Abstract
To address an existing controversy concerning the presence of HIV-1-specific antibodies of the IgA isotype in the female genital tract secretions of highly-exposed but persistently seronegative (HEPSN) women, 41 samples of plasma and cervicovaginal lavage (CVL) fluid were distributed to six laboratories for their blinded evaluation using ELISA with 10 different HIV-1 antigens, chemiluminescence-enhanced Western blots (ECL-WB), and virus neutralization. HIV-specific IgG or IgA antibodies in plasma samples from HEPSN women were absent or detectable only at low levels. In CVL, 11/41 samples displayed low levels of reactivity in ELISA against certain antigens. However, only one sample was positive in two of five laboratories. All but one CVL sample yielded negative results when analyzed by ECL-WB. Viral neutralizing activity was either absent or inconsistently detected in plasma and CVL. Plasma and CVL samples from 26 HIV-1-infected women were used as positive controls. Irrespective of the assays and antigens used, the results generated in all laboratories displayed remarkable concordance in the detection of HIV-1-specific antibodies of the IgG isotype. In contrast, IgA antibodies to HIV-1 antigens were not detected with consistency, and where present, IgA antibodies were at markedly lower levels than IgG. Although HIV-neutralizing activity was detected in plasma of all HIV-1-infected women, only a few of their CVL samples displayed such activity. In conclusion, frequent HIV-1 sexual exposure does not stimulate uniformly detectable mucosal or systemic HIV-1-specific responses, as convincingly documented in the present blindly performed study using a broad variety of immunological assays. Although HIV-1-infection leads to vigorous IgG responses in plasma and CVL, it does not stimulate sustained IgA responses in either fluid.
Introduction
The correlates of protection against mucosal acquisition and control of HIV-1 infection have not been clearly defined. Humoral factors, innate immunity, and specific antibodies present in external secretions, as well as cytotoxic lymphocytes distributed in mucosal tissues, have been considered in the prevention and local limitation of HIV-1 and SIV at mucosal sites of viral entry.1–4 The protective effect of systemic or locally administered monoclonal, virus-neutralizing antibodies of the IgG isotype against vaginal viral challenge has been most convincingly demonstrated in the macaque-SHIV model.5–7 Furthermore, pentameric IgM, polymeric IgA, and secretory IgA HIV-1-specific antibodies can neutralize HIV-1 and inhibit in vitro transcytosis of HIV-1 through monolayers of epithelial cells and mediate intraepithelial virus neutralization.8–13
The protective role of HIV-1-specific antibodies of the IgA isotype in secretions of the genital tract (vaginal washes and semen) was also inferred from several studies of HIV-1-exposed but persistently seronegative (HEPSN) female sex workers and males.8,14–29 These reports suggest that HIV-1-specific IgA antibodies may interact with, and probably neutralize, free HIV-1 in mucosal secretions, as well as HIV-1 within certain populations of cells that internalize IgA due to the presence of IgA-specific cellular receptors.10–13,30 In contrast, other investigators have not detected such mucosal antibodies in several similar cohorts of HEPSN women from the United States and Africa.31–33 In our previous studies addressing potential methodological problems,34 samples of rectal washes from 30 HIV-1-infected and healthy controls were sent blindly to six US and European laboratories for evaluation of HIV-1-specific IgA and IgG antibodies. The results clearly indicated that, although the detection of IgG antibodies in different laboratories is comparable with respect to their frequency and levels, the measurement of HIV-1-specific IgA antibodies displays marked variability and often yielded false-positive results.35–37 Furthermore, although in rectal washes the levels of total IgA were much higher than those of IgG, HIV-1- specific antibodies were mainly of the IgG isotype.34 The subsequent extension of analogous studies to sera and other external secretions (tears, saliva, urine, semen, and vaginal and nasal washes) indicated that in HIV-1-infected individuals, humoral IgA responses to HIV-1 in sera and all secretions examined are less frequent, and when present, occur at significantly lower levels than those of IgG.1,34,38–43 Interestingly, low or absent IgA responses were reported in external secretions of HIV-1-infected chimpanzees44 and SIV-infected macaques.45 Obviously, in striking contrast to other mucosally encountered microbial infections,46,47 HIV-1 and SIV do not induce vigorous specific IgA responses in any body fluid examined. A mechanism involved in this selective hyporesponsiveness in the IgA isotype to the HIV-1 infection has been recently elucidated.48
The purpose of this report was to evaluate, in a blinded fashion, plasma and cervicovaginal lavage (CVL) samples collected from HIV-1-infected and HEPSN sex workers for the presence of HIV-1-specific IgG and IgA antibodies, using a broad spectrum of HIV-1 antigens and immunochemical reagents in a variety of conventional assays (ELISA, chemiluminescence-enhanced Western blotting, and virus neutralization) in the six participating laboratories.
Materials and Methods
Subjects
The 67 subjects for this study were selected from participants in a high risk cohort of 600 barworkers HIV Superinfection Study (HISIS study) that had been recruited in the year 2000 and were followed up every 3 months for 5 years. This cohort was set up to study HIV superinfection and correlates of protection from HIV-1-infection and was conducted in the Mbeya Region of Southwestern Tanzania. Detailed descriptions of the cohort have been published elsewhere.49,50 The initial HIV-1 prevalence was 67% and within the remaining 198 HEPSN women, the average incidence of HIV-1 acquisition over the first 3 years was 7% per year. The 67 samples for this sub-study were collected during a follow-up visit 4 years after enrollment. The mean age was 30 years (range 21–40 years); there was no difference in the mean age between HIV-1-infected and uninfected women. For the HEPSN women, the mean CD4+ T cell count was 842/μl (range 513–1464/μl). In the group of HIV-seropositive women who had been infected for at least 4 years, the mean CD4 count was 367/μl (range 56–1049/μl).
Forty-one sera and corresponding CVL samples were from HEPSN women who remained HIV-1-uninfected despite continuous high-risk behavior for HIV-1 over the last 4–6 years. Inclusion criteria for this group were that they had worked at least for the last 4 years as bar workers with regular sexual activity and without regular condom usage. To assess their sexual activity, several strategies were developed, such as trimonthly structured interviews, diary cards, and focus group discussions.50 As positive controls, plasma and CVL samples were collected from 26 HIV-infected women who were randomly selected from the same HISIS cohort. The HISIS study was approved by the local and national Ethical Review Board of Tanzania. During the study, all participants received health care that included treatment of all acute infectious diseases, screening and treatment of sexually transmitted diseases, and since 2003, clotrimoxazole prophylaxis for opportunistic infections in women with CD4 T-cell counts below 200/μl. Since 2005, antiretroviral treatment has been available for all participants with AIDSdefining symptoms or CD4 counts below 200/μl. Samples obtained during the course of this study were collected from individuals who were not on antiretroviral therapy.
Collection of samples
Blood was collected by venipuncture into Sarstedt monovette tubes containing citrate phosphate dextrose adenine to obtain plasma. The CVL specimens were obtained by vigorous flushing of the cervix and vagina with 5 ml sterile saline; the washings were subsequently collected from the posterior vaginal vault.49–51 The CVL was promptly frozen and held at −40°C until aliquoted, refrozen, and sent to the individual labs. Protease inhibitors were not added to the specimens on collection.
ELISA for immunoglobulin (Laboratories A and B)
The total IgG, IgA, or IgM concentration in plasma and CVL was quantitated by ELISA as described.34,42,52 Briefly, high protein-binding 96-well microplates (Nalge Nunc International, Rochester, NY) were coated with 1 μg/ml goat polyclonal anti-human IgA, IgG, or IgM (Jackson ImmunoResearch Laboratories, West Grove, PA), and then blocked with 5% goat serum in PBS-0.05% Tween 20. Duplicates of 2-fold serially diluted samples and a human Immunoglobulin (Ig) reference serum (Human Immunoglobulin Calibrator; Binding Site, Birmingham, U.K.) were added to the plates and maintained overnight at 4°C. The captured Ig was detected after consecutive incubations with biotin-labeled goat F(ab')2 specific for human IgA, IgG, or IgM (BioSource, Camarillo, CA), horseradish (HRP)-labeled avidin (Sigma, St. Louis, MO), H2O2 in O-phenylenediamine (Sigma), and 1 M H2SO4 stop solution. After recording the absorbance at 490 nm, the concentration of total IgA, IgG or IgM was interpolated from four-parameter standard curves constructed with the DeltaSoft 3 program (BioMettalics, Princeton, NJ). The sensitivity of the assay was less than 1 ng/ml.
Antigens and assays for HIV-1-specific antibodies
Laboratory A
The antigen used in the capture ELISA was the HIV-1 consensus B (ConB) gp120.53–55 The HIV-1 ConB env sequence was codon-optimized to enhance protein expression in vitro as previously described53,56,57 and is available at Genbank under accession number DQ 667594. The ConB gp120 was produced by transfecting 293T17 embryonic kidney cells (ATCC, Manassas, VA) with the corresponding plasmid (ConB env with a premature stop-codon immediately prior to the gp160 cleavage site) using FuGene6 (Roche Applied Science, Indianapolis, IN). After 72 h, the culture supernatant was harvested and clarified by centrifugation and 0.22 μm filtration. The gp120 was purified using lectin chromatography with agarose-immobilized (α-1,3) mannose-specific Galanthus nivalis (Vector laboratories, Burlingame, CA) and 500 mM alpha-methyl mannoside (Vector) as eluant. After dialysis in PBS, the purity of the gp120 was confirmed by gel electrophoresis and Western blotting with HIV-1-positive human serum. The concentration was determined with the BCA protein assay kit (Pierce Biotechnology, Rockford, IL).
ELISA
The gp120-specific antibody in specimens was measured using previously described methods.34,42,52 Briefly, MaxiSorp 96-well plates (Nalge Nunc) were coated overnight at room temperature with 1 μg/ml ConB gp120. For generation of standard curves, two rows of each plate were instead coated with 1 μg/ml goat polyclonal F(ab')2 anti-human IgG, IgA, or IgM. The following day, plates were washed and blocked as above. Serial dilutions of CVL or plasma specimens and positive control human serum containing HIV-specific IgA or IgG34,42 were placed in the gp120-coated portions. The wells for the standard curve were loaded with known amounts of purified colostral IgA58 (for IgA assays with CVL) or the above-described human Ig reference serum (for plasma IgA and IgG assays). After an overnight incubation at 4°C, the plates were developed as above, and the concentration of gp120-specific IgG or IgA in samples was similarly determined from four-parameter standard curves. Samples were designated anti-HIV antibody positive if the concentrations were 2 SD above the mean of the values obtained with negative controls. The concentration of gp120-specific antibody was divided by the concentration of total IgA or IgG in the sample to determine the percent of specific antibody within each isotype.
Chemiluminescence-enhanced Western blot (ECL-WB) analysis34,42,59,60
Nitrocellulose HIV-1 strips (Maxim Biomedical, Rockville, MD) were used. The strips contain 9 HIV-1 proteins derived from HIV-1IIIB-infected H9 T cells. Separate strips were incubated overnight at 4°C with appropriately diluted samples (1:1,000 to 1:5,000 for plasma, and 1:100 to 1:200 for CVL) in SuperBlock buffer. Samples that reacted too strongly were retested at 5- to 10-fold greater dilutions. The following day, strips were developed using the above biotinylated antibodies, neutravidin-peroxidase (Southern Biotech, Birmingham, AL), and SuperSignal chemiluminescent substrate (Pierce). Imaging and scoring of band intensity was done as previously described.34,42 Sera from HIV-1-infected or healthy individuals were used as positive and negative controls.
Neutralizing assay
Viral inhibitory activity in plasma and CVL samples were analyzed as previously described using the TZM-bl cell line.53,61 Briefly, TZM-bl 104 cells per well were incubated overnight in 96-well tissue culture plates to reach 60% confluency. In separate plates (TC Microwell Nunclon D; Nunc), CVL or plasma specimens were serially-diluted in 10% FCS/DMEM to a final volume of 120 μl/well. Positive and negative control plasma and CVL samples were included in every assay to ensure validity and reproducibility of the results. The diluted samples were incubated for 1 h with an equal volume of medium containing 80 μg/mL DEAE-dextran61 and recombinant HIV-1NL4.3 at a concentration of 3000 infectious particles/ml. A 100 μl volume of each sample-virus mixture was then transferred to plated TZM-bl cells. At least 6 wells per plate were incubated with virus and medium alone to assess the maximum infectivity and background, respectively. After incubation for 48 h at 37°C in 5% CO2, the media was removed and 70 μl of Reporter lysins buffer (Promega, Madison, WI) was added to each well. The plates were frozen at −70°C and subjected to two freeze-thaw cycles. Luciferase activity was measured using a Perkin-Elmer luminometer after transferring 20 μl cell lysate to a white 96 well Clinicplate (Thermo Electron, Vantaa, Finland) and adding 100 μl per well of Luciferase Assay System substrate (Promega).61 The reduction of viral infectivity was calculated by comparing the average relative light units in wells cultured with virus alone. The highest dilution of a sample that inhibited viral infection by 50% was regarded as the neutralization titer.
