Abstract
Background
This study compared the pharmacokinetics, postprandial glycemic response, and pain associated with intradermal lispro insulin delivery using a microneedle with that of a conventional catheter.
Subjects and Methods
Five subjects with type 1 diabetes were administered a bolus infusion of lispro insulin using a 9-mm-long subcutaneous catheter (control treatment) and a 0.9-mm-long microneedle (study treatment), followed by consumption of a standardized meal. Blood samples were periodically assayed for plasma glucose and free insulin levels.
Results
Intradermal insulin infusion using microneedles reached peak insulin concentrations in approximately half the time and led to greater reduction in plasma glucose levels than subcutaneous catheters. Microneedles were also significantly less painful than the catheters.
Conclusion
The rapid pharmacokinetics and minimally invasive nature of intradermal insulin infusion using microneedles provide significant potential for improved diabetes management.
Introduction
Recombinant human insulin analog preparations are currently the most effective therapy available for patients with diabetes who require insulin.1 Insulin is conventionally given in both a bolus and basal manner. Insulin is typically given subcutaneously either by injection with a hypodermic needle and syringe or by infusion through a subcutaneous catheter connected to a patient-operated pump. For patients with diabetes receiving insulin via injection, a bolus dose of rapid-acting insulin is delivered at mealtime, when a rapid increase in serum glucose ensues. In the fasting state, however, a small amount of insulin is required to maintain a state of euglycemia. This small amount of insulin, or basal dose, is a slower-releasing insulin and is delivered once or twice daily. For patients with diabetes using an insulin pump, only a rapid-acting insulin analog is utilized. Similar to those on subcutaneous insulin injections, those on pumps deliver bolus infusions of insulin prior to meals. For their basal dose, however, patients with diabetes on insulin pumps receive continuous insulin at low rates over the entire day.
Human insulin exists in a hexameric form in which six insulin molecules self-assemble into a metastable structure that dissociates slowly into individual and physiologically active insulin molecules.2,3 However, this dissociation is relatively slow, such that the subcutaneous injection of regular human insulin does not reach peak levels until 2–3 h after injection. Therefore, regular insulin administered shortly before a meal does not reach its peak level until well after blood glucose (BG) levels have risen.4
Novel forms of analog insulin preparations have had small changes made to the primary structure of the insulin molecule. For the rapid-acting insulin preparations, these changes prevent insulin molecule aggregation and allow for maintenance of insulin in a monomeric state.5 This accelerates insulin absorption into the bloodstream and enables peak insulin concentration to be achieved 45–60 min after subcutaneous injection.6 Use of analog rapid-acting insulin preparations has been shown to significantly improve clinical outcomes.7,8
While increased insulin pharmacokinetics (PK) have typically been achieved by altering the insulin molecule, it may also be possible to expedite insulin PK by changing the route of administration from the conventional subcutaneous route.9 The intradermal route could enable more rapid insulin absorption into the bloodstream because of skin's increased vascularity compared with the subcutaneous space.10,11 Use of the intradermal route, however, has historically been unattractive because conventional intradermal injection is difficult, unreliable, and unlikely to be performed by diabetes patients themselves.
To address this limitation, we have developed micron-dimension needles called microneedles that simply and reliably administer insulin to the dermis in a minimally invasive manner.12 Unlike the conventional intradermal Mantoux injection method, in which hypodermic needles are precisely inserted into the skin at a shallow angle almost parallel to the skin surface, microneedles are short enough that they can be inserted perpendicular to the skin surface and reliably remain within the skin.
Microneedles may address another limitation of current insulin therapy by improving patient compliance. Many diabetes patients find insulin injection unpleasant, inconvenient, and painful, and some experience fear and apprehension associated with needle use.13–16 Microneedles may reduce this pain, fear, and apprehension because their small size not only causes less physical pain, but also appears less threatening than a hypodermic needle or catheter.17–20
In this report, we present the first peer-reviewed study in human subjects to test the hypothesis that intradermal insulin infusion using microneedles leads to more rapid PK, improved postprandial glycemic control, and less pain than subcutaneous catheter-based infusion in subjects with type 1 diabetes.
