Abstract
Background: Processed meat and fish have been shown to be associated with the risk of advanced prostate cancer, but few studies have examined diet after prostate cancer diagnosis and risk of its progression.
Objective: We examined the association between postdiagnostic consumption of processed and unprocessed red meat, fish, poultry, and eggs and the risk of prostate cancer recurrence or progression.
Design: We conducted a prospective study in 1294 men with prostate cancer, without recurrence or progression as of 2004–2005, who were participating in the Cancer of the Prostate Strategic Urologic Research Endeavor and who were followed for an average of 2 y.
Results: We observed 127 events (prostate cancer death or metastases, elevated prostate-specific antigen concentration, or secondary treatment) during 2610 person-years. Intakes of processed and unprocessed red meat, fish, total poultry, and skinless poultry were not associated with prostate cancer recurrence or progression. Greater consumption of eggs and poultry with skin was associated with 2-fold increases in risk in a comparison of extreme quantiles: eggs [hazard ratio (HR): 2.02; 95% CI: 1.10, 3.72; P for trend = 0.05] and poultry with skin (HR: 2.26; 95% CI: 1.36, 3.76; P for trend = 0.003). An interaction was observed between prognostic risk at diagnosis and poultry. Men with high prognostic risk and a high poultry intake had a 4-fold increased risk of recurrence or progression compared with men with low/intermediate prognostic risk and a low poultry intake (P for interaction = 0.003).
Conclusions: Our results suggest that the postdiagnostic consumption of processed or unprocessed red meat, fish, or skinless poultry is not associated with prostate cancer recurrence or progression, whereas consumption of eggs and poultry with skin may increase the risk.
INTRODUCTION
Approximately 2.1 million men currently live with prostate cancer in the United States, and an estimated 186,000 new cases were diagnosed in 2008. Over 90% of new cases are diagnosed in the localized or regional stages and have a 5-y survival approaching 100%. In contrast, the 5-y survival of prostate cancer patients with distant metastases is only 32% (1). Thus, identification of modifiable factors that affect the progression of prostate cancer is important for prostate cancer patients and public health.
Prostate cancer is a heterogeneous disease, and factors that affect its occurrence may differ from factors that affect its progression. For example, processed or cured meats are more strongly associated with increased risk of advanced or metastatic prostate cancer than of total prostate cancer (2–8). Similarly, fish intake may not be associated with risk of total prostate cancer, but is inversely associated with risk of metastatic prostate cancer and prostate cancer mortality (9, 10). Fewer prospective studies have assessed poultry or egg consumption and prostate cancer risk, and the results have been largely inconclusive, and no studies have examined postdiagnostic intake of these items in relation to prostate cancer progression (11–13).
In addition, saturated fat intake may be positively associated with prostate cancer mortality or biochemical recurrence after radical prostatectomy, and we previously reported a decreased risk of prostate cancer progression associated with high postdiagnostic fish and tomato sauce intake in the Health Professionals Follow-Up Study (14–17). These findings support the hypothesis that dietary factors may affect the progression of prostate cancer.
The aim of this study was to prospectively analyze the associations between postdiagnostic processed and unprocessed red meat, fish, poultry, and egg consumption and the risk of prostate cancer recurrence or progression in the Diet and Lifestyle substudy of the Cancer of the Prostate Strategic Urologic Research Endeavor [CapSURE (CapSURE is a trademarked name)]. We hypothesized that consumption of unprocessed and processed red meat may increase the risk of prostate cancer recurrence or progression because of the high saturated fat content of such meats (18) and that consumption of fish may decrease the risk of recurrence or progression through beneficial effects on inflammatory pathways (19). For comparison, we examined poultry and eggs—animal products with lower levels of saturated fat and long chain n−3 fatty acids—and hypothesized that consumption of these foods would not be associated with prostate cancer recurrence or progression.
SUBJECTS AND METHODS
Study population
Men in this study were participants in the Diet and Lifestyle substudy of CaPSURE. Details of CaPSURE were published previously (20, 21). Starting in 1995, men with biopsy-proven prostate cancer were invited to participate at 31 sites throughout the United States and asked to complete a questionnaire on sociodemographic characteristics, comorbidities, health-related quality of life, and use of health services. Follow-up questionnaires were mailed every 6 mo. Clinical data were collected by a certified urologist at baseline and at each subsequent clinic visit, including history of prostate cancer diagnosis, biopsies, pathological results, and treatments. During 2004 and 2005, active members at 25 CaPSURE sites were invited to participate in the Diet and Lifestyle substudy. Invitations were mailed to 5570 participants, and 2467 participants accepted. The baseline survey included questions on sociodemographic factors, medical history, physical activity, and a semiquantitative food-frequency questionnaire (FFQ). The distribution of sociodemographic and prognostic factors did not differ across the 25 CaPSURE sites. Completed surveys were received from 87% of the men who accepted the invitation. This study was approved by the Committee on Human Research, the Institutional Review Board of the University of California, San Francisco.
Dietary assessment
The FFQ included 127 food and beverage items plus supplements. A portion size was specified for each item, and participants were asked how often they had consumed that amount of the item on average over the past year (<1 time/mo, 1–3 times/mo, 1 time/wk, 2–4 times/wk, 5–6 times/wk, 1 time/d, 2–3 times/d, 4–5 times/d, or ≥6 times/d). Intake of each food item was calculated by multiplying the frequency of consumption by the portion size specified. Participants were asked whether they consumed less, the same amount, or more of each food item relative to before their diagnosis of prostate cancer. Additional questions addressed the type of fat used when cooking, the frequency of fried food consumption, and an open-ended section for any foods eaten frequently that were not included in the multiple-choice section.
We defined 5 food groups for this analysis: processed red meat, unprocessed red meat, fish, poultry, and eggs. Processed red meat included hot dogs, bacon, and processed meats (bologna, salami, sausage, etc). Unprocessed red meat included hamburgers, liver, beef, pork, or lamb as a sandwich or mixed dish (stew, casserole, lasagna, etc), and beef, pork, or lamb as a main dish (steak, roast, ham, etc). Fish included canned tuna fish, dark-meat fish (salmon, mackerel, bluefish, swordfish, sardines, etc), other fish, and shrimp, lobster, or scallops as a main dish. Poultry included skinless chicken or turkey and chicken or turkey with skin. We included eggs as a separate category because their nutrient composition differs from that of other poultry products.
Outcome assessment and follow-up
Data on prostate cancer progression were collected from medical records by study investigators and included pathologic results, staging, and primary and subsequent treatments. After receiving the participants' permission, self-reported hospitalizations were verified through hospital records. Procedures performed, length of stay, discharge diagnosis, and discharge status were recorded. The National Death Index and Bureau of Vital Statistics were checked through 10 July 2008 for mortality data, and records were reviewed by a study physician to verify the date, primary cause, and location of death.
We used the following clinical variables from physicians' reports: diagnostic biopsy Gleason sum (2–10), prostate-specific antigen (PSA) at diagnosis (0-10.0, 10.1–20.0, or > 20.0 ng/mL), clinical T stage at diagnosis (T1, T1a-c; T2, T2a-c; T3, T3a-c, T4, T4a-b), and primary and subsequent treatments. Treatments were categorized as follows: radical prostatectomy (RP), radiation therapy (RT), hormone therapy (ADT), and other. Other treatments included cryosurgery, trans-urethral microwave thermotherapy, second line medications, and watchful waiting. Watchful waiting was included in “other treatment” because it was uncommon in this study population (n = 47, 3.6%). We categorized each participant by prognostic risk based on the D'Amico risk categories as follows (22): high (PSA > 20 ng/mL or Gleason sum = 8–10 or T stage T3a+), intermediate [PSA = 10.1–20 ng/mL or Gleason sum = 7 or secondary 4–5 pattern or T stage T2b/T2c (2002) or T2b (1997)], or low (PSA ≤ 10 ng/mL and Gleason sum = 2–6 and T stage = T1/T2a).