Laboratory B
ELISA
Concentrations of HIV-1-specific IgA and IgG in plasma and CVL were measured using a chromogenic ELISA as previously described34 with the following modifications. Plates were coated with aldrithiol-2-inactivated Clade C HIV-1TZA virus (from Dr. Jeff Lifson, AIDS and Cancer Program, Frederick, MD) at 200 ng capsid per well after lysis with 0.25% v/v Triton X-100. In preliminary analyses with HISIS blinded plasma samples, this Clade C viral lysate was deemed optimal to Clade B HIV-1MN lysate (Advanced Biotechnologies Inc, Columbia, MD) because IgA antibodies were more frequently observed against the former. For TZA IgG ELISA, the standard was human polyclonal anti-HIV-1 p24 IgG antibody (ImmunoDiagnostics, Woburn, MA). For the IgA ELISA, the standard was IgG-depleted, pooled HIV-1-positive human serum that had been calibrated relative to the IgA in the above human Ig serum reference standard by coating different portions of a plate with anti-human IgA antibody or viral lysate. All CVL or plasma samples were depleted of IgG as described34 before performing ELISA for IgA antibodies. Plasma was considered positive for HIV-1-specific IgG or IgA if it contained a concentration >mean concentration + 3 SD obtained for plasma from 20 low-risk HIV-1-uninfected individuals. A CVL sample was considered positive if the specific activity (ng anti-HIV IgG or IgA per μg of total IgG or IgA, respectively) was >mean specific activity + 3SD obtained for 10 CVL from low risk HIV-uninfected women (provided by co-authors at the University of Alabama at Birmingham).
Laboratory C
ELISA
Measurement of gp41-specific antibodies was performed by chromagenic ELISA as described34,62,63 using 384-well Maxisorp plates (Nalgene/NUNC #460518) coated with 2 μg/ml gp41 peptide 1 (WGCSGKLICTTAVPW) or gp41 peptide 2 (NEQELLELDKWASLW) in pH 9 carbonate buffer. The peptides were synthesized as 4-branch MAPS peptides that include a SGGRGG spacer at the C-terminal residue to distance the gp41 sequence from the MAPS (CPC Scientific; San Jose, CA). A serum sample from an HIV-1-seropositive individual was included as a positive control on each plate. The 2F5 antibody reacted with peptide 2 but not peptide 1, as expected. Plasma samples were tested 2–3 times in dilutions ranging from 1:32 to 1:4096. The CVL were tested at 1:8 to 1:1024 dilutions. The reciprocal endpoint titers were determined as the last sample dilution that produced a 3-fold greater absorbance than that of the sample diluent.
Laboratory D
ELISA
An HIV-1 Clade C recombinant gp12096ZM651 protein (NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH) was used to coat plates at 100 ng per well. Plasma and CVL were tested by kinetic ELISA (kELISA) adapted from a previously described assay for measurement of antibodies to the influenza virus.34,64 The kELISA measures substrate activation every 9 sec over the first 5 min of the enzymatic assay and plots the change in color per minute as mOD/min using a Thermomax microplate reader (Molecular Devices, Sunnyvale, CA). With other mucosal samples, the kELISA has been found to reduce background and increase sensitivity.34,64 Uncoated wells were included on each plate to determine background, which was subtracted from the result. A standard curve with the 91BU003 HIV-1-positive serum specimen (NIH AIDS Research and Reference Reagent Program, contributed by Dr. Harvey Holmes) was included in each assay as a measure of sensitivity and reproducibility. Biotinylated goat anti-human IgA and IgG conjugates (Biosource) were used with streptavidin-HRP and ABTS (Sigma) to develop plates.
Laboratory E
ELISA
Nunc Maxisorp microtiter plates were coated overnight at 4°C with 0.1 μg/well HIV-1 rgp41 (Sorin Biomedica, Torino, Italy) or the above Clade C rgp12096ZM651 protein at 0.05 μg/well (for IgA assays) or 0.025 μg/well (for IgG assays) diluted in 50 mM pH 9.5 carbonate buffer. The rgp41 protein was produced in Escherichia coli and comprises the extracellular region without the fusogenic peptide at the amino terminus.65 The following day, both Env-coated and uncoated plates were blocked for 1 h at 37°C with PBS containing 5% skim milk (Sigma), 5% FBS (Biochrom, Berlin, Germany), and 0.1% Tween 20 (Sigma). Duplicate 2-fold dilutions of heat-inactivated specimens were added at 50 μl/well to all plates. Positive and negative controls were plasma or CVL samples from HIV-1-infected and healthy HIV-1-unexposed Africans, respectively (a gift from Dr. G. Pancino, Institut of Pasteur, Paris, France). After 1 h at 37°C, the plates were washed and treated for 1 h at room temperature with 100 μl/well of 1:5,000 biotinylated goat anti-human IgA or 1:2,000 anti-human IgG (Southern Biotech, Birmingham, AL), then 1:3,000 HRP-conjugated streptavidin (Vector). Plates were developed by adding 100 μl/well of TMB (KPL, Gaithersburg, MD) and stopping the reaction with 50 μl/well of 10% H2SO4 after 5 or 13 min for IgG or IgA assays, respectively. Absorbance was read at 450 nm using a BioRad Microplate Reader (Hercules, CA). The absorbance values recorded for uncoated plates were subtracted from those on the Env-coated plates. Cut-offs for each sample dilution were subsequently determined by calculating the mean “adjusted” absorbance + 3 SD for negative controls. The reciprocal endpoint titer of antibody in plasma or CVL from the HISIS cohort was defined as the last sample dilution that produced an absorbance greater than the cut-off.
Virus isolation and titration
A primary virus named CC.1 was obtained as previously described65,66 after co-culturing equal numbers of phytohemagglutinin (PHA)-activated peripheral blood mononuclear cells (PBMC) from HIV-1-infected and uninfected African subjects. The PBMC were co-cultured for 3 weeks in RPMI-1640 medium supplemented with 20% FBS and IL-2. Once a week, fresh PHA-activated PBMC were added to the cultures. Positive cultures were identified using a p24 ELISA kit (AALTO Bio Reagents Ltd, Dublin, Ireland). The infectious activity (TCID 50/ml) of the selected CC.1 viral stock was determined by seeding 5-fold dilutions of virus into six parallel wells of a round-bottomed microtiter plate (Nunc, Roskilde, Denmark) that contained 105 resting PBMC in 75 μl medium supplemented with PHA and 10 U/ml rIL-2 (Amersham, Buchinghamshire, UK). After 2 h, the cultures were washed and resuspended in IL-2-containing medium. After incubation for 7–9 days, the wells were scored positive or negative based on p24 content. The Reed–Muench formula was then used to calculate the TCID50/ml.
Neutralizing assay
Viral inhibitory activity in plasma or CVL was evaluated using PBMC and primary virus as described with slight modifications.12,25–28 Due to the low volume of samples, only one R5 primary strain (CC.1) was used. In brief, 75 μl of CC.1 viral supernatant (containing 19 or 56 TCID50) was incubated with 75 μl diluted specimen for 1 h. The virus/sample mixture was then added to 2 × 105 PHA-activated PBMC and incubated for 2 h. The cultures were washed, maintained in medium for 7–9 days, then evaluated for supernatant p24. Each set of assays was performed with 26 plasma and 14 CVL from HIV-unexposed Africans as negative controls. A pool of 3 neutralizing anti-HIV-1 monoclonal antibodies (TRIMAB) was used as a positive control. Due to possible interference of the mucosal components with neutralizing assays, all CVL were enriched for Ig by precipitation in 55% (NH4) 2SO4 followed by gel-filtration on a Bio-Select SEC 400 column (BioRad) and concentration with an Amicon Ultra Centrifugal filter MW 30,000 (Millipore Corporation, Bedford, MA). The CVL were reconstituted with medium to their original volume before analysis. The mean p24 value obtained with a specific sample dilution was compared to the mean p24 values from six corresponding replicates in the absence of sample. The values are expressed as IC50 (last sample dilution resulting in 50% inhibition of viral infection).
Laboratory F
Neutralizing assay
Virus neutralization was measured using a recombinant virus assay that involves a single round of infection.67,68 Pseudotyped viral particles were produced by cotransfection of HEK-293 cells with a replication-defective retroviral vector containing a luciferase gene along with env expression vectors containing plasma-derived viral envelope sequences. The pseudovirus stocks were incubated with serial dilutions of heat-inactivated plasma or nonheat-inactivated CVL for 1 h and then used to infect cells expressing CD4, CCR5, and CXCR4 (CD4 + /CCR5 + /CXCR4 + /U87). The ability of antibody in the plasma or CVL to neutralize HIV-1 infectivity was assessed by measuring inhibition of luciferase activity 72 h after viral inoculation as compared to a control infection with murine leukemia virus envelope (aMLV) pseudotyped virus.
Statistics
Calculations of geometric means and statistical comparisons were done using the Statview 5 computer program (Amazon.com). GraphPad Prism software (La Jolla, CA) was used to construct Tukey box plots. Correlation analyses were done by Spearman rank using Fisher's r-to-z conversion for p values. The levels of Ig or specific antibody in HIV-1-infected and HEPSN subjects were compared using the Mann–Whitney U Test. The frequencies of antibody-positive samples were compared using the Fisher's Exact Test. Results were considered significant if p values <0.05 were obtained.
Results
Levels of total Ig in plasma and CVL
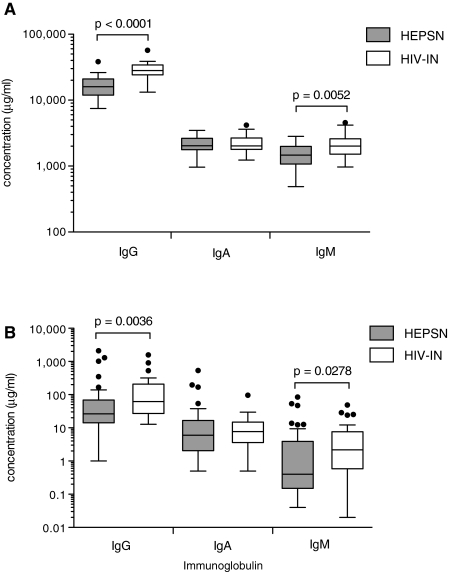
Levels of total IgG, IgM, and IgA were determined by ELISA in plasma (Laboratory A) and in CVL (Laboratories A and B). Results presented in Fig. 1A indicate that HIV-1-infected women had significantly increased plasma levels of total IgG and IgM, but similar levels of IgA, when compared to HEPSN women. The IgG values in the HEPSN Tanzanian women were substantially increased over normal values in the United States.42 In CVL, the levels of total IgG and IgM, but not IgA, were also higher in HIV-1-infected women when compared to HEPSN women (Fig. 1B). In contrast to the Ig levels in plasma, CVLs displayed much greater variability. This is probably due to the fact that samples were collected irrespective of the stage of the menstrual cycle. It is well known that the levels of Ig in CVL are hormonally dependent and display remarkable variation related to the stage of the menstrual cycle at which the sample is collected.69
FIG. 1.
Immunoglobulin concentrations. Total IgG, IgA, and IgM were measured by ELISA in (A) plasma and (B) CVL from the 41 HEPSN women and 26 HIV-1-infected women. Concentrations are presented as Tukey box plots with outliers denoted by circles. The Mann–Whitney rank sum test was used to compare concentrations of IgG, IgA, or IgM in the two groups of women.
HIV-1-specific antibodies
Three independent assays (ELISA, ECL-WB, and virus neutralization) with laboratory-specific variations described in Methods were used to determine the presence of HIV-1-specific antibodies of the IgG and IgA isotypes in the plasma and CVL of the 26 HIV-1-infected and 41 HEPSN women.
HIV-1-infected women
HIV-1 Env-specific antibodies of the IgG isotype were present in the plasma and CVL of all HIV-1-infected women irrespective of the assays and antigens used in Laboratories A–E (Tables 1 and 3), with the exception of a response to gp 41 peptide 2 used in Laboratory C, where only 8 of 26 samples were positive (Table 1). Using ECL–WB, the results indicated broad reactivity and strong intensity of the nine bands measured (Table 3). Three laboratories performed neutralization assays and two of the three readily detected antibodies to their representative strains. The exception was a primary clade C strain to which only three of the HIV-1-infected woman had detectable antibody. By ELISA, HIV-1-specific antibodies of the IgA isotype were routinely detected in plasma specimens of HIV-1-infected women, with the exception of the gp41 peptide 1 (Table 1). The titers of specific antibodies varied among laboratories due to the differences in antigens that were used for plate coating and secondary antibodies, but in general, the titers of IgA antibodies were considerably lower than those of IgG. Using ECL-WB, 25/26 plasma samples were positive for IgA antibodies but most samples had fewer positive bands of lower intensity (Table 3).
Table 1.
HIV-1 Protein- or Peptide-Specific Antibodies Detected in Plasma Using ELISA
| |
Laboratory A |
Laboratory B |
Laboratory C |
Laboratory D |
Laboratory E |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Clade B gp120 |
Clade C HIV lystate |
gp41 peptide 1 |
gp41 peptide 2 |
Clade C gp120 |
Clade B gp41 |
Clade C gp120 |
||||||
| |
IgG |
IgA |
IgG |
IgA |
IgG |
IgA |
IgG |
IgG |
IgA |
IgG |
IgA |
IgG |
IgA |
| (% of total) | (μg/ml) | (reciprocal endpoint titer) | (mOD/min) | (reciprocal endpoint titer) | |||||||||
| HIV-1 infected subjects | |||||||||||||
| 1 | 0.47 | 0.21 | 2,352.2 | 18.3 | > 4,096 | – | – | 523.9 | 306.8 | >640 | >640 | >640 | >640 |
| 2 | 1.07 | 0.41 | 7,198.7 | 51.4 | > 4,096 | – | >4,096 | 542.7 | 76.9 | >640 | >640 | >640 | >640 |
| 3 | 1.69 | 0.04 | 707.8 | – | > 4,096 | – | 1,024 | 492.8 | 70.8 | >640 | >640 | >640 | >640 |
| 4 | 0.39 | 0.07 | 5,652.0 | 6.4 | > 4,096 | – | – | 632.9 | 127.6 | >640 | >640 | >640 | >640 |
| 5 | 1.34 | – | 11,315.6 | 159.8 | > 4,096 | – | 512 | 558.2 | – | >640 | >640 | >640 | >640 |
| 6 | 1.72 | 0.24 | 757.2 | 1.2 | > 4,096 | 512 | – | 634.7 | 365.4 | >640 | >640 | >640 | >640 |
| 7 | 1.43 | 0.04 | 2,966.6 | 9.3 | > 4,096 | – | – | 598.8 | 18.9 | >640 | >640 | >640 | >640 |
| 8 | 3.24 | 0.07 | 1,240.4 | 3.1 | > 4,096 | – | – | 599.5 | 25.9 | >640 | >640 | >640 | >640 |
| 9 | 1.36 | – | 701.2 | – | > 4,096 | – | – | 829.5 | 17.5 | >640 | >640 | >640 | >640 |
| 10 | 1.49 | 0.06 | 4,792.2 | 2.3 | > 4,096 | – | – | 890.2 | 111.7 | >640 | >640 | >640 | >640 |
| 11 | 1.26 | 0.03 | 1,406.9 | 1.2 | > 4,096 | – | – | 582.6 | 334.4 | >640 | >640 | >640 | >640 |
| 12 | 1.34 | – | 1,542.4 | 1.2 | > 4,096 | – | – | 454.8 | – | >640 | nd | >640 | nd |
| 13 | 1.83 | – | 7,566.2 | 6.9 | > 4,096 | – | – | 520.9 | 15.0 | >640 | >640 | >640 | >640 |
| 14 | 1.28 | 0.24 | 2,062.4 | 0.8 | > 4,096 | – | >4,096 | 757.8 | 101.6 | >640 | >640 | >640 | >640 |
| 15 | 3.10 | 0.25 | 11,987.1 | 155.4 | > 4,096 | – | >4,096 | 485.5 | 159.4 | >640 | >640 | >640 | >640 |
| 16 | 0.95 | – | 238.1 | – | > 4,096 | – | – | 636.7 | 11.4 | >640 | nd | >640 | >640 |
| 17 | 1.95 | 0.28 | 18,561.1 | 81.2 | > 4,096 | >4,096 | – | 733.6 | 583.3 | >640 | >640 | >640 | >640 |
| 18 | 1.28 | 0.12 | 330.5 | – | > 4,096 | – | – | 654.3 | 55.0 | >640 | >640 | >640 | >640 |
| 19 | 2.32 | – | 2,152.6 | – | > 4,096 | – | >4,096 | 752.7 | 26.3 | >640 | 640 | >640 | >640 |
| 20 | 2.46 | – | 391.0 | – | > 4,096 | – | – | 629.2 | 13.6 | >640 | nd | >640 | >640 |
| 21 | 0.40 | 0.10 | 4,089.0 | 16.8 | > 4,096 | – | – | 642.8 | 35.1 | >640 | >640 | >640 | >640 |
| 22 | 1.79 | – | 6,420.4 | 8.0 | > 4,096 | – | – | 537.3 | 32.1 | >640 | >640 | >640 | >640 |
| 23 | 1.16 | 0.26 | 1,149.7 | – | > 4,096 | – | – | 627.1 | 26.1 | >640 | >640 | >640 | >640 |
| 24 | 1.66 | – | 2,852.9 | 22.9 | > 4,096 | – | – | 676.2 | 112.5 | >640 | 640 | >640 | >640 |
| 25 | 1.12 | – | 852.0 | – | > 4,096 | – | 4,096 | 829.7 | – | >640 | >640 | >640 | >640 |
| 26 | 1.39 | 0.19 | 1,146.7 | 1.2 | > 4,096 | – | 128 | 869.3 | 28.5 | >640 | >640 | >640 | >640 |
| HEPSN subjects | |||||||||||||
| 1 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 2 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 3 | – | – | 1.4 | – | – | – | – | – | – | – | – | – | – |
| 4 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 5 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 6 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 7 | – | – | – | – | – | – | – | – | – | >640 | 20 | – | – |
| 8 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 9 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 10 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 11 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 12 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 13 | – | – | – | – | 512 | – | 128 | – | – | – | – | – | – |
| 14 | – | – | – | – | – | – | – | – | – | – | 40 | – | – |
| 15 | – | – | – | – | – | – | 512 | 35.8 | – | – | – | – | – |
| 16 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 17 | – | – | – | – | – | – | – | 16.2 | – | – | – | – | – |
| 18 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 19 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 20 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 21 | – | – | – | – | 1024 | – | 512 | – | – | – | 20 | – | – |
| 22 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 23 | – | – | – | – | 512 | – | – | – | – | – | – | – | – |
| 24 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 25 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 26 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 27 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 28 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 29 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 30 | – | – | – | – | – | – | – | – | – | – | – | – | |
| 31 | – | – | – | – | – | – | 256 | – | – | – | – | – | – |
| 32 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 33 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 34 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 35 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 36 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 37 | – | – | – | – | – | – | – | – | – | – | 640 | – | – |
| 38 | – | – | – | – | – | – | 256 | – | – | – | – | – | – |
| 39 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 40 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 41 | – | – | – | – | – | – | – | 49.3 | – | – | – | – | – |
Values presented are for specimens that were reported to contain significant anti-HIV-1 antibody. Dashes denote the samples which were negative. In Laboratory B, 0/41 HEPSN plasma tested positive for gp41-specific IgA using an ELISA with gp41MN capture antigen (not shown). nd: not done.
Table 3.
HIV-1-Specific Antibodies Detected in Plasma By ECL-WB
| |
gp160 |
gp120 |
p66 |
p55 |
p51 |
gp41 |
p31 |
p24 |
p17 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG | IgA | IgG | IgA | IgG | IgA | IgG | IgA | IgG | IgA | IgG | IgA | IgG | IgA | IgG | IgA | IgG | IgA | |
| HIV-1 infected subjects | ||||||||||||||||||
| 1 | 3.0 | – | 3.0 | 1.0 | 3.0 | 1.0 | – | – | 3.0 | 1.0 | 3.0 | – | 3.0 | 2.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| 2 | 2.0 | 2.0 | 1.0 | – | 2.0 | 2.0 | – | – | 1.0 | 2.0 | 3.0 | 3.0 | 1.0 | – | 3.0 | 3.0 | 3.0 | 3.0 |
| 3 | 2.0 | – | 1.0 | 1.0 | 2.0 | – | – | – | 2.0 | – | 2.0 | – | 1.0 | 1.0 | 3.0 | – | 1.0 | – |
| 4 | 2.0 | – | 1.0 | – | 2.0 | – | 1.0 | – | 2.0 | – | 1.0 | – | 1.0 | – | 2.0 | 3.0 | 2.0 | 1.0 |
| 5 | 2.0 | – | 2.0 | – | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | – | 1.0 | – | 3.0 | 3.0 | 2.0 | 2.0 |
| 6 | 2.0 | 1.0 | 2.0 | – | 2.0 | 1.0 | – | – | 2.0 | 1.0 | 2.0 | 1.0 | 1.0 | – | 2.0 | 2.0 | 2.0 | – |
| 7 | 2.0 | – | 1.0 | – | 1.0 | – | 1.0 | – | 1.0 | – | 2.0 | – | 1.0 | – | 3.0 | 2.0 | 1.0 | – |
| 8 | 3.0 | – | 3.0 | – | 3.0 | – | – | – | 3.0 | – | 3.0 | – | 1.0 | – | 1.0 | – | 1.0 | – |
| 9 | 3.0 | – | 3.0 | – | 3.0 | – | – | – | 2.0 | – | 3.0 | – | 3.0 | – | 3.0 | 1.0 | 3.0 | – |
| 10 | 3.0 | – | 3.0 | – | 3.0 | – | – | – | 2.0 | – | 3.0 | – | 3.0 | – | 3.0 | 1.0 | 1.0 | – |
| 11 | 3.0 | – | 3.0 | 1.0 | 3.0 | – | – | – | 3.0 | – | 2.0 | – | 2.0 | – | 3.0 | – | 3.0 | – |
| 12 | 3.0 | – | 3.0 | – | 3.0 | – | – | – | 3.0 | – | 3.0 | – | 2.0 | – | 3.0 | – | 2.0 | 3.0 |
| 13 | 2.0 | – | 1.0 | – | 1.0 | 1.0 | 1.0 | – | 1.0 | – | 2.0 | – | 2.0 | 1.0 | 3.0 | 2.0 | 2.0 | 1.0 |
| 14 | 3.0 | – | 2.0 | 2.0 | 2.0 | – | – | – | 2.0 | – | 3.0 | – | 2.0 | – | 3.0 | – | 3.0 | – |
| 15 | 2.0 | 2.0 | 1.0 | – | 1.0 | 2.0 | – | – | 1.0 | 2.0 | 2.0 | 3.0 | 1.0 | 3.0 | 3.0 | 3.0 | 2.0 | 2.0 |
| 16 | 2.0 | – | 1.0 | – | 3.0 | – | – | 2.0 | – | 2.0 | – | 2.0 | – | 2.0 | 1.0 | 1.0 | – | |
| 17 | 2.0 | 1.0 | 1.0 | – | 3.0 | 2.0 | 1.0 | 1.0 | 3.0 | 2.0 | 3.0 | 3.0 | 1.0 | 1.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| 18 | 2.0 | 2.0 | 1.0 | – | 3.0 | – | – | – | 3.0 | – | 2.0 | – | 2.0 | – | 2.0 | 1.0 | 1.0 | 1.0 |
| 19 | 2.0 | – | 1.0 | – | 3.0 | 1.0 | – | – | 2.0 | – | 3.0 | – | 2.0 | – | 3.0 | 1.0 | 2.0 | – |
| 20 | 2.0 | – | 1.0 | – | 1.0 | 1.0 | – | – | – | – | – | – | 1.0 | – | 1.0 | 1.0 | – | – |
| 21 | 2.0 | – | 1.0 | – | 3.0 | 2.0 | 1.0 | – | 2.0 | – | 3.0 | – | 2.0 | 1.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| 22 | 3.0 | – | 3.0 | – | 3.0 | – | 1.0 | – | 3.0 | – | 3.0 | – | 2.0 | 2.0 | 3.0 | 3.0 | 3.0 | 2.0 |
| 23 | 3.0 | – | 3.0 | 1.0 | 0.5 | – | – | – | – | – | 3.0 | – | 0.5 | – | 2.0 | 1.0 | – | 1.0 |
| 24 | 3.0 | – | 2.0 | – | 2.0 | – | 1.0 | – | 2.0 | – | 3.0 | – | 2.0 | – | 3.0 | 3.0 | 3.0 | 3.0 |
| 25 | 3.0 | – | 3.0 | – | 3.0 | 1.0 | 2.0 | – | 3.0 | 1.0 | 3.0 | 1.0 | 3.0 | – | 3.0 | 1.0 | 3.0 | 1.0 |
| 26 | 3.0 | 1.0 | 3.0 | – | 3.0 | – | 3.0 | – | 2.0 | – | 3.0 | 1.0 | 3.0 | – | 3.0 | 1.0 | 3.0 | – |
| HEPSN subjects | ||||||||||||||||||
| 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | 1.0 | – | – | – | |
| 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| 3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2.0 | – | – | – |
| 4 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 5 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 6 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 7 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 8 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 9 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 10 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 11 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 12 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 13 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 14 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 15 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 16 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 17 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 18 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 19 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 20 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 21 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 22 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 23 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 24 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 25 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 3.0 | – | – |
| 26 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 27 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 28 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 29 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 30 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 31 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 32 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 33 | – | – | – | – | – | – | – | – | – | – | – | 2.0 | – | – | – | – | – | – |
| 34 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2.0 | – | – |
| 35 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 36 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 37 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 38 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1.0 | – | – | – |
| 39 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 40 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 41 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
Assays were performed in Laboratory A as described in the Materials and Methods. Shown is the relative intensity of bands reacting with viral proteins, with greatest intensity indicated by a value of 3.0. Dashes denote the samples that were negative.