Subjects and Methods
Microneedle device
Hollow microneedles (Fig. 1) were fabricated by pulling fire-polished type I borosilicate glass pipettes (BF150-86-15, Sutter Instrument, Novato, CA) with a micropipette puller (model P-97, Sutter Instrument). Programmable puller parameters of pull = 40, velocity = 10, heat = 580, time = 150, and pressure = 500 allowed reproducible production of microneedles with tip properties strong enough to insert into skin without breakage. The pulled needles were then beveled at a 30° angle using a beveler (BV-10 beveler, 104D fine bevel plate, Sutter Instrument) producing hollow microneedles with an oval-shaped opening. Because of this oval shape, the effective radius of the needle opening was determined by averaging the lengths of the long and short axes of the needle tip opening. The effective tip opening radii of the microneedles were between 60 and 80 μm. The microneedles were then cleaned in an ultrasonic deionized water bath (model SW-34, Sonicwise Ultrasonics, San Diego, CA) for 2 min by bubbling air through the needles dipped in water via an air-filled syringe (10 mL, Becton Dickinson, Franklin Lakes, NJ) and tubing (2C5685, Baxter, Deerfield, IL) connected to the non-tip needle end. The needles were placed in an autoclavable micropipette storage bell jar (World Precision Instruments, Sarasota, FL) and dried for 2 h in an oven (VC-300, Grieve Corp., Round Lake, IL) at 180°C followed by steam sterilization in an autoclave (Scientific Series 3021-S, AMSCO, Erie, PA).
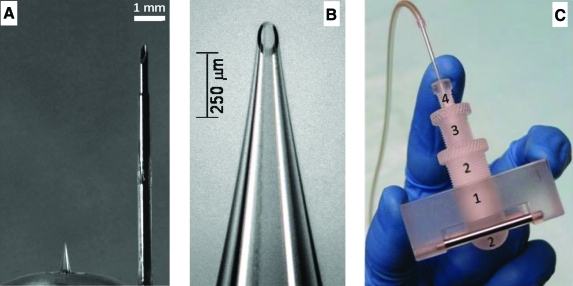
FIG. 1.
Microneedle device used for intradermal insulin administration. (A) A 900-μm-long hollow microneedle (left) next to a 9-mm insulin infusion set catheter (right). (B) A magnified view of the microneedle showing the beveled tip opening. (C) The microneedle holder and insertion device, consisting of the (1) device base structure, (2) microneedle holder, (3) microneedle insertion depth controller, and (4) set screw. Color images available online at www.liebertonline.com/dia.
To control the insertion of microneedles into the skin, a custom rotary device was fabricated by modifying previously used designs.12 As seen in Figure 1C, the device consisted of four parts positioned concentrically within each other so as to allow the glass microneedle to pass through. The rectangular block (piece 1) allowed the user to hold and stabilize the device on the skin's surface. The ball-shaped end of piece 2 helped minimize skin deflection during microneedle insertion. Only the microneedle length protruding beyond the convex surface was inserted into skin. Piece 3 was threaded into piece 2, and these parts together served as the depth-controlling structures. The threads were calibrated such that each 360° turn of piece 3 moved the microneedle tip 800 μm in its axial direction. Graded markings on pieces 2 and 3 allowed the microneedle to be precisely “drilled” into the skin at the desired depth. During this process, microneedle length could be controlled with ± 10 μm accuracy, although insertion depth into the skin was probably an order of magnitude less accurate because of the variable deformation of the skin during microneedle insertion. The fourth and final piece served as a set-screw that helped keep the needle firmly positioned within the device. A rubber gasket (pipette seal PS-15, Warner Instruments, Hamden, CT) was placed between pieces 1 and 2 to ensure the needle was secured tightly within the device. The non-tip end of the needle extended beyond piece 1 and was connected to tubing. The microneedle was inserted into skin at a 90° angle by placing the convex end of the device on the skin followed by carefully inserting the microneedle to the desired depth into the skin.
