Abstract
Members of the Bcl-2 family play key roles as proapoptotic (e.g., Bax) and antiapoptotic (e.g., Bcl-xL) regulators of programmed cell death. We previously identified the mitochondrial potassium channel Kv1.3 as a novel target of Bax. Incubating Kv1.3-positive isolated mitochondria with Bax triggered apoptotic events, whereas Kv1.3-deficient mitochondria were resistant to this stimulus. Mutation of Bax at lysine 128 (BaxK128E) abrogated its effects on Kv1.3 and the induction of apoptotic changes in mitochondria. These data indicate a toxin-like action of Bax on Kv1.3 to trigger at least some of the mitochondrial changes typical for apoptosis. To gain insight into the mechanism of Bax–Kv1.3 interaction, we mutated Glu158 of Bcl-xL (corresponding to K128 in Bax) to lysine. This substitution turned Bcl-xL proapoptotic. Transfection of double knockout (Bax−/−/Bak−/−) mouse embryonic fibroblasts (DKO MEFs) with either wild-type Bax, BaxK128E, or Bcl-xLE158K showed that apoptosis induced by various stimuli was defective in DKO MEFs and BaxK128E-transfected cells, but was recovered upon transfection with Bcl-xLE158K or wild-type Bax. Both wild-type Bax and BaxK128E can form similar ion-conducting pores upon incorporation into planar lipid bilayers. Our results point to a physiologically relevant interaction of Bax with Kv1.3 and further indicate a crucial role of a distinct lysine in determining the proapoptotic character of Bcl2-family proteins.
Keywords: ion channels, Bax, Bcl2-like proteins, apoptosis
Mitochondria mediate apoptosis by releasing proapoptotic factors such as cytochrome c, apoptosis-inducing factor (AIF), second mitochondria-derived activator of caspase (Smac/Diablo), HtrA2, and endonucleases.1 The release of these factors is the direct consequence of the activation of proapoptotic proteins from the Bcl-2 family, in particular Bax and Bak. Various proteins in the Bcl-2 family are key positive or negative regulators of apoptosis. They share conserved regions called Bcl-2 homology domains (BH1, BH2, and BH3). Biochemical and subcellular fractionation studies localized the antiapoptotic members, such as Bcl-2 and Bcl-xL, at the outer mitochondrial membranes (OMMs) and at the endoplasmic reticulum and nuclear membranes. Proapoptotic factors either reside at the OMM, as is true for Bak, or are cytosolic proteins in unstimulated cells, as is the case for Bax, Bid, and Bad.
Although it is very well known that Bax and Bak are crucial for the induction of apoptosis in many systems, the molecular details of Bax-mediated apoptotic events are still unclear (see, e.g., Antignani and Youle2). A diversity of stimuli have been suggested as initiators of the activation of Bax and Bak,3, 4 including direct stimulation by BH3-only proteins such as Bid or Bim.5, 6 Bax activation is believed to be a highly regulated, multistep process involving mitochondrial translocation and oligomerization, and this process ultimately leads to mitochondrial dysfunction and apoptosis.7, 8 Upon the induction of apoptosis, Bax migrates to the mitochondria, where it is integrated into the outer membrane as a monomer. Bax monomers then oligomerize and may form large pores in the OMM.8 In its inactive form, Bak is constitutively present in mitochondrial membranes and is released from inhibitory binding partners upon apoptotic stimulation.
Bax and Bid, as well as Bcl-xL and Bcl-2, form pores in synthetic lipid vesicles and membranes.9, 10, 11, 12. The ability of Bax to form ion-conducting pores in synthetic and mitochondrial membranes suggests that such pores are involved in the release of cytochrome c.13, 14, 15 Bax however might work as a twin-arginine translocation (Tat)-like protein rather than as an always-open ion-conducting pore in the OMM.2 Bcl-2-like proteins have also been shown to interact with some mitochondrial components. Evidence points to interactions between Bax and the machinery involved in mitochondrial fission and fusion (see, e.g., Youle and Karbowski16 and Scorrano17). Bcl-xL interacts with the adenine nucleotide translocator, an important component of the permeability transition pore complex, and it has been proposed that this interaction prevents cell death.18 It has also been suggested that Bcl-2 as well as Bax interact with the voltage-dependent anion channel.19, 20
We have recently shown that OMM-integrated Bax directly interacts with and inhibits an inner membrane-located mitochondrial potassium channel, Kv1.3,21 in lymphocytes.22 Kv1.3 has been shown to be expressed in different tissues and cell types, including brain, lymphocytes, macrophages, liver, and skeletal muscle.23 The absence of Kv1.3 in lymphocytes prevents Bax-induced release of cytochrome c, as well as changes in mitochondrial membrane potential and the production of reactive oxygen species (ROS). In a previous study we showed that wild-type (WT) Bax inhibits Kv1.3 and induces the release of cytochrome c from isolated mitochondria, whereas Bax with a single-point mutation (K128E) does not induce this effect.22 According to a model of the structure of the membrane-integrated Bax monomer, at least amino acids 127 and 128, located between the fifth and sixth helices of Bax, protrude from the OMM into the intermembrane space.8 We determined that Bax binds to the vestibule region of the channel via lysine 128 and preincubation of Bax with recombinant Kv1.3 prevents its proapoptotic effects in isolated mitochondria.24 The physiological relevance of Kv1.3 for apoptosis was illustrated by the facts that knockdown of Kv1.3 expression in human peripheral blood lymphocytes impaired apoptosis in these cells, and expression of mitochondria-targeted Kv1.3 was sufficient to sensitize apoptosis-resistant CTLL-2T lymphocytes, which lack Kv channels.22
In this study, we test the function of BaxK128E in a cell system and analyze the mechanism of Bax–Kv1.3 interactions using a mutant of Bcl-xL as a model. We report that mutation of lysine 128 in Bax to glutamate (Glu; Bax K128E) abrogates the proapoptotic function of Bax in a cellular context. The critical role of lysine 128 in Bax for the induction of apoptosis is demonstrated by the finding that exchange of Glu158 of Bcl-xL (corresponding to K128 in Bax) with lysine converts Bcl-xL into a proapoptotic protein.
Results
Previous findings22 demonstrated that Bax requires lysine 128 to induce apoptotic events in isolated mitochondria. The mutation of lysine 128 in Bax prevents the release of cytochrome c and the depolarization of the mitochondrial membrane, events that were observed after the incubation of isolated mitochondria with WT Bax. These findings indicate that the highly conserved positively charged lysine of Bax plugs the pore of the mitochondrial potassium channel Kv1.3, and thereby initiates mitochondrial changes during apoptosis. Antiapoptotic proteins Bcl-2 and Bcl-xL in various species contain a negative charge in the position corresponding to that of K128 in Bax: amino acid (aa) 158 for Bcl-xL (Figure 1). In these proteins, a conserved lysine/arginine precedes this glutamate. Thus, whereas in proapoptotic Bcl-2-like proteins an isolated positive charge is found at aa 128, in antiapoptotic Bcl-2-like proteins the positive charge at aa 157 is counterbalanced by a negative charge at position 158, resulting in a net charge of zero in antiapoptotic proteins at this site. The absence of a net positive charge at this site is expected to prevent inhibition of Kv1.3 by the antiapoptotic Bcl-2-like proteins, even if they are in close contact with each other.
Figure 1.
