1, which translocates to the nucleus. The apurinic endonuclease 1 is also cleaved by (Hu)gzmK. On the other hand, caspase-3 activation is undetectable.14, 15, 16 (Hu)gzmK may also cleave the tumor suppressor, p53, thus sensitizing tumor cells for apoptosis induction17 and may process a vasolin-containing protein, thus contributing to endoplasmic reticulum stress and caspase-independent cytotoxicity.18 Compared with the proposed cytotoxic role for gzmK, the protease has been found by immunoassays19 to be elevated in the plasma of patients with sepsis20 and in the bronchoalveolar fluid of patients with asthma and viral pneumonias,21 suggesting that gzmK may have additional biological functions.
Perf−/− mice demonstrate the critical role of the granule-secretory pathway in the recovery from lymphocytic choriomeningitis virus (LCMV) infection.22 However, compared with perf, the contribution of gzmA, gzmB and orphan gzms in the host defense against this pathogen is controversial.23, 24 After intraperitoneal (i.p.) challenge with LCMV, gzmB−/− and gzmAxB−/− mice clear the virus, although somewhat less vigorously than wild-type (wt) mice. A recent study showed that (Mo)gzmK is expressed as mRNA by ex vivo LCMV-immune Tc cells, independent of their expression of gzmA and/or gzmB.25 GzmK has been suggested to contribute to the clearance of influenza virus in mice,26, 27 but overall, the biological function(s) of this gzm family member remains incompletely characterized. The purpose of this report is to re-examine the cytotoxic activity of (Mo)gzmK and, because of its similar substrate specificity to (Hu/Mo)gzmA, to determine whether the protease has pro-inflammatory effects.
Results
LCMV infection in mice is readily controlled in the absence of gzmA and B
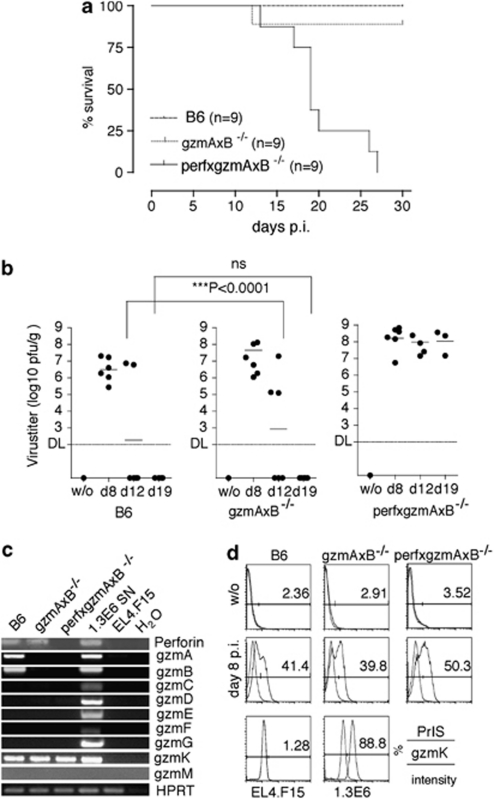
Previous studies have demonstrated that although perf is essential for optimal control of LCMV infection,22 both gzmA and B have a marginal role.23, 24. To verify this supposition, we compared survival and viral titers in mice lacking gzmA and B (gzmAxB−/−) with those without perf and the two gzms (perfxgzmAxB−/−). After challenge with 1 × 105 p.f.u LCMV-WE, all perfxgzmAxB−/− (9/9) died, but only 1/9 gzmAxB−/− and none of the wt B6 mice succumbed to the virus during the 30-day observation period (Figure 1a). At day 8 after inoculation, hepatic virus titers were similarly increased in WT, as well as in gzmAxB−/− and triple ko mice with somewhat higher levels in ko mouse strains (Figure 1b). However, although the level of virus gradually declined to background levels in gzmAxB−/− and B6 mice, no reduction of virus load was observed in the liver of perfxgzmAxB−/− mice during the entire observation period (Figure 1b). The data are consistent with previous studies23 emphasizing that the control of LCMV infection, including viral elimination, is strictly dependent on perf but that neither gzmA nor gzmB are obligatory participants.
Figure 1.
(a) Survival of wild-type, gzmAxB−/− and perfxgzmAxB−/− mice infected with LCMV-WE. Groups (nine mice each) of B6 (dashed line) or gzmAxB−/−(dotted line) or perfgzmAxB−/− (line) mice were infected with 1 × 105 p.f.u. LCMV-WE i.p., and survival was monitored for 30 days. (b) LCMV-WE replication in the liver of WT, gzmAxB−/− and perfxgzmAxB−/− mice. Groups of B6, gzmAxB−/− and perfxgzmAxB−/− (18 mice per strain) recipients were infected with 1 × 105 p.f.u. LCMV-WE i.p. Six mice from each group were killed at 8, 12 and 19 days p.i. Virus titers of individual mice were determined by the plaque titer assay as described in the ‘Materials and Methods' section. (c) Expression of mRNA in ex vivo LCMV-immune CD8-enriched Tc cells from WT, gzmAxB−/− and perfxgzmAxB−/− mice. Total mRNA was isolated from ex vivo LCMV-immune CD8-enriched Tc cells (day 8 p.i.) from wild-type, gzmAxB−/− and perfxgzmAxB−/− mice, previously infected with 1 × 105 p.f.u. LCMV-WE i.p. Samples were analyzed by RT-PCR for their expression of transcripts using specific primers for gzmA-G, gzmK, gzmM, Prf1 and Hprt1. (d) Intracellular expression of gzm and perf protein in ex vivo LCMV-immune CD8-enriched Tc cells (day 8 p.i.). Ex vivo LCMV-immune CD8-enriched Tc cells (day 8 p.i.) of wt, gzmAxB−/− and perfxgzmAxB−/− mice, infected with 1 × 105 p.f.u. LCMV-WE i.p. were analyzed by FACS for intracellular expression of gzmK (blue line) using the respective rabbit anti-K immune serum and the control pre-immune serum (red line)
Gzm K is expressed in LCMV-immune Tc cells from wt and gzmAxB-deficient mice
To test the assumption that other gzms besides gzmA and B might contribute to perf-mediated control of LCMV infection in gzmAxB−/− mice, virus-immune Tc cells (day 8 post infection (p.i.)) were evaluated for the expression of perf and gzm-specific mRNAs and their respective intracellular proteins. As reported previously,25 virus-immune Tc cells from B6, gzmAxB−/− and perfxgzmAxB−/− mice expressed similar levels of the gzmK transcript and the mRNA for gzmA, gzmB and perf expected for the respective ko mice (Figure 1c). No transcripts for gzmC–G and gzmM were detectable (for gzmM, also see Supplementary Figure 1C). To ensure that various effector populations expressed the gzmK protein, its presence in CD8+ Tc cells of uninfected and LCMV-infected mice (day 8 p.i.) was determined using a recently developed rabbit anti-recombinant (rec.) (Mo)gzmK antibody. Although gzmK was undetectable in Tc cells from non-infected mice, Tc cells from all three infected mouse strains contained similar levels of the protease (Figure 1d). As expected, only Tc cells from LCMV-immune B6 but not from gzmAxB−/− and perfxgzmAxB−/− mice expressed gzmA and gzmB proteins (data not shown).
