Abstract
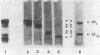
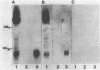
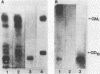
A search for compounds from intestinal mucosa of pigs carrying and not carrying blood group A-active substances (A+ and A- pigs, respectively) capable of binding cholera toxin (CT) was performed. Glycolipid extracts from a pool of pig intestinal mucosa resolved in thin-layer chromatography (TLC) revealed the presence of six to eight compounds capable of binding 125I-CT, two of them running as the ganglioside standards GM1 and GD1b. When intestinal mucosa glycolipids from single pigs were assayed by TLC for CT-binding capacity, two different patterns of labeling were observed. The main difference was at the level of compounds running below GD1b. The A+ pigs but not the A- pigs showed CT binding at this level. The major CT-binding compound detected only in A+ pigs was purified and some properties were determined. After TLC developed with different solvent systems, the purified compound bound CT and also immunoreacted with anti-A and anti-AB antisera but not with anti-B antiserum. The compound was also able to inhibit the hemagglutination of human A erythrocytes caused by anti-A antiserum, but inhibition was not observed with the B-anti-B or O (H)-Ulex europaeus lectin systems. A partial chemical characterization indicated that the active compound is a neutral glycosphingolipid containing glucose, fucose, galactose, and hexosamine. The existence of a blood group-active substance(s) able to interact with CT may help to explain the relationship between ABO blood groups and the diarrheal disease caused by infection with Vibrio cholerae.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariga T., Sekine M., Yu R. K., Miyatake T. Disialogangliosides in bovine adrenal medulla. J Biol Chem. 1982 Mar 10;257(5):2230–2235. [PubMed] [Google Scholar]
- Barua D., Paguio A. S. ABO blood groups and cholera. Ann Hum Biol. 1977 Sep;4(5):489–492. doi: 10.1080/03014467700002481. [DOI] [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- Chaudhuri A., DasAdhikary C. R. Possible role of blood-group secretory substances in the aetiology of cholera. Trans R Soc Trop Med Hyg. 1978;72(6):664–665. doi: 10.1016/0035-9203(78)90031-7. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A., De S. Cholera and blood-groups. Lancet. 1977 Aug 20;2(8034):404–404. doi: 10.1016/s0140-6736(77)90332-4. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973 Aug 28;12(18):3547–3558. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- Cumar F. A., Barra H. S., Maccioni H. J., Caputto R. Sulfation of glycosphingolipids and related carbohydrates by brain preparations from young rats. J Biol Chem. 1968 Jul 25;243(14):3807–3816. [PubMed] [Google Scholar]
- Cumar F. A., Maggio B., Caputto R. Ganglioside-cholera toxin interactions: a binding and lipid monolayer study. Mol Cell Biochem. 1982 Aug 6;46(3):155–160. doi: 10.1007/BF00239664. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fukuta S., Magnani J. L., Twiddy E. M., Holmes R. K., Ginsburg V. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect Immun. 1988 Jul;56(7):1748–1753. doi: 10.1128/iai.56.7.1748-1753.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass R. I., Holmgren J., Haley C. E., Khan M. R., Svennerholm A. M., Stoll B. J., Belayet Hossain K. M., Black R. E., Yunus M., Barua D. Predisposition for cholera of individuals with O blood group. Possible evolutionary significance. Am J Epidemiol. 1985 Jun;121(6):791–796. doi: 10.1093/oxfordjournals.aje.a114050. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hansson G. C., Karlsson K. A., Larson G., Samuelsson B. E., Thurin J., Bjursten L. M. Detection of blood group type glycosphingolipid antigens on thin-layer plates using polyclonal antisera. J Immunol Methods. 1985 Oct 24;83(1):37–42. doi: 10.1016/0022-1759(85)90055-9. [DOI] [PubMed] [Google Scholar]
- Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981 Jul 30;292(5822):413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Lindblad M., Fredman P., Svennerholm L., Myrvold H. Comparison of receptors for cholera and Escherichia coli enterotoxins in human intestine. Gastroenterology. 1985 Jul;89(1):27–35. doi: 10.1016/0016-5085(85)90741-3. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Svennerholm L. Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect Immun. 1973 Aug;8(2):208–214. doi: 10.1128/iai.8.2.208-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamori M., Shimomura J., Nagai Y. Specific binding of cholera toxin to rat erythrocytes revealed by analysis with a fluorescence-activated cell sorter. J Biochem. 1985 Mar;97(3):729–735. doi: 10.1093/oxfordjournals.jbchem.a135112. [DOI] [PubMed] [Google Scholar]