Sequence homology between pro- and anti-apoptotic proteins from various species. The T-Coffee algorithm was used for multiple alignments. ‘*' Indicates identity, ‘:' indicates conserved, and ‘.' indicates semiconserved substitutions. (a) Sequence alignment of human antiapoptotic Mcl-1, Bcl-2, and Bcl-xL, as well as that of proapoptotic Bax and Bak. The highly conserved lysine residue in Bax (K128) that is crucial for interaction with Kv1.322 is highlighted in light gray. In the corresponding position, Bak contains a glycine; however, adjacent to this G there is a histidine (in light gray). Furthermore, Bak molecules from other species are characterized by an arginine residue in the same position (not shown). The amino acid at position 158 is a highly conserved, negatively charged glutamate (in dark gray) in both Bcl-xL (b) and Bcl-2 (c) from differing species. Accession numbers for sequences from the ExPASy databases are reported
On the basis of these considerations, and to gain insight into the mechanism of Kv1.3–Bax interaction, we replaced Glu158 in Bcl-xL with a lysine. Bcl-xLE158K caused a marked inhibition of Kv1.3 in patch-clamp experiments on Jurkat T lymphocytes (Figure 2A), comparable to that caused by WT Bax (Figure 2B) (IC50 of 5 nM for Bcl-xLE158K; IC50 of 4 nM for WT Bax). In contrast, neither WT Bcl-xL nor mutant BaxK128E or GST altered Kv1.3 currents (Figures 2C–E, and Szabó et al.22). Bax and margatoxin (MgTx) competed for the same binding site on Kv1.3 (Figure 2F), further supporting a specific interaction of Bax with the pore region of Kv1.3 and the notion that proapoptotic Bcl-2-like proteins mimic the interactions of Kv1.3-inhibiting toxins.24
Figure 2.
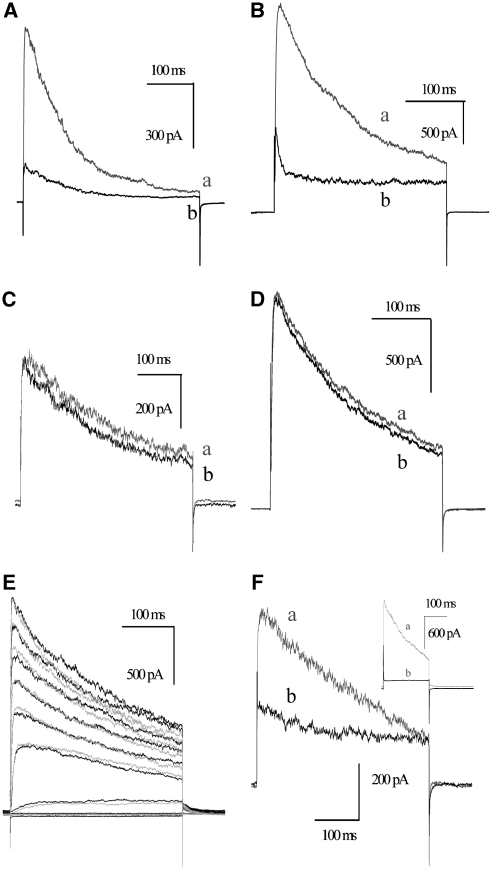
Effect of Bax and Bcl-xL mutants on Kv1.3 current. Representative experiments with Jurkat cells are shown. (A) Mutant Bcl-xLE158K (gray, control; black, after addition of 20 nM GST-Bcl-xLE158K) inhibited current within 450 s (IC50 5 nM, n=3). (B) In the same experimental setup, the addition of wild-type Bax inhibited current conduction (gray, control; black, 315 s after the addition of 15 nM GST-Bax). (C) BaxK128E does not reduce Kv1.3 current (gray, control; black, 450 s after addition of 20 nM recombinant GST-BaxK128E to bath). (D) The addition of 40 nM GST (a, control trace; b, 10 min after addition) does not decrease the amplitude of Jurkat whole-cell Kv1.3 current elicited by applying voltage pulses to +70 mV from a holding potential of −50 mV every 45 s. (E) Results of a representative experiment in which voltage pulses ranging from −110 to +90 mV were applied at 20-mV steps. Traces show currents recorded in the absence (gray) or presence (black) of 50 nM wild-type GST-Bcl-xL (10 min after addition). (F) BaxK128E and MgTx compete for the same binding site. Jurkat lymphocytes were incubated for 20 min with 10 nM BaxK128E. Please note that BaxK128E was added in solution where it may have a conformation allowing its docking to the rim of the channel; however, in the absence of the critical lysine residue that plugs the pore, inhibition does not take place. The seal was established, and the current was recorded at +70 mV in the absence (gray) and presence (black) of 12 nM MgTx in the same experiment. In other experiments, 6 nM MgTx caused a rapid, complete inhibition of Kv1.3 (see inset). Shown are representative studies from at least three independent experiments
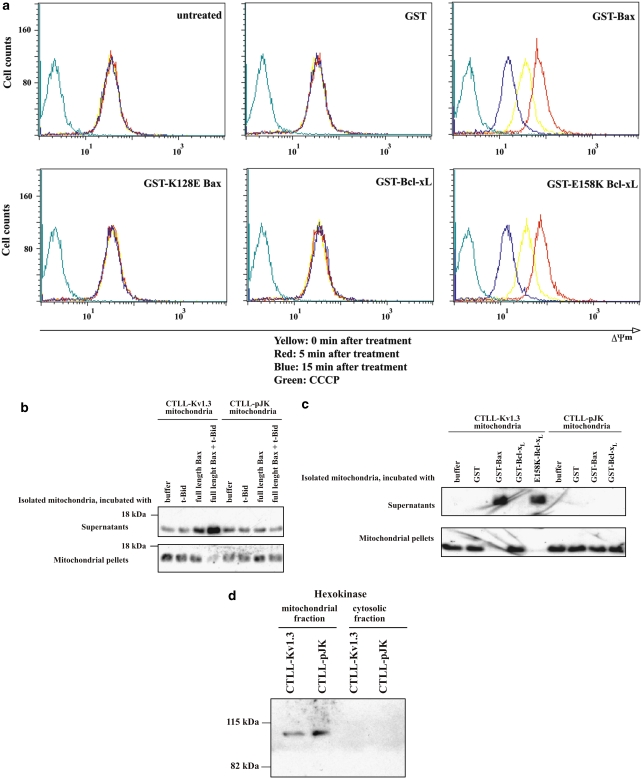
To show that mutant Bcl-xL induces changes in mitochondria similar to those induced by WT Bax, we incubated purified Jurkat mitochondria with recombinant GST-Bax (lacking the C-terminal transmembrane domain and therefore able to insert into the membrane25), GST-Bcl-xL, GST-Bcl-xLE158K, or GST as a control. Recombinant GST-Bax and full-length Bax induced very similar effects on isolated mitochondria.22 Recombinant GST-Bax triggers a hyperpolarization followed by a decrease of the potential of the inner mitochondrial membrane (IMM; Figure 3a). The electrochemical gradient for K+ predicts that K+ should enter the matrix through a potassium channel located in the IMM. If the influx of K+ becomes inhibited, hyperpolarization is expected. Depolarization, which followed hyperpolarization, was inhibited by cyclosporin A (CSA) and, thus, was probably mediated by the opening of the mitochondrial permeability transition pore (PTP) (see Supplementary Material in Szabó et al.22). The transmembrane potential of Kv1.3-containing Jurkat mitochondria was not altered by incubation with GST alone, GST-BaxK128E,22 or GST-Bcl-xL (Figure 3a). In contrast, marked hyperpolarization followed by depolarization was observed when mitochondria were treated with GST-Bcl-xLE158K (Figure 3a). Likewise, the mutant GST-Bcl-xLE158K, similar to GST-Bax and truncated Bid (tBid)-preactivated full-length Bax (Figure 3b), triggered a massive release of mitochondrial cytochrome c, whereas GST-Bcl-xL had no effect (Figure 3c; see Figure 3d for purity control). Importantly, the proapoptotic effects of Bax and GST-Bcl-xLE158K were abrogated in mitochondria isolated from CTLL-2/pJK cells lacking Kv1.3 (Figures 3b and c). This finding indicates that the Bcl-xLE158K, similar to Bax, exerts its action by regulating Kv1.3 activity in mitochondria isolated from CTLL-2/Kv1.3 cells.