LCMV-immune Tc cells that express gzmK and lack gzmA and gzmB are non-cytotoxic
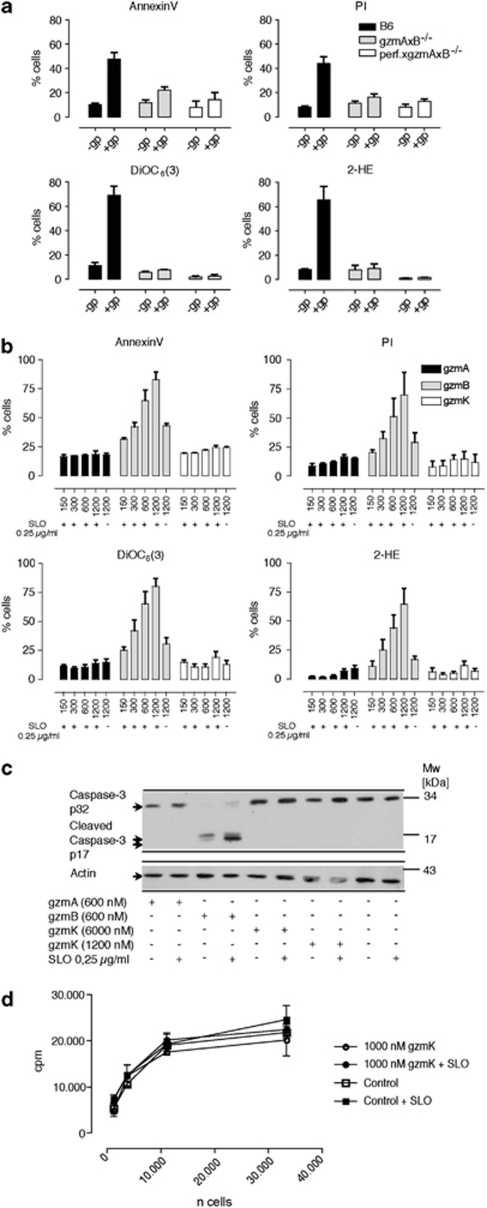
To determine whether LCMV-immune gzmAxB−/− Tc cells that primarily expressed gzmK induce apoptosis in mouse embryonic fibroblast (MEF) cells, we measured multiple parameters, including phosphatidylserine (PS) externalization, propidium iodide (PI) uptake, mitochondrial depolarization (ΔΨm) and reactive oxygen species (ROS) generation. Despite the fact that LCMV-immune B6 and gzmAxB−/− mice both readily controlled LCMV infection, only ex vivo-derived LCMV-immune B6 Tc cells induced cell death in target cells in vitro as defined by the above-mentioned parameters (Figure 2a). On the other hand, LCMV-immune gzmAxB−/− Tc cells were only marginally cytotoxic, whereas LCMV-immune perfxgzmAxB−/− Tc cells were completely inactive. Conversely, LCMV-immune gzmAxB−/− NK cells, which expressed perf and gzmM, but no gzmK, showed residual cytotoxic activity, which was however at least five times lower than that of gzmA−/− mice (Supplementary Figure 1A).
Figure 2.
(a) Induction of pro-apoptotic markers by ex vivo LCMV-immune Tc cells from WT, gzmAxB−/− and perfxgzmAxB−/− mice. Gp-33-pulsed or untreated MEF target cells were incubated with ex vivo LCMV-immune CD8-enriched Tc cells of the indicated strain (4 h, 10 : 1 effector/target ratio). Subsequently, PS exposure on the plasma membrane (annexin-V-FITC) and PI uptake, and in parallel, ΔΨm loss (DiOC6) and ROS generation (2-HE) were analyzed by three-color flow cytometry in the cell population negative for CD8 expression as described in the ‘Materials and Methods' section. Data in the panels are represented as mean±S.E.M. of three independent experiments. (b) rec. (Mo)gzmA and rec. (Mo)gzmK are not cytotoxic: analyses of apoptotic parameters during (Mo)gzmA, (Mo)gzmB and (Mo)gzmK-induced cell death in EL4 cells. EL4 cells were incubated with the indicated amounts of gzms in the presence of a sublytic dose of SLO for 4 h, and the highest amount of gzm was also probed without SLO indicated by 1200 nM – SLO. Subsequently, PS exposure on the plasma membrane (annexin-V-FITC) and PI uptake, and in parallel, ΔΨm loss (DiOC6) and ROS generation (2-HE) were analyzed by three-color flow cytometry. (c) EL4 cells were either incubated or not incubated with the indicated amounts of rec. (Mo)gzmA, rec. (Mo)gzmB and rec. (Mo)gzmK in the presence and absence of SLO for 1 h at 37°C. Subsequently, lysates were prepared and caspase-3 and cleaved caspase-3 were analyzed by WB, with actin serving as control. (d) Survival assay of EL4 cells treated with rec. (Mo)gzmK in the presence and absence of SLO as described in the ‘Materials and Methods' section
Recombinant (Mo) gzmK and gzmA are not cytotoxic
To compare the cytotoxic potential of isolated rec. (Mo)gzmK with rec. (Mo)gzmA and B, EL4 cells were incubated with increasing concentrations of the proteases (150–1200 nM) delivered by streptolysin O (SLO), and cell death was determined by PI uptake, annexin reactivity (PS exposure), Δψ and ROS generation. Although neither (Mo)gzmA nor (Mo)gzmK elicited a cytotoxic response at all concentrations, (Mo)gzmB showed the predicted concentration-dependent induction of cell death (Figure 2b), including cleavage of caspase-3 (Figure 2c). To ensure that rec. (Mo)gzmA and rec. (Mo)gzmK manifested enzymatic activity and to verify their predicted differences in cleavage specificity,12 their amidolytic activities were compared against four different chromogenic substrates. Both gzms were proteolytically active, with each exhibiting distinct preferences for the four related chromogenic substrates, especially Tos-Gly-pro-Arg-pNA (Table 1). Finally, a cell survival assay was performed28 to exclude the possibility that gzmK might induce cell death that is undetected by the parameters mentioned above. Re-growth of EL4 cells was unimpaired for targets incubated only with the rec. (Mo)gzmK (1000 nM) or after the protease was delivered by SLO (Figure 2d).
