Abstract
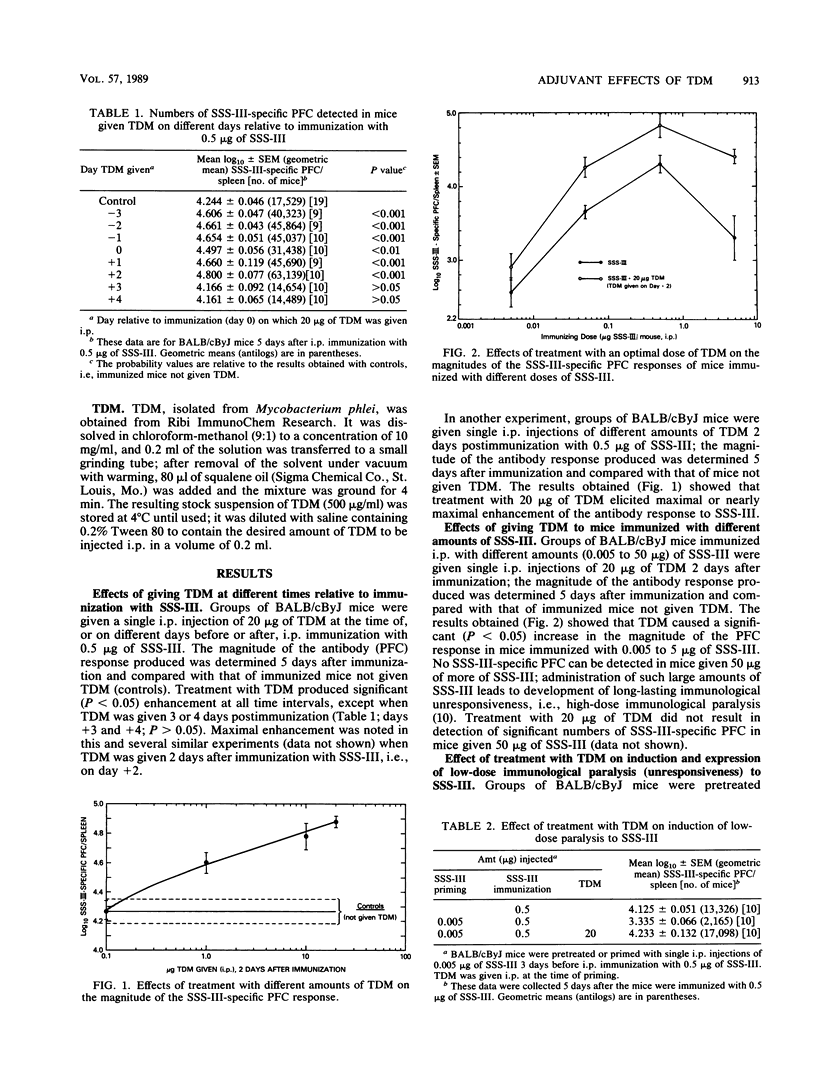
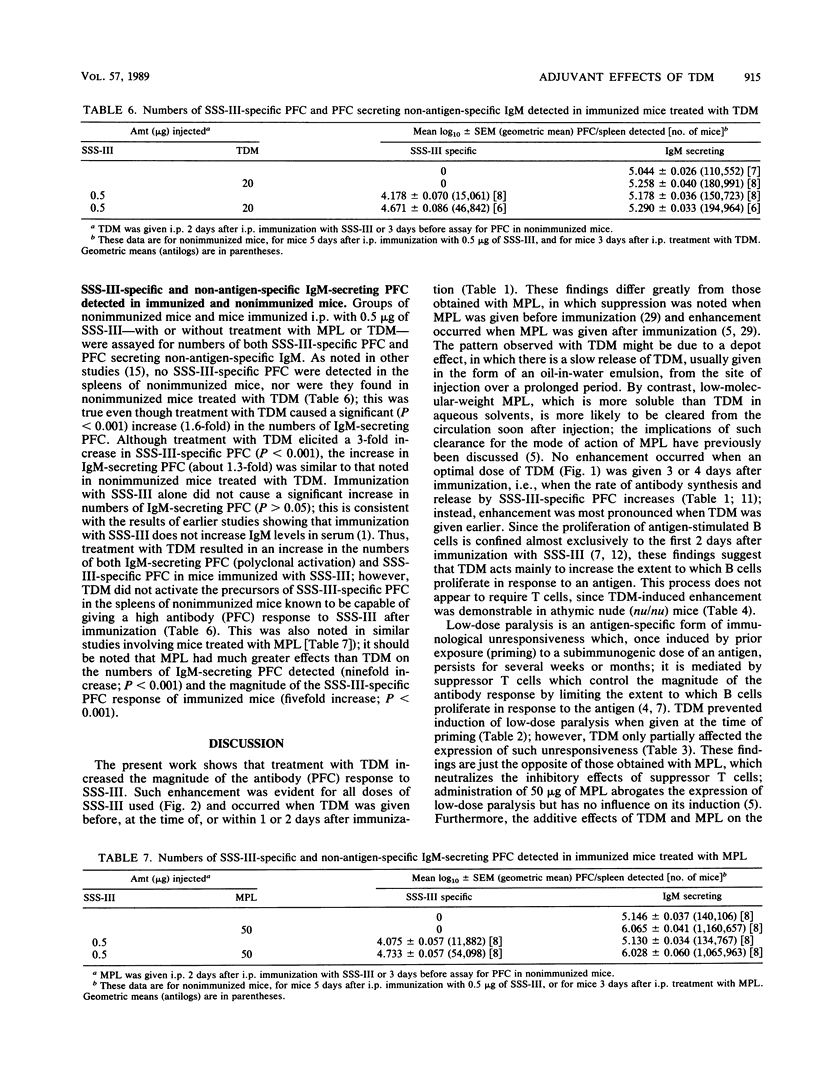
Treatment with trehalose dimycolate (TDM) increases the magnitude of the immunoglobulin M (IgM) antibody response of mice to type III pneumococcal polysaccharide (SSS-III). Such enhancement is demonstrable over a wide range of immunizing doses and does not require thymus-derived (T) cells to be elicited. Although young adult mice immunized with SSS-III do not usually make anti-SSS-III antibodies of the IgG1 and IgG3 classes, antibodies of one or both isotypes were produced after immunization and treatment with TDM and/or monophosphoryl lipid A (MPL); the additive nature of the effect produced by both TDM and MPL suggests that the two immunomodulators act by different mechanisms. TDM and MPL have different effects on the induction and expression of low-dose immunological paralysis, a form of unresponsiveness known to be mediated by suppressor T cells. The relevance of these findings to the modes of action of TDM and MPL is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsbaugh D. F., Hansen C. T., Prescott B., Stashak P. W., Asofsky R., Baker P. J. Genetic control of the antibody response to type 3 pneumococcal polysaccharide in mice. II. Relationship between IgM immunoglobulin levels and the ability to give an IgM antibody response. J Exp Med. 1974 Jun 1;139(6):1499–1512. doi: 10.1084/jem.139.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma M., Suzutani T., Sazaki K., Yoshida I., Sakuma T., Yoshida T. Role of interferon in the augmented resistance of trehalose-6,6'-dimycolate-treated mice to influenza virus infection. J Gen Virol. 1987 Mar;68(Pt 3):835–843. doi: 10.1099/0022-1317-68-3-835. [DOI] [PubMed] [Google Scholar]
- Baker P. H., Stashak P. W. Quantitative and qualitative studies on the primary antibody response to pneumococcal polysaccharides at ehe cellular level. J Immunol. 1969 Dec;103(6):1342–1348. [PubMed] [Google Scholar]
- Baker P. J., Amsbaugh D. F., Stashak P. W., Caldes G., Prescott B. Direct evidence for the involvement of T suppressor cells in the expression of low-dose paralysis to type III pneumococcal polysaccharide. J Immunol. 1982 Mar;128(3):1059–1062. [PubMed] [Google Scholar]
- Baker P. J., Amsbaugh D. F., Stashak P. W., Caldes G., Prescott B. Regulation of the antibody response to pneumococcal polysaccharide by thymus-derived cells. Rev Infect Dis. 1981 Mar-Apr;3(2):332–341. doi: 10.1093/clinids/3.2.332. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Hiernaux J. R., Fauntleroy M. B., Prescott B., Cantrell J. L., Rudbach J. A. Inactivation of suppressor T-cell activity by nontoxic monophosphoryl lipid A. Infect Immun. 