Figure 3.
Effect of Bax and Bcl-xL mutants on mitochondrial membrane potential and release of cytochrome c. (a) Point mutation of glutamic acid 158 to a lysine converts the antiapoptotic protein Bcl-xL into a protein that triggers an initial hyperpolarization followed by depolarization in isolated Jurkat mitochondria. Wild-type Bax causes the same alterations in mitochondrial transmembrane potential, whereas GST-Bcl-xL or GST were without effect. CCCP, carbonyl cyanide 3-chlorophenylhydrazone. (b) Full-length Bax (20 nM) preincubated with tBid (0.1 nM) induces release of cytochrome c only from Kv1.3-positive CTLL-2/Kv1.3 mitochondria. (c) Recombinant Bax and Bcl-xLE158K induce the release of cytochrome c only from Kv1.3-positive CTLL-2/Kv1.3 mitochondria, whereas GST-Bcl-xL has no effect. (b, c) Here, isolated mitochondria were incubated with the proteins as indicated or left untreated. The release of cytochrome c and the amount of cytochrome c remaining in the mitochondrial fraction were determined by western blotting. (d) Mitochondria were purified as for the above experiments and aliquots of the mitochondrial and cytosolic fractions were blotted with anti-hexokinase antibodies and developed with alkaline phosphatase-coupled secondary antibodies and ECL. The blots reveal the purity of the preparations and show that the fraction containing the released cytochrome c does not contain the mitochondrial hexokinase. Displayed are representative studies from at least three independent experiments
To test the function of the mutant Bcl2-family proteins in intact cells, we transfected mouse embryonic fibroblasts (MEFs) deficient for both Bax and Bak (double knockout (DKO) MEFs) with expression vectors for WT Bax, Bax K128E, or Bcl-xLE158K. Bax/Bak-deficient cells are widely used for determining the effect of mutant Bcl-2 proteins in a cellular context and on isolated mitochondria (see, e.g., Nie et al.4 and Gavathiotis et al.5). The transfection of DKO MEFs with WT Bax or Bcl-xLE158K restored apoptosis induced by staurosporine, etoposide, or sphingomyelinase, whereas transfection with BaxK128E failed to restore apoptosis in these cells (Figures 4a–c). The cytochrome c release induced by staurosporine in Bax or mutant Bcl-xLE158K-expressing MEF DKO cells was inhibited by CSA (Figure 4d), suggesting a possible involvement of the PTP in this experimental setup. To avoid a possible proapoptotic effect of protein overexpression per se, the stoichiometry of transfection was adjusted to yield amounts of protein close to those found in untransformed WT cells. The protein levels were determined in the transfected and stimulated cells by western blot and fluorescence-activated cell sorter (FACS; which permits us to determine the expression of Bax in the population also investigated for apoptosis) and were similar to the levels of Bax endogenously expressed by WT MEFs (Figures 4a and b).
Figure 4.
Bax and Bcl-xLE158K, but not BaxK128E, restore mitochondrial cytochrome c release and apoptosis in Bax/Bak double knockout (DKO) mouse embryonic fibroblasts (MEFs). (a) Transfection of wild-type Bax or Bcl-xLE158K into Bax/Bak DKO MEFs restores the release of cytochrome c upon stimulation with staurosporine to levels that are comparable to those seen in wild-type MEFs. In contrast, BaxK128E does not restore the staurosporine-triggered release of cytochrome c by Bax/Bak-deficient cells. The expression levels of transfected wild-type Bax and BaxK128E, as shown on western blots, are somewhat lower than those of endogenous Bax in wild-type MEFs (loading control with anti-actin antibody is shown in the lower row). Expression levels of transfected Bax or BaxK128E in individual cells are displayed in panel (b) and also exclude overexpression of Bax. (b) Transfection of wild-type Bax or Bcl-xLE158K together with Kv1.3 into Bax/Bak DKO MEFs restores apoptosis upon stimulation with staurosporine to levels that are comparable to those seen in wild-type MEFs treated with staurosporine. In contrast, BaxK128E does not restore staurosporine-triggered apoptosis in DKO MEFs. The results of FACS studies using Cy3-coupled anti-Bax antibodies show that the expression levels of wild-type (WT) Bax or BaxK128E in the transfected cells are slightly lower than those in WT MEFs; this finding is also indicated by the western blots of panel (a). To identify cells that were transfected with Bcl-xLE158K, we co-transfected EGFP-actin. Apoptosis was determined by FITC or Cy3-Annexin staining. (c) Transfection of E158KBcl-xL and WT-Bax together with Kv1.3 restores apoptosis in MEF DKOs treated with etoposide (100 μM for 12) or sphingomyelinase (1 U/ml, 6 h incubation), whereas BaxK128E or Bcl-xL fail to restore death. Almost all cells transfected with Bcl-xLE158K and WT-Bax died upon treatment with etoposide or sphingomyelinase, whereas untransfected cells (lower left quadrant) or transfection of BaxK128E or Bcl-xL did not result in significant induction of apoptosis. Transfected cells were detected by staining with FITC-coupled anti-Bax or anti-Myc 9E10 antibodies. Apoptosis was measured by Cy3-labelled Annexin V and FACS analysis. (d) MEF DKO cells were transfected with constructs coding for the indicated proteins and were selected as described in the Materials and Methods section. Staurosporine-induced apoptosis resulted in cytochrome c release in Bax and Bcl-xLE158K-expressing cells. Cytochrome c release was prevented by incubation of the cells with 2 μM cyclosporine A (CSA). The data are representative for three independent experiments with very similar results. (e) Co-immunoprecipitation experiments reveal an association of WT Bax with Kv1.3 after co-transfection into Bax/Bak-double deficient cells (DKO), whereas BaxK128E does not associate with co-transfected Kv1.3. The upper blots show Bax (left) or Kv1.3 (right) immunoprecipitates blotted with anti-Bax or anti-Kv1.3 antibodies. The lower blots were developed with the same antibody that was used for immunoprecipitation to control amounts of the immunoprecipitated protein. Wild-type MEFs were transfected with Kv1.3 only and served as controls. The cells were stimulated with 1 μM staurosporine after co-transfection, lysed, and the association of Bax or BaxK128E with Kv1.3 was determined as above. All data are representative for three experiments with similar results
To confirm that Bax interacts with Kv1.3 via lysine 128, we co-transfected Kv1.3 and BaxK128E or WT Bax into MEF DKO cells, induced apoptosis with staurosporine, lysed cells, and determined co-immunoprecipitation of the proteins. The results (Figure 4e) show that the BaxK128E mutant failed to bind Kv1.3, whereas WT Bax and Kv1.3 interacted after induction of apoptosis.
To exclude that the lack of proapoptotic effects of mutant BaxK128E in DKO MEFs (Figure 4) and in CTLL-2 cells22 may be caused by alterations of the membrane-insertion capability and/or of the pore-forming properties of Bax by the mutation, we addressed whether the Bcl-2-family proteins used in our studies can efficiently insert into mitochondria. Figure 5a illustrates that mutant recombinant Bax and Bcl-xL are able to insert into MEF DKO-isolated mitochondria as efficiently as the WT recombinant Bax and Bcl-xL, as assayed by alkaline extraction. Activation and oligomerization of Bax can be detected by analyzing resistance of Bax to trypsin.26 In particular, following these events, amino acids at the N-terminus of Bax become susceptible to trypsin digestion: the rest of the protein is protected, giving rise to truncated Bax of 15 kDa. In our system, in mitochondria isolated from DKO MEF cells, the recombinant Bax WT and Bax mutant equally give rise to such a truncated form (revealed using an antibody against aa 1–171 of Bax (Δ21)), indicating that the mutated version of Bax is still able to form higher-order oligomers (Figure 5b). In accordance, BaxK128E is able to induce the release of Smac/Diablo from these mitochondria, similar to Bax WT, whereas the release of cytochrome c is impaired when using mutant Bax (Figure 5c). Smac/Diablo is released not only from isolated mitochondria using recombinant proteins, but also in MEF DKO cells transfected with mutant Bax (Figure 5d; see Figure 5e for purity control of cytosolic fraction). Importantly, the effects of recombinant GST-Bax and full-length Bax22 on isolated mitochondria and those of Bax expressed in MEF cells are the same (Figures 3 and 4). These results further indicate the ability of Bax and of BaxK128E to form oligomers in the outer membrane and to allow the exit of Smac/Diablo, but also point to another event, that is, inhibition of Kv1.3 by Bax, necessary for the release of cytochrome c. This compulsory proapoptotic event does not take place with BaxK128E (see Figure 4c), or in the absence of Kv1.3.22
Figure 5.
Membrane insertion and oligomerization capacity is not altered in BaxK128E. (a) Alkaline extraction does not remove GST-Bax, GST-BaxK128E, GST-Bcl-xL, and GST-Bcl-xLE158K (20 nM each) inserted into mitochondria isolated from DKO MEFs. Mitochondrial fractions and supernatants obtained following centrifugation are shown. Purity of supernatants was assessed using anti-Tom22 antibody. (b) GST-Bax and GST-BaxK128E (20 nM each) were incubated with mitochondria isolated from DKO MEFs and subjected to trypsin treatment where indicated. The N-terminal part is cleaved off as revealed by an antibody against N-terminus (upper panel). GST-Bax (45 kDa) and Bax (22 kDa) are both present in the untreated sample (first lane). Both WT and mutant Bax molecules were protected against complete cleavage as indicated by recognition of the 15 kDa fragment by an antibody against an epitope corresponding to aa 1–171 of Bax (Δ21). Where indicated, tBid was also added (5 nM) and increased insertion/activation of Bax (middle panel). As a control for the efficiency of trypsinization, the same blot was re-probed with an antibody against Tom22, an outer membrane protein, which is anchored to the cytoplasm-facing side of the membrane only by a short helix (lower panel). (c) Cytochrome c is released from isolated DKO MEF mitochondria by GST-Bax, but to much less extent by GST-BaxK128E, whereas Smac/Diablo is released equally as assessed by stripping and re-blotting of the same membrane with anti-Smac antibody. (d) DKO MEF cells were transfected and treated as in Figure 4c and then assessed for released Smac/Diablo content in the supernatant fraction. (e) Mitochondria from DKO MEFs were purified and aliquots of the mitochondrial and cytosolic fractions were blotted with anti-Tom20 or anti-Tim23 antibodies and developed with alkaline phosphatase-coupled secondary antibodies and ECL. The blots reveal the purity of the preparations and show that the cytosolic fractions do not contain the mitochondrial proteins Tom or Tim
The notion that interaction of Kv1.3 with Bax is important for the release of cytochrome c but not for the oligomerization of Bax, is further indicated in Figures 6a and b, where in isolated mitochondria, Smac/Diablo and HtrA2/Omi release occurs independently of the presence of Kv1.3 upon incubation with recombinant Bax, suggesting that interaction of the channel with Bax is involved mainly in cytochrome c mobilization rather than in its translocation through the OMM. We tested proapoptotic factor release also in intact CTLL-2/pJK and CTLL-2/Kv1.3 cells challenged with staurosporine. In accordance with data of Figures 3, and 6a and b, cytochrome c is released in a Kv1.3-dependent manner, whereas Smac release occurred also in the absence of the channel. The release of Smac was insensitive to CSA (Figure 6c). In contrast, the release of AIF and endoG required expression of Kv1.3 and was absent in CTLL-2-pJK treated with staurosporine (Figure 6c).
Figure 6.
Kv1.3-dependent and -independent release of proapoptotic factors from mitochondria in CTLL-2 cells. Smac/Diablo (a) and HtrA2/Omi (b) are released by GST-Bax and tBid independently of Kv1.3 expression. Mitochondria were isolated from CTLL/pJK and CTLL/Kv1.3 cells and were incubated with 5 nM GST or GST-Bax. Mitochondria were pelleted and the release of Smac/Diablo and HtrA2/Omi into the supernatants was visualized by western blotting. Shown are representative results from at least three independent experiments. Purity of supernatants was controlled as in Figure 3d (not shown). (c) CTLL-2/pJK and CTLL-2/Kv1.3 cells were left untreated (c) or treated with staurosporine (ST) and lysed. Mitochondria were separated from cytosolic fraction and both fractions were assayed for the release of cytochrome c, Smac, EndoG, and AIF. In the case of Smac, fractions obtained from cells incubated with cyclosporine A (CSA) and ST (ST+CSA) were loaded. Purity of cytosolic fractions from the same experiment was tested using an antibody against the outer mitochondrial protein Tom20
Finally, we tested whether recombinant Bax WT and BaxK128E may form channels by incorporating these proteins into planar lipid bilayer. The BaxK128E was able to insert into liposomes and oligomerize as assayed by crosslinking with disuccinimidyl suberate (Figure 7a). Both WT and mutant Bax proteins formed channels in this system only when added at very high (up to 16 nM; WT: n=28; mutant: n=20) concentrations. Although the activities differed, the differences were within the range of the variability displayed by Bax channels from one experiment to the other. Note in Figure 7b that the amplitude of the concentration gradient-driven current passing through the pores at zero applied voltage (the condition believed to prevail at the OMM) and the kinetic behavior (current traces) are similar for the WT and mutant channels.
Figure 7.
GST-Bax and GST-BaxK128E form pores in planar lipid bilayers. (a) GST-BaxK128E becomes incorporated into liposomes and forms high-molecular-weight (MW) oligomers as revealed by crosslinking with 1 mM disuccinimydil suberate (DSS) (second lane) and western blot. GST-BaxK128E was incubated with 2% octylglycoside (for 30 min) to induce oligomer formation and added to liposomes. The proteoliposomes were either left untreated (left lane) or incubated with 1 mM DSS (2 h at 4°C; right lane). Aliquots containing 330 μg asolectin and 20 pmol GST-BaxK128E/lane were pelleted by centrifugation (30 min, 4°C, 14 000 × g), solubilized, and loaded onto SDS-PAGE. Monomeric GST-BaxK128E (45 kDa) is indicated by an arrow. In the right lane, high-MW oligomeric forms of the fusion protein are detected. (b) Two exemplary bilayer experiments with 400:100 mM (cis/trans) KCl are shown. Representative 10-s current trace segments recorded at the indicated voltages. Current-voltage (I–V) curves, constructed from the same experiments and fitted with third order polynomial functions (not shown) indicated that the x axis intercepts are −13 mV for wild type (WT) and −25 mV for mutant Bax; these intercepts correspond to weak cation selectivity (PK/PCl of 2.7 for wild-type and 7.1 for mutant). The results are representative for at least three independent studies with very similar results
Discussion
In this work we provide insights into the mechanisms of action of the proapoptotic Bcl-2-family protein Bax, namely the inhibition of mitochondrial Kv1.3. We also show that an inactive mutant of Bax forms ion-conducting channels similar to those formed by WT Bax, a finding indicating that Bax does not exert its action during apoptosis merely by forming ion-conducting oligomers.