Table 1. Amidolytic activity of (Mo)gzmA and (Mo)gzmK on chromogenic substrates.
| Substrates | gzmA | gzmK |
|---|---|---|
| Specific activity Units/μg protein | ||
| Pro-Phe-Arg-pNA | 107 | 115 |
| Tos-Gly-Pro-Arg-pNA | 9 | 120 |
| H-D-Ile-Pro-Arg-pNA | 25 | 31 |
| Bz-Arg-pNA | 0 | 3 |
Recombinant (Mo)gzmK induces macrophages to release IL-1β
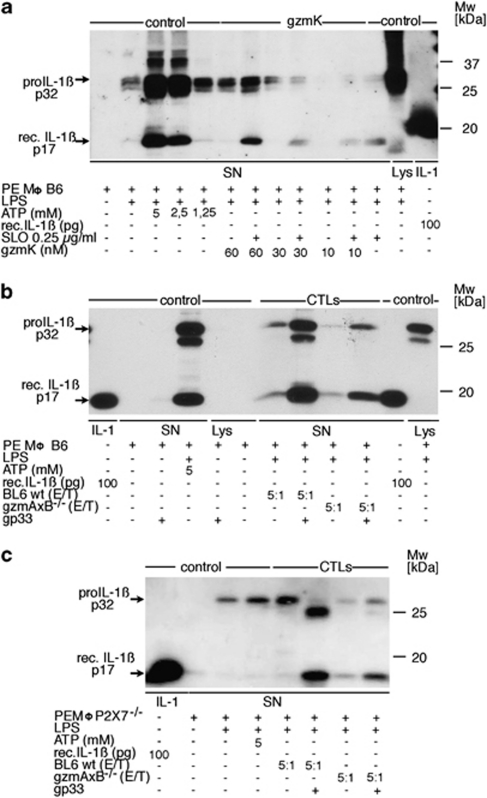
Although rec. (Mo)gzmA and rec. (Mo)gzmK have different substrate specificities, we speculated that the tryptase-like activity of (Mo)gzmK might mimic (Mo)gzmA in its capacity to induce pre-activated PEMØs to express and secrete mature IL-1β. In this regard, rec. (Mo)gzmK was added to LPS-primed PEMØs in the presence or absence of SLO. The delivery of gzmK by SLO induces concentration-dependent processing of pro-IL-1β, requiring doses >30 nM (Figure 3a and Supplementary Figure 2). SLO alone was also observed to induce detectable, although minor maturation/secretion of IL-1β (Figure 3a and Supplementary Figure 2). At higher concentrations of gzmK (600 and 1000 nM), the protease alone was sufficient to induce release of IL-1β to levels observed for cells treated with the gzm and SLO. Finally, the inactive pro-form of rec. (Mo)gzmK did not alter maturation/secretion of IL-1β (Supplementary Figure 2) eliminating the possibility that the gzm was inducing the cytokine through a non-proteolytic mechanism.
Figure 3.
(a) Induction of IL-1β release by rec. (Mo)gzmK. Adherent B6 PEMØs, previously sensitized with thioglycollate in vivo and challenged with LPS in vitro were incubated with the indicated amounts of gzmK in presence or absence of 0.25 μg/ml SLO, or ATP as positive control, for 3 h and SNs were subjected to WB analysis using anti-IL-1β-specific Ab. Aliquots of the following preparations were used as standards for WB analysis: SNs of ATP-sensitized pre-activated B6 PEMØs; SNs of thioglycollate-pre-sensitized and LPS-challenged B6 PEMØs. (b) Induction of IL-1β release by ex vivo CD8 cells expressing gzmK. Thioglycollate-pre-sensitized and LPS-challenged adherent B6 PEMØs were incubated with ex vivo-derived LCMV-immune Tc cells from B6 or gzmAxB ko mice at an E/T ratio of 5 : 1 in the presence or absence of the LCMV-specific peptide gp33. SN was taken after 6 h and subjected to WB analysis using anti-IL-1β-specific Ab. As control, SN from thioglycollate-pre-sensitized and LPS-challenged B6 PEMØs, and subsequently stimulated with 5 mM ATP, was analyzed accordingly. Aliquots of the following preparations were used as standards for WB analysis: rec. IL-1βs, p17; SN of ATP-sensitized pre-activated wt B6 PEMØs; SN of wt thioglycollate-pre-sensitized and LPS-activated B6 PEMØs. (c) Induction of IL-1β release by ex vivo CD8 cells expressing gzmK by targets lacking the P2X7 receptor. Experimental procedure was conducted as described in panel b, but with macrophages from P2X7−/− mice. Controls were prepared individually for this experiment with P2X7−/− macrophages (SN of ATP-sensitized pre-activated PEMØs; SN of wt thioglycollate-pre-sensitized and LPS-activated PEMØs)
LCMV-immune Tc cells of B6 and gzmAxB−/− mice induce IL-1β release from wt and P2X7−/− macrophages
LCMV-immune B6 and gzmAxB−/− Tc cells were added to LPS-primed PEMØs in the presence or absence of LCMV-specific immunogenic peptide, gp33. In the absence of gp33, LCMV-immune B6 and gzmAxB−/− Tc cells induced only marginal processing and release of IL-1β in PEMØs at an E/T ratio of 5 : 1. In contrast, gp33-pulsed targets responded to LCMV-immune B6 and gzmAxB−/− Tc cells by secreting substantial amounts of IL-1β (p17; Figure 3b). To exclude the possibility that Tc cell-induced IL-1β secretion was due to ATP released from stressed PEMØs,29, 30 the response of cells from mice lacking the ATP sensor (P2X7 receptor) was used under similar conditions. Although treatment with ATP (5 mM) did not result in secretion of active IL-1β, wt and gzmAxB−/− Tc cells readily induced the release of mature IL-1β from P2X7-deficient PEMØs to an extent similar to wt PEMØs (Figure 3c).