1988 May;56(5):1076–1083. doi: 10.1128/iai.56.5.1076-1083.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Hiernaux J. R., Fauntleroy M. B., Stashak P. W., Prescott B., Cantrell J. L., Rudbach J. A. Ability of monophosphoryl lipid A to augment the antibody response of young mice. Infect Immun. 1988 Dec;56(12):3064–3066. doi: 10.1128/iai.56.12.3064-3066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Prescott B., Stashak P. W., Amsbaugh D. F. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. 3. Studies on the average avidity of the antibody produced by specific plaque-forming cells. J Immunol. 1971 Sep;107(3):719–724. [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. I. Dose-response studies and the effect of prior immunization on the magnitude of the antibody response. Immunology. 1971 Apr;20(4):469–480. [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. II. Studies on the relative rate of antibody synthesis and release by antibody-producing cells. Immunology. 1971 Apr;20(4):481–492. [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Regulation of the antibody response to type 3 pneumococcal polysaccharide. II. Mode of action of thymic-derived suppressor cells. J Immunol. 1974 Jan;112(1):404–409. [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Prescott B. Use of erythrocytes sensitized with purified pneumococcal polysaccharides for the assay of antibody and antibody-producing cells. Appl Microbiol. 1969 Mar;17(3):422–426. doi: 10.1128/am.17.3.422-426.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekierkunst A., Yarkoni E., Flechner I., Morecki S., Vilkas E., Lederer E. Immune response to sheep red blood cells in mice pretreated with mycobacterial fractions. Infect Immun. 1971 Sep;4(3):256–263. doi: 10.1128/iai.4.3.256-263.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauntleroy M. B., Rudbach J. A., Prescott B., Baker P. J. Increased activation of antigen-primed or memory B cells by bacterial lipopolysaccharide. Cell Immunol. 1987 Jul;107(2):263–272. doi: 10.1016/0008-8749(87)90235-8. [DOI] [PubMed] [Google Scholar]
- Goren M. B. Immunoreactive substances of mycobacteria. Am Rev Respir Dis. 1982 Mar;125(3 Pt 2):50–69. doi: 10.1164/arrd.1982.125.3P2.50. [DOI] [PubMed] [Google Scholar]
- Gottlieb C. F. Application of transformations to normalize the distribution of plaque-forming cells. J Immunol. 1974 Jul;113(1):51–57. [PubMed] [Google Scholar]
- Grand-Perret T., Lepoivre M., Petit J. F., Lemaire G. Macrophage activation by trehalose dimycolate requirement for an expression signal in vitro for antitumoral activity; biochemical markers distinguishing primed and fully activated macrophages. Eur J Immunol. 1986 Apr;16(4):332–338. doi: 10.1002/eji.1830160403. [DOI] [PubMed] [Google Scholar]