The data obtained with Bcl-xLE158K are consistent with the model we recently proposed for the action of Bax.22 WT Bax inhibits Kv1.3, a potassium-selective channel of the IMM, and exhibits actions similar to those exerted by margatoxin and charybdotoxin, which block the channel with high specificity.22 These toxins possess a crucial lysine that blocks the channel by binding to the ring of four aspartate residues of the channel vestibule. Position 128 in Bax, which folds out of the OMM after insertion,8 corresponds to a highly conserved lysine. If lysine 128 were important for the proapoptotic action of Bax via inhibition of Kv1.3, we expect that introducing a positive charge at the corresponding position in Bcl-xL turns this antiapoptotic protein into a blocker of Kv1.3 with proapoptotic effects. Our results confirm this hypothesis and show that the addition of Bcl-xLE158K to isolated mitochondria efficiently blocks Kv1.3 and induces hyperpolarization and the release of cytochrome c. DKO MEFs were used to verify the effect of these mutations in a cellular context. The fact that mutant Bcl-xLE158K inhibits Kv1.3 and is sufficient to restore cell death in cells lacking Bax and Bak strongly indicates that the crucial lysine residue mediates inhibition of the channel; it may also be important for the physical interaction between Bax and Kv1.3. A single amino acid residue seems to be sufficient to mediate strong protein–protein interactions as previously shown by the observation that, for instance, a point mutation of K27 in agitoxin reduces the affinity of the toxin for the KcsA K+ channel 130-fold. In agreement, data shown in Figure 4e indicate that BaxK128E does not interact with Kv1.3.
The existence of various potassium channels in the IMM of different tissues has been reported (see, e.g., Zoratti et al.27 and references therein). Mitochondrial potassium channels permit K+ transport into the mitochondrial matrix, regulating thus mitochondrial volume homeostasis, changes in mitochondrial membrane potential, respiration, pH gradient, and synthesis of ROS.28 In various systems, open potassium channels seem to keep the PTP closed.29 Kv1.3 was recently found in mitochondria of prostate cancer and breast cancer cell lines PC3 and MCF-7,24 in hippocampal neurons,30 and in astrocytes.29 The observation that the ring of negatively charged amino acids in the pore of potassium channels is structurally highly conserved in all voltage-gated potassium channels (see, e.g., Rauer et al.31) suggests that the action of Bax may not be limited to Kv1.3.
Mutations at K128 in Bax and at E158 in Bcl-xL have not been previously reported, but other point mutations have been shown to alter the activity of Bax and Bcl-xL. Recently, Gavathiotis et al.5 described a point mutation of Bax (K21E) that identifies a novel structural location of Bax activation. Cysteine 62 of Bax was shown to be critical for its conformational activation and proapoptotic activity in response to H2O2-induced apoptosis.4 These results, like ours, point to the importance of a single amino acid for protein–protein interaction and for the proapoptotic efficiency of Bax. Lysine 128 in Bax and the Glu158 in Bcl-xL are not located within the BH3 domain, making therefore unlikely that these amino acids are involved in the dimerization process of Bax or Bcl-xL. With regard to the possibility that Bax mutations may cause alterations in the release of cytochrome c, Heimlich et al.32 reported that the Bax-induced release of cytochrome c from mitochondria depends on α-helices 5 and 6, a region comprising K128. However, the effect of these deletions in intact cells has not been investigated. Regarding Bcl-xL, a G138A mutation abrogates the ability of Bcl-xL to dimerize with Bax and prevents the inhibition of cell death induced by deprivation of IL-3 or dexamethasone in the murine prolymphocytic IL-3-dependent cell line F5.12.33 It should be noted that we mutated Bcl-xL to analyze the role of a critical lysine in Bax for the induction of apoptosis. Our studies demonstrate that a point mutation in Bcl-xL converts the protein into a proapoptotic protein, whereas the mutation of Bax at K128 prevents the induction of apoptosis by Bax. Thus, lysine 128 in Bax plays a crucial role in the induction of apoptosis in a cellular model. The findings further support the notion that Bax induces apoptosis via the direct inhibition of a mitochondrial potassium channel by plugging the pore with K128. We would like to point out that we do not propose Bcl-xL to interact with Kv1.3 in vivo; the Bcl-xL mutant was used as a tool to reinforce the evidence concerning the importance of the lysine residue in Bax.
Recombinant Bax can directly insert into membranes with the α-helices 5 and 6, and it can also spontaneously oligomerize (see, e.g., Antonsson et al.25). In vitro experiments have shown that Bax oligomers are able to conduct cytochrome c.14, 25, 34 These findings, and the observation that some proteins from the Bcl-2 family form ion-conducting pores upon reconstitution in artificial membranes,10, 11, 12, 13 suggest that cytochrome c may cross the OMM through an aqueous channel formed by assembled Bax monomers, Bak monomers, or hetero-oligomers of both proteins. Our findings do not contradict this hypothesis, but indicate that a Kv1.3-dependent event is necessary for the release of cytochrome c upon the interaction of Bax with mitochondria. Formation of Bax oligomers and pore formation by Bax in the OMM may well occur independently of Kv1.3, as suggested by the release of Smac/Diablo and HtrA2/Omi occurring independently of Kv1.3. On the other hand, the Bax mutant is efficiently inserted and activated in mitochondria (Figure 5), and is able to form oligomers and ion channels and mediate the release of Smac/Diablo and HtrA2/Omi (Figures 5, 6, 7); yet, cytochrome c is not released with mutant Bax and cell death is impaired (Figure 4). The notion that Bax itself is not sufficient alone for mediating the efflux of cytochrome c from mitochondria has been also demonstrated by other investigations on the roles of cardiolipin35 and Opa-136 in controlling the release of cytochrome c from mitochondria. Similar to cytochrome c, the release of AIF and EndoG are Kv1.3 dependent (Figure 6). AIF and EndoG have been proposed to be released in a ROS-dependent way from mitochondria.37, 38 Given that Kv1.3 inhibition by Bax leads to an increase in ROS release,22 one possible explanation for our findings is that Kv1.3 is only involved in ROS-mediated mobilization/release of proapoptotic factors. Further work is required to prove this hypothesis.
Bax and mutant Bcl-xL expressed in MEF DKOs induced a CSA-sensitive cytochrome c release, pointing to a possible involvement of PTP in this process, at least in our experimental setup. CSA may have additional effects in intact cells, and data by Schinzel et al.39 obtained in cyclophilin-D-less Bax and Bak containing MEFs argue against the involvement of PTP in staurosporine-induced apoptosis. Thus, the role of PTP in MEF death is controversial and further work is required to definitively exclude or prove its role.
In summary, we demonstrate for the first time a single-point mutation in Bcl-xL that converts the protein into a proapoptotic protein, both in experiments with isolated mitochondria and in MEF cells. Our work addresses the mode of action of a single-point mutant of Bax in a cellular context, and shows that the Bax mutant loses its ability to mediate cell death. Further work is required to understand how general this feature of Bax action is and to further investigate the role of Kv channels and/or other potassium channels in general in Bax-mediated apoptosis.40
Materials and Methods
Cell cultures
Jurkat lymphocytes and CTLL-2 cells were grown in RPMI-1640 supplemented with 10% fetal calf serum, 10 mM HEPES (pH 7.4), 2 mM -glutamine, 1 mM sodium pyruvate, 100 μM nonessential amino acids, 100 units/ml penicillin, 100 μg/ml streptomycin (all from Life Technologies, Karlsruhe, Germany), and 50 μM β-mercaptoethanol. Mouse interleukin-2 (IL-2) (4 units/ml; Roche Diagnostics, Mannheim, Germany) was added daily to CTLL-2 cells (American Type Culture Collection (ATCC), Manassas, VA, USA). DKO MEFs and control cells (both kindly provided by L Scorrano) were grown in Dulbecco's modified Eagle's medium (DMEM; Gibco, Milan, Italy) supplemented as above (without HEPES). If indicated, we added 2 μM CSA.