The levels of HIV-1-specific IgG antibodies were lower in CVL from HIV-1-infected women in all assays (Tables 2 and 4) due to the lower levels of total IgG than in corresponding plasma samples. Nevertheless, by ELISA, IgG HIV-1-specific antibodies were routinely detected in all five laboratories (Table 2). Using ECL–WB, IgG antibodies were also readily detectable though with slightly less breadth and intensity than in the plasma (Table 4). The HIV-1-specific neutralizing activity activity in CVLs without contaminating blood was at a low level with a maximum of 7 of 21 samples (Table 5). In addition to viruses used in Laboratories A and F, the neutralizing activity of all specimens was also evaluated using an African clade C primary virus HIV#CC.1 (Laboratory E). All plasma samples were tested at 1:60 dilution and CVL samples at 1:10 dilution against this virus. Three plasma samples showed an IC50 ranging from 1:80 to 1:126, and five CVL samples neutralized HIV#CC.1 with IC50 ranging from 1:10 to >1:17. No sample reached an IC90 at the tested dilutions. The lower level of neutralizing activity could be due to the use of a primary virus that is more difficult to be neutralized, as compared to pseudoviruses, even though this virus matched the clade most commonly circulating in Tanzania.
Table 2.
HIV-1 Protein- or Peptide-Specific Antibodies Detected in CVL Using ELISA
| |
Laboratory A |
Laboratory B |
Laboratory C |
Laboratory D |
Laboratory E |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Clade B gp120 |
Clade C HIV lystate |
gp41 peptide 1 |
gp41 peptide 2 |
Clade C gp120 |
Clade B gp41 |
Clade C gp120 |
|||||||
| |
IgG |
IgA |
IgG |
IgA |
IgG |
IgA |
IgG |
IgA |
IgG |
IgA |
IgG |
IgA |
IgG |
IgA |
| (% of total) | (% of total) | (reciprocal endpoint titer) | (mOD/min) | (reciprocal endpoint titer) | ||||||||||
| HIV-1 infected subjects | ||||||||||||||
| 1 | 1.35 | 0.28 | 4.1 | 0.30 | >1024 | – | 512 | – | >1267 | 265.4 | >320 | >16 | >320 | >16 |
| 2 | 3.67 | 0.26 | 21.1 | 0.93 | >1024 | – | 128 | – | >1267 | 23.8 | >320 | 8 | >320 | >16 |
| 3 | 1.29 | – | 1.2 | – | 512 | – | – | – | 114.7 | – | >320 | – | >320 | – |
| 4 | 0.37 | – | 1.8 | – | 128 | – | – | – | 10.2 | – | 320 | – | 80 | – |
| 5 | 0.47 | 0.06 | 2.1 | 1.31 | >1024 | – | 128 | – | 614.5 | 8.7 | >320 | – | >320 | 8 |
| 6 | 1.99 | 0.31 | 3.0 | – | >1024 | – | – | – | 484.7 | – | >320 | – | >320 | – |
| 7 | 1.37 | 0.17 | 9.3 | 0.15 | >1024 | – | – | – | 845.2 | 2.2 | >320 | 2 | >320 | 16 |
| 8 | 1.86 | 0.07 | 6.4 | – | >1024 | – | – | – | 381.1 | – | >320 | ≥16 | >320 | 4 |
| 9 | 0.57 | – | 0.7 | – | 64 | – | – | – | 25.9 | – | nd | – | nd | nd |
| 10 | 0.77 | 0.14 | 5.5 | 0.03 | 512 | – | – | – | 444.6 | 4.0 | ≥320 | – | >320 | 2 |
| 11 | 1.06 | 0.08 | 4.2 | – | 128 | – | – | – | 54.0 | 7.9 | ≥320 | – | 80 | – |
| 12 | 1.98 | – | 1.1 | – | >1024 | – | – | – | 73.5 | – | >320 | – | >320 | – |
| 13 | 1.13 | 0.14 | 23.4 | 0.29 | 256 | – | – | – | 46.9 | 1.3 | >320 | 2 | >320 | – |
| 14 | 1.12 | – | 5.2 | – | 256 | – | – | – | 537.8 | – | >320 | – | 80 | – |
| 15 | 0.69 | 0.22 | 10.9 | 2.08 | >1024 | – | 256 | – | >1267 | 32.3 | >320 | >16 | >320 | >16 |
| 16 | 0.48 | 0.07 | 0.3 | – | 16 | – | – | – | 12.9 | – | 20 | – | 20 | – |
| 17 | 0.42 | 0.28 | 17.7 | 1.32 | >1024 | – | – | – | >340 | 7.2 | >320 | – | >320 | – |
| 18 | 1.12 | – | 1.0 | – | 256 | – | – | – | 117.5 | – | >320 | – | 320 | – |
| 19 | 1.69 | – | 3.1 | – | 256 | – | 128 | – | >1267 | 3.9 | >320 | 2 | >320 | >16 |
| 20 | 2.71 | 0.16 | 3.3 | – | nd | – | nd | – | 71.8 | – | >320 | – | >320 | – |
| 21 | 0.33 | – | 8.5 | 0.03 | 256 | – | – | – | 22.2 | 3.3 | >320 | – | 80 | – |
| 22 | 0.94 | 0.16 | 9.8 | 0.37 | >1024 | – | 8 | – | 593.7 | 3.5 | >320 | – | >320 | >16 |
| 23 | 0.18 | 0.43 | 0.1 | – | 32 | – | – | – | 18.4 | 4.8 | 20 | – | 5 | 8 |
| 24 | 2.14 | 0.06 | 10.4 | 0.16 | >1024 | – | – | – | 255.3 | 7.1 | ≥320 | – | >320 | – |
| 25 | 0.82 | 0.40 | 1.6 | – | >1024 | – | 8 | – | 74.7 | – | >320 | 8 | >320 | – |
| 26 | 1.08 | 0.20 | 3.4 | – | >1024 | – | 8 | – | 225.6 | – | >320 | – | >320 | >16 |
| HEPSN subjects | ||||||||||||||
| 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 4 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 5 | – | 0.12 | – | – | – | – | – | – | – | – | – | – | – | – |
| 6 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 7 | – | – | – | – | – | – | – | – | – | – | nd | – | nd | nd |
| 8 | – | – | – | – | – | – | – | – | – | – | – | 2 | 5 | 4 |
| 9 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 10 | – | 0.85 | – | – | – | – | – | – | – | – | – | – | – | – |
| 11 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 12 | – | 0.26 | – | – | – | – | – | – | – | – | – | – | – | – |
| 13 | – | 0.32 | – | – | – | – | – | – | – | – | – | – | – | – |
| 14 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 15 | – | – | – | – | – | 16 | – | – | – | – | – | – | – | – |
| 16 | – | – | 0.07 | – | – | – | – | – | – | – | – | – | – | – |
| 17 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 18 | – | – | – | – | – | – | – | – | – | – | 5 | – | – | – |
| 19 | – | 0.53 | – | – | – | – | – | – | – | – | – | – | – | – |
| 20 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 21 | – | – | 0.08 | – | – | – | – | – | – | – | – | – | – | – |
| 22 | – | – | – | – | 8 | 8 | – | – | – | – | nd | – | – | – |
| 23 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 24 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 25 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 26 | – | – | – | – | 16 | 8 | 32 | – | – | – | 20 | 8 | 5 | 2 |
| 27 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 28 | – | – | – | – | – | – | – | – | 19.5 | – | – | – | – | – |
| 29 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 30 | – | – | – | – | – | – | 16 | – | – | – | 80 | 2 | 20 | – |
| 31 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 32 | – | – | – | – | nd | nd | nd | nd | – | – | – | – | – | – |
| 33 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 34 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 35 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 36 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 37 | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – |
| 38 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 39 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 40 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 41 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
Assays were performed as described in the Materials and Methods. Values shown are for specimens which were found to contain significant HIV-1-specific antibodies. Dashes denote the CVL that were negative. nd: not done.
Table 4.
HIV-1-Specific Antibodies Detected in CVL By ECL-WB
| |
gp160 |
gp120 |
p66 |
p55 |
p51 |
gp41 |
p31 |
p24 |
p17 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG | IgA | IgG | IgA | IgG | IgA | IgG | IgA | IgG | IgA | IgG | IgA | IgG | IgA | IgG | IgA | IgG | IgA | |
| HIV-1 infected subjects | ||||||||||||||||||
| 1 | – | – | – | – | 1.0 | – | – | – | 1.0 | – | – | – | – | – | 1.0 | – | 1.0 | – |
| 2 | 3.0 | 2.0 | 3.0 | 1.0 | 3.0 | 1.0 | 1.0 | – | 3.0 | 1.0 | 3.0 | 1.0 | 2.0 | – | 3.0 | 2.0 | 3.0 | 1.0 |
| 3 | 3.0 | – | 3.0 | – | 2.0 | – | – | – | 2.0 | – | 2.0 | – | 1.0 | – | 3.0 | – | 1.0 | – |
| 4 | 1.0 | – | – | – | 2.0 | – | 2.0 | – | – | – | – | – | – | – | 3.0 | – | 2.0 | – |
| 5 | 1.0 | – | 1.0 | – | 1.0 | – | – | – | – | – | – | – | 1.0 | – | – | – | 1.0 | – |
| 6 | 3.0 | – | 3.0 | – | 3.0 | – | – | – | 3.0 | – | 3.0 | – | – | – | 3.0 | – | 3.0 | – |
| 7 | 3.0 | – | 3.0 | – | 1.0 | – | 1.0 | – | 1.0 | – | 3.0 | – | 2.0 | – | 3.0 | 1.0 | 1.0 | – |
| 8 | 3.0 | – | 3.0 | – | 2.0 | – | – | – | 2.0 | – | 3.0 | – | 1.0 | – | 1.0 | – | 1.0 | – |
| 9 | 3.0 | – | 3.0 | – | 2.0 | – | – | – | – | – | 1.0 | – | – | – | 2.0 | – | 1.0 | – |
| 10 | 1.0 | – | 1.0 | – | 1.0 | – | – | – | – | – | – | – | – | – | 1.0 | – | – | – |
| 11 | 3.0 | – | 2.0 | – | 3.0 | – | – | – | 3.0 | – | 2.0 | – | 2.0 | – | 3.0 | – | 3.0 | – |
| 12 | 3.0 | – | 3.0 | – | 3.0 | – | – | – | 3.0 | – | 3.0 | – | 2.0 | – | 3.0 | – | 3.0 | 2.0 |
| 13 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1.0 | – | 1.0 | – |
| 14 | 2.0 | – | – | – | 2.0 | – | – | – | 2.0 | – | – | – | 1.0 | – | 2.0 | – | 1.0 | – |
| 15 | 2.0 | 1.0 | 1.0 | – | 2.0 | 2.0 | 1.0 | – | 2.0 | 2.0 | 2.0 | 2.0 | 1.0 | 3.0 | 3.0 | 3.0 | 1.0 | 1.0 |
| 16 | 1.0 | – | – | – | 1.0 | – | – | – | – | – | – | – | – | – | 1.0 | – | – | – |
| 17 | 2.0 | – | 1.0 | – | 2.0 | – | 1.0 | – | 2.0 | – | 2.0 | – | – | – | 3.0 | 2.0 | 2.0 | – |
| 18 | 1.0 | – | – | – | 2.0 | – | – | – | 2.0 | – | – | – | 2.0 | – | 3.0 | – | 1.0 | – |
| 19 | 3.0 | – | 3.0 | – | 2.0 | – | – | – | 2.0 | – | 3.0 | – | 2.0 | – | 3.0 | – | 2.0 | – |
| 20 | 1.0 | – | – | – | 1.0 | – | – | – | – | – | – | – | 1.0 | – | 2.0 | – | 1.0 | – |
| 21 | 2.0 | – | 2.0 | – | 2.0 | – | – | – | 2.0 | – | 1.0 | – | 1.0 | – | 3.0 | – | 3.0 | – |
| 22 | 3.0 | – | 3.0 | – | 3.0 | 1.0 | 2.0 | – | 3.0 | 1.0 | 3.0 | – | 3.0 | 1.0 | 3.0 | 3.0 | 3.0 | – |
| 23 | 1.0 | – | – | – | – | – | – | – | – | – | – | – | – | – | 1.0 | – | – | – |
| 24 | 2.0 | – | 1.0 | – | 2.0 | – | – | – | 1.0 | – | 2.0 | – | – | – | 3.0 | 1.0 | 3.0 | – |
| 25 | 3.0 | – | 2.0 | – | 1.0 | – | – | – | – | – | 1.0 | – | 1.0 | – | 1.0 | – | – | – |
| 26 | 1.0 | – | – | – | 1.0 | – | – | – | 1.0 | – | 2.0 | – | – | – | 1.0 | – | 1.0 | – |
| HEPSN subjects | ||||||||||||||||||
| 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 4 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 5 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 6 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1.0 | – | – | – |
| 7 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 8 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 9 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 10 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 11 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 12 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 13 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 14 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 15 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 16 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 17 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 18 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 19 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1.0 | – | – | – |
| 20 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 21 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 22 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 23 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 24 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 25 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 26 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 27 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 28 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 29 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1.0 |
| 30 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 31 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 32 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 33 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 34 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 35 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 36 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 37 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 38 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 39 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 40 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 41 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
Assays were performed in Laboratory A as described in the Materials and Methods. Shown is the relative intensity of bands reacting with viral proteins, with greatest intensity indicated by a value of 3.0. Dashes denote the samples that were negative.