- King C. A., Van Heyningen W. E. Deactivation of cholera toxin by a sialidase-resistant monosialosylganglioside. J Infect Dis. 1973 Jun;127(6):639–647. doi: 10.1093/infdis/127.6.639. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Nalin D. R., Rennels M. B., Hornick R. B., Sotman S., Van Blerk G., Hughes T. P., O'Donnell S., Barua D. Genetic susceptibility to cholera. Ann Hum Biol. 1979 Jul-Aug;6(4):369–374. doi: 10.1080/03014467900003751. [DOI] [PubMed] [Google Scholar]
- Magnani J. L., Smith D. F., Ginsburg V. Detection of gangliosides that bind cholera toxin: direct binding of 125I-labeled toxin to thin-layer chromatograms. Anal Biochem. 1980 Dec;109(2):399–402. doi: 10.1016/0003-2697(80)90667-3. [DOI] [PubMed] [Google Scholar]
- McKibbin J. M. Fucolipids. J Lipid Res. 1978 Feb;19(2):131–147. [PubMed] [Google Scholar]
- Mestrallet M. G., Bennun F. R., Maggio B., Cumar F. A. Tryptophan fluorescence properties of cholera toxin upon interacting with ganglioside GD1b. J Neurosci Res. 1984;12(2-3):335–341. doi: 10.1002/jnr.490120220. [DOI] [PubMed] [Google Scholar]
- Mestrallet M. G., Cumar F. A., Caputto R. Trisialoganglioside synthesis by a chicken brain sialyltransferase. Comparative study with the similar reaction for the synthesis of disialoganglioside. Mol Cell Biochem. 1977 Jul 5;16(2):63–70. doi: 10.1007/BF01732045. [DOI] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Mechanism of action of choleragen and E. coli heat-labile enterotoxin: activation of adenylate cyclase by ADP-ribosylation. Mol Cell Biochem. 1981 Jul 7;37(2):75–90. doi: 10.1007/BF02354931. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Suzuki M., Inagaki F., Yamakawa T., Suzuki A. A new ganglioside showing choleragenoid-binding activity in mouse spleen. J Biochem. 1987 Apr;101(4):825–835. doi: 10.1093/oxfordjournals.jbchem.a121949. [DOI] [PubMed] [Google Scholar]
- Nishimura K. Phytosphingosine is a characteristic component of the glycolipids in the vertebrate intestine. Comp Biochem Physiol B. 1987;86(1):149–154. doi: 10.1016/0305-0491(87)90190-8. [DOI] [PubMed] [Google Scholar]
- Nores G. A., Caputto R. Inhibition of the UDP-N-acetylgalactosamine: GM3, N-acetylgalactosaminyl transferase by gangliosides. J Neurochem. 1984 May;42(5):1205–1211. doi: 10.1111/j.1471-4159.1984.tb02773.x. [DOI] [PubMed] [Google Scholar]
- SAMBASIVARAO K., MCCLUER R. H. THIN-LAYER CHROMATOGRAPHIC SEPARATION OF SPHINGOSINE AND RELATED BASES. J Lipid Res. 1963 Jan;4:106–108. [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Sahyoun N., Shatila T., LeVine H., 3rd, Cuatrecasas P. Skeletal association of the cholera toxin receptor in rat erythrocytes. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1216–1222. doi: 10.1016/s0006-291x(81)80141-6. [DOI] [PubMed] [Google Scholar]
- Sjöquist J., Meloun B., Hjelm H. Protein A isolated from Staphylococcus aureus after digestion with lysostaphin. Eur J Biochem. 1972 Sep 25;29(3):572–578. doi: 10.1111/j.1432-1033.1972.tb02023.x. [DOI] [PubMed] [Google Scholar]
- Skipski V. P., Smolowe A. F., Barclay M. Separation of neutral glycosphingolipids and sulfatides by thin-layer chromatography. J Lipid Res. 1967 Jul;8(4):295–299. [PubMed] [Google Scholar]
- Slomiany B. L., Slomiany A. Branched blood group A-active fucolipids of hog gastric mucosa. Biochim Biophys Acta. 1977 Mar 25;486(3):531–540. doi: 10.1016/0005-2760(77)90103-5. [DOI] [PubMed] [Google Scholar]
- Smith E. L., Mckibbin J. M., Breimer M. E., Karlsson K. A., Pascher I., Samuelson B. E. Identification of a novel hepraglycosylceramide with two fucose residues and a terminal hexosamine. Biochim Biophys Acta. 1975 Jul 22;398(1):84–91. doi: 10.1016/0005-2760(75)90171-x. [DOI] [PubMed] [Google Scholar]
- Strombeck D. R., Harrold D. Binding of cholera toxin to mucins and inhibition by gastric mucin. Infect Immun. 1974 Dec;10(6):1266–1272. doi: 10.1128/iai.10.6.1266-1272.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]