Transfections
The lipofectamine technique was used as described by the supplier (Invitrogen, Carlsbad, CA, USA) to co-transfect DKO MEFs with 2 μg/106 cells pJK/Kv1.3 and 5 μg each of the expression vectors pcDNA-Bax, pJK-K128E Bax, or pJK-E158K Bcl-xL so that the MEFs would express WT Bax, K128E Bax, or E158 K Bcl-xL. Expression of Kv1.3 was confirmed by FACS analysis (not shown). The pJK plasmid contains a single chain antibody with a Myc-Tag. The transfected MEF cells were sorted using anti-Myc-Tag antibodies and magnetic beads 36 h after transfection and then incubated for another 24 h. Alternatively, MEF cells were co-transfected with 2 μg pcDNA-EGFP-actin so that transfected cells could be detected or the cells were stained with FITC-coupled anti-Myc 9E10 antibodies to identify transfected cells (for transfection with pJK-E158K Bcl-xL). Dead cells were removed after 12 h, and the cells were then cultured in DMEM supplemented as above for an additional 36 h and used for functional tests. For CTLL-2 cells we obtained stable clones by transfecting CTLL-2 cells with 40 μg/107 cells of pJK/Kv1.3 or pJK plasmids by electroporation and culturing them with 800 μg/ml G418 (Sigma, Deisenhofen, Germany). All experiments except for selection of clones were performed with the bulk cell population. All cultures were re-established from frozen stocks after 4 weeks of growth. Cells were depleted of IL-2 before all experiments to avoid interference by signaling from the IL-2 receptor. To this end, cells were washed in 132 mM NaCl, 20 mM HEPES (pH 7.4), 5 mM KCl, 1 mM CaCl2, 0.7 mM MgCl2, and 0.8 mM MgSO4 (H/S) and allowed to recover for 3 h in cell culture medium without IL-2.
Recombinant proteins
Bax (amino acids 1–170) was cloned into pGEX-3X as GST fusion protein, expressed in BL21A1, and purified from bacterial lysates using glutathione-sepharose. Bacteria were lysed in 50 ml of 25 mM HEPES (pH 7.4), 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X-100, 125 mM NaCl, 10 mM each NaF, Na3VO4, and sodium pyrophosphate, 10 μM each aprotinin and leupeptin (A/L), and 1 mg/ml lysozyme. Samples were incubated on ice for 15 min so that lysis could be completed, and DNA was degraded with 5 μg/ml DNAaseI in 30 mM MgCl2 for 30 min. After insoluble material had been pelleted by a 50-min centrifugation at 11 000 × g at 4°C, the GST fusion proteins in the supernatant were immobilized by incubation with 300 μl glutathione sepharose (GE Healthcare, Chalfont St. Giles, UK) for 1 h at 4°C. The beads were extensively washed so that any detergents could be eliminated and 20 mM glutathione was added to elute the fusion protein. Glutathione was removed by dialysis and the samples were concentrated to 1 ml by several centrifugation rounds through size-exclusion columns (cutoff, 10 000 Da; Viva Science/Sartorius, Göttingen, Germany). The K128E mutant of Bax and the E158K mutant of Bcl-xL were obtained by a site-directed mutagenesis PCR technique (Stratagene, La Jolla, CA, USA), cloned into pGEX-3X, and expressed and purified as a GST fusion protein as above. In the present study we used a GST-ΔC-Bax, which lacks the C-terminal transmembrane domain and does not require activation by tBid, for insertion into the membrane and induction of cytochrome c release.25
Cellular apoptosis assays
To detect the release of mitochondrial cytochrome c and Smac, transfected MEF cells were either left untreated or treated with staurosporine for 12 h, washed in cold HEPES/saline, incubated for 30 min at 4°C in 0.3 M sucrose, 10 mM TES (pH 7.4), and 0.5 mM EGTA (TES buffer) and then Dounce-homogenized. Nuclei and unbroken cells were pelleted by centrifugation for 5 min at 600 × g and 4°C. Supernatants were centrifuged at 6000 × g for 10 min at 4°C. The supernatants were used to detect released cytochrome c or Smac/Diablo, and the pellets were used to detect mitochondrial cytochrome c. Proteins were separated on 15% SDS-PAGE, blotted onto a nitrocellulose membrane, and developed with a monoclonal mouse anti-cytochrome c antibody (clone 7H8.2C12; BD Biosciences Pharmingen, San Diego, CA, USA) or with anti-Smac antibody and the Tropix ECL system (Bedford, MA, USA). Morphological signs of apoptosis, such as DNA condensation, nuclear fragmentation, and blebbing, were detected by staining (non-permeabilized) cells with 4 μg/ml propidium iodide and 4 μg/ml ethidium bromide. This method also differentiated apoptotic and necrotic cells. To detect cytochrome c, AIF, Smac, Omi, and EndoG release in intact CTLL-2 cells, the cells were treated with 1 μM staurosporine with or without 2 μM CSA for 12 h. After incubation, the cells were collected, washed with phosphate-buffered saline (PBS), re-suspended in 150 μl of TES buffer, and incubated on ice. Cells were broken with an electric homogenizer (60 s). Unbroken cells were separated by centrifugation at 600 × g for 10 min at 4°C. Mitochondria were separated from cytosol by centrifugation at 7000 × g at 4°C for 10 min. Mitochondria were re-suspended in TES buffer. Both cytosolic fraction and mitochondria were analyzed by western blot.
Alkaline extraction and trypsin treatment
Mitochondria were isolated as described by Szabò et al.22 1 × 106 DKO MEFs cells/sample were washed with PBS and centrifuged for 10 min at room temperature at 450 × g. The cells were then re-suspended in 1.5 ml of TES Buffer and incubated in ice for 30 min. After 150 strokes with a glass/teflon potter, intact cells were pelleted by centrifugation for 10 min at 4°C at 600 × g. Mitochondria were separated by centrifugation of the resulting supernatant at 6000 × g for 10 min at 4°C and washed in 500 μl of buffer 1 (50 mM PIPES-KOH (pH 7.4), 50 mM KCl, 2 mM MgCl2, 2 mM EGTA, 10 μg/ml A/L, 2 mM ATP, 10 mM phosphocreatine, 5 mM succinate, and 50 μg/ml creatine kinase). Mitochondria were then incubated with GST-Bax, or GST-K128E Bax, or GST-Bcl-XL or GST-E158K Bcl-XL (20 nM each) for 30 min on ice. Na2CO3 (0.1 M final concentration) was added and samples were incubated for further 30 min on ice. Mitochondrial fraction and supernatant were separated by centrifugation at 20 000 × g for 10 min at 4°C. To assess Bax activation/oligomerization, isolated mitochondria were treated with trypsin (0.51 mg/ml final concentration) for 1.5 h at 30°C. Reaction was stopped by addition of equal volume of buffer 1 containing twofold concentrated protease inhibitors. Mitochondrial fraction and supernatant were separated by centrifugation at 20 000 × g for 10 min at 4°C and the former was assayed for the presence of Bax by western blot, using N-20 antibody (Santa Cruz, Santa Cruz, CA, USA) produced against a peptide mapping the N-terminus of Bax or Δ21 antibody (Santa Cruz) against an epitope corresponding to amino acids 1–171 of Bax.