In vivo treatment of mice with IL-1RA inhibits virus elimination
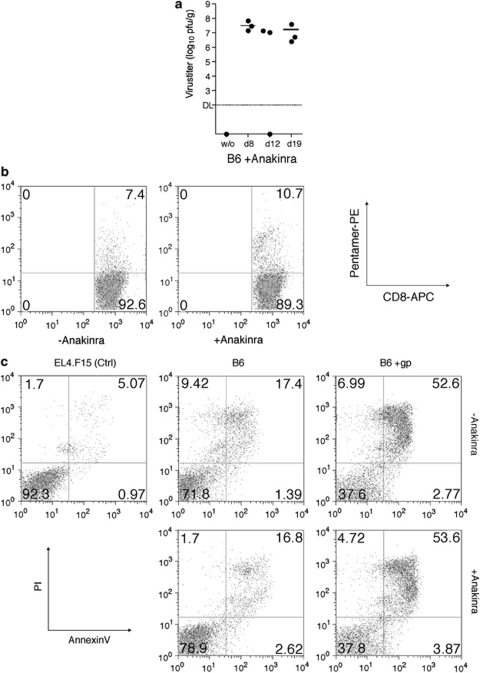
To explore the possibility that IL-β participates in the control of LCMV infection, B6 mice were treated with IL-1Ra (Anakinra; see the study by So et al.31) and virus titers were monitored in the liver on days 8, 12 and 19. In contrast to mock-treated and virus-infected B6 mice (Figure 1b), recipients treated with Anakinra were unable to eliminate LCMV (Figure 4a). This outcome was not due to the absence of activated Tc cells because the ratio of LCMV-specific Tc is comparable for untreated and treated LCMV-infected mice (Figure 4b). Furthermore, LCMV-immune Tc cells taken from the same Anakinra-treated mice showed pro-apoptotic activity similar to mock-treated Tc (Figure 4c).
Figure 4.
(a) Anakinra inhibits recovery from LCMV infection. B6 mice received Anakinra before infection (1 × 105 p.f.u. LCMV-WE i.p) and then on every third day thereafter. Three mice from each group were killed at 8, 12 and 19 days and hepatic viral replication determined. (b) Anakinra-treated mice infected with LCMV express levels of LCMV-specific Tc cells similar to untreated controls. Mice were first treated with Anakinra (200 μg) 1 day before infection and then every third day. After killing (day 8), splenic CD8 cells were isolated and virus-specific Tc cells were determined by pentamer staining. The livers from these mice were used to generate data reported in panel a. (c) Anakinra-treated and control Tc cells mediate similar apoptotic effects against EL4 cells. Gp-33-pulsed or untreated EL4 target cells were incubated with ex vivo LCMV-immune CD8-enriched Tc cells (4 h, 10 : 1). Subsequently, PS exposure on the plasma membrane (annexin-V-FITC) and PI uptake were analyzed by flow cytometry
Discussion
The observation that perf−/− mice die from LCMV infection clearly indicates that the pore-forming protein perf is essential for Tc cell (NK)-mediated control of this virus.22 However, as perf functions chiefly to deliver gzms and other granule components into target cells, an understanding of the role(s) of these proteins in the anti-viral response and associated tissue damage is crucial. For example, LCMV is cleared in gzmA−/− and gzmB−/− mice, although recovery is delayed in the latter compared with wt controls,24 and LCMV-associated hepatitis seems to require the presence of gzmA and gzmB.23 We have observed in this study that the course of virus infection was similar, but not identical for gzmAxB−/− and B6 mice, indicating that other granule proteins are possible participants.2 As Tc cells are essential to control LCMV infection and gzmK is expressed in virus-immune gzmAxB−/− Tc cells that retain this capacity, one could speculate that gzmK together with perf may contribute to the host response that results in the elimination of the virus.
Although the molecular basis for a gzmK-mediated anti-viral response is uncertain, based on past observations, the tryptase is predicted to clear LCMV by inducing infected cells to undergo apoptosis. By studying the human system, the Fan group has shown that rec. (Hu)gzmK elicits multiple pro-apoptotic pathways that could participate in anti-viral immunity. Their laboratory has reported that the gzm directly processes Bid, which releases cytochrome c and endonuclease G, resulting in the activation of a mitochondrial-dependent cell death pathway, although without caspase-3 activation.14 Rec. (Hu)gzmK, similar to rec. (Hu)gzmA, has also been reported to cleave SET, which occurs in association with single-stranded DNA nicks in target cells.15 Furthermore, rec. (Hu)gzmK has been reported to cleave p53 into three products (namely p40, p35 and p13), which apparently possess pro-apoptotic activities. In addition, these fragments are proposed to augment the cytotoxic activity of gzmK, although other biological effects cannot be excluded.17 Finally, (Hu)gzmK was shown to cleave a vasolin-containing protein resulting in accelerated endoplasmic reticulum stress and caspase-independent cytotoxicity of tumor cells.18 In marked contrast, this study demonstrates that ex vivo-derived LCMV-immune Tc cells which are positive for gzmK but negative for gzmA and gzmB expressed only marginal pro-apoptotic/cytotoxic activities, especially when measured by sensitive markers that assess plasma membrane and mitochondrial integrity, as well as by a target cell-survival assay. This finding agrees with reports showing that LCMV- and ectromelia-immune gzmAxB−/− Tc cells show marginal pro-apoptotic activity.25, 32, 33 More strikingly, rec. (Mo)gzmK, like (Mo)gzmA,8 completely failed to induce cell death at concentrations that suffice for gzmB-induced apoptosis. The combined results suggest that (Mo)gzmK, through non-cytotoxic processes, might be one component in the complex of molecular events that contribute to the elimination of LCMV. Notably, NK cells from infected gzmAxB−/− mice, which did not express gzmK as expected from previous studies,34 showed low but significant cytotoxic activity, which may be due to the expression of gzmM. However, the possibility that LCMV infection in these ko mice is controlled by NK cells is less likely, as NK cells have been shown before to be unable on their own to control LCMV infection, even if they fully express the exocytosis pathway.