Table 5.
HIV-1-Neutralizing Activity in Plasma and CVL
| |
Laboratory A |
Laboratory E |
Laboratory F |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
NL4.3 |
CC.1 |
NL4.3 |
JRCSF |
SF162 |
aMLV |
||||
| Plasma | CVL | Plasma | CVL | Plasma | Plasma | Plasma | CVL | Plasma | CVL | |
| HIV-1 infected subjects | ||||||||||
| 1 | 1,000 | 12 | <60 | – | 2,586 | 87 | 5,846 | <10 | 2,586 | <10 |
| 2 | 1,620 | 12 | <60 | – | 7,271 | 96 | 25,870 | 24 | <20 | <10 |
| 3 | 540 | – | <60 | – | 2,442 | 807 | 19,884 | <10 | 23 | <10 |
| 4 | 540 | – | <60 | – | 1,683 | 50 | 2,549 | <10 | 25 | <10 |
| 5 | 120 | – | <60 | >17.1 | 562 | 84 | 5,155 | 13 | 20 | <10 |
| 6 | 540 | – | <60 | – | 2,113 | 656 | 5,479 | <10 | 278 | <10 |
| 7 | 180 | nd | <60 | 14.3 | 1,334 | 400 | 10,474 | <10 | 25 | <10 |
| 8 | 1,000 | – | <60 | – | 3,805 | 141 | 22,693 | 22 | 54 | 18 |
| 9 | 180 | – | <60 | nd | 966 | 53 | 4,891 | 43 | 29 | <10 |
| 10 | 180 | – | <60 | – | 545 | 82 | 3,709 | <10 | <20 | <10 |
| 11 | 360 | nd | <60 | – | 662 | 140 | 1,045 | <10 | <20 | <10 |
| 12 | 180 | – | <60 | – | 638 | 443 | 1,100 | 13 | 23 | <10 |
| 13 | 180 | – | <60 | – | 179 | 157 | 1,343 | <10 | 26 | <10 |
| 14 | 300 | – | <60 | – | 794 | 33 | 737 | 51 | 30 | 25 |
| 15 | 180 | – | 111.2 | – | 477 | 82 | 12,790 | <10 | 36 | <10 |
| 16 | 60 | – | <60 | – | 474 | 41 | 2,236 | 39 | 22 | <10 |
| 17 | 180 | 54 | <60 | >17.1 | 1,056 | 108 | 9,851 | <10 | <20 | <10 |
| 18 | 60 | – | <60 | >17.1 | 532 | 58 | 2,282 | <10 | 32 | <10 |
| 19 | 180 | – | 80.0 | – | 1,846 | 675 | 4,304 | 30 | 367 | <10 |
| 20 | 180 | – | <60 | – | 1,135 | 45 | 11,213 | <10 | <20 | <10 |
| 21 | 180 | – | <60 | – | 507 | 79 | 568 | <10 | <20 | <10 |
| 22 | 1,000 | – | <60 | – | 7,270 | 190 | 8,292 | <10 | 29 | <10 |
| 23 | 180 | – | <60 | – | 819 | 644 | 2,994 | <10 | 22 | <10 |
| 24 | 60 | – | 126.8 | – | 315 | 58 | 1,750 | <10 | <20 | <10 |
| 25 | 500 | – | <60 | 10.0 | 1,126 | 207 | 8,434 | 17 | <20 | <10 |
| 26 | 500 | – | <60 | – | 1,161 | 435 | 2,605 | 28 | 32 | <10 |
| HEPSN subjects | ||||||||||
| 1 | – | – | <60 | – | 38 | 41 | 30 | 290 | 28 | <10 |
| 2 | – | – | <60 | – | 29 | 23 | 37 | <10 | 30 | <10 |
| 3 | – | – | <60 | nd | 33 | 23 | 25 | 92 | <20 | <10 |
| 4 | – | – | <60 | – | 48 | 33 | 35 | <10 | 36 | <10 |
| 5 | – | – | <60 | – | 31 | <20 | 73 | <10 | 21 | <10 |
| 6 | – | – | <60 | – | 47 | 26 | 31 | 12 | 30 | <10 |
| 7 | – | – | <60 | nd | 22 | <20 | 22 | <10 | <20 | <10 |
| 8 | – | – | <60 | – | 40 | 24 | 22 | <10 | <20 | <10 |
| 9 | – | – | <60 | – | 27 | 22 | 20 | <10 | 21 | <10 |
| 10 | 60 | nd | <60 | >17.1 | 32 | 27 | 23 | <10 | <20 | <10 |
| 11 | 60 | – | <60 | – | 21 | 30 | 29 | 13 | 21 | <10 |
| 12 | 60 | – | <60 | – | 28 | <20 | 23 | <10 | <20 | <10 |
| 13 | – | – | <60 | – | 34 | <20 | 24 | <10 | 28 | <10 |
| 14 | – | – | <60 | – | 28 | 24 | 39 | 16 | 23 | <10 |
| 15 | – | – | <60 | – | 38 | 26 | 33 | 63 | 34 | <10 |
| 16 | – | – | <60 | – | <20 | <20 | 30 | <10 | 30 | <10 |
| 17 | – | – | <60 | – | 23 | 21 | 34 | 13 | 30 | <10 |
| 18 | – | – | <60 | – | <20 | <20 | <20 | <10 | <20 | <10 |
| 19 | – | – | <60 | >17.1 | 42 | 31 | 32 | 17 | 28 | <10 |
| 20 | – | – | <60 | >17.1 | 35 | <20 | 21 | <10 | 30 | <10 |
| 21 | – | – | <60 | – | <20 | <20 | 22 | 11 | <20 | <10 |
| 22 | – | – | <60 | – | 21 | 22 | <20 | <10 | <20 | <10 |
| 23 | – | – | <60 | – | <20 | <20 | <20 | <10 | <20 | <10 |
| 24 | – | – | <60 | – | <20 | <20 | 22 | 11 | <20 | <10 |
| 25 | – | – | <60 | – | 41 | 27 | 24 | <10 | 33 | <10 |
| 26 | – | – | <60 | – | 27 | <20 | 32 | <10 | 26 | <10 |
| 27 | – | – | <60 | – | 26 | 22 | 32 | 75 | 26 | <10 |
| 28 | – | – | <60 | – | 287 | 136 | 696 | 31 | 23 | 11 |
| 29 | – | – | <60 | – | 27 | 21 | <20 | 42 | 22 | <10 |
| 30 | – | – | <60 | – | 26 | 23 | 27 | <10 | 20 | <10 |
| 31 | – | – | <60 | – | 36 | 23 | 24 | 11 | 27 | <10 |
| 32 | – | – | <60 | – | <20 | <20 | 23 | 20 | <20 | <10 |
| 33 | – | – | <60 | – | 25 | 23 | 24 | <10 | <20 | <10 |
| 34 | – | – | <60 | – | 27 | <20 | 27 | <10 | <20 | <10 |
| 35 | – | – | <60 | – | 28 | <20 | 21 | <10 | <20 | <10 |
| 36 | – | – | <60 | – | 63 | 65 | 62 | 33 | 36 | <10 |
| 37 | – | – | <60 | – | 20 | 21 | 22 | 12 | <20 | <10 |
| 38 | – | – | <60 | – | 29 | 24 | 29 | 15 | 27 | <10 |
| 39 | 60 | – | <60 | – | 29 | 33 | 36 | 13 | 23 | <10 |
| 40 | – | – | <60 | – | 30 | 26 | 28 | <10 | 31 | <10 |
| 41 | – | – | <60 | – | <20 | <20 | <20 | 11 | <20 | <10 |
The values shown represent the last reciprocal dilution that inhibited viral infection by 50% as described for each laboratory in the Materials and Methods. Dashes denote samples which failed to neutralize virus. nd: not done.
In CVL samples from HIV-1-infected women, HIV-1-specific IgA antibodies were detected in approximately one-half of the samples by ELISA, but at levels that represented only a small percentage of the total IgA. Based on the data from Laboratory A, HIV-1-specific IgG antibodies represent ∼1.41% of total plasma IgG and ∼1.22% of total IgG in CVL, while for IgA, the values are ∼0.2% for plasma and ∼0.24% for CVL. The similarity of percentages calculated for HIV-1- specific IgG and IgA in plasma and CVL suggests that the majority of both of these antibody isotypes in the latter fluid are derived from the circulation rather than local production. By ECLWB, only three CVL samples were IgA-positive with more than one band detected (Table 4). Virus-neutralizing activity was detectable in all plasma samples that were positive for HIV-1-specific antibodies by both ELISA and ECL-WB. Their titers ranged from the dilution 1:60 – 1:1620 (Table 5). A comparison of the levels of gp140-binding antibodies in μg/ml with titers of neutralizing antibodies in individual plasma samples did not indicate any correlation (e.g., S2 and S44). Low levels on neutralizing activity were detectable in only three of 24 samples of the vaginal lavage fluid tested (L48, L1, and L6) (Table 5).
HEPSN women
Using ELISA, HIV-1-specific antibodies of the IgG or IgA isotype were either absent from the majority of plasma and CVL samples or detectable, in comparison to HIV-1-infected women, at extremely low levels (Tables 1 and 2). Importantly, samples marginally positive in one laboratory were negative in other laboratories and vice versa. In plasma, antibodies of the IgA isotype were not detectable in any of the samples analyzed, with the exception of four samples (S7, 14, 21, and 37), which displayed positivity for gp 41 but not for gp 120. Laboratory E found low titers of gp41 protein-specific IgA in 4/41 HEPSN plasma, but Laboratory B reported 0/41 HEPSN plasma had IgA antibodies against a similar gp41 protein (Table 1 legend). Similarly, Laboratory D found low levels of Clade C gp120-specific IgG in 3/41 HEPSN plasma, but using the same gp120 protein, Laboratory E reported 0/41 HEPSN plasma were positive for IgG antibodies (Table 1).
In CVL (Table 2), five of 41 samples were positive for IgA antibodies specific for gp 120 in Laboratory A, three for peptide 1 in Laboratory C, and four for gp 41 in Laboratory E. As in plasma samples, there was no concordance of positive samples among laboratories, which may be attributable to the differences in antigens used in ELISA. The absence or low levels of IgG or IgA antibodies to various HIV-1 antigens, detectable by ELISA, were corroborated by ECL-WB (Tables 3 and 4). In sharp contrast to HIV-1-infected women, none of the plasma or CVL samples from HEPSN women were observed to contain anti-gp120 (or gp160) antibodies of either the IgA or IgG isotypes (Tables 3 and 4). Further, only 1/41 HEPSN plasma (S33) and 0/41 HEPSN CVL had IgA that reacted against gp41 (Tables 3 and 4). Indeed, only 1/41 HEPSN CVL (L29) appeared to contain HIV-specific IgA, and this reactivity was against a single viral protein (p17 gag; Table 4). Similarly, none of the plasma or CVL from HEPSN women was observed by ECL-WB to contain IgG specific for gp160, gp120, or gp41, although three plasmas and two CVLs (all from different subjects) did display a low intensity IgG reaction against the HIV-1 p24 gag protein (Tables 3 and 4).