Insulin administration study design
This study was an open-label, within-subject, controlled design. Five subjects with type 1 diabetes (Table 1) who were managed with an insulin pump and were in good glycemic control were recruited to participate in the study. In order to be included, subjects were required to have type 1 diabetes for at least 2 years, be using a conventional Food and Drug Administration–approved insulin pump with lispro insulin for the past year, have mean hemoglobin A1c levels ≤8% for the past year, and a body mass index within the 85th percentile for their age. Subjects were excluded if they had type 2 diabetes, had acanthosis nigricans, had a clinically significant major organ system disease, were on glucocorticoid therapy, had an insulin requirement of ≥150 U/day, had an illness on the day of the study, or were pregnant or breastfeeding. Subjects refrained from intense physical activity for at least 2 days prior to the study visits. All subjects provided informed consent (or written assent for subjects under age 18 together with parental consent) prior to participation. The study was approved by the Emory University (Atlanta, GA) Institutional Review Board and was carried out in accordance with the Declaration of Helsinki protocol at Emory Children's Center Diabetes Clinic.
Table 1.
Demographics of Study Subjects
| Parameter | Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 |
|---|---|---|---|---|---|
| Age (years) | 43 | 38 | 11 | 19 | 18 |
| Race | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian |
| Gender | Female | Male | Male | Male | Male |
| Mean HbA1c (%)a | 6.5 | 6.2 | 6.9 | 6.3 | 6.9 |
| Weight (kg) | 63.5 | 78.0 | 45.2 | 80.8 | 72.6 |
| BMI (kg/m2) | 23.3 | 25.5 | 19.7 | 26.6 | 21.8 |
| Time since diagnosis (years) | 30.0 | 28.5 | 5.5 | 10.75 | 14.5 |
| Duration of pump use (years) | 12.2 | 7.5 | 3.9 | 7.4 | 5.0 |
| Length of pump catheter (mm) | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 |
| Mean insulin per day (units) | 40.0 | 45.0 | 36.0 | 80.0 | 52.0 |
| ICR (units/g) | 1:12.5 | 1:7.5 | 1:5 | 1:5 | 1:7.5 |
Mean hemoglobin A1c (HbA1c) over the past year.
BMI, body mass index; ICR, insulin-to-carbohydrate ratio.
The study consisted of two visits, the first being the control visit, in which a bolus infusion of U-100 lispro insulin (Humalog®, Eli Lilly, Indianapolis, IN) was administered abdominally using a 9-mm subcutaneous catheter (Paradigm® Quick-set®, Medtronic MiniMed, Northridge, CA) and an insulin pump (Paradigm, Medtronic MiniMed). The insulin dose was based on the subject's glucose level and the amount of insulin required for a 75-g carbohydrate meal based on the subject's own insulin-to-carbohydrate-ratio. During the second visit, the same insulin dose was administered using diluted U-50 lispro insulin and a 900-μm-long hollow borosilicate glass microneedle (Fig. 1) connected to a syringe pump (model NE-1000, New Era Systems, Farmingdale, NY) set to a flow rate of 1 mL/min. Although each subject received the same insulin dose on each of his or her visits, the injected volume was twice as big during the microneedle-based infusion because of the use of diluted insulin solution. The infusion was also slower during the microneedle visit, but was completed within 1 min in all cases.