We defined an event of prostate cancer recurrence or progression (hereafter referred to as “progression” for brevity) as the first of the following events: prostate cancer death, bone metastases from prostate cancer, biochemical recurrence, or initiation of secondary treatment. An outcome of bone metastases was defined as physician report of 1) distant prostate cancer progression to bone, 2) a positive bone scan, 3) radiation for metastasis at a bone site, or 4) M1b stage in TNM staging. Biochemical recurrence was defined as 2 consecutive PSA concentrations ≥0.2 ng/mL ≥8 wk after radical prostatectomy or 3 consecutive increases in PSA above the postradiation nadir after radiation therapy. Secondary treatment was defined as treatment initiated ≥6 mo after primary treatment ended. Secondary treatment was included in our outcome definition because, among men who have undergone primary treatment, initiation of secondary treatment is indicative of biochemical or clinical evidence of disease recurrence (23, 24). The date of prostate cancer progression was assigned as the first of the following: prostate cancer death, diagnosis of bone metastases from prostate cancer, date of second PSA increase for radical prostatectomy patients, midpoint between date of postradiation nadir and first PSA increase for radiation patients, or date of initiation of secondary treatment.
Analysis population and exclusion criteria
To be included in this analysis, men had to have completed the baseline Diet and Lifestyle CaPSURE survey between April 2004 and November 2005 (n = 2134). We excluded men with advanced or metastatic disease at diagnosis (n = 139) and men with no treatment information (n = 36). We also excluded men with no follow-up beyond their Diet and Lifestyle survey (n = 251) and men whose prostate cancer progressed before they completed the survey (n = 365). Last, we excluded men who reported an unreasonable energy intake (<800 or >4200 kcal/d) (n = 49), which resulted in 1294 men for analysis.
Statistical methods
We analyzed the associations between the 4 meat groups and eggs and time to prostate cancer progression using Cox proportional hazard regression models. We used hazards ratios (HRs) and 95% CIs to estimate the relative risk of progression. Person-time was calculated for each participant from the date they completed the Diet and Lifestyle questionnaire until the date of disease progression, nonprostate cancer death, last contact, or end of follow-up (10 July 2008), whichever occurred first. Dietary intakes were divided into quantiles based on the distribution of intakes in the study population. Relative risks were calculated by comparing the risk of progression for men in the upper quantiles relative to men in the lowest quantile.
Our basic model included indicator variables for the quartiles of the food group or item of interest, age at diagnosis (<60, 60–69.9, 70–79.9, or ≥80 y), energy intake (kcal/d), and time from diagnosis to questionnaire. Our second model additionally adjusted for predictors of progression in this study population, including primary treatment (RP, RT, ADT, or other), body mass index (BMI; in kg/m2; <18.5, 18.5–24.9, 25–29.9, or ≥30), nonvigorous activity (metabolic equivalent hours/wk), Gleason sum at diagnosis (2–10), and PSA at diagnosis (0–10.0, 10.1–20.0, or >20.0 ng/mL). We examined models adjusting for other food groups (meats other than the main exposure, fruit, grains, sweets, vegetables, and dairy products), clinical T stage at diagnosis, smoking, race, education, income, marital status, vigorous activity, and frequency of fried food intake in addition to the abovementioned variables, and our results did not change materially. In addition, we considered confounding by tomato products and cruciferous vegetables, because these items have been shown to be inversely associated with prostate cancer incidence or progression in prior studies; however, the results were unaffected (14, 15, 17, 25, 26). We added energy-adjusted saturated fat to our final models using the nutrient residual method to examine whether saturated fat from the food group of interest explained any of the association between the food group and prostate cancer progression (27). Last, we used the median value of each quantile as an ordinal score variable to test for evidence of linear trends in our final models (28).
We examined whether the associations for any meat group or eggs and risk of progression were modified by BMI, prognostic risk at diagnosis, or time from diagnosis to questionnaire. BMI was considered as a potential effect modifier based on Strom et al (15), who observed shorter progression-free survival after prostatectomy among obese men who consumed a high-saturated-fat diet before diagnosis compared with lean men who consumed a high-saturated-fat diet before diagnosis. We considered effect modification by prognostic risk because prostate cancer has a heterogeneous natural history, and we hypothesized that dietary factors may have a different association with progression of aggressive compared with nonaggressive prostate cancer. We generated cross-product terms between each item and potential effect modifier, entered the cross-product terms in our multivariate models, and tested the significance of the cross-product terms' regression coefficients using Wald tests (29). If there was evidence of a significant interaction, we created indicator variables for each unique combination of effect modifier and quartile of meat or egg intake and included them in a multivariate model to compare the risk of progression in each group with a common reference.
To assess whether our results were affected by reverse causation (ie, higher-risk patients ate more or less of a food item after their diagnosis than did lower-risk patients out of concern for their disease prognosis), we compared self-reported change in consumption of individual meat and egg items after prostate cancer diagnosis across prognostic risk categories using Pearson chi-square and Fisher's exact tests.
We were concerned that health-conscious men would choose healthier diets and would also be more likely to have routine PSA monitoring and/or seek secondary treatment. It was possible that the inclusion of biochemical recurrence and second treatment in our outcome definition would introduce positive confounding between healthy dietary factors and risk of progression and negative confounding between unhealthy dietary factors and risk of progression. To address this possibility, we performed secondary analyses excluding events defined by initiation of secondary treatment, of which we had no biological evidence of recurrence preceding the secondary treatment (n = 38); our results did not change materially. We defined biological evidence of recurrence as any PSA concentration ≥0.2 ng/mL after radical prostatectomy or any PSA concentration ≥0.3 ng/mL above the posttreatment nadir after radiation or other forms of treatment.
We were also concerned that watchful waiters could be misclassified as events of progression if they initiated treatment during follow-up because of anxiety rather than because of an objective change in their disease state. However, our effect estimates were materially unchanged after excluding watchful waiters (n = 47). Therefore, the results reported include all 127 events for the 1294 men. Statistical tests were 2-sided and were performed at the 0.05 level of significance. We used SAS (version 9.1; SAS Institute, Cary, NC) for all analyses.
RESULTS
We identified 127 events of prostate cancer progression among 1294 men with a diagnosis of localized or regional prostate cancer during 2610 person-years of follow-up. Comparison of the men included in our analysis (n = 1294) with the men who were initially invited (n = 5570) showed that the participants were more likely to be white (95.6% compared with 90.6%), more likely to have a better prognosis (Gleason sum 2–6: 68.9% compared with 63.5%; PSA 0–10 ng/mL: 83.2% compared with 74.4%), and more likely to have a radical prostatectomy as their primary treatment (63% compared with 53%). The median year of diagnosis was 2002, and half of the participants were diagnosed between 2000 and 2003. Initiation of secondary treatment accounted for 57% (n = 72) of events, biochemical recurrence accounted for 39% (n = 49) of events, and metastases to bone and death from prostate cancer each accounted for 2% (n = 3) of events.