Offering a major paradigm shift in characterizing the functionality of the granule secretory pathway, we have learned that isolated (Mo)gzmA and ex vivo virus-immune mouse Tc cells containing gzmA induce IL-1β release from LPS-sensitized PEMØs.8, 35 As gzmA may contribute to host defense by inducing pro-inflammatory cytokines in pathogen-infected cells targeted by the granule secretory process, we speculated that (Mo)gzmK, displaying a tryptase-like activity similar but not identical to (Mo)gzmA,12 might also be an inducer of pro-inflammatory cytokines. Accordingly, we show in this study for the first time that nanomolar concentrations of rec. (Mo)gzmK and LCMV-immune Tc containing gzmK, but neither gzmA nor gzmB, induce the maturation and secretion of IL-1β in LPS-sensitized PEMØs. The fact that gzmK displays a highly restricted substrate specificity that only partially overlaps with (Mo)gzmA,12 suggests that (Mo)gzmK may augment (Mo)gzmA-induced pro-inflammatory processes by differentially cleaving the same or distinct specific substrates. This assumption is supported by the fact that B6 Tc cells, which express gzmA and gzmK together with gzmB, have a greater capacity than gzmAxB−/− Tc cells to induce IL-1β release from LPS-sensitized PEMØs.
Pro-IL-1β is produced in response to danger or pathogen-associated molecular patterns such as LPS and can be processed to the mature and secreted cytokine by the NLRP3 inflammasome, a caspase-1-activating cytosolic protein complex.30 The NLRP3 inflammasome is not only activated by numerous stimuli such as bacterial toxins and uric acid crystals but also by extracellular ATP released from stressed or damaged cells. This endogenous danger signal is sensed by the purinergic receptor P2X7, a ligand-gated ion channel.29 Our results show that the release of IL-1β of PEMØs induced by B6 and gzmAxB−/− Tc was not affected by the absence of the P2X7 receptor. This result suggests that Tc-induced IL-1β processing is not due to ATP derived from damaged target cells, and therefore, strengthening the concept that pro-inflammatory gzms such as gzmK participate in inflammasome activation.
Overall, the data show that neither gzmA nor gzmB are essential to control LCMV infection in mice. This observation, which supports previous work,23, 24 is intriguing especially when combined with results of this study indicating that ex vivo anti-LCMV Tc cells from gzmAxB−/− mice exhibit markedly reduced cytotoxicity in vitro. Similar to these Tc cells that primarily contain (Mo)gzmK, rec. (Mo)gzmK delivered by SLO also failed to mount the anticipated cytotoxic response. Thus, the anti-viral effects of in vivo anti-LCMV Tc cells does not correlate with their in vitro cytolytic activity, but rather is mediated through non-cytotoxic pathways, such as inflammasome activation or uncharacterized biological effects.36
The possibility that (Mo)gzmK induces IL-1β release which aids in LCMV clearance is further supported by the observation that mice treated with Anakinra are unable to control replication, even though the ratio of LCMV-immune Tc cells generated under these conditions, including their cytotoxic potential was similar to that of untreated infected B6 mice (Figures 4b and c). These findings differ from a recent study in which IL-1R−/− mice, challenged with influenza virus, were unable to generate virus-specific Tc.37 This discrepancy may be due to the different approaches used to reduce IL-1β in the two systems. In IL-1R−/− mice, IL-1β is expected to be unable to mediate its myriad biological effects through IL-1R including the induction of Tc. The blockade produced by Anakinra is predicted to be incomplete allowing the induction of virus-specific Tc cells, but curtailing the anti-viral activity capacity of the cytokine during the effector phase of the host response. Although (Mo)gzmK, similar to (Hu)gzmH,36 might mediate direct antiviral effects that contribute to viral clearance, together the evidence supports the existence of a (Mo)gzmK-induced pro-inflammatory pathway that controls intracellular pathogens.
In comparison with (Mo)gzmK, isolated (Hu)gzmK has been reported to be directly cytotoxic in vitro when delivered by SLO or adenoviral particles.14 However, others have been unable to demonstrate the rec. (Hu)gzmk is cytotoxic in vitro when delivered by SLO to Hela cell targets at concentrations reaching 1000 nM (personal communication, Niels Bovenschen). Using intact Tc primarily containing (Mo)gzmK, we have verified that (Mo)gzmK delivered in vitro is not cytotoxic. A similar approach will be necessary to reconcile whether (Hu)gzmK is cytotoxic.
Future studies that use gzmK-deficient mice will be necessary to verify whether LCMV infection is controlled by gzmK and/or by other granule-associated components and whether the putative anti-viral activity of gzmK is linked to the induction of pro-inflammatory cytokines in macrophage subsets or to other non-cytotoxic pathways. In contrast to LCMV, other viruses such as ectromelia depend on cytolytic gzmB.38 Thus, depending on the virus and the tissue distribution of infection, Tc cells may use individual gzms to kill infected targets and use one or more of the gzms to disrupt their life cycle by non-cytolytic means.
Materials and Methods
Mouse strains
The following strains were maintained at the Max-Planck-Institut für Immunobiologie, Freiburg (Germany): C57BL/6 (B6; wt); strains deficient for gzmA (gzmA−/−), gzmA and gzmB (gzmAxB−/−) and perf, gzmA and gzmB deficient (perfxgzmAxB−/−), were bred on the B6 background.23 Mice deficient for P2X7 (P2X7−/−) were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Animal studies were conducted in accordance with the ethics guidelines of the Federation of European Laboratory Animal Science Association.