Prior to each visit, subjects underwent overnight fasting and were required to turn off their insulin pumps 45 min before starting the study. At the start of the study, blood was sampled from an intravenous catheter placed in the subject's anetcubital fossa. For both treatment methods, immediately after the bolus infusion of insulin, subjects consumed a standardized mixed-meal (75 g of carbohydrates, 12 g of protein, and 14 g of fat) in less than 10 min. Capillary glucose (FreeStyle Lite™, Abbott Laboratories, Abbott Park, IL) and venous blood sampling were performed periodically over a period of 3.5 h or until the subject became hypoglycemic (capillary glucose <70 mg/dL) or hyperglycemic (capillary glucose >300 mg/dL). Collected samples were assayed for plasma glucose and plasma free insulin levels (Esoterix, Calabasas Hills, CA). Subjects were asked to rate the pain associated with microneedle/catheter insertion and insulin infusion using a visual analog pain scale (VAS) ranging from 0 mm (no pain) to 100 mm (worst possible pain). Statistical analyses to compare pharmacokinetic parameters and pain scores were performed using two-tailed, paired Student's t tests with P < 0.05 considered significant.
Results
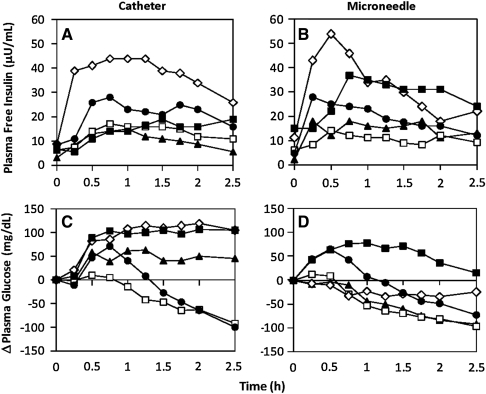
We first tested the hypothesis that microneedles lead to faster PK and improved postprandial glycemic control (Fig. 2). The study population of five subjects consisted of two adults, two adolescents, and one child (Table 1). Figure 2A shows the plasma free insulin levels in these subjects over time after insulin administration by subcutaneous catheter infusion. Insulin levels initially rose and then either were maintained at a relatively high level or declined somewhat over the 2.5-h measurement period. In Figure 2B, insulin levels are shown after intradermal microneedle-based administration. In this case, insulin levels rose faster and generally declined faster.
FIG. 2.
Pharmacokinetic and pharmacodynamic response of five diabetes subjects administered insulin: subject 1 (solid triangle; 43 years old, administered 6 units), subject 2 (open square; 38 years old, administered 10 units), subject 3 (open diamond; 11 years old, administered 15 units of lispro insulin), subject 4 (solid square; 19 years old, administered 15 units), and subject 5 (solid circle; 18 years old, administered 10 units). Plasma free insulin levels are shown for (A) subcutaneous catheter-based and (B) intradermal microneedle-based insulin infusion for the five study subjects over a 2.5-h interval after insulin administration. Change (Δ) in plasma glucose levels after (C) cutaneous catheter-based and (D) intradermal microneedle-based insulin infusion is also shown for the five subjects. Although the study was carried out over a 3.5-h interval, the data were analyzed up to 2.5 h because some trials had to be stopped at this time point because of hypoglycemia.
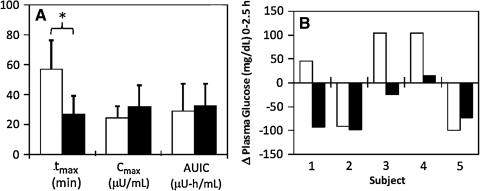
These data are summarized in Figure 3A. Subcutaneous catheter PK achieved peak insulin concentration (Cmax-catheter = 24.6 ± 7.5 μU/mL) within approximately 1 h (tmax-catheter = 57 ± 20 min), in agreement with the literature.6 In contrast, intradermal microneedle infusion of the same insulin doses achieved a 31% higher peak insulin concentration (Cmax-microneedle = 32.2 ± 14.0 μU/mL) in less than half the time (tmax-microneedle = 27 ± 13 min). In this small sample size, the difference in Cmax was not significant (Student's t test, P > 0.05), but the difference in tmax was significant (Student's t test, P = 0.01). The area under the insulin curve (AUIC) was not significantly different between the microneedle (AUICmicroneedle = 32.6 ± 14.8 μU-h/mL) and catheter (AUICcatheter = 29.3 ± 18.2 μU-h/mL) (Student's t test, P > 0.05). These data demonstrate that although both treatments led to similar relative insulin bioavailability, intradermal microneedle-based infusion led to much faster absorption.