Cytochrome c and Smac/Diablo release from isolated mitochondria
Mitochondria (obtained from 1 × 106 CTLL-2 or MEF cells) were isolated as described above and were incubated for 30 min on ice in buffer 1 with 5 nM GST-Bax, or 5 nM K128E GST-Bax, or 5 nM GST, or 5 nM tBid, or 15 nM GST-Bcl-2, or 15 nM GST-Bcl-xL, or 15 nM E158K GST-Bcl-xL, or 0.1 nM tBid + 20 nM full-length Bax (tBid and full length Bax were co-incubated for 15 min at 37°C prior to addition to the mitochondria to achieve activation of Bax) so that chimeric proteins could bind to mitochondria. The very low concentration of tBid is sufficient to activate full-length Bax in vitro, but too low to stimulate low amounts of Bax and Bak associating with the outer membrane of isolated mitochondria. The mitochondria were then pelleted and re-suspended in pre-warmed buffer 1 (37°C) and incubated for 5 min. The reaction was terminated by the addition of one volume of ice-cold buffer 1, centrifugation at 20 000 × g at 4°C. The supernatants were transferred into new tubes. Reducing SDS sample buffer was added to the supernatants and the pellets. The samples (pellet and supernatant) were analyzed for the release of cytochrome c or Smac/Diablo by western blotting.
Membrane potential in isolated mitochondria
The membrane potential of isolated mitochondria was determined by incubating purified mitochondria from Jurkat cells with 5 nM GST-Bax, or 5 nM GST-BaxK128E, or 5 nM GST, or 5 nM tBid, or 15 nM GST-Bcl-xL, or 15 nM GST-Bcl-xL E158K in buffer 1 supplemented with 10 nM 3,3(′)-dihexyloxacarbocyanine iodide (DioC6(3)), for 30 min at 4°C. Mitochondria were then pelleted and re-suspended in pre-warmed buffer 2 containing 10 nM DioC6(3), and the transmembrane potential (Δψm) was determined by flow cytometry. Hyperpolarization increases the accumulation of DioC6(3) in mitochondria, as indicated by a right shift of the fluorescence signal; depolarization is indicated by a left shift. Complete depolarization of mitochondria was achieved by incubation with 1 μM carbonyl cyanide 3-chlorophenylhydrazone (CCCP) for 10 min at room temperature and served as a control for the integrity of the mitochondria. The experiments were repeated with the Δψm indicator tetramethylrhodamine ethyl ester (TMRE) and yielded very similar results.
Flow cytometry studies
To identify cell death in pJK-K158E Bax-transfected cells, the cells were stained with FITC-coupled anti-Myc9E10 antibodies to detect the Myc-epitope present on the single chain antibody, which is also encoded by the pJK vector. Death was detected by staining with Cy3-labelled Annexin. Samples were analyzed using FACS-Calibur (Becton-Dickinson, Franklin Lakes, NJ, USA).
Co-immunoprecipitation
For the co-immunoprecipitation experiments, transfected MEFs were stimulated with staurosporine, lysed in 4% CHAPS, 5 mM MgCl2, 137 mM KCl, 1 mM EDTA, 1 mM EGTA, 20 mM Tris-HCl (pH 8.0), and 10 μg/ml aprotinin and leupeptin, and Bax/Bax K128E or Kv1.3 were immunoprecipitated from the lysates employing protein A/G agarose. The immunoprecipitates were washed six times in the lysis buffer, separated by 7.5 or 12.5% SDS-PAGE, blotted, and developed with anti-Kv1.3 or anti-Bax antibodies. An aliquot of the immunoprecipitates was blotted with the immunoprecipitating antibody to test for similar amounts of protein in all samples. Control immunoprecipitates were performed with isotype-matched irrelevant antibodies. Specificity of immunoprecipitations was proven with CTLL-pJK cells lacking Kv1.3 expression (data not shown).
Patch-clamp experiments
Whole-cell currents were recorded with an EPC 7 amplifier (List, Darmstadt, Germany (filter, 1 kHz; sampling rate, 5 kHz)), as described in Szabò et al.21 in Jurkat cells. Leak currents were not subtracted. The bath solution was composed of 150 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2, and 10 mM HEPES (pH 7.3). The intracellular solution contained 134 mM KCl, 1 mM CaCl2, 2 mM MgCl2, 10 mM EGTA, and 10 mM HEPES (pH 7.3). Intracellular voltages are reported, and outward currents are plotted upwards.
Planar lipid bilayer
A Warner Instruments (Hamden, CT, USA) BC-525C electrophysiological planar bilayer apparatus was used. Bilayers with a capacity of approximately 150 to 200 pF were prepared by painting a decane/chloroform solution of soybean asolectin (Sigma, Milan, Italy), partially purified by precipitation with cold acetone from a chloroform solution, across a 250-μm hole in a polystyrene cuvette. The inside of the cuvette constituted the trans compartment. The standard experimental medium contained 100 or 150 mM KCl, 0.5 mM EGTA, and 8 mM HEPES (pH 7.5). After the planar membrane had formed, salt concentration gradients were produced by adding equal volumes of the standard medium (in the trans compartment) and a medium with the same composition except for a higher concentration of KCl (in cis). The contents of both chambers were stirred by magnetic bars when necessary. Connections to the electrodes were provided by agar bridges. Protein was added to the cis side. Voltages reported are those of the cis chamber, and current is considered positive when carried by cations flowing from the cis compartment to the trans compartment. Output was recorded with a 10-KHz bandwidth on videotape using a Medical Systems (New York, NY, USA) PCM-2 interface. The data were later analyzed offline using the pCLAMP program set (Molecular Devices, Sunnyvale, CA, USA).
Acknowledgments
We are grateful for help with some experiments to U De Marchi, P Cusin, and M Zenere. The studies were supported by Italian Association for Cancer Research (AIRC to IS (Grant 5118) and MZ) and DFG Grant Gu 335/13-3.
Glossary
- AIF
apoptosis-inducing factor
- CSA
cyclosporine A
- DKO
double knockout deficient for Bax and Bak
- FACS
fluorescence-activated cell sorter
- Glu
glutamate
- IMM
inner mitochondrial membrane
- I-V plot
current–voltage relationship
- Kv
voltage-gated potassium channel
- MEF
mouse embryonic fibroblast
- Mgtx
margatoxin
- OMM
outer mitochondrial membrane
- PTP
permeability transition pore
- ROS
reactive oxygen species
- Smac/Diablo
second mitochondria-derived activator of caspase
- tBid
truncated Bid
- Tat
twin-arginine translocation
- WT
wild type
- IL-2
interleukin-2
- ATCC
American Type Culture Collection
- DioC6(3)
3,3(′)-dihexyloxacarbocyanine iodide
- DMEM
Dulbecco's modified Eagle's medium
- PBS
phosphate-buffered saline
- CCCP
carbonyl cyanide 3-chlorophenylhydrazone
- TMRE
tetramethylrhodamine ethyl ester
The authors declare no conflict of interest.