Virus
The LCMV (strain WE), kindly provided by O. Utermöhlen (Cologne, Germany) was expanded in L929 fibroblasts, as described previously.25 The virus-specific epitope gp33 (kavynfatatc; Neosystem, Strasbourg, France) for H-2Db was used for target labeling in cytotoxicity assays.25
Disease model
Mice were infected i.p. with 105 p.f.u. of LCMV-WE according to established protocols.25, 39
IL-1Ra (Anakinra) treatment
Mice were injected 1 day before infection with 105 p.f.u. LCMV-WE with 200 μg IL-1Ra (Anakinra, Kineret Amgen, Breda, The Netherlands) in 200 μl PBS and from then on every third day with the same dose until the end of experiment.31
Virus titers
Aliquots of liver tissues were homogenized and used for determination of virus titers as described elsewhere.39
Generation of ex vivo CD8+ cells
On day 8 p.i., CD8+ cells were positively selected from the spleen using α-CD8-MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) with an autoMACS (Miltenyi Biotec) and resuspended in MEM/5% FCS before use in cytotoxic assays as described previously.25 Detection of LCMV-immune CTL was conducted by double staining with CD8 antibody (BD Pharmingen, San Diego, CA, USA; clone 53–67) and gp33-labeled pentamers (ProImmune, Oxford, UK) as described previously.32
Cell lines, cell culture and reagents
The mouse cell lines 1.3E6 (Tc cell line) and EL4.F15 (thymoma), and MEF cell lines were cultured as described previously.25, 40 rec. (Mo)gzmK was produced in Escherichia coli B834 (DE3) using the pET-21a vector and purified as described previously.41 Rabbit immune serum specific for (Mo)gzmK was generated as described for (Mo)gzmB.28
Analysis of pro-apoptotic processes
Cell death induced by ex vivo CD8-enriched Tc cells or by isolated gzms in the presence or absence of SLO, was analyzed as described previously.32 In brief, target cells were pre-treated with LCMV-immunodominant peptide gp33 for 2 h before incubation with ex vivo-derived LCMV-immune CTL at 10 : 1 E/T cell ratio for 4 h at 37°C, 7% CO2. Alternatively, target cells were incubated with the indicated concentration of rec. gzms with or without SLO for 4 h. Subsequently, different apoptotic parameters were tested in the target population (CD8−) by FACS using a FACSCalibur (BD Pharmingen) and FLOWJO (Ashland, OR, USA) software as described previously.8, 25 In short, for the analysis of cell membrane and mitochondrial membrane pertubations. PS exposure and PI uptake were analyzed by FACS using the annexin-V-FITC kit obtained from BD Pharmingen. Mitochondrial membrane potential was measured with the fluorescent probe 3,3′-dihexyloxacarbocyanine iodide (DiOC6(3); (Molecular Probes, Eugene, OR, USA) and ROS generation with 2-hydroxyethidium (Molecular Probes). Furthermore, cell populations were analyzed for cell surface markers and intracellular gzm expression as described elsewhere,40 analyzed by FACS using a FACSCalibur (BD Pharmingen) and FLOWJO software.
Survival assay
Survival of target cells after their incubation with rec. (Mo)gzmK in the presence and absence of SLO was analyzed as described previously.42 In brief, target cells were incubated with 1000 nM rec. (Mo)gzmK in the presence or absence of SLO for 4 h. Thereafter, 3H-thymidine was added and cells were incubated for 10 h at 37°C, 5% CO2, without removing rec. (Mo)gzmK and SLO. Subsequently, cells were harvested and target cell survival was quantified by 3H-thymidine incorporation as described previously.42 This assay produces similar results as clonogenic survival assays in agar plates.25
Enzymatic assays
Amidolytic activity of gzms using the following chromogenic substrates (Bachem, Weil am Rhein, Germany) was performed as described previously:43 Bz-Arg-pNA, H-D-Pro-Phe-Arg-NA, Ac-Ile-Glu-Pro-Asp-pNA and Tos-Gly-Pro-Arg-pNA.
RT-PCR
Total RNA was extracted from up to 5 × 106 ex vivo CD8+ cells, using the QIAshredder spin columns, the RNAeasy mini kit and the RNAse-free DNAse kit (all from Qiagen, Hilden, Germany) according to the manufacturer's instructions, and specific transcripts were amplified. The sense/antisense primers for gzmA, gzmC, gzmD, gzmE, gzmF, gzmG and Hprt1 are described in the studies by Martin et al.40 and Revell et al.,44 respectively. Sense/antisense primers for gzmB, gzmK and gzmM are described in the study by Pardo et al.25 Primers for perf are described in the study by Balkow et al.23
IL-1β release assay
B6 mice were injected i.p. with 2 ml 4% thioglycollate (Sigma, Steinheim, Germany) in LPS-free water, 3 days before removal of PEMØs. On day 3 p.i., mice were killed and their peritoneum was washed with 10 ml of PBS to collect PEMØs. Cell suspension was washed, and 8 × 105 cells/well were plated in a 12-well plate (500 μl volume/MEM without FCS) and incubated for 1 h at 37°C, 5% CO2. Subsequently, PEMØs were sensitized with 100 ng/ml LPS (Salmonella minnesota R595) and incubated for 2 h at 37°C, 5% CO2. Thereafter, the supernatant was discarded and PEMØs were challenged either with LCMV-immune ex vivo Tc cells or with rec. gzms (300 μl volume/MEM without FCS) in the presence or absence of SLO, for 4–6 h at 37°C, 5% CO2.
Western blot analysis
The inactive and active form of caspase-3, as well as actin and IL-1β was determined by western blotting under reducing conditions. Rabbit polyclonal anti-human caspase-3 IgG (active and inactive) was obtained from Cell Signaling Technology (Danvers, MA, USA), mouse anti-actin IgG was from MP Biomedicals (Aurora, OH, USA) and goat anti-IL-1β was obtained from R&D Systems (Minneapolis, MN, USA). Blots were then stained with horseradish peroxidase-conjugated goat anti-rabbit IgG or goat anti-rabbit IgG or goat anti-mouse IgG (all purchased from Jackson ImmunoResearch Laboratories Inc., Suffolk, UK), followed by enhanced chemiluminescence (ECL Western Blotting Analysis System; GE Healthcare, München, Germany).