FIG. 3.
Pharmacokinetic and pharmacodynamic parameters of the 9-mm catheter (open columns) and 900-μm-long microneedle (solid columns). (A) Pharmacokinetic parameters time to peak insulin concentration (tmax), peak insulin concentration (Cmax), and area under the insulin curve (AUIC) for subcutaneous catheter and intradermal microneedle treatments. The average tmax value for the intradermal microneedle was significantly smaller than that for the subcutaneous catheter control, indicating rapid absorption of insulin. The AUICs were similar, indicating comparable relative bioavailability. *P < 0.05. (B) Comparison of the net change in plasma glucose levels between the two treatment methods for each of the five subjects 2.5 h after insulin administration. Overall, intradermal microneedle injection was more effective than subcutaneous catheter infusion in reducing plasma glucose levels either to or below baseline glucose levels.
The pharmacodynamic response is shown after subcutaneous catheter infusion in Figure 2C and after intradermal microneedle injection in Figure 2D. As summarized in Figure 3B, three out of five subcutaneous catheter treatments did not lead to BG reduction over the entire study period, whereas all intradermal microneedle treatments led to reduction either at or below initial glucose levels. Average changes in BG levels for microneedle and control treatments over the 2.5-h period were ΔBGmicroneedle = − 55 ± 49 mg/dL and ΔBGcatheter =+ 13 ± 102 mg/dL. Thus, although subjects were administered the same insulin dose and started at similar baseline glucose levels, intradermal microneedles were more effective than subcutaneous catheters in reducing postprandial glucose levels. Overall, intradermal microneedle infusion enabled faster onset and more rapid achievement of euglycemia.
In addition to improved PK and pharmacodynamics, we also hypothesized that microneedles can improve patient compliance by being less painful than catheters. To test this hypothesis, we asked the subjects to rate the pain associated with insertion of the microneedle/catheter into the skin as well as the pain associated with injecting the insulin. Insertion of microneedles (VASmicroneedle-insert = 8 ± 9 mm) was reported to be significantly less painful than catheter insertion (VAScatheter-insert = 40 ± 13 mm) (Student's t test, P = 0.02). There was no significant difference in pain scores for infusion using microneedles versus catheters (VASmicroneedle-infuse = 28 ± 15 mm and VAScatheter-infuse = 17 ± 16 mm; Student's t test, P > 0.05). All subjects considered microneedles to be less painful than catheters.
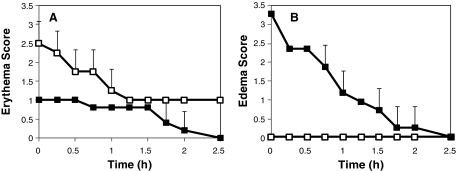
To further characterize the local skin response to intradermal microneedle injection relative to subcutaneous catheter infusion, we measured skin erythema and edema at the site of administration (Fig. 4). As shown in Figure 4A, moderate erythema was observed in the skin into which a catheter was introduced, which subsided to slight erythema after 2.5 h. In contrast, skin punctured by microneedles had only slight erythema initially, which completely disappeared within 2.5 h. Skin erythema after catheter treatment was significantly greater than after microneedle treatment (analysis of variance, P = 0.002). This is consistent with the minimally invasive nature of microneedles and may enable improved patient acceptance.
FIG. 4.
Local skin reaction at the site of insulin administration. (A) Erythema and (B) edema scores for subcutaneous 9-mm catheter (open symbols) and intradermal 900-μm-long microneedle (solid symbols) treatment sites using the Dermal Draize scale. Intradermal microneedle injection caused significantly less erythema compared with subcutaneous catheter infusion. The characteristic intradermal bleb of insulin solution formed after microneedle injection caused an elevated edema score that subsided over the course of approximately 2 h. Data are mean ± SD values (n = 5).