A comparison of the participants' characteristics, by the highest to the lowest quartiles of the meat groups and eggs, are presented in Table 1. Processed red meat and egg consumption were positively associated with less healthy lifestyle behaviors and worse clinical characteristics, including higher mean BMI, smoking, and Gleason sum 8–10 at diagnosis. Men in the highest quartile of unprocessed red meat also had a higher mean BMI than did men in the lowest quartile of unprocessed red meat. Men in the highest quartiles of fish and poultry were younger at diagnosis than were men in the lowest quartiles of those items. In addition, men in the highest quartile of poultry were more likely to have radical prostatectomy as their primary treatment, and men in the highest quartile of fish were more likely to have other forms of treatment compared with men in the lowest quartiles of those items.
TABLE 1.
Characteristics of 1294 men with prostate cancer in the Diet and Lifestyle substudy of CaPSURE (Cancer of the Prostate Strategic Urologic Research Endeavor) comparing extreme quartiles (Q) of intakes of processed and unprocessed red meat, fish, poultry, and eggs (2004–2008)1
| Processed red meat |
Unprocessed red meat |
Fish |
Poultry |
Eggs2 |
||||||
| Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | |
| Median intake (servings/wk)3 | 0 | 5.0 | 0.9 | 7.0 | 0.4 | 4.3 | 1.0 | 5.5 | 0.4 | 5.5 |
| No. of participants | 310 | 337 | 324 | 303 | 348 | 347 | 380 | 346 | 319 | 176 |
| Total person-years | 603 | 677 | 618 | 634 | 750 | 655 | 782 | 660 | 631 | 347 |
| Age at diagnosis (y) | 65.7 ± 8.74 | 65.6 ± 8.1 | 65.7 ± 7.8 | 64.6 ± 8.5 | 66.0 ± 8.2 | 64.4 ± 8.15 | 67.1 ± 7.3 | 62.8 ± 8.45 | 64.7 ± 8.0 | 64.1 ± 8.0 |
| BMI (kg/m2) | 26.2 ± 3.3 | 27.9 ± 4.45 | 26.5 ± 3.5 | 27.9 ± 4.35 | 27.2 ± 3.8 | 27.2 ± 4.0 | 27.3 ± 3.7 | 27.8 ± 4.2 | 26.5 ± 3.6 | 28.3 ± 4.75 |
| Nonvigorous activity (MET-h/wk) | 11.5 ± 14.5 | 12.3 ± 15.1 | 11.5 ± 13.6 | 11.7 ± 14.8 | 10.8 ± 14.5 | 12.5 ± 14.6 | 9.8 ± 13.5 | 11.9 ± 13.9 | 11.8 ± 14.6 | 13.0 ± 15.4 |
| Smoking (%) | ||||||||||
| Current | 3.6 | 6.5 | 5.9 | 7.9 | 6.9 | 5.2 | 6.6 | 3.8 | 6.6 | 9.7 |
| Past | 51.3 | 59.45 | 52.8 | 53.5 | 49.4 | 55.0 | 55.8 | 57.6 | 49.2 | 60.85 |
| Never | 45.2 | 32.15 | 41.1 | 37.3 | 42.8 | 38.9 | 36.6 | 37.9 | 42.6 | 30.05 |
| Unknown | 0.0 | 2.15 | 0.3 | 1.3 | 0.9 | 0.9 | 1.1 | 0.9 | 1.6 | 0.05 |
| Race (%) | ||||||||||
| White | 95.8 | 96.4 | 93.8 | 96.0 | 97.4 | 94.05 | 97.1 | 94.5 | 95.3 | 96.0 |
| African American | 2.3 | 2.4 | 4.6 | 1.7 | 2.0 | 2.3 | 1.8 | 3.8 | 2.5 | 2.8 |
| Other | 1.9 | 1.2 | 1.5 | 2.3 | 0.6 | 3.85 | 1.1 | 1.7 | 2.2 | 1.1 |
| Gleason sum at diagnosis (%) | ||||||||||
| 2–6 | 65.2 | 67.4 | 66.4 | 69.3 | 68.4 | 69.2 | 67.9 | 70.2 | 71.8 | 67.1 |
| 7 | 28.7 | 23.4 | 25.0 | 22.8 | 26.4 | 21.9 | 25.3 | 23.7 | 23.5 | 21.0 |
| 8–10 | 4.8 | 8.05 | 6.8 | 7.6 | 4.6 | 6.6 | 5.5 | 4.6 | 3.1 | 9.75 |
| Unknown | 1.3 | 1.2 | 1.9 | 0.3 | 0.6 | 2.35 | 1.3 | 1.5 | 1.6 | 2.3 |
| PSA at diagnosis (%) | ||||||||||
| 0–10 ng/mL | 80.3 | 84.9 | 81.8 | 81.9 | 84.2 | 81.8 | 82.1 | 83.2 | 84.3 | 81.3 |
| 10.1–20.0 ng/mL | 12.9 | 10.1 | 11.1 | 11.9 | 10.6 | 10.4 | 12.1 | 11.0 | 10.3 | 11.4 |
| ≥20 ng/mL | 3.2 | 3.9 | 4.3 | 3.3 | 2.6 | 4.3 | 2.6 | 3.5 | 1.9 | 3.4 |
| Unknown | 3.6 | 1.2 | 2.8 | 3.0 | 2.6 | 3.5 | 3.2 | 2.3 | 3.5 | 4.0 |
| Primary treatment (%) | ||||||||||
| Radical prostatectomy | 61.6 | 58.5 | 61.7 | 64.0 | 60.6 | 59.7 | 57.9 | 67.65 | 65.2 | 64.8 |
| Radiation therapy | 24.5 | 24.6 | 24.1 | 21.8 | 24.1 | 27.4 | 25.8 | 20.2 | 22.6 | 22.7 |
| Hormonal therapy | 5.8 | 7.1 | 5.6 | 5.6 | 4.3 | 7.5 | 7.4 | 5.8 | 5.0 | 6.3 |
| Other | 8.1 | 9.8 | 8.6 | 8.6 | 10.9 | 5.55 | 9.0 | 6.4 | 7.2 | 6.3 |
MET-h, metabolic equivalent task hours; PSA, prostate-specific antigen.
The numbers of persons per tertile for eggs are not even because of the distribution of egg intake in the study population; ≈45% consumed ≤1 egg/wk, 41% consumed 3 eggs/wk, and 14% consumed >3 eggs/wk.
One processed red meat serving = 1 hot dog, 2 slices of bacon, or 1 slice of processed meats (sausage, salami, bologna, etc). One unprocessed red meat serving = 85–142 g (3–5 oz) of liver; 1 hamburger patty; beef, pork, or lamb as a sandwich or mixed dish; or 113–170 g (4–6 oz) beef, pork, or lamb as a main dish. One fish serving = 85–113 g (3–4 oz) of canned tuna fish, dark-meat fish, other fish, or shrimp, lobster, or scallops as a main dish. One poultry serving = 113–170 g (4–6 oz) of chicken or turkey with or without skin. One egg serving = one whole egg.
Mean ± SD (all such values).
P for trend <0.05; estimated by using the median intakes of each quartile continuously in linear regression models for continuous variables and in logistic regression models for categorical variables.
A comparison of select dietary habits of the participants, by highest to lowest quartiles of the meat groups and eggs, are presented in Table 2. After energy intake was adjusted for, men in the highest quartile of processed red meat, unprocessed red meat, or eggs consumed more of all of these items than did men in the lowest quartiles. Men in the highest quartile of processed red meat also consumed more poultry and less tomato products than did men in the lowest quartile of processed red meat. In contrast, men in the highest quartiles of fish or poultry had healthier dietary habits, including more fish, poultry, and cruciferous vegetables, than did men in the lowest quartiles of fish or poultry. Men in the highest quartile of fish also consumed more tomato products and less unprocessed red meat than did men in the lowest quartile of fish. Last, men in the highest quartiles of unprocessed red meat, fish, or poultry consumed less dairy products than did men in the lowest quartiles of those items.
TABLE 2.
Select dietary habits of 1294 men with prostate cancer in the Diet and Lifestyle substudy of CaPSURE (Cancer of the Prostate Strategic Urologic Research Endeavor) comparing extreme quartiles (Q) of intakes of processed and unprocessed red meat, fish, poultry, and eggs (2004–2008)1
| Processed red meat |
Unprocessed red meat |
Fish |
Poultry |
Eggs2 |
||||||
| Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | |
| No. of participants | 310 | 337 | 324 | 303 | 348 | 347 | 380 | 346 | 319 | 176 |
| Total person-years | 603 | 677 | 618 | 634 | 750 | 655 | 782 | 660 | 631 | 347 |
| Energy intake (kcal/d) | 1768 ± 5783 | 2255 ± 5754 | 1670 ± 554 | 2386 ± 5984 | 1867 ± 641 | 2173 ± 6224 | 1738 ± 536 | 2256 ± 6324 | 1818 ± 615 | 2200 ± 6464 |
| Processed red meat (servings/wk)5 | 0.2 ± 0.3 | 5.8 ± 2.94 | 1.7 ± 2.4 | 3.0 ± 2.74 | 2.5 ± 2.5 | 2.6 ± 2.8 | 2.6 ± 2.6 | 2.6 ± 2.9 | 1.7 ± 2.3 | 4.0 ± 3.84 |
| Unprocessed red meat (servings/wk)6 | 3.0 ± 2.8 | 4.4 ± 2.44 | 1.2 ± 0.8 | 6.7 ± 2.34 | 4.0 ± 2.9 | 3.4 ± 2.44 | 3.7 ± 2.7 | 3.9 ± 2.5 | 3.1 ± 2.5 | 4.2 ± 2.64 |
| Fish (servings/wk)7 | 2.4 ± 2.2 | 2.2 ± 1.9 | 2.5 ± 2.4 | 2.2 ± 2.0 | 0.6 ± 0.4 | 4.6 ± 2.14 | 1.7 ± 1.6 | 2.6 ± 2.14 | 2.3 ± 2.3 | 2.3 ± 1.7 |
| Poultry (servings/wk)8 | 3.2 ± 2.6 | 2.5 ± 1.84 | 2.8 ± 2.5 | 2.8 ± 2.0 | 2.3 ± 2.0 | 3.3 ± 2.34 | 0.9 ± 0.5 | 5.0 ± 2.44 | 2.8 ± 2.3 | 3.2 ± 2.6 |
| Eggs (servings/wk)9 | 2.2 ± 3.4 | 3.5 ± 3.84 | 2.4 ± 3.5 | 3.1 ± 3.34 | 2.5 ± 2.9 | 2.8 ± 3.5 | 2.4 ± 2.4 | 2.9 ± 3.3 | 0.3 ± 0.3 | 7.9 ± 5.34 |
| Dairy products (servings/wk)10 | 14.7 ± 11.3 | 14.3 ± 8.7 | 15.5 ± 11.2 | 12.7 ± 8.44 | 15.7 ± 11.3 | 13.0 ± 8.14 | 16.5 ± 11.2 | 13.1 ± 8.54 | 15.3 ± 11.3 | 14.9 ± 8.5 |
| Tomato products (servings/wk)11 | 5.1 ± 4.1 | 4.4 ± 3.34 | 5.1 ± 4.3 | 4.6 ± 3.7 | 4.0 ± 3.3 | 5.5 ± 3.84 | 4.3 ± 3.5 | 4.9 ± 3.8 | 4.7 ± 3.7 | 4.9 ± 4.2 |
| Cruciferous vegetables (servings/wk)12 | 3.2 ± 2.7 | 2.8 ± 2.8 | 3.2 ± 3.9 | 3.3 ± 2.9 | 2.2 ± 2.0 | 4.0 ± 4.04 | 2.2 ± 2.1 | 3.6 ± 3.04 | 3.1 ± 3.6 | 3.8 ± 3.4 |
Servings/wk were standardized to 2000 kcal/d.
The numbers of persons per tertile of eggs is not equal because of the distribution of egg intake in the study population; ≈45% consumed ≤1 egg/wk, 41% consumed 3 eggs/wk, and 14% consumed >3 eggs/wk.
Mean ± SD (all such values).
P for trend <0.05; estimated by using the median intakes of each quartile continuously in linear regression models for continuous variables and in logistic regression models for categorical variables.
Processed red meat includes bacon, hot dogs, and processed meats (sausage, salami, bologna, etc).
Unprocessed red meat includes the following: liver; hamburger; beef, pork, or lamb as a mixed dish; and beef, pork, or lamb as a main dish.
Fish includes canned tuna fish, dark-meat fish, other fish, and shrimp, lobster, or scallops as a main dish.
Poultry includes chicken or turkey with skin and chicken or turkey without skin.
Eggs includes whole eggs.
Dairy products include skim or low-fat milk, whole milk, cream, sour cream, yogurt, cottage or ricotta cheese, cream cheese, other cheese, margarine added to food or bread, and butter added to food or bread.
Tomato products include tomatoes, tomato juice, tomato sauce, and pizza.
Cruciferous vegetables include broccoli, cabbage or coleslaw, cauliflower, Brussels sprouts, kale, mustard greens, and chard.
The relative risks of prostate cancer progression by quartiles of the meat groups and eggs are presented in Table 3. We observed no evidence of an association between processed red meat, unprocessed red meat, or fish with prostate cancer progression. The HRs (95% CIs) for the comparison of the highest with the lowest quartiles were as follows: 1.30 (0.78, 2.17) for processed red meat, 0.95 (0.55, 1.66) for unprocessed red meat, and 1.13 (0.70, 1.84) for fish after adjustment for sociodemographic and clinical risk factors. We observed an increased risk of prostate cancer progression associated with higher poultry intake that was not statistically significant [HR for quartile 4 (Q4) compared with quartile 1 (Q1): 1.55; 95% CI: 0.91, 2.66]. In addition, we observed a significant 2-fold increased risk of prostate cancer progression among men in the highest quartile of egg intake compared with men in the lowest quartile (HR: 2.02; 95% CI: 1.10, 3.72), which appeared to be limited to the highest level of intake.
TABLE 3.
Postdiagnostic meat and egg consumption and relative risk of prostate cancer progression among 1294 men with prostate cancer in the Diet and Lifestyle substudy of CaPSURE (Cancer of the Prostate Strategic Urologic Research Endeavor): 2004–20081
| Quartile of intake |
|||||
| 1 | 2 | 3 | 4 | P for trend2 | |
| Median processed red meat intake | 0.0 | 1.0 | 2.5 | 5.0 | 0.18 |
| No. of events/no. of participants | 29/310 | 32/372 | 21/275 | 45/337 | |
| Total person-years | 603 | 765 | 565 | 677 | |
| Model 13 | 1.0 (ref) | 1.01 (0.61, 1.68)4 | 0.88 (0.50, 1.56) | 1.48 (0.91, 2.43) | |
| Model 256 | 1.0 (ref) | 0.91 (0.54, 1.54) | 0.89 (0.50, 1.61) | 1.30 (0.78, 2.17) | |
| Median unprocessed red meat intake | 0.9 | 2.5 | 4.5 | 7.0 | 0.65 |
| No. of events/no. of participants | 30/324 | 38/371 | 26/296 | 33/303 | |
| Total person-years | 618 | 746 | 612 | 634 | |
| Model 1 | 1.0 (ref) | 1.12 (0.69, 1.81) | 0.89 (0.52, 1.53) | 1.02 (0.60, 1.76) | |
| Model 2 | 1.0 (ref) | 1.09 (0.67, 1.77) | 0.80 (0.44, 1.43) | 0.95 (0.55, 1.66) | |
| Median fish intake | 0.4 | 1.3 | 1.9 | 4.3 | 0.46 |
| No. of events/no. of participants | 36/348 | 21/275 | 34/324 | 36/347 | |
| Total person-years | 750 | 551 | 654 | 655 | |
| Model 1 | 1.0 (ref) | 0.80 (0.47, 1.38) | 1.17 (0.73, 1.88) | 1.23 (0.77, 1.97) | |
| Model 2 | 1.0 (ref) | 0.78 (0.45, 1.37) | 1.07 (0.65, 1.73) | 1.13 (0.70, 1.84) | |
| Median poultry intake | 1.0 | 2.0 | 3.0 | 5.5 | 0.17 |
| No. of events/no. of participants | 33/380 | 24/224 | 35/344 | 35/346 | |
| Total person-years | 782 | 474 | 694 | 660 | |
| Model 1 | 1.0 (ref) | 1.36 (0.79, 2.31) | 1.29 (0.80, 2.10) | 1.42 (0.85, 2.37) | |
| Model 2 | 1.0 (ref) | 1.51 (0.87, 2.61) | 1.29 (0.78, 2.13) | 1.55 (0.91, 2.66) | |
| Median egg intake | 0.4 | 1.0 | 3.0 | 5.5 | 0.05 |
| No. of events/no. of participants | 24/319 | 27/267 | 51/532 | 25/176 | |
| Total person-years | 631 | 548 | 1084 | 347 | |
| Model 1 | 1.0 (ref) | 1.30 (0.75, 2.26) | 1.21 (0.74, 1.97) | 2.07 (1.16, 3.70) | |
| Model 2 | 1.0 (ref) | 1.17 (0.66, 2.07) | 1.06 (0.63, 1.77) | 2.02 (1.10, 3.72) | |
Median intakes are reported as servings/wk. One processed red meat serving = 1 hot dog, 2 slices of bacon, or 1 slice of processed meats (sausage, salami, bologna, etc). One unprocessed red meat serving = 85–142 g (3–5 oz) of liver; 1 hamburger patty; beef, pork, or lamb as a sandwich or mixed dish; or 113–170 g (4–6 oz) beef, pork, or lamb as a main dish. One fish serving = 85–113 g (3–4 oz) of canned tuna fish, dark meat fish, other fish, or shrimp, lobster, or scallops as a main dish. One poultry serving = 113–170 g (4–6 oz) of chicken or turkey with or without skin. One egg serving = one whole egg. ref, reference.
Calculated from a Wald test of the regression coefficient of an ordinal variable by using the median of each quartile in a multivariate model adjusted for age at diagnosis, energy intake, time from diagnosis to questionnaire, Gleason sum at diagnosis, prostate-specific antigen at diagnosis, primary treatment, BMI, and nonvigorous activity.
Model 1 was adjusted for age at diagnosis (<60, 60–69.9, 70–79.9, and ≥80 y), energy intake (kcal/d), and time from diagnosis to questionnaire.
Hazard ratio; 95% CI in parentheses (all such values).
Model 2 was adjusted for age at diagnosis (<60, 60–69.9, 70–79.9, and ≥80 y), energy intake (kcal/d), time from diagnosis to questionnaire, primary treatment (radical prostatectomy, radiation therapy, hormone therapy, or other), BMI (in kg/m2; <18.5, 18.5–24.9, 25–29.9, and ≥30), nonvigorous activity (metabolic equivalent hours/wk), Gleason sum at diagnosis (2–10), and prostate-specific antigen at diagnosis (0–10.0, 10.1–20.0, and >20.0 ng/mL). Fifty-five (4.3%) participants were missing data on covariates and were not included in the model.
Additional adjustment for other food groups, clinical T stage at diagnosis, smoking, race, education, income, marital status, vigorous physical activity, and frequency of intakes of fried food, tomato products, and cruciferous vegetables did not materially change the effect estimates.
To further explore the borderline significant association for poultry, we analyzed poultry with and without skin separately (Table 4). Consumption of skinless poultry was not associated with risk of prostate cancer progression. In contrast, men in the highest tertile of poultry with skin had more than a doubling in risk of prostate cancer progression compared with men in the lowest tertile after adjustment for sociodemographic factors, clinical characteristics, and skinless poultry. Furthermore, there was evidence of a strong linear trend (HR: 2.26; 95% CI: 1.36, 3.76, P for trend = 0.003).
TABLE 4.
Postdiagnostic consumption of poultry with skin and skinless poultry and risk of prostate cancer progression among 1294 men with prostate cancer in the Diet and Lifestyle substudy of CaPSURE (Cancer of the Prostate Strategic Urologic Research Endeavor): 2004–20081
| Tertile of intake |
||||
| 1 | 2 | 3 | P for trend2 | |
| Median poultry with skin intake | 0.0 | 1.0 | 3.0 | 0.003 |
| No. of events/no. of participants | 48/584 | 46/473 | 33/237 | |
| Total person-years | 1183 | 980 | 447 | |
| Model 13 | 1.0 (ref)4 | 1.19 (0.78, 1.84) | 2.23 (1.39, 3.59) | |
| Model 256 | 1.0 (ref) | 1.22 (0.78, 1.92) | 2.26 (1.36, 3.76) | |
| Median skinless poultry intake | 0.4 | 1.0 | 3.0 | 0.87 |
| No. of events/no. of participants | 35/344 | 42/391 | 50/559 | |
| Total person-years | 691 | 804 | 1115 | |
| Model 1 | 1.0 (ref) | 1.27 (0.80, 1.99) | 1.03 (0.64, 1.63) | |
| Model 2 | 1.0 (ref) | 1.53 (0.95, 2.48) | 1.20 (0.73, 1.96) | |
Median intakes are reported as servings/wk; 113–170 g (4–6 oz) = 1 serving. ref, reference.
Calculated from a Wald test of the regression coefficient of an ordinal variable by using the median of each quartile in a multivariate model adjusted for age at diagnosis, energy intake, time from diagnosis to questionnaire, Gleason sum at diagnosis, prostate-specific antigen at diagnosis, primary treatment, BMI, nonvigorous activity, and other poultry.
Model 1 is adjusted for age at diagnosis (<60, 60–69.9, 70–79.9, ≥80 y), energy intake (kcal/d), time from diagnosis to questionnaire, and other poultry.
Hazard ratio; 95% CI in parentheses (all such values).
Model 2 was adjusted for age at diagnosis (<60, 60–69.9, 70–79.9, and ≥80 y), energy intake (kcal/d), time from diagnosis to questionnaire, primary treatment (radical prostatectomy, radiation therapy, hormone therapy, or other), BMI (in kg/m2; <18.5, 18.5–24.9, 25–29.9, and ≥30), nonvigorous activity (metabolic equivalent hours/wk), Gleason sum at diagnosis (2–10), prostate-specific antigen at diagnosis (0–10.0, 10.1–20.0, and >20.0 ng/mL), and other poultry. Fifty-five percent (4.3%) of participants were missing data on covariates and were not included in the model.
Additional adjustment for other food groups, clinical T stage at diagnosis, smoking, race, education, income, marital status, vigorous physical activity, and frequency of intakes of fried food, tomato products, and cruciferous vegetables did not materially change the effect estimates.
Adjustment for saturated fat did not materially change the effect estimates for poultry with skin, skinless poultry, fish, or eggs. The point estimates for the comparison of extreme quartiles for processed and unprocessed red meat decreased after adjustment for saturated fat, but remained statistically nonsignificant; the HR for processed red meat was 1.17 (95% CI: 0.68, 2.01) and for unprocessed red meat was 0.79 (95% CI: 0.41, 1.98).
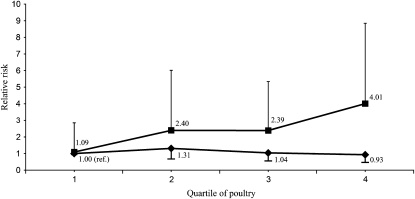
We observed an interaction between prognostic risk, total poultry, and risk of prostate cancer progression. Greater poultry intake was associated with increased risk of progression among men with high prognostic risk, but there was no association among men with low/intermediate prognostic risk (P for interaction = 0.003) (Figure 1). Men with high prognostic risk disease and in the highest quartile of poultry had a 4-fold increased risk of progression compared with men with low/intermediate prognostic risk disease and low poultry intake (HR: 4.01; 95% CI: 1.82, 8.85). There was no evidence of a significant interaction between prognostic risk and consumption of any other meat group or eggs. In addition, no interactions were observed between BMI or time from diagnosis to questionnaire and any of the meat groups or eggs.
FIGURE 1.
Relative risk of prostate cancer progression by prognostic risk and postdiagnostic consumption of poultry among 1294 men with prostate cancer in the Diet and Lifestyle substudy of CaPSURE (Cancer of the Prostate Strategic Urologic Research Endeavor, 2004–2008). P for interaction = 0.003 derived by using a Wald test of the coefficient of the cross-product term between poultry and prognostic risk in a Cox proportional hazards regression model adjusted for the main effects of poultry and prognostic risk (low/intermediate compared with high), age at diagnosis, energy intake, time from diagnosis to questionnaire, Gleason sum at diagnosis, prostate-specific antigen at diagnosis, primary treatment, BMI, and nonvigorous activity. ▪, high prognostic risk (prostate-specific antigen >20 ng/mL or Gleason sum = 8–10 or T stage T3a+); n = 200 participants (49 events). ♦ , low/intermediate prognostic risk; n = 1065 participants (73 events). Prognostic risk was missing for 29 participants (5 events).
We observed no significant associations between prognostic risk and self-reported change in diet after diagnosis for any processed or unprocessed red meat item, fish item, eggs, or poultry with skin. Men with high prognostic risk were somewhat less likely to report change in consumption of skinless poultry after diagnosis compared with men with a low or intermediate prognostic risk. Approximately 8% of high-risk men reported eating more skinless poultry and none reported eating less compared with before their diagnosis of prostate cancer, whereas 10% of low-risk men reported eating more skinless poultry and 2% of low-risk men reported eating less compared with before their diagnosis (P value = 0.03).
In secondary analyses excluding the 38 men who initiated secondary treatment of which we did not have biological evidence of recurrence (defined as at least one posttreatment PSA ≥ 0.2 ng/mL after radical prostatectomy or at least one PSA ≥ 0.3 ng/mL above posttreatment nadir after radiation or other treatment), the results for egg consumption remained positive but became nonsignificant (HR for Q4 compared with Q1: 1.47; 95% CI: 0.72, 2.98), whereas the positive associations observed for total poultry (HR for Q4 compared with Q1: 1.80; 95% CI: 0.95, 3.41) and poultry with skin [HR for tertile (T) 3 compared with T1: 2.72; 95% CI: 1.51, 4.89) were strengthened.
DISCUSSION
We observed no association between postdiagnostic consumption of processed or unprocessed red meat, fish, or skinless poultry and risk of prostate cancer progression among 1294 men with a diagnosis of localized prostate cancer and followed for an average of 2 y. However, postdiagnostic consumption of poultry with skin and whole eggs were associated with 2-fold increases in risk of prostate cancer progression.
We acknowledge that our study had several limitations, including a short follow-up, a small number of prostate cancer deaths or metastases, and a lack of prediagnostic dietary data. The Diet and Lifestyle substudy of CaPSURE has yet to accrue many events of prostate cancer metastases or death. Thus, we included biochemical recurrence and initiation of secondary treatment in our outcome definition to improve statistical power, but also because biochemical recurrence within 2 y of primary treatment is highly predictive of prostate cancer metastases and death, and secondary treatment is administered to patients with biochemical or clinical evidence of recurrence (23, 24, 30–32). This outcome is appropriate in our study population because watchful waiting was rare (n = 47); only 6 (4.7%) of our events occurred in watchful waiters, and the exclusion of all watchful waiters did not materially change our results. In addition, our results did not change after we excluded men who initiated the secondary treatment of which we had no biological evidence of recurrence, which suggested that anxiety after prostate cancer diagnosis did not confound our observed associations.
Men were recruited into our study after diagnosis of prostate cancer, so we were unable to collect prediagnostic dietary information. As a result, we could not examine the association between these items and risk of prostate cancer. Most of the previous studies that examined poultry or eggs and risk of prostate cancer reported no association (13). However, risk factors for incidence of total prostate cancer may differ from risk factors for advanced or fatal disease and thus it is plausible that poultry with skin and eggs may increase the risk of advanced prostate cancer or prostate cancer progression, but are not associated with risk of total prostate cancer (33). It is difficult to distinguish factors that affect initiation of aggressive prostate cancer from factors that affect progression of the disease in epidemiologic studies. However, because the 5-y survival of men with advanced prostate cancer is only 35%, it is important to identify modifiable factors that may prevent either the occurrence of advanced prostate cancer or its progression.
We are aware of only one prior study of postdiagnostic consumption of whole foods and risk of prostate cancer progression. Our collaborative group examined the postdiagnostic intake of grains, vegetables, fruit, red meat, milk, fish, tomato sauce, and fresh tomato products and prostate cancer progression among 1202 men with localized or regional prostate cancer in the Health Professionals Follow-Up Study. In that study, red meat was not associated with the risk of prostate cancer progression, and fish was inversely associated with risk of progression (17).
Our results for processed and unprocessed red meat are consistent with the prior study. However, we did not observe an association between fish intake and risk of prostate cancer progression. Two cohort studies have reported an inverse association between fish intake and advanced or metastatic prostate cancer and another reported an inverse association between fish intake and prostate cancer mortality (9, 10, 12). The inconsistent results may reflect unmeasured genetic differences in the populations or variation in the type and amount of fish consumed. For example, Hedelin et al (34) reported an interaction between salmon-type fish and a variant in the COX-2 gene (rs5275: +6365 T/C) where, among men with the variant allele, consuming salmon-type fish more than once per week was associated with a 72% decreased risk of prostate cancer compared with men who never consumed salmon-type fish, but no association was observed among men with the wild-type genotype.
Our analyses of poultry and eggs were exploratory, because no studies have examined the postdiagnostic intake of these items and risk of prostate cancer progression (11, 13). However, in 2007, an international panel (35) concluded there was a possible positive association between total poultry and prostate cancer risk, and Michaud et al (6) reported a positive association between poultry with skin and metastatic prostate cancer but an inverse association between skinless poultry and metastatic prostate cancer. Our results agree with these findings and, although we cannot rule out chance or confounding, our results did not change after we controlled for known sociodemographic, dietary, or clinical risk factors for prostate cancer incidence or mortality, and we observed a significant linear trend for poultry with skin.
On the basis of previous literature, we hypothesized that meat items high in saturated fat may increase the risk of prostate cancer progression. However, saturated fat from poultry with skin did not explain our observed association between poultry with skin and prostate cancer progression. An alternative mechanism that may explain our observation for poultry with skin is a high intake of heterocyclic amines.
Heterocyclic amines are mutagens present at much higher concentrations in well-done poultry than in other meats (36, 37). We had no information on meat-preparation methods, but poultry with skin may be more likely to be broiled or grilled than skinless poultry, which results in higher concentrations of heterocyclic amines (37). Heterocyclic amines induce prostate, colon, and mammary adenocarcinomas in rats and have been shown to covalently bind and damage DNA in cultured human prostate tissue and primary prostate cells (38–42). In epidemiologic studies, consumption of cooked meats, particularly grilled meat, and heterocyclic amines have been associated with an increased risk of prostate and other cancers, although a few studies reported no association (8, 43–49).
A plausible mechanism that may explain our observed association between eggs and prostate cancer progression is high dietary choline. Egg consumption is a determinant of plasma choline, and higher plasma choline was recently reported to be associated with a greater risk of prostate cancer (50, 51). Malignant prostate cells have higher choline concentrations than do healthy cells, and choline kinase is overexpressed in prostate cancer (52–54). In addition, because of the increased uptake of choline by progressing prostate tumors, radiolabeled choline is used to identify early prostate cancer recurrence (55). No studies have examined dietary choline and prostate cancer risk or progression; however, higher dietary choline has been associated with an increased risk of colorectal adenoma in women (56).
The strengths of our study included our prospective design and our comprehensive clinical, dietary, and sociodemographic data. All of our clinical information was collected by certified urologists and included important risk factors for prostate cancer progression, including Gleason sum at diagnosis, PSA at diagnosis, and treatment. We were also able to control for many known and potential risk factors for prostate cancer progression, such as age, race, BMI, smoking, education, and physical activity.
Overall, our results support the hypothesis that diet may influence the progression of prostate cancer among men with localized disease. In particular, consumption of poultry with skin and eggs may be associated with an increased risk of prostate cancer progression.
Acknowledgments
The authors' responsibilities were as follows—JMC and ELR: developed the analysis plan; ELR and AP: analyzed the data; ELR: drafted the manuscript; MJS: provided significant consultation; and PRC, JMC, and JMB: designed the study, obtained funding, and collected the data. All authors critically reviewed the manuscript and approved its final version. None of the authors had any personal or financial conflicts of interest.
REFERENCES
- 1.Cancer Statistics Review SEER. 1975-2005. : Ries L, Melbert D, Krapcho M, et al., Bethesda, MD: National Cancer Institute, 2007 [Google Scholar]
- 2.Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Fat and meat intake and prostate cancer risk: the multiethnic cohort study. Int J Cancer 2007;121:1339–45 [DOI] [PubMed] [Google Scholar]
- 3.Rohrmann S, Platz EA, Kavanaugh CJ, Thuita L, Hoffman SC, Helzlsouer KJ. Meat and dairy consumption and subsequent risk of prostate cancer in a US cohort study. Cancer Causes Control 2007;18:41–50 [DOI] [PubMed] [Google Scholar]
- 4.Schuurman AG, van den Brandt PA, Dorant E, Goldbohm RA. Animal products, calcium and protein and prostate cancer risk in The Netherlands Cohort Study. Br J Cancer 1999;80:1107–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez C, McCullough ML, Mondul AM, et al. Meat consumption among black and white men and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev 2006;15:211–6 [DOI] [PubMed] [Google Scholar]
- 6.Michaud DS, Augustsson K, Rimm EB, Stampfer MJ, Willet WC, Giovannucci E. A prospective study on intake of animal products and risk of prostate cancer. Cancer Causes Control 2001;12:557–67 [DOI] [PubMed] [Google Scholar]
- 7.Kolonel LN. Fat, meat, and prostate cancer. Epidemiol Rev 2001;23:72–81 [DOI] [PubMed] [Google Scholar]
- 8.Sinha R, Park Y, Graubard BI, et al. Meat and meat-related compounds and risk of prostate cancer in a large prospective cohort study in the United States. Am J Epidemiol 2009;170:1165–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Augustsson K, Michaud DS, Rimm EB, et al. A prospective study of intake of fish and marine fatty acids and prostate cancer. Cancer Epidemiol Biomarkers Prev 2003;12:64–7 [PubMed] [Google Scholar]
- 10.Chavarro JE, Stampfer MJ, Hall MN, Sesso HD, Ma J. A 22-y prospective study of fish intake in relation to prostate cancer incidence and mortality. Am J Clin Nutr 2008;88:1297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Platz E, Giovannucci E. Prostate cancer. : Schottenfeld D, Fraumeni JF, Cancer epidemiology and prevention. 3rd ed. New York, NY: Oxford University Press, 2006:1128–50 [Google Scholar]
- 12.Terry PD, Rohan TE, Wolk A. Intakes of fish and marine fatty acids and the risks of cancers of the breast and prostate and of other hormone-related cancers: a review of the epidemiologic evidence. Am J Clin Nutr 2003;77:532–43 [DOI] [PubMed] [Google Scholar]
- 13.Dagnelie PC, Schuurman AG, Goldbohm RA, Van den Brandt PA. Diet, anthropometric measures and prostate cancer risk: a review of prospective cohort and intervention studies. BJU Int 2004;93:1139–50 [DOI] [PubMed] [Google Scholar]
- 14.Meyer F, Bairati I, Shadmani R, Fradet Y, Moore L. Dietary fat and prostate cancer survival. Cancer Causes Control 1999;10:245–51 [DOI] [PubMed] [Google Scholar]
- 15.Strom SS, Yamamura Y, Forman MR, Pettaway CA, Barrera SL, DiGiovanni J. Saturated fat intake predicts biochemical failure after prostatectomy. Int J Cancer 2008;122:2581–5 [DOI] [PubMed] [Google Scholar]
- 16.Bairati I, Meyer F, Fradet Y, Moore L. Dietary fat and advanced prostate cancer. J Urol 1998;159:1271–5 [PubMed] [Google Scholar]
- 17.Chan JM, Holick CN, Leitzmann MF, et al. Diet after diagnosis and the risk of prostate cancer progression, recurrence, and death (United States). Cancer Causes Control 2006;17:199–208 [DOI] [PubMed] [Google Scholar]
- 18.USDA What's in the food you eat search tool. 3rd ed Washington, DC: USDA Agricultural Research Service, 2009 [Google Scholar]
- 19.Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n−3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr 2004;79:935–45 [DOI] [PubMed] [Google Scholar]
- 20.Cooperberg MR, Broering JM, Litwin MS, et al. The contemporary management of prostate cancer in the United States: lessons from the cancer of the prostate strategic urologic research endeavor (CapSURE), a national disease registry. J Urol 2004;171:1393–401 [DOI] [PubMed] [Google Scholar]