Acknowledgments
This study was supported in part by the Excellence Initiative of the German Federal and State Governments (GSC-4, Spemann Graduate School) and by the Centre for Biological Signaling Studies (bioss, EXC 294), both supported by the Excellence Initiative of the German Federal and State Governments, and the Deutsche Forschungsgemeinschaft (BO-1933 and GRK1104). CF was supported through 5RO1AI04494-03. JP was supported by Grant SAF2008-02139 from the Ministerio de Ciencia e Innovación (Spain) and Grant PI076/08 by Gobierno de Aragón. We thank Anton Grubisic, Aynur Ekiciler, Thomas Stehle, Bettina Hartmann, Inga Clausen, Dominik Wieland, Jüri Habicht and Christiane Brenner for their expert technical assistance. We also thank Sucharit Bhakdi for his generous gift of streptolysin O and Marina Freudenberg and Chris Galanos for providing LPS.
Glossary
- gzm
granzyme
- Hu
human
- IL-1β
interleukin-1 beta
- IL-1Ra
IL-1 receptor antagonist (Anakinra)
- i.p.
intraperitoneal
- ko
knockout
- LCMV
lymphocytic choriomeningitis virus
- MEF
mouse embryonic fibroblast
- Mo
mouse
- NK
natural killer
- PEMØs
peritoneal macrophages
- perf
perforin
- p.i.
post infection
- rec.
recombinant
- SET
nucleosome assembly protein
- SLO
streptolysin O
- Tc
T-killer cell
- wt
wild type
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by J Tschopp
Supplementary Material
References
- Barry M, Bleackley RC. Cytotoxic T lymphocytes: all roads lead to death. Nat Rev Immunol. 2002;2:401–409. doi: 10.1038/nri819. [DOI] [PubMed] [Google Scholar]
- Grossman WJ, Revell PA, Lu ZH, Johnson H, Bredemeyer AJ, Ley TJ. The orphan granzymes of humans and mice. Curr Opin Immunol. 2003;15:544–552. doi: 10.1016/s0952-7915(03)00099-2. [DOI] [PubMed] [Google Scholar]
- Kramer M, Simon MM. Are proteinases functional molecules of T cells. Immunol Today. 1987;8:140–143. doi: 10.1016/0167-5699(87)90141-1. [DOI] [PubMed] [Google Scholar]
- Hayes MP, Berrebi GA, Henkart PA. Induction of target cell DNA release by the cytotoxic T lymphocyte granule protease granzyme A. J Exp Med. 1989;170:933–946. doi: 10.1084/jem.170.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Kraut RP, Aebersold R, Greenberg AH. A natural killer cell granule protein that induces DNA fragmentation and apoptosis. J Exp Med. 1992;175:553–566. doi: 10.1084/jem.175.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sower LE, Froelich CJ, Allegretto N, Rose PM, Hanna WD, Klimpel GR. Extracellular activities of human granzyme A. Monocyte activation by granzyme A versus alpha-thrombin. J Immunol. 1996;156:2585–2590. [PubMed] [Google Scholar]
- Irmler M, Hertig S, MacDonald HR, Sadoul R, Becherer JD, Proudfoot A, et al. Granzyme A is an interleukin 1 beta-converting enzyme. J Exp Med. 1995;181:1917–1922. doi: 10.1084/jem.181.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metkar SS, Menaa C, Pardo J, Wang B, Wallich R, Freudenberg M, et al. Human and mouse granzyme A induce a proinflammatory cytokine response. Immunity. 2008;29:720–733. doi: 10.1016/j.immuni.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Beresford PJ, Xia Z, Greenberg AH, Lieberman J. Granzyme A loading induces rapid cytolysis and a novel form of DNA damage independently of caspase activation. Immunity. 1999;10:585–594. doi: 10.1016/s1074-7613(00)80058-8. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- Anthony DA, Andrews DM, Chow M, Watt SV, House C, Akira S, et al. A role for granzyme M in TLR4-driven inflammation and endotoxicosis. J Immunol. 2010;185:1794–1803. doi: 10.4049/jimmunol.1000430. [DOI] [PubMed] [Google Scholar]
- Bovenschen N, Quadir R, van den Berg AL, Brenkman AB, Vandenberghe I, Devreese B, et al. Granzyme K displays highly restricted substrate specificity that only partially overlaps with granzyme A. J Biol Chem. 2009;284:3504–3512. doi: 10.1074/jbc.M806716200. [DOI] [PubMed] [Google Scholar]
- Shi L, Kam CM, Powers JC, Aebersold R, Greenberg AH. Purification of three cytotoxic lymphocyte granule serine proteases that induce apoptosis through distinct substrate and target cell interactions. J Exp Med. 1992;176:1521–1529. doi: 10.1084/jem.176.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Zhang H, Guo Y, Fan Z. Granzyme K directly processes Bid to release cytochrome c and endonuclease G leading to mitochondria-dependent cell death. J Biol Chem. 2007;282:12104–12111. doi: 10.1074/jbc.M611006200. [DOI] [PubMed] [Google Scholar]
- Zhao T, Zhang H, Guo Y, Zhang Q, Hua G, Lu H, et al. Granzyme K cleaves the nucleosome assembly protein SET to induce single-stranded DNA nicks of target cells. Cell Death Differ. 2007;14:489–499. doi: 10.1038/sj.cdd.4402040. [DOI] [PubMed] [Google Scholar]
- Guo Y, Chen J, Zhao T, Fan Z. Granzyme K degrades the redox/DNA repair enzyme Ape1 to trigger oxidative stress of target cells leading to cytotoxicity. Mol Immunol. 2008;45:2225–2235. doi: 10.1016/j.molimm.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Hua G, Wang S, Zhong C, Xue P, Fan Z. Ignition of p53 bomb sensitizes tumor cells to granzyme K-mediated cytolysis. J Immunol. 2009;182:2152–2159. doi: 10.4049/jimmunol.0802307. [DOI] [PubMed] [Google Scholar]
- Guo Y, Chen J, Shi L, Fan Z. Valosin-containing protein cleavage by granzyme K accelerates an endoplasmic reticulum stress leading to caspase-independent cytotoxicity of target tumor cells. J Immunol. 2010;185:5348–5359. doi: 10.4049/jimmunol.0903792. [DOI] [PubMed] [Google Scholar]
- Bade B, Lohrmann J, ten Brinke A, Wolbink AM, Wolbink GJ, ten Berge IJ, et al. Detection of soluble human granzyme K in vitro and in vivo. Eur J Immunol. 2005;35:2940–2948. doi: 10.1002/eji.200526249. [DOI] [PubMed] [Google Scholar]