In contrast to HIV-1-infected women, HIV-1-neutralizing activity was present at low levels in plasma samples from four HEPSN women in Laboratory A and one sample in Laboratory F. In CVL, one sample was positive in Laboratory E, and five samples (titers >33 against SF162 virus) in Laboratory F. Because we did not have a large volume of CVL samples to selectively remove immunoglobulins, we cannot ascribe the low level of neutralizing activity to the presence of HIV-1-neutralizing antibodies. However, because no correlation was found between the neutralizing activity and ELISA or ECL-WB, it is probable that other soluble factors of innate immunity may be involved in the low level of or reduction of infectivity.
Discussion
There are three areas in which this paper extends or reinforces previous observations. The first is that with current technology, six laboratories with expertise in the field of mucosal immunity could find no evidence that HEPSN women made a consistently detectable mucosal or systemic humoral response to HIV-1. The second is that the mucosal response to HIV-1 in the female genital tract is primarily in the IgG with little, if any, IgA isotype and appears to reflect transudation of systemic antibody probably combined with local production or active transport in the vaginal lumen. These results confirm and extend several earlier reports,1,16,34,36,39–43,70 indicating the dominance of IgG over IgA HIV-1-specific responses in both plasma and genital tract secretions. The third is that the results obtained clearly indicate that CVL and plasma collected from HEPSN women do not contain reliably detectable HIV-1-specific IgA or IgG antibodies.
The assessment of mucosal immune responses starts with the collection methodology.51 Small volumes of collected external secretions and low levels of immunoglobulins51 as compared to plasma may not allow rigorous evaluation on all samples; the levels of immunoglobulins of all isotypes in external secretions are usually 100 to 1000 times lower than in serum or plasma.51 Furthermore, even in the same type of secretion (in this case CVL), the levels of total immunoglobulins are highly variable due to dilution with rinsing fluid, the method of collection,51 and the stage of the menstrual cycle.69 Due to the presence of endogenous or exogenous (bacterial) enzymes in external secretions, immunoglobulins may be proteolytically degraded considering that no protease inhibitors were used and, therefore, escape the measurements. In the present study, collections of CVL were made without consideration of the menstrual cycle-dependent variations in the total immunoglobulin levels. Consequently, some of the samples of CVL were visibly contaminated with blood. Further, the samples are nonhomogeneous and innate immune factors are present in external secretions, including SLPI, lactoferrin, lysozyme, and other soluble substances that exhibit viral inhibtion.4 Consequently, all assays and particularly virus neutralization should be performed in parallel on samples selectively depleted of immunoglobulins by immunosorbtion. It is conceivable that HIV-1-neutralization activity at the low dilution (e.g., 1:10–1:20) of CVL, as determined in our studies, does not reflect antibody-mediated neutralization. Importantly, almost all samples of CVL collected from HSPSN women displayed either weak or no neutralization activity, although other humoral factors and levels of immunoglobulins comparable to fluids collected from HIV-1-infected women were likely to be present. Obviously, samples from repeated collections and reliable removal of immunoglobulins by immunosorbtion will be required to prove unequivocally that the neutralization is indeed antibody-mediated. Additionally, in evaluation of antigen-specific humoral responses in external secretions, the level of total immunoglobulins should be determined to generate meaningful results.
Results of this blindly performed study clearly demonstrate that the plasma as well as CVL collected from HSPSN female sex workers do not contain uniformly and easily detectable HIV-1-specific antibodies or neutralizing activities. There was a sharp contrast in responses in HEPSN women to high IgG and low IgA responses detectable in plasma and CVL from HIV-1-infected women. In 41 samples of CVL from HEPSN women, extremely low levels of IgG and/or IgA antibodies specific for some of HIV-1 antigens were detected in a few samples. Importantly, samples that displayed marginal positivity in one laboratory were not considered positive in other laboratories. These data confirm and extend other previous reports,31–33 but are at variance with several reports indicating the presence of HIV-1-specific antibodies of the IgA isotype in external secretions and sera or plasma of HIV-1 HEPSN individuals.8,9,12–19,21–29,72
Although there is no obvious explanation for this marked discrepancy, differences in the source of HIV-1-derived antigens used for coating of plates are of considerable importance in the ELISA-generated results. However, in a recent report, a gp41-specific neutralizing monoclonal IgA antibody was generated from an HEPSN subject, thus demonstrating that, although rare, antibodies of such specificity could be generated and block in vitro HIV-1 transcytosis through epithelial cells.11,12 However, the HEPSN subject was, by definition, seronegative; therefore, it is unlikely that the resistance to HIV-1 infection was mediated by antibodies.
Results based on ELISA are prone to frequent false-positivity.35–37 This may be due to the presence of S-IgA antibodies to insect or mammalian cells, which are used for the production of HIV-1 antigens.34,35,59,60 This potential problem can be avoided by using peptides or a highly sensitive and discriminating ECL-WB in which individual HIV-1-specific antigens can be easily distinguished from other HIV-1-irrelevant antigens. The consistent use of known negative control samples can also aid in the prevention of this type of discrepancy. With regard to the second conclusion, the similarity in the percentages of HIV-1-specific vs. total IgG and IgA levels in plasma and CVL suggests that a considerable proportion of antibodies in the latter fluid is derived from the circulatory pool, rather than from local synthesis in the genital tract. This possibility is further supported by several studies,52,73–75 indicating that systemic immunization results in the detection of plasma-derived antigen-specific antibodies in 21 secretions of both the male and female genital tracts, but not in other external secretions such as saliva, tears, and intestinal fluid. Extrapolation of these findings to HIV-1 suggests that systemic immunization with relevant HIV-1 antigens may provide protective humoral responses in the secretions of the genital, but not the lower intestinal tract.52,73–75 Furthermore, results generated independently in six laboratories, using a broad spectrum of HIV-1-derived antigens, three methods of specific antibody detection, and performed as a blinded study, displayed a remarkable concordance in all participating laboratories with respect to the detection of HIV-1-specific antibodies of the IgG isotype in both plasma and CVL of HIV-1-infected women.
Although present in plasma of most of the HIV-1-infected women, the levels of IgA antibodies specific for HIV-1 antigens were markedly lower than those of IgG when expressed as titers, μg/ml, or percentages of total IgG and IgA. In both plasma as well as CVL, HIV-1-specific antibodies of the IgA isotype represented a significantly lower percentage of total IgA when compared to IgG. These results confirm and extend several previous studies, including those from our laboratories, in which low levels of HIV-1-specific antibodies of the IgA isotype in HIV-1-infected patients have been reported.1,34,36,38,40–43,70 Importantly, low or absent IgA responses have also been described in HIV-1-infected chimpanzees44 and SIV-infected macaques.45 A mechanism involved in this selective suppression of HIV-1-specific responses in the IgA isotype have been recently elucidated by Xu et al.48 It appears that HIV-1 evades virus-specific IgA and IgG2 responses by shuttling negative factor (Nef) from infected macrophages to mucosal and systemic B cells through long-range intercellular conduits. As a result, B cell switching to IgA and IgG2 production is suppressed by Nef. Alternatively, the extensive mucosal replication of HIV-1 and a profound depletion of CD4+ T cells involved in immunoglobulin-isotype switching and the generation of antibody diversity through somatic hypermutation76 during acute infection may alter the capacity to mount an IgA response to new antigens. The possibilities that IgA antibodies to HIV-1 are not detectable due to the formation of HIV-1-IgA immune complexes in blood and external secretions77 or that tolerance is induced seem less likely. During acute infection, IgM and IgG, but only limited IgA responses to HIV-1 gp41 and gp120 have been detected in sera by ELISA and in peripheral blood lymphocytes by ELISPOT.42
Although the levels of total IgA in plasma and external secretions are not altered in HIV-1-infected individuals,42 it has not been determined whether low or absent IgA responses to HIV-1 extend to other mucosally encountered antigens. Such studies will be of considerable importance because it has been demonstrated that the intestinal absorption of microbial antigens, such as lipopolysaccharide of gram-negative bacteria, is increased in HIV-1-infected patients and is responsible for chronic activation of the immune system.78 Extensive studies have demonstrated that secretory IgA (S-IgA) antibodies to such antigens inhibit absorption of these antigens from mucosal surfaces by noninflammatory means.79 Consequently, the paucity of IgA responses specific for microbial antigens is likely to compromise mucosal defense mechanisms. S-IgA as the dominant Ig isotype in most external secretions displays several structurally and functionally advantageous features when compared to IgG and IgM.79 In this respect, HIV-1 is markedly different from other viral and bacterial mucosal infections that stimulate vigorous albeit transient IgA.46,47 For example, mucosal immunization with a live influenza virus vaccine and infections with hepatitis B, cytomegalo, Epstein–Barr, or varicella viruses stimulate IgA responses shortly after immunization or acute infection, which, however, may not persist in the chronic stage.80–84 The lack of a vigorous or sustained IgA response to HIV-1 may have particular implications for the development of protective immunity although the antigen presented in a vaccine may differ considerably from the events during natural infection.
Unlike IgG or IgA, S-IgA is especially resistant to the endogenous and exogenous (bacterial) proteolytic enzymes present in abundance not only in the gastrointestinal tract, but also in the oral cavity, respiratory tract, CVL fluids, and semen.79,85 Proteases present in the latter fluid promptly digest IgM and monomeric IgA, but have little effect on S-IgA.86 Furthermore, S-IgA composed of both dimers and tetramers displays four to eight antigen-binding sites, which in turn greatly increase the avidity of such antibodies over monomeric IgG or IgA, due to the bonus effect of multivalency, as documented by highly effective virus neutralization.79,87 Because locally produced S-IgA is composed of polymeric IgA with J chain and secretory component acquired during selective and active epithelial transcytosis,88 IgA has the potential to neutralize not only free viruses in plasma and external secretions, but also viruses present in epithelial cells, although neutralizing antibodies may display their effect without inhibition of epithelial transcytosis.92 However, because of the absence or presence at very low levels of HIV-1 binding antibodies of the IgA isotype in both sera and CVL, it is improbable that usually even lower levels of neutralizing IgA antibodies would be involved in an effective HIV-1 neutralization activity of vaginal fluid in vivo and be related to the resistance of HEPSN women to HIV-1 infection. Obviously, these unique properties would make S-IgA-mediated protective responses a highly desirable goal. Therefore, the immunization approaches that would result in the induction of IgA responses to HIV-1 has provided a promising target for effective vaccination.
Acknowledgments
These studies were supported by the US PHS program projects 5 P01 AI28147 and 1 P01 AI1083027, and Grants DK64400, AI058896, 1 R21 AI083613, Vanderbilt Vaccine Clinical Trials Unit (HVTN) grant AI47985, Grant No. 201433 from European Commission/Seventh Framework, GCE No. 53030 and No. PP108144 from Bill and Melinda Gates Foundation, the European Commission through Grants ICA_CT-1999-10007, ICA_CT-2002-10048, and VZ MSM 0021620812 (Czech Republic). The authors also thank the MMRP team in Tanzania for providing precious samples of the HISIS cohort. Finally, special thanks go to Maria Crenshaw, Weston Assisya, Oliver Hoffmann, Erica Sanga, and Mary Rusizoka.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Artenstein AW. VanCott TC. Sitz KV, et al. Mucosal immune responses in four distinct compartments of women infected with human immunodeficiency virus type 1: A comparison by site and correlation with clinical information. J Infect Dis. 1997;175:265–271. doi: 10.1093/infdis/175.2.265. [DOI] [PubMed] [Google Scholar]
- 2.Moog C. Immune responses that correlate with HIV-1 protection? AIDS. 2008;22:1461–1462. doi: 10.1097/QAD.0b013e3282fdf625. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen M. Pean P. Lopalco L, et al. HIV-specific antibodies but not T-cell responses are associated with protection in seronegative partners of HIV-1-infected individuals in Cambodia. J Acquir Immune Defic Syndr. 2006;42:412–419. doi: 10.1097/01.qai.0000222289.97825.35. [DOI] [PubMed] [Google Scholar]
- 4.Novak RM. Donoval BA. Graham PJ, et al. Cervicovaginal levels of lactoferrin, secretory leukocyte protease inhibitor, and RANTES and the effects of coexisting vaginoses in human immunodeficiency virus (HIV)-seronegative women with a high risk of heterosexual acquisition of HIV infection. Clin Vaccine Immunol. 2007;14:1102–1107. doi: 10.1128/CVI.00386-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baba TW. Liska V. Hofmann–Lehmann R, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 6.Mascola JR. Stiegler G. VanCott TC, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 7.Veazey RS. Shattock RJ. Pope M, et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 8.Devito C. Hinkula J. Kaul R, et al. Cross-clade HIV-1-specific neutralizing IgA in mucosal and systemic compartments of HIV-1-exposed, persistently seronegative subjects. J Acquir Immune Defic Syndr. 2002;30:413–420. doi: 10.1097/00042560-200208010-00007. [DOI] [PubMed] [Google Scholar]
- 9.Hocini H. Belec L. Iscaki S, et al. High-level ability of secretory IgA to block HIV type 1 transcytosis: Contrasting secretory IgA and IgG responses to glycoprotein 160. AIDS Res Hum Retroviruses. 1997;13:1179–1185. doi: 10.1089/aid.1997.13.1179. [DOI] [PubMed] [Google Scholar]
- 10.Huang YT. Wright A. Gao X. Kulick L. Yan H. Lamm ME. Intraepithelial cell neutralization of HIV-1 replication by IgA. J Immunol. 2005;174:4828–4835. doi: 10.4049/jimmunol.174.8.4828. [DOI] [PubMed] [Google Scholar]
- 11.Tudor D. Derrien M. Diomede L, et al. HIV-1 gp41-specific monoclonal mucosal IgAs derived from highly exposed but IgG-seronegative individuals block HIV-1 epithelial transcytosis and neutralize CD4+ cell infection: An IgA gene and functional analysis. Mucosal Immunol. 2009;5:412–416. doi: 10.1038/mi.2009.89. [DOI] [PubMed] [Google Scholar]
- 12.Shen R. Drelichman ER. Bimczok D, et al. GP41-specific antibody blocks cell-free HIV type 1 transcytosis through human rectal mucosa and model colonic epithelium. J Immunol. 2010;184:3648–3655. doi: 10.4049/jimmunol.0903346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang YT. Wright A. Gao X. Kulick L. Yan H. Lamm ME. Intraepithelial cell neutralization of HIV-1 replication by IgA. J Immunol. 2005;174:4828–4835. doi: 10.4049/jimmunol.174.8.4828. [DOI] [PubMed] [Google Scholar]
- 14.Beyrer C. Artenstein AW. Rugpao S, et al. Epidemiologic and biologic characterization of a cohort of human immunodeficiency virus type 1 highly exposed, persistently seronegative female sex workers in northern Thailand. Chiang Mai HEPS Working Group. J Infect Dis. 1999;179:59–67. doi: 10.1086/314556. [DOI] [PubMed] [Google Scholar]
- 15.Broliden K. Hinkula J. Devito C, et al. Functional HIV-1 specific IgA antibodies in HIV-1 exposed, persistently IgG seronegative female sex workers. Immunol Lett. 2001;79:29–36. doi: 10.1016/s0165-2478(01)00263-2. [DOI] [PubMed] [Google Scholar]
- 16.Buchacz K. Parekh BS. Padian NS, et al. HIV-specific IgG in cervicovaginal secretions of exposed HIV-uninfected female sexual partners of HIV-infected men. AIDS Res Hum Retroviruses. 2001;17:1689–1693. doi: 10.1089/08892220152741388. [DOI] [PubMed] [Google Scholar]
- 17.Devito C. Hinkula J. Kaul R, et al. Cross-clade HIV-1-specific neutralizing IgA in mucosal and systemic compartments of HIV-1-exposed, persistently seronegative subjects. J Acquir Immune Defic Syndr. 2002;30:413–420. doi: 10.1097/00042560-200208010-00007. [DOI] [PubMed] [Google Scholar]
- 18.Hasselrot K. Saberg P. Hirbod T, et al. Oral HIV-exposure elicits mucosal HIV neutralizing antibodies in uninfected men who have sex with men. AIDS. 2009;23:329–333. doi: 10.1097/QAD.0b013e32831f924c. [DOI] [PubMed] [Google Scholar]
- 19.Hirbod T. Broliden K. Mucosal immune responses in the genital tract of HIV-1-exposed uninfected women. J Intern Med. 2007;262:44–58. doi: 10.1111/j.1365-2796.2007.01822.x. [DOI] [PubMed] [Google Scholar]
- 20.Hirbod T. Reichard C. Hasselrot K, et al. HIV-1 neutralizing activity is correlated with increased levels of chemokines in saliva of HIV-1-exposed uninfected individuals. Curr HIV Res. 2008;6:28–33. doi: 10.2174/157016208783571964. [DOI] [PubMed] [Google Scholar]
- 21.Horton RE. Ball TB. Wachichi C, et al. Cervical HIV-specific IgA in a population of commercial sex workers correlates with repeated exposure but not resistance to HIV. AIDS Res Hum Retroviruses. 2009;25:83–92. doi: 10.1089/aid.2008.0207. [DOI] [PubMed] [Google Scholar]
- 22.Kaul R. Plummer F. Clerici M. Bomsel M. Lopalco L. Broliden K. Mucosal IgA in exposed, uninfected subjects: Evidence for a role in protection against HIV infection. AIDS. 2001;15:431–432. doi: 10.1097/00002030-200102160-00026. [DOI] [PubMed] [Google Scholar]
- 23.Kaul R. Trabattoni D. Bwayo JJ, et al. HIV-1-specific mucosal IgA in a cohort of HIV-1-resistant Kenyan sex workers. AIDS. 1999;13:23–29. doi: 10.1097/00002030-199901140-00004. [DOI] [PubMed] [Google Scholar]
- 24.Lo Caputo S. Trabattoni D. Vichi F, et al. Mucosal and systemic HIV-1-specific immunity in HIV-1-exposed but uninfected heterosexual men. AIDS. 2003;17:531–539. doi: 10.1097/00002030-200303070-00008. [DOI] [PubMed] [Google Scholar]
- 25.Mazzoli S. Lopalco L. Salvi A, et al. Human immunodeficiency virus (HIV)-specific IgA and HIV neutralizing activity in the serum of exposed seronegative partners of HIV-seropositive persons. J Infect Dis. 1999;180:871–875. doi: 10.1086/314934. [DOI] [PubMed] [Google Scholar]
- 26.Mazzoli S. Trabattoni D. Lo Caputo S, et al. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat Med. 1997;3:1250–1257. doi: 10.1038/nm1197-1250. [DOI] [PubMed] [Google Scholar]
- 27.Miyazawa M. Lopalco L. Mazzotta F. Lo Caputo S. Veas F. Clerici M. The 'immunologic advantage' of HIV-exposed seronegative individuals. AIDS. 2009;23:161–175. doi: 10.1097/QAD.0b013e3283196a80. [DOI] [PubMed] [Google Scholar]
- 28.Pastori C. Barassi C. Piconi S, et al. HIV neutralizing IgA in exposed seronegative subjects recognise an epitope within the gp41 coiled-coil pocket. J Biol Regul Homeost Agents. 2000;14:15–21. [PubMed] [Google Scholar]
- 29.Belec L. Ghys PD. Hocini H, et al. Cervicovaginal secretory antibodies to human immunodeficiency virus type 1 (HIV-1) that block viral transcytosis through tight epithelial barriers in highly exposed HIV-1-seronegative African women. J Infect Dis. 2001;184:1412–1422. doi: 10.1086/324375. [DOI] [PubMed] [Google Scholar]
- 30.Wright A. Yan H. Lamm ME. Huang YT. Immunoglobulin A antibodies against internal HIV-1 proteins neutralize HIV-1 replication inside epithelial cells. Virology. 2006;356:165–170. doi: 10.1016/j.virol.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dorrell L. Hessell AJ. Wang M, et al. Absence of specific mucosal antibody responses in HIV-exposed uninfected sex workers from the Gambia. AIDS. 2000;14:1117–1122. doi: 10.1097/00002030-200006160-00008. [DOI] [PubMed] [Google Scholar]
- 32.Fiore JR. Laddago V. Lepera A, et al. Limited secretory-IgA response in cervicovaginal secretions from HIV-1 infected, but not high risk seronegative women: Lack of correlation to genital viral shedding. New Microbiol. 2000;23:85–92. [PubMed] [Google Scholar]
- 33.Skurnick JH. Palumbo P. DeVico A, et al. Correlates of nontransmission in US women at high risk of human immunodeficiency virus type 1 infection through sexual exposure. J Infect Dis. 2002;185:428–438. doi: 10.1086/338830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright PF. Kozlowski PA. Rybczyk GK, et al. Detection of mucosal antibodies in HIV type 1-infected individuals. AIDS Res Hum Retroviruses. 2002;18:1291–1300. doi: 10.1089/088922202320886334. [DOI] [PubMed] [Google Scholar]
- 35.Jackson S. Prince S. Kulhavy R. Mestecky J. False positivity of enzyme-linked immunosorbent assay for measurement of secretory IgA antibodies directed at HIV type 1 antigens. AIDS Res Hum Retroviruses. 2000;16:595–602. doi: 10.1089/088922200309016. [DOI] [PubMed] [Google Scholar]
- 36.Raux M. Finkielsztejn L. Salmon–Ceron D, et al. Comparison of the distribution of IgG and IgA antibodies in serum and various mucosal fluids of HIV type 1-infected subjects. AIDS Res Hum Retroviruses. 1999;15:1365–1376. doi: 10.1089/088922299310070. [DOI] [PubMed] [Google Scholar]
- 37.Raux M. Finkielsztejn L. Salmon–Ceron D, et al. Development and standardization of methods to evaluate the antibody response to an HIV-1 candidate vaccine in secretions and sera of seronegative vaccine recipients. J Immunol Methods. 1999;222:111–124. doi: 10.1016/s0022-1759(98)00188-4. [DOI] [PubMed] [Google Scholar]
- 38.Alexander R. Mestecky J. Neutralizing antibodies in mucosal secretions: IgG or IgA? Curr HIV Res. 2007;5:588–593. doi: 10.2174/157016207782418452. [DOI] [PubMed] [Google Scholar]
- 39.Belec L. Meillet D. Gaillard O, et al. Decreased cervicovaginal production of both IgA1 and IgA2 subclasses in women with AIDS. Clin Exp Immunol. 1995;101:100–106. doi: 10.1111/j.1365-2249.1995.tb02284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haimovici F. Mayer KH. Anderson DJ. Quantitation of HIV-1-specific IgG, IgA, and IgM antibodies in human genital tract secretions. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:185–191. doi: 10.1097/00042560-199707010-00001. [DOI] [PubMed] [Google Scholar]
- 41.Lu FX. Predominate HIV-1-specific IgG activity in various mucosal compartments of HIV-1-infected individuals. Clin Immunol. 2000;97:59–68. doi: 10.1006/clim.2000.4910. [DOI] [PubMed] [Google Scholar]
- 42.Mestecky J. Jackson S. Moldoveanu Z, et al. Paucity of antigen-specific IgA responses in sera and external secretions of HIV-type 1-infected individuals. AIDS Res Hum Retroviruses. 2004;20:972–988. doi: 10.1089/aid.2004.20.972. [DOI] [PubMed] [Google Scholar]
- 43.Wolff H. Mayer K. Seage G. Politch J. Horsburgh CR. Anderson D. A comparison of HIV-1 antibody classes, titers, and specificities in paired semen and blood samples from HIV-1 seropositive men. J Acquir Immune Defic Syndr. 1992;5:65–69. [PubMed] [Google Scholar]