Figure 4B shows that subcutaneous catheter infusion resulted in no skin edema. In contrast, intradermal microneedle injection caused moderate edema, which was significantly greater than the catheter control (analysis of variance, P < 0.0001). However, this edema did not appear to be associated with an inflammatory response. Instead, it had the characteristic appearance of a fluid bleb formed after intradermal injection due to displacement of the skin surface by the insulin solution injected into the skin. Consistent with the literature,17 the bleb resorbed and disappeared within approximately 2 h. There were no adverse events during the study.
Discussion
This is the first reported study to demonstrate that microneedle-based intradermal infusion leads to more rapid insulin PK and tighter glucose control compared with subcutaneous catheters. The microneedle delivery also resulted in significantly less pain in children, adolescents, and adults. We believe this is due to targeting of microneedle-based infusion to the papillary dermal region, which has a rich capillary and lymphatic network allowing for rapid insulin absorption.10,11 These results, although in a small number of subjects, suggest that intradermal microneedles may provide improved glucose control and more efficient utilization of insulin, both of which are critical to reducing diabetes complications.21 Future studies in larger patient populations will have to be performed to further strengthen these initial findings. The results also suggest that insulin administration using microneedles could increase patient compliance, particularly among children and adolescents, who often omit insulin injections because of fear, pain, anxiety, and inconvenience associated with hypodermic needles and subcutaneous catheters.13–16 We conclude that intradermal microneedles may enable better postprandial glucose control with increased patient compliance and improved health outcomes.
Acknowledgments
This work was supported in part by the Emory Egelston Seed Grant Program. Human studies were carried out at the Emory Children's Center. Microneedle fabrication and data analysis were carried out at the Center for Drug Design, Development and Delivery and the Institute for Bioengineering and Biosciences at the Georgia Institute of Technology.
Author Disclosure Statement
M.R.P. serves as a consultant and is an inventor on patents licensed to companies developing microneedle-based products. This potential conflict of interest has been disclosed and is being managed by the Georgia Institute of Technology and Emory University. J.G. and E.I.F. declare no conflicts of interest exist.
References
- 1.Vajo Z. Fawcett J. Duckworth WC. Recombinant DNA technology in the treatment of diabetes: insulin analogs. Endocr Rev. 2001;22:706–717. doi: 10.1210/edrv.22.5.0442. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174–183. doi: 10.1056/NEJMra040832. [DOI] [PubMed] [Google Scholar]
- 3.Dodd SW. Havel HA. Kovach PM. Lakshminarayan C. Redmon MP. Sargeant CM. Sullivan GR. Beals JM. Reversible adsorption of soluble hexameric insulin onto the surface of insulin crystals cocrystallized with protamine: an electrostatic interaction. Pharm Res. 1995;12:59–67. doi: 10.1023/a:1016231019793. [DOI] [PubMed] [Google Scholar]
- 4.Derewenda U. Derewenda Z. Dodson GG. Hubbard RE. Korber F. Molecular structure of insulin: the insulin monomer and its assembly. BMJ. 1989;45:4–18. doi: 10.1093/oxfordjournals.bmb.a072320. [DOI] [PubMed] [Google Scholar]
- 5.Heinemann L. Starke AAR. Heding L. Jensen I. Berger M. Action profiles of fast onset insulin analogues. Diabetologia. 1990;33:384–386. doi: 10.1007/BF00404644. [DOI] [PubMed] [Google Scholar]