- Rucevic M, Fast LD, Jay GD, Trespalcios FM, Sucov A, Siryaporn E, et al. Altered levels and molecular forms of granzyme K in plasma from septic patients. Shock. 2007;27:488–493. doi: 10.1097/01.shk.0000246905.24895.e5. [DOI] [PubMed] [Google Scholar]
- Bratke K, Klug A, Julius P, Kuepper M, Lommatzsch M, Sparmann G, et al. Granzyme K: a novel mediator in acute airway inflammation. Thorax. 2008;63:1006–1011. doi: 10.1136/thx.2007.091215. [DOI] [PubMed] [Google Scholar]
- Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Balkow S, Kersten A, Tran TT, Stehle T, Grosse P, Museteanu C, et al. Concerted action of the FasL/Fas and perforin/granzyme A and B pathways is mandatory for the development of early viral hepatitis but not for recovery from viral infection. J Virol. 2001;75:8781–8791. doi: 10.1128/JVI.75.18.8781-8791.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac AJ, Dye JM, Quinn DG. Control of lymphocytic choriomeningitis virus infection in granzyme B deficient mice. Virology. 2003;305:1–9. doi: 10.1006/viro.2002.1754. [DOI] [PubMed] [Google Scholar]
- Pardo J, Wallich R, Martin P, Urban C, Rongvaux A, Flavell RA, et al. Granzyme B-induced cell death exerted by ex vivo CTL: discriminating requirements for cell death and some of its signs. Cell Death Differ. 2008;15:567–579. doi: 10.1038/sj.cdd.4402289. [DOI] [PubMed] [Google Scholar]
- Jenkins MR, Kedzierska K, Doherty PC, Turner SJ. Heterogeneity of effector phenotype for acute phase and memory influenza A virus-specific CTL. J Immunol. 2007;179:64–70. doi: 10.4049/jimmunol.179.1.64. [DOI] [PubMed] [Google Scholar]
- Jenkins MR, Trapani JA, Doherty PC, Turner SJ. Granzyme K expressing cytotoxic T lymphocytes protects against influenza virus in granzyme AB(/) mice. Viral Immunol. 2008;21:341–346. doi: 10.1089/vim.2008.0036. [DOI] [PubMed] [Google Scholar]
- Pardo J, Wallich R, Ebnet K, Iden S, Zentgraf H, Martin P, et al. Granzyme B is expressed in mouse mast cells in vivo and in vitro and causes delayed cell death independent of perforin. Cell Death Differ. 2007;14:1768–1779. doi: 10.1038/sj.cdd.4402183. [DOI] [PubMed] [Google Scholar]
- Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol. 2009;71:333–359. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by Anakinra in acute gout. Arthritis Res Ther. 2007;9:R28. doi: 10.1186/ar2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo J, Bosque A, Brehm R, Wallich R, Naval J, Mullbacher A, et al. Apoptotic pathways are selectively activated by granzyme A and/or granzyme B in CTL-mediated target cell lysis. J Cell Biol. 2004;167:457–468. doi: 10.1083/jcb.200406115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo J, Aguilo JI, Anel A, Martin P, Joeckel L, Borner C, et al. The biology of cytotoxic cell granule exocytosis pathway: granzymes have evolved to induce cell death and inflammation. Microbes Infect. 2009;11:452–459. doi: 10.1016/j.micinf.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Chowdhury D, Lieberman J. Death by a thousand cuts: granzyme pathways of programmed cell death. Annu Rev Immunol. 2008;26:389–420. doi: 10.1146/annurev.immunol.26.021607.090404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froelich CJ, Pardo J, Simon MM. Granule-associated serine proteases: granzymes might not just be killer proteases. Trends Immunol. 2009;30:117–123. doi: 10.1016/j.it.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Andrade F, Fellows E, Jenne DE, Rosen A, Young CS. Granzyme H destroys the function of critical adenoviral proteins required for viral DNA replication and granzyme B inhibition. EMBO J. 2007;26:2148–2157. doi: 10.1038/sj.emboj.7601650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullbacher A, Waring P, Tha Hla R, Tran T, Chin S, Stehle T, et al. Granzymes are the essential downstream effector molecules for the control of primary virus infections by cytolytic leukocytes. Proc Natl Acad Sci USA. 1999;96:13950–13955. doi: 10.1073/pnas.96.24.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode M, Balkow S, Sobek V, Brehm R, Martin P, Kersten A, et al. Perforin and Fas act together in the induction of apoptosis, and both are critical in the clearance of lymphocytic choriomeningitis virus infection. J Virol. 2004;78:12395–12405. doi: 10.1128/JVI.78.22.12395-12405.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Wallich R, Pardo J, Mullbacher A, Munder M, Modolell M, et al. Quiescent and activated mouse granulocytes do not express granzyme A and B or perforin: similarities or differences with human polymorphonuclear leukocytes. Blood. 2005;106:2871–2878. doi: 10.1182/blood-2005-04-1522. [DOI] [PubMed] [Google Scholar]
- Wilharm E, Parry MA, Friebel R, Tschesche H, Matschiner G, Sommerhoff CP, et al. Generation of catalytically active granzyme K from Escherichia coli inclusion bodies and identification of efficient granzyme K inhibitors in human plasma. J Biol Chem. 1999;274:27331–27337. doi: 10.1074/jbc.274.38.27331. [DOI] [PubMed] [Google Scholar]
- Pardo J, Balkow S, Anel A, Simon MM. The differential contribution of granzyme A and granzyme B in cytotoxic T lymphocyte-mediated apoptosis is determined by the quality of target cells. Eur J Immunol. 2002;32:1980–1985. doi: 10.1002/1521-4141(200207)32:7<1980::AID-IMMU1980>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Simon MM, Hoschutzky H, Fruth U, Simon HG, Kramer MD. Purification and characterization of a T cell specific serine proteinase (TSP-1) from cloned cytolytic T lymphocytes. EMBO J. 1986;5:3267–3274. doi: 10.1002/j.1460-2075.1986.tb04638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell PA, Grossman WJ, Thomas DA, Cao X, Behl R, Ratner JA, et al. Granzyme B and the downstream granzymes C and/or F are important for cytotoxic lymphocyte functions. J Immunol. 2005;174:2124–2131. doi: 10.4049/jimmunol.174.4.2124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.