- 44.Israel ZR. Marx PA. Nonclassical mucosal antibodies predominate in genital secretions of HIV-1 infected chimpanzees. J Med Primatol. 1995;24:53–60. doi: 10.1111/j.1600-0684.1995.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 45.Schafer F. Kewenig S. Stolte N, et al. Lack of simian immunodeficiency virus (SIV) specific IgA response in the intestine of SIV infected rhesus macaques. Gut. 2002;50:608–614. doi: 10.1136/gut.50.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holmgren J. Svennerholm A–M. Mucosal immunity to bacteria. In: Mestecky J, editor; Bienenstock J, editor; Lamm M, editor; Mayer L, editor; McGhee J, editor; Strober W, editor. Mucosal Immunology, 3rd. Edn. Elsevier/Academic Press; Amsterdam: 2005. pp. 783–797. [Google Scholar]
- 47.Murphy BR. Mucosal immunity to viruses. In: Mestecky J, editor; Bienenstock J, editor; Lamm ME, editor; Mayer L, editor; McGhee JR, editor; Strober W, editor. Mucosal Immunology, 3rd. Ed. Elsevier/Academic Press; Amsterdam: 2005. pp. 799–813. [Google Scholar]
- 48.Xu W. Santini PA. Sullivan JS, et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat Immunol. 2009;10:1008–1017. doi: 10.1038/ni.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riedner G. Hoffmann O. Rusizoka M, et al. Decline in sexually transmitted infection prevalence and HIV incidence in female barworkers attending prevention and care services in Mbeya Region, Tanzania. AIDS. 2006;20:609–615. doi: 10.1097/01.aids.0000210616.90954.47. [DOI] [PubMed] [Google Scholar]
- 50.Hoffmann O. Zaba B. Wolff B, et al. Methodological lessons from a cohort study of high risk women in Tanzania. Sex Transm Infect. 2004;80(Suppl 2):ii69–73. doi: 10.1136/sti.2004.011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jackson S. Mestecky J. Moldoveanu Z. Spearman P. Appendix I. Collection, processing of human mucosal secretions. In: Mestecky J, editor; Bienenstock J, editor; Lamm ME, editor; Mayer L, editor; McGhee JR, editor; Strober W, editor. Mucosal Immunology, 3rd ed. Elsevier/Academic Press; Amsterdam: 2005. pp. 1829–1839. [Google Scholar]
- 52.Moldoveanu Z. Huang WQ. Kulhavy R. Pate MS. Mestecky J. Human male genital tract secretions: Both mucosal and systemic immune compartments contribute to the humoral immunity. J Immunol. 2005;175:4127–4136. doi: 10.4049/jimmunol.175.6.4127. [DOI] [PubMed] [Google Scholar]
- 53.Kothe DL. Decker JM. Li Y, et al. Antigenicity and immunogenicity of HIV-1 consensus subtype B envelope glycoproteins. Virology. 2007;360:218–234. doi: 10.1016/j.virol.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kothe DL. Li Y. Decker JM, et al. Ancestral and consensus envelope immunogens for HIV-1 subtype C. Virology. 2006;352:438–449. doi: 10.1016/j.virol.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 55.Gaschen B. Taylor J. Yusim K, et al. Diversity considerations in HIV-1 vaccine selection. Science. 2002;296:2354–2360. doi: 10.1126/science.1070441. [DOI] [PubMed] [Google Scholar]
- 56.Gao F. Li Y. Decker JM, et al. Codon usage optimization of HIV type 1 subtype C gag, pol, env, and nef genes: In vitro expression and immune responses in DNA-vaccinated mice. AIDS Res Hum Retroviruses. 2003;19:817–823. doi: 10.1089/088922203769232610. [DOI] [PubMed] [Google Scholar]
- 57.Haas J. Park EC. Seed B. Codon usage limitation in the expression of HIV-1 envelope glycoprotein. Curr Biol. 1996;6:315–324. doi: 10.1016/s0960-9822(02)00482-7. [DOI] [PubMed] [Google Scholar]
- 58.Mestecky J. Kilian M. Immunoglobulin A (IgA) Methods Enzymol. 1985;116:37–75. doi: 10.1016/s0076-6879(85)16005-2. [DOI] [PubMed] [Google Scholar]
- 59.Constantine NT. Bansal J. Zhang X. Hyams KC. Hayes C. Enhanced chemiluminescence as a means of increasing the sensitivity of Western blot assays for HIV antibody. J Virol Methods. 1994;47:153–164. doi: 10.1016/0166-0934(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 60.Mohamed OA. Ashley R. Goldstein A. McElrath J. Dalessio J. Corey L. Detection of rectal antibodies to HIV-1 by a sensitive chemiluminescent western blot immunodetection method. J Acquir Immune Defic Syndr. 1994;7:375–380. [PubMed] [Google Scholar]
- 61.Montefiori DC, editor; Ed C, editor. Evaluating Neutralizing Antibodies against HIV, SIV, SHIV in Luciferase Reporter Gene Assays. John Wiley and Sons, Inc.; 2004. [DOI] [PubMed] [Google Scholar]
- 62.Bradney CP. Sempowski GD. Liao HX. Haynes BF. Staats HF. Cytokines as adjuvants for the induction of anti-human immunodeficiency virus peptide immunoglobulin G (IgG) and IgA antibodies in serum and mucosal secretions after nasal immunization. J Virol. 2002;76:517–524. doi: 10.1128/JVI.76.2.517-524.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Staats HF. Alam SM. Scearce RM, et al. In vitro and in vivo characterization of anthrax anti-protective antigen and anti-lethal factor monoclonal antibodies after passive transfer in a mouse lethal toxin challenge model to define correlates of immunity. Infect Immun. 2007;75:5443–5452. doi: 10.1128/IAI.00529-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boyce TG. Gruber WC. Coleman–Dockery SD, et al. Mucosal immune response to trivalent live attenuated intranasal influenza vaccine in children. Vaccine. 1999;18:82–88. doi: 10.1016/s0264-410x(99)00183-8. [DOI] [PubMed] [Google Scholar]
- 65.Clerici M. Butto S. Saresella M, et al. Immune activation in Africa is environmentally driven and is associated with upregulation of CCR5. AIDS. 2000;14:2083–2092. doi: 10.1097/00002030-200009290-00003. [DOI] [PubMed] [Google Scholar]
- 66.Pastori C. Weiser B. Barassi C, et al. Long lasting CCR5 internalization by antibodies in a subset of long term non progressors: A possible protective effect against disease progression. Blood. 2006;107:4825–4833. doi: 10.1182/blood-2005-06-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whitcomb JM. Huang W. Fransen S, et al. Development and characterization of a novel single-cycle recombinant-virus assay to determine human immunodeficiency virus type 1 coreceptor tropism. Antimicrob Agents Chemother. 2007;51:566–575. doi: 10.1128/AAC.00853-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Richman DD. Wrin T. Little SJ. Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci USA. 2003;100:4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kutteh WH. Prince SJ. Hammond KR. Kutteh CC. Mestecky J. Variations in immunoglobulins and IgA subclasses of human uterine cervical secretions around the time of ovulation. Clin Exp Immunol. 1996;104:538–542. doi: 10.1046/j.1365-2249.1996.36742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Belec L. Dupre T. Prazuck T, et al. Cervicovaginal overproduction of specific IgG to human immunodeficiency virus (HIV) contrasts with normal or impaired IgA local response in HIV infection. J Infect Dis. 1995;172:691–697. doi: 10.1093/infdis/172.3.691. [DOI] [PubMed] [Google Scholar]
- 71.Williams SB. Flanigan TP. Cu–Uvin S, et al. Human immunodeficiency virus (HIV)-specific antibody in cervicovaginal lavage specimens obtained from women infected with HIV type 1. Clin Infect Dis. 2002;35:611–617. doi: 10.1086/342201. [DOI] [PubMed] [Google Scholar]
- 72.Burnett PR. VanCott TC. Polonis VR. Redfield RR. Birx DL. Serum IgA-mediated neutralization of HIV type 1. J Immunol. 1994;152:4642–4648. [PubMed] [Google Scholar]
- 73.Bouvet JP. Belec L. Pires R. Pillot J. Immunoglobulin G antibodies in human vaginal secretions after parenteral vaccination. Infect Immun. 1994;62:3957–3961. doi: 10.1128/iai.62.9.3957-3961.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mestecky J. Moldoveanu Z. Russell MW. Immunologic uniqueness of the genital tract: challenge for vaccine development. Am J Reprod Immunol. 2005;53:208–214. doi: 10.1111/j.1600-0897.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 75.Russell MW. Mestecky J. Humoral immune responses to microbial infections in the genital tract. Microbes Infect. 2002;4:667–677. doi: 10.1016/s1286-4579(02)01585-x. [DOI] [PubMed] [Google Scholar]
- 76.Honjo T. Kinoshita K. Muramatsu M. Molecular mechanisms of class switch recombination: Linkage with somatic hypermutation. Annu Rev Immunol. 2002;20:165–196. doi: 10.1146/annurev.immunol.20.090501.112049. [DOI] [PubMed] [Google Scholar]
- 77.Tomaras GD. Yates NL. Liu P, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: Virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol. 2008;82:12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brenchley JM. Price DA. Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 79.Russell MW. Kilian M. Biological activities of IgA. In: Mestecky J, editor; Bienenstock J, editor; Lamm ME, editor; Mayer L, editor; McGhee JR, editor; Strober W, editor. Mucosal Immunology, 3rd. ed. Elsevier/Academic Press; Amsterdam: 2005. pp. 267–289. [Google Scholar]
- 80.Nomura M. Imai M. Tsuda F, et al. Immunoglobulin A antibody against hepatitis B core antigen in the acute and persistent infection with hepatitis B virus. Gastroenterology. 1985;89:1109–1113. doi: 10.1016/0016-5085(85)90217-3. [DOI] [PubMed] [Google Scholar]
- 81.Engelhard D. Weinberg M. Or R, et al. Immunoglobulins A, G, and M to cytomegalovirus during recurrent infection in recipients of allogeneic bone marrow transplantation. J Infect Dis. 1991;163:628–630. doi: 10.1093/infdis/163.3.628. [DOI] [PubMed] [Google Scholar]
- 82.Fachiroh J. Schouten T. Hariwiyanto B, et al. Molecular diversity of Epstein-Barr virus IgG and IgA antibody responses in nasopharyngeal carcinoma: A comparison of Indonesian, Chinese, and European subjects. J Infect Dis. 2004;190:53–62. doi: 10.1086/421245. [DOI] [PubMed] [Google Scholar]
- 83.Hadar T. Tovi F. Sidi J. Sarov B. Sarov I. Detection of specific IgA antibodies to varicella zoster virus in serum of patients with Ramsay Hunt syndrome. Ann Oto Rhinol Laryngol. 1990;99:461–465. doi: 10.1177/000348949009900609. [DOI] [PubMed] [Google Scholar]
- 84.Moldoveanu Z. Clements ML. Prince SJ. Murphy BR. Mestecky J. Human immune responses to influenza virus vaccine administered by systemic or mucosal routes. Vaccine. 1995;13:1006–1012. doi: 10.1016/0264-410x(95)00016-t. [DOI] [PubMed] [Google Scholar]
- 85.Kilian M. Russell MW. Microbial evasion of IgA functions. In: Mestecky J, editor; Bienenstock J, editor; Lamm ME, editor; Mayer L, editor; McGhee JR, editor; Strober W, editor. Mucosal Immunology, 3rd. ed. Elsevier/Academic Press; Amsterdam: 2005. pp. 291–303. [Google Scholar]
- 86.Tjokronegoro A. Sirisinha S. Degradation of immunoglobulins by secretions of human reproductive tracts. J Reprod Fertil. 1974;38:221–225. doi: 10.1530/jrf.0.0380221. [DOI] [PubMed] [Google Scholar]
- 87.Renegar KB. Jackson GD. Mestecky J. In vitro comparison of the biologic activities of monoclonal monomeric IgA, polymeric IgA, and secretory IgA. J Immunol. 1998;160:1219–1223. [PubMed] [Google Scholar]
- 88.Kaetzel CS. Mostov K. Immunoglobulin transport, the polymeric immunoglobulins receptor. In: Mestecky J, editor; Bienenstock J, editor; Lamm ME, editor; Mayer L, editor; McGhee JR, editor; Strober W, editor. Mucosal Immunology, 3rd. ed. Elsevier/Academic Press; Amsterdam: 2005. pp. 211–250. [Google Scholar]
- 89.Belec L. Ghys PD. Hocini H, et al. Cervicovaginal secretory antibodies to human immunodeficiency virus type 1 (HIV-1) that block viral transcytosis through tight epithelial barriers in highly exposed HIV-1-seronegative African women. J Infect Dis. 2001;184:1412–1422. doi: 10.1086/324375. [DOI] [PubMed] [Google Scholar]
- 90.Bomsel M. Heyman M. Hocini H, et al. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity. 1998;9:277–287. doi: 10.1016/s1074-7613(00)80610-x. [DOI] [PubMed] [Google Scholar]
- 91.Moja P. Tranchat C. Tchou I, et al. Neutralization of human immunodeficiency virus type 1 (HIV-1) mediated by parotid IgA of HIV-1-infected patients. J Infect Dis. 2000;181:1607–1613. doi: 10.1086/315420. [DOI] [PubMed] [Google Scholar]
- 92.Chomont N. Hocini H. Gody JC, et al. Neutralizing monoclonal antibodies to human immunodeficiency virus type 1 do not inhibit viral transcytosis through mucosal epithelial cells. Virology. 2008;370:246–254. doi: 10.1016/j.virol.2007.09.006. [DOI] [PubMed] [Google Scholar]