Footnotes
Edited by L Scorrano
References
- Wyllie AH, Golstein P. More than one way to go. Proc Natl Acad Sci USA. 2001;98:11–13. doi: 10.1073/pnas.98.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antignani A, Youle RJ. How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane. Curr Opin Cell Biol. 2006;18:685–689. doi: 10.1016/j.ceb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- Nie C, Tian C, Zhao L, Petit PX, Mehrpour M, Chen Q. Cysteine 62 of Bax is critical for its conformational activation and its proapoptotic activity in response to H2O2-induced apoptosis. J Biol Chem. 2008;283:15359–15369. doi: 10.1074/jbc.M800847200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, et al. Stepwise activation of Bax and Bak by tBid, Bim and Puma initiates mitochondrial apoptosis. Mol Cell. 2009;36:487–499. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annis MG, Soucie EL, Dlugosz PJ, Cruz-Aguado JA, Penn LZ, Leber B, et al. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 2005;24:2096–2103. doi: 10.1038/sj.emboj.7600675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel SL, Azimov R, Pawlowski K, Godzik A, Kagan BL, Reed JC. Ion channel activity of the BH3 only Bcl-2 family member, BID. J Biol Chem. 1999;274:21932–21936. doi: 10.1074/jbc.274.31.21932. [DOI] [PubMed] [Google Scholar]
- Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, et al. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- Schlesinger PH, Gross A, Yin XM, Yamamoto K, Saito M, Waksman G, et al. Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic Bcl-2. Proc Natl Acad Sci USA. 1997;94:11357–11362. doi: 10.1073/pnas.94.21.11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn AJ, Velez P, Schendel SL, Liang H, Muchmore SW, Fesik SW, et al. Bcl-x(L) forms an ion channel in synthetic lipid membranes. Nature. 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- Saito M, Korsmeyer SJ, Schlesinger PH. Bax-dependent transport of cytochrome c reconstituted in pure liposomes. Nat Cell Biol. 2000;2:553–555. doi: 10.1038/35019596. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- Martinez-Caballero S, Deajan LM, Kinnally MS, Oh KJ, Mannella CA, Kinnally KW. Assembly of the mitochondrial apoptosis-induced channel, MAC. J Biol Chem. 2009;284:12235–12245. doi: 10.1074/jbc.M806610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol. 2005;8:657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- Scorrano L. Proteins that fuse and fragment mitochondria in apoptosis: con-fissing a deadly con-fusion. J Bioenerg Biomembr. 2005;37:165–170. doi: 10.1007/s10863-005-6572-x. [DOI] [PubMed] [Google Scholar]
- Brenner C, Cadiou H, Vieira HL, Zamzami N, Marzo I, Xie Z, et al. Bcl-2 and Bax regulate the channel activity of the mitochondrial adenine nucleotide translocator. Oncogene. 2000;19:329–336. doi: 10.1038/sj.onc.1203298. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Chandel NS, Li XX, Schumacker PT, Colombini M, Thompson CB. Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proc Natl Acad Sci USA. 2000;97:4666–4671. doi: 10.1073/pnas.090082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Narita M, Tsujimoto Y. Bcl-2 proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- Szabò I, Bock J, Jekle A, Soddemann M, Adams C, Lang F, et al. A novel potassium channel in lymphocyte mitochondria. J Biol Chem. 2005;280:12790–12798. doi: 10.1074/jbc.M413548200. [DOI] [PubMed] [Google Scholar]
- Szabó I, Bock J, Grassmé H, Soddemann M, Wilker B, Lang F, et al. Mitochondrial potassium channel Kv1.3 mediates Bax-induced apoptosis in lymphocytes. Proc Natl Acad Sci USA. 2008;105:14861–14866. doi: 10.1073/pnas.0804236105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, et al. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev. 2005;57:473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- Gulbins E, Sassi N, Grassmè H, Zoratti M, Szabò I. Role of Kv1.3 mitochondrial potassium channel in apoptotic signalling in lymphocytes. Biochim Biophys Acta. 2010;1797:1251–1259. doi: 10.1016/j.bbabio.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Antonsson B, Montessuit S, Lauper S, Eskes R, Martinou JC. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem J. 2000;345:271–278. [PMC free article] [PubMed] [Google Scholar]
- Lucken-Ardjomande S, Montessuit S, Martinou JC. Bax activation and stress-induced apoptosis delayed by the accumulation of cholesterol in the mitochondrial membranes. Cell Death Differ. 2008;15:484–493. doi: 10.1038/sj.cdd.4402280. [DOI] [PubMed] [Google Scholar]
- Zoratti M, De Marchi U, Gulbins E, Szabò I. Novel channels of the mitochondrial inner membrane. Biochim Biophys Acta. 2009;1787:351–363. doi: 10.1016/j.bbabio.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Szewczyk A, Kajma A, Malinska D, Wrzosek A, Bednarczyk P, Zabłocka B, et al. Pharmacology of mitochondrial potassium channels: the dark side of the field. FEBS Lett. 2010;584:2063–2069. doi: 10.1016/j.febslet.2010.02.048. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Debska-Vielhaber G, Siemen D. Interaction of the mitochondrial potassium channels with the permeability transition pore. FEBS Lett. 2010;584:2005–2012. doi: 10.1016/j.febslet.2009.12.038. [DOI] [PubMed] [Google Scholar]
- Bednarczyk P, Kowalczyk JE, Beresewicz M, Dołowy K, Szewczyk A, Zabłocka B. Identification of a voltage-gated potassium channel in gerbil hippocampal mitochondria. Biochim Biophys Res Commun. 2010;397:614–620. doi: 10.1016/j.bbrc.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Rauer H, Pennington M, Cahalan M, Chandy KG. Structural conservation of the pores of calcium-activated and voltage-gated potassium channels determined by a sea anemone toxin. J Biol Chem. 1999;274:21885–21892. doi: 10.1074/jbc.274.31.21885. [DOI] [PubMed] [Google Scholar]
- Heimlich G, McKinnon AD, Bernardo K, Brdiczka D, Reed JC, Kain R, et al. Bax-induced cytochrome c release from mitochondria depends on alpha-helices-5 and -6. Biochem J. 2004;378:247–255. doi: 10.1042/BJ20031152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson CB, et al. Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc Natl Acad Sci USA. 1995;92:7834–7838. doi: 10.1073/pnas.92.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epand RF, Martinou JC, Montessuit S, Epand RM, Yip CM. Direct evidence for membrane pore formation by the apoptotic protein Bax. Biochem Biophys Res Comm. 2002;298:744–749. doi: 10.1016/s0006-291x(02)02544-5. [DOI] [PubMed] [Google Scholar]
- Ott M, Zhivotovsky B, Orrenius S. Role of cardiolipin in cytochrome c release from mitochondria. Cell Death Differ. 2007;14:1243–1247. doi: 10.1038/sj.cdd.4402135. [DOI] [PubMed] [Google Scholar]
- Pellegrini L, Scorrano L. A cut short to death: Parl and Opa1 in the regulation of mitochondrial morphology and apoptosis. Cell Death Differ. 2007;14:1275–1284. doi: 10.1038/sj.cdd.4402145. [DOI] [PubMed] [Google Scholar]
- Norberg E, Orrenius S, Zhivotovsky B. Mitochondrial regulation of cell death: processing of apoptosis-inducing factor (AIF) Biochem Biophys Res Comm. 2010;396:95–100. doi: 10.1016/j.bbrc.2010.02.163. [DOI] [PubMed] [Google Scholar]
- Kim JS, Lee JH, Jeong WW, Choi DH, Cha HJ, Kim do H, et al. Reactive oxygen species-dependent EndoG release mediates cisplatin-induced caspase-independent apoptosis in human head and neck squamous carcinoma cells. Int J Cancer. 2008;122:672–680. doi: 10.1002/ijc.23158. [DOI] [PubMed] [Google Scholar]
- Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, et al. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabò I, Zoratti M, Gulbins E. FEBS Lett. 2010. pp. 2049–2056. [DOI] [PubMed]