- 6.Brange J. The new era of biotech insulin analogues. Diabetologia. 1997;40(Suppl 2):S48–S53. doi: 10.1007/s001250051400. [DOI] [PubMed] [Google Scholar]
- 7.Ischii H. Anderson JH., Jr Yamamura A. Tikeuchi M. Ikeda I. Improvement of glycemic control and quality-of-life by insulin lispro therapy: assessing benefits by ITR-QOL questionnaires. Diabetes Res. 2008;81:169–178. doi: 10.1016/j.diabres.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Griffen SC. Oostema K. Stanhope KL. Graham J. Styne DM. Glaser N. Cummings DE. Connors MH. Havel PJ. Administration of Lispro insulin with meals improves glycemic control, increases circulating leptin, and suppresses ghrelin, compared with regular/NPH insulin in female patients with type 1 diabetes. J Clin Endocrinol Metab. 2006;91:485–491. doi: 10.1210/jc.2005-1338. [DOI] [PubMed] [Google Scholar]
- 9.Heinemann L. Future directions for insulin therapy and diabetes treatment. Endocrinol Metab Clin North Am. 2007;36:69–79. doi: 10.1016/s0889-8529(07)80012-0. [DOI] [PubMed] [Google Scholar]
- 10.Prausnitz MR. Gill HS. Park JH. Microneedles for drug delivery. In: Rathbone MJ, editor; Hadgraft J, editor; Roberts JS, editor; Lane ME, editor. Modified Release Drug Delivery. Informa Healthcare; New York: 2008. pp. 295–309. [Google Scholar]
- 11.Heinemann L. Hompesch M. Kapitza C. Harvey N. Ginsberg B. Pettis RJ. Intra-dermal insulin lispro application with a new microneedle delivery system led to a substantially more rapid insulin absorption than subcutaneous injection [abstract P909] Diabet Med. 2006;23:200–410. [Google Scholar]
- 12.Wang PM. Cornwell M. Hill J. Prausnitz MR. Precise microinjection into skin using hollow microneedles. J Invest Dermatol. 2006;126:1080–1087. doi: 10.1038/sj.jid.5700150. [DOI] [PubMed] [Google Scholar]
- 13.Hanas R. Reducing injection pain in children and adolescents with diabetes: a review of indwelling catheters. Pediatr Diabetes. 2004;5:102–111. doi: 10.1111/j.1399-543X.2004.00048.x. [DOI] [PubMed] [Google Scholar]
- 14.Mollema ED. Snock FJ. Hune RJ. van der Ploeg HM. Phobia of self-injecting and self-testing in insulin-treated diabetes patients: opportunities for screening. Diabet Med. 2001;18:671–674. doi: 10.1046/j.1464-5491.2001.00547.x. [DOI] [PubMed] [Google Scholar]
- 15.Zambanini A. Newson RB. Maisey M. Feher MD. Injection related anxiety in insulin-treated diabetes. Diabetes Res Clin Pract. 1999;46:239–246. doi: 10.1016/s0168-8227(99)00099-6. [DOI] [PubMed] [Google Scholar]
- 16.Hanas R. Ludvigsson J. Experience of pain from insulin injections and needle-phobia in young patients with IDDM. Pract Diabetes Int. 1997;14:95–99. [Google Scholar]
- 17.Gupta J. Felner EI. Prausnitz MR. Minimally invasive insulin delivery in subjects with type 1 diabetes using hollow microneedles. Diabetes Technol Ther. 2009;11:329–337. doi: 10.1089/dia.2008.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanna M. Mihic M. Modi P. Painless intradermal delivery of insulin: the novel ClickSoft microinjection device. Drug Deliv Technol. 2009;9:22–27. [Google Scholar]
- 19.Nordquist L. Roxhed N. Griss P. Stemme G. Novel microneedle patches for active insulin delivery are efficient in maintaining glycaemic control: an initial comparison with subcutaneous administration. Pharm Res. 2007;24:1381–1388. doi: 10.1007/s11095-007-9256-x. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y. Gao Y. Qin G. Zhang S. Qiu Y. Li F. Xu B. Sustained release of insulin through skin by intradermal microdelivery system. Biomed Microdevices. 2010;12:665–671. doi: 10.1007/s10544-010-9419-0. [DOI] [PubMed] [Google Scholar]
- 21.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]