- 21.Lubeck DP, Litwin MS, Henning JM, et al. The CaPSURE database: a methodology for clinical practice and research in prostate cancer. CaPSURE Research Panel. Cancer of the Prostate Strategic Urologic Research Endeavor. Urology 1996;48:773–7 [DOI] [PubMed] [Google Scholar]
- 22.D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998;280:969–74 [DOI] [PubMed] [Google Scholar]
- 23.Agarwal PK, Sadetsky N, Konety BR, Resnick MI, Carroll PR. Cancer of the Prostate Strategic Urological Research E. Treatment failure after primary and salvage therapy for prostate cancer: likelihood, patterns of care, and outcomes. Cancer 2008;112:307–14 [DOI] [PubMed] [Google Scholar]
- 24.Grossfeld GD, Li YP. DP PL, Carroll PR. Patterns of failure after primary local therapy for prostate cancer and rationale for secondary therapy. Urology 2002;60:57–62, discussion 62–3 [DOI] [PubMed] [Google Scholar]
- 25.Chan JM, Gann PH, Giovannucci EL. Role of diet in prostate cancer development and progression. J Clin Oncol 2005;23:8152–60 [DOI] [PubMed] [Google Scholar]
- 26.Fradet Y, Meyer F, Bairati I, Shadmani R, Moore L. Dietary fat and prostate cancer progression and survival. Eur Urol 1999;35:388–91 [DOI] [PubMed] [Google Scholar]
- 27.Willett WC. Nutritional epidemiology. 2nd ed. New York, NY: Oxford University Press, 1998 [Google Scholar]
- 28.Rosner B. Fundamentals of Biostatistics, 6th ed. Belmont, CA: Thomson Brooks/Cole, 2006 [Google Scholar]
- 29.Holford T. Multivariate Methods in Epidemioloy. New York, NY: Oxford University Press, 2002 [Google Scholar]
- 30.Ray ME, Thames HD, Levy LB, et al. PSA nadir predicts biochemical and distant failures after external beam radiotherapy for prostate cancer: a multi-institutional analysis. Int J Radiat Oncol Biol Phys 2006;64:1140–50 [DOI] [PubMed] [Google Scholar]
- 31.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999;281:1591–7 [DOI] [PubMed] [Google Scholar]
- 32.Swindle PW, Kattan MW, Scardino PT. Markers and meaning of primary treatment failure. Urol Clin North Am 2003;30:377–401 [DOI] [PubMed] [Google Scholar]
- 33.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the Health Professionals Follow-Up Study. Int J Cancer 2007;121:1571–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hedelin M, Chang ET, Wiklund F, et al. Association of frequent consumption of fatty fish with prostate cancer risk is modified by COX-2 polymorphism. Int J Cancer 2007;120:398–405 [DOI] [PubMed] [Google Scholar]
- 35.World Cancer Research Fund, American Institute for Cancer Research Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC: World Cancer Research Fund and American Institute for Cancer Research, 2007 [Google Scholar]
- 36.Bogen KT, Keating GAUS. dietary exposures to heterocyclic amines. J Expo Anal Environ Epidemiol 2001;11:155–68 [DOI] [PubMed] [Google Scholar]
- 37.Sinha R, Rothman N, Brown ED, et al. High concentrations of the carcinogen 2-amino-1-methyl-6-phenylimidazo- [4,5-b]pyridine (PhIP) occur in chicken but are dependent on the cooking method. Cancer Res 1995;55:4516–9 [PubMed] [Google Scholar]
- 38.Martin FL, Cole KJ, Muir GH, et al. Primary cultures of prostate cells and their ability to activate carcinogens. Prostate Cancer Prostatic Dis 2002;5:96–104 [DOI] [PubMed] [Google Scholar]
- 39.Williams JA, Martin FL, Muir GH, Hewer A, Grover PL, Phillips DH. Metabolic activation of carcinogens and expression of various cytochromes P450 in human prostate tissue. Carcinogenesis 2000;21:1683–9 [DOI] [PubMed] [Google Scholar]
- 40.Ghoshal A, Preisegger KH, Takayama S, Thorgeirsson SS, Snyderwine EG. Induction of mammary tumors in female Sprague-Dawley rats by the food-derived carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and effect of dietary fat. Carcinogenesis 1994;15:2429–33 [DOI] [PubMed] [Google Scholar]
- 41.Stuart GR, Holcroft J, de Boer JG, Glickman BW. Prostate mutations in rats induced by the suspected human carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Cancer Res 2000;60:266–8 [PubMed] [Google Scholar]
- 42.Ito N, Hasegawa R, Sano M, et al. A new colon and mammary carcinogen in cooked food, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Carcinogenesis 1991;12:1503–6 [DOI] [PubMed] [Google Scholar]
- 43.Kampman E, Slattery ML, Bigler J, et al. Meat consumption, genetic susceptibility, and colon cancer risk: a United States multicenter case-control study. Cancer Epidemiol Biomarkers Prev 1999;8:15–24 [PubMed] [Google Scholar]
- 44.Sinha R, Rothman N. Role of well-done, grilled red meat, heterocyclic amines (HCAs) in the etiology of human cancer. Cancer Lett 1999;143:189–94 [DOI] [PubMed] [Google Scholar]
- 45.Augustsson K, Skog K, Jagerstad M, Dickman PW, Steineck G. Dietary heterocyclic amines and cancer of the colon, rectum, bladder, and kidney: a population-based study. Lancet 1999;353:703–7 [DOI] [PubMed] [Google Scholar]
- 46.Muscat JE, Wynder EL. The consumption of well-done red meat and the risk of colorectal cancer. Am J Public Health 1994;84:856–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Norrish AE, Ferguson LR, Knize MG, Felton JS, Sharpe SJ, Jackson RT. Heterocyclic amine content of cooked meat and risk of prostate cancer. J Natl Cancer Inst 1999;91:2038–44 [DOI] [PubMed] [Google Scholar]
- 48.Mills PK, Beeson WL, Phillips RL, Fraser GE. Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer 1989;64:598–604 [DOI] [PubMed] [Google Scholar]
- 49.Koutros S, Cross AJ, Sandler DP, et al. Meat and meat mutagens and risk of prostate cancer in the Agricultural Health Study. Cancer Epidemiol Biomarkers Prev 2008;17:80–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johansson M, Van Guelpen B, Vollset SE, et al. One-carbon metabolism and prostate cancer risk: prospective investigation of seven circulating B vitamins and metabolites. Cancer Epidemiol Biomarkers Prev 2009;18:1538–43 [DOI] [PubMed] [Google Scholar]
- 51.Konstantinova SV, Tell GS, Vollset SE, Ulvik A, Drevon CA, Ueland PM. Dietary patterns, food groups, and nutrients as predictors of plasma choline and betaine in middle-aged and elderly men and women. Am J Clin Nutr 2008;88:1663–9 [DOI] [PubMed] [Google Scholar]
- 52.Glunde K, Jacobs MA, Bhujwalla ZM. Choline metabolism in cancer: implications for diagnosis and therapy. Expert Rev Mol Diagn 2006;6:821–9 [DOI] [PubMed] [Google Scholar]
- 53.Ramirez de Molina A, Gallego-Ortega D, Sarmentero-Estrada J, et al. Choline kinase as a link connecting phospholipid metabolism and cell cycle regulation: implications in cancer therapy. Int J Biochem Cell Biol 2008;40:1753–63 [DOI] [PubMed] [Google Scholar]
- 54.Kwee SA, DeGrado TR, Talbot JN, Gutman F, Coel MN. Cancer imaging with fluorine-18-labeled choline derivatives. Semin Nucl Med 2007;37:420–8 [DOI] [PubMed] [Google Scholar]
- 55.Apolo AB, Pandit-Taskar N, Morris MJ. Novel tracers and their development for the imaging of metastatic prostate cancer. J Nucl Med 2008;49:2031–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cho E, Willett WC, Colditz GA, et al. Dietary choline and betaine and the risk of distal colorectal adenoma in women. J Natl Cancer Inst 2007;99:1224–31 [DOI] [PMC free article] [PubMed] [Google Scholar]