- Grand-Perret T., Petit J. F., Lemaire G. Modifications induced by activation to tumor cytotoxicity in the protein secretory activity of macrophages. J Leukoc Biol. 1986 Jul;40(1):1–19. doi: 10.1002/jlb.40.1.1. [DOI] [PubMed] [Google Scholar]
- Jones J. M., Amsbaugh D. F., Stashak P. W., Prescott B., Baker P. J., Alling D. W. Kinetics of the antibody response to type III pneumococcal polysaccharide. I. Evidence that suppressor cells function by inhibiting the recruitment and proliferation of antibody-producing cells. J Immunol. 1976 Mar;116(3):647–656. [PubMed] [Google Scholar]
- Kierszenbaum F., Walz D. R. Proliferative responses of central and peripheral rat lymphocytes elicited by cord factor (trehalose 6,6'-dimycolate). Infect Immun. 1981 Jul;33(1):115–119. doi: 10.1128/iai.33.1.115-119.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire G., Tenu J. P., Petit J. F., Lederer E. Natural and synthetic trehalose diesters as immunomodulators. Med Res Rev. 1986 Jul-Sep;6(3):243–274. doi: 10.1002/med.2610060302. [DOI] [PubMed] [Google Scholar]
- Pasanen V. J., Asofsky R., Baker P. J. Synthesis of two classes of antibody, gammaM and gammaG or gammaM and gammaA, by identical cells. Amplification of the antibody response to pneumococcal polysaccharide type III. J Exp Med. 1979 May 1;149(5):1227–1237. doi: 10.1084/jem.149.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribi E. Beneficial modification of the endotoxin molecule. J Biol Response Mod. 1984;3(1):1–9. [PubMed] [Google Scholar]
- Saito R., Tanaka A., Sugiyama K., Azuma I., Yamamura Y. Adjuvant effect of cord factor, a mycobacterial lipid. Infect Immun. 1976 Mar;13(3):776–781. doi: 10.1128/iai.13.3.776-781.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Haq A., Ahmad S., Lederer E. Vaccination of rabbits against Entamoeba histolytica with aqueous suspensions of trehalose-dimycolate as the adjuvant. Infect Immun. 1985 Jun;48(3):634–637. doi: 10.1128/iai.48.3.634-637.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomai M. A., Solem L. E., Johnson A. G., Ribi E. The adjuvant properties of a nontoxic monophosphoryl lipid A in hyporesponsive and aging mice. J Biol Response Mod. 1987 Apr;6(2):99–107. [PubMed] [Google Scholar]
- Uhr J. W., Möller G. Regulatory effect of antibody on the immune response. Adv Immunol. 1968;8:81–127. doi: 10.1016/s0065-2776(08)60465-4. [DOI] [PubMed] [Google Scholar]
- Vieira P., Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol. 1988 Feb;18(2):313–316. doi: 10.1002/eji.1830180221. [DOI] [PubMed] [Google Scholar]



