Abstract
Females are less sensitive to the hypertensive effects of angiotensin II compared to males, although the molecular mechanisms responsible are unknown. We hypothesize that differential activation of angiotensin II, Ang (1–7), AT1, AT2, and mas levels in the renal cortex of male and female spontaneously hypertensive rats contribute to sex differences in the blood pressure response to angiotensin II infusion. Males had a greater increase in blood pressure following angiotensin II infusion than females (males: 150±2 to 186±3 mmHg; females: 137±3 to 160±4 mmHg; p<0.05). Angiotensin II infusion resulted in comparable increases in plasma and renal cortical angiotensin II levels in both sexes. Renal cortical Ang (1–7) levels were higher in female rats under basal conditions (195±10 vs. 67±11 ng/gram cortex, p<0.05) and following angiotensin II infusion (281±25 vs. 205±47 ng/gram cortex, p<0.05) compared to male rats. In the renal cortex of male rats, angiotensin II infusion decreased AT1 protein expression and increased AT2 expression with no change in mas expression. In female rats there was an increase in mas receptor protein expression with angiotensin II infusion although AT1 and AT2 expression were unchanged. Male and female rats were then treated with the Ang (1–7) mas receptor antagonist, A-779, in the absence and presence of angiotensin II. A-779 equalized the blood pressure response to angiotensin II in males and females (blood pressure at the end of treatment: males, 166±4; females, 164±5 mmHg). In conclusion, Ang (1–7) contributes to the sex difference in angiotensin II-induced increases in blood pressure in spontaneously hypertensive rats.
Keywords: proteinuria, gender, SHR, blood pressure, renin angiotensin system, Ang (1–7), mas receptor
Introduction
Male spontaneously hypertensive rats (SHR) have elevated blood pressure, albuminuria, and renal inflammation compared to age-matched female SHR 1–3. Reckelhoff et al. reported that treatment of male and female SHR with the angiotensin converting enzyme (ACE) inhibitor enalapril reduced blood pressures to similar levels in both sexes, indicating that both the hypertension and the sex difference in blood pressure was renin angiotensin system (RAS) mediated 3. The RAS is a key system in controlling blood pressure and kidney function, and over-activation of the RAS contributes to hypertension 3. There are two Ang II receptors: AT1 and AT2. Activation of AT1 receptors, the “classical pathway”, mediate most well-known biological functions of Ang II, including vasoconstriction, oxidative stress, and inflammation 4, 5. Activation of the “non-classical pathway” (Ang (1–7), AT2, mas receptors), oppose AT1-mediated effects leading to vasodilation, improved renal blood flow, and enhanced pressure natriuresis 6, 7. Ang (1–7) effects are thought to be primarily mediated by the G-protein-coupled receptor mas 8, 9.
There are known sex differences in the expression levels of RAS components and functional responses to angiotensin (Ang) II-infusion. Young male SHR have higher levels of AT1 mRNA and protein expression in the kidney, aorta, and mesenteric arteries 1, 10, while AT2 mRNA expression is higher in females 10. Although not previously examined in SHR, Ang (1–7) levels tend to be higher in hypertensive female congenic mRen(2). Lewis rats compared to males 11. There are also pronounced sex differences in the blood pressure responses of normotensive male and female experimental animals to exogenous Ang II infusion. Male Sprague-Dawley rats and C57/BL6J mice have a more robust pressor response to Ang II infusion compared to females 12–16.
The molecular mechanism(s) accounting for sex differences in response to Ang II are unknown; however, a differential balance in the expression and activation of the classical and non-classical RAS may contribute. We hypothesized that greater non-classical RAS activation limits the hypertensive actions of Ang II in female SHR. Thus, the first goal of this study was to assess expression levels of the primary components of the classical (AT1 and Ang II) and non-classical (AT2, mas receptor, Ang (1–7)) RAS in the renal cortex of male and female SHR. Animals were studied under basal conditions and following Ang II infusion to determine how direct stimulation of the RAS alters that balance of the classical and non-classical RAS. Male and female SHR were studied as an experimental model of hypertension that mimics the human condition in that young men tend to have higher blood pressures than women and tend to become hypertensive earlier than women (based on statistics from the National Center for Health Statistics). We found that following Ang II infusion, female SHR had greater Ang (1–7) levels in the renal cortex and an increase in mas receptor expression that was not evident in males. Based on these observations, additional studies were designed to test the hypothesis that enhanced mas receptor activation in female SHR contributes to a lower blood pressure in response to chronic Ang II infusion.
Methods
Animals
Male and female SHR were used in this study (Harlan Laboratories, Indianapolis, IN). All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved and monitored by the Medical College of Georgia Institutional Animal Care and Use Committee. A subset of male and female SHR (n=12–13, respectively) were implanted with telemetry transmitters (Data Sciences, St. Paul, MN) at 11-weeks of age while under ketamine/xylazine (50 mg/kg / 10 mg/kg, i.p.) anesthesia. Rats were allowed one week to recover before they were placed on telemetry receivers for measurement of baseline blood pressure. After 5 days, 6 male and 7 female SHR received osmotic minipumps (Alzet, Cupertino, CA) implanted s.c. while anesthetized with isoflurane (1.5%) to deliver Ang II (Phoenix, Burlingame, CA) at a dose of 200 ng/kg/min for 14 days. Separate groups of SHR, 6 males and 6 females, received osmotic minipumps implanted s.c. to deliver the Ang (1–7) mas receptor antagonist D-Alanine-[Ang-(1–7)] (A-779) at a dose of 48 μg/kg/hr (Bachem, Torrance, CA). Rats were placed on telemetry receivers to measure blood pressure for 1 week and then received a second minipump to deliver Ang II (200 ng/kg/min) beginning on day 8 of the A-779 treatment. Rats receiving Ang II alone or in combination with A-779 were placed in metabolic cages weekly for 24-hour urine collection and at the end of 2 weeks of Ang II infusion kidneys were processed for immunohistochemical analysis.
Histological and Immunohistochemical analysis
Briefly, kidneys were perfusion fixed and prepared as previously described for CD68 to assess macrophage infiltration (ED-1; Serotec, Kidlington, Oxford, UK), CD3 for T cell infiltration or stained with periodic acid-Schiff (PAS) or trichrome blue 1. To evaluate renal lesions, stained sections were viewed with an Olympus BX40 microscope (Olympus America, Melville, NY) on bright-field setting fitted with a digital camera (Olympus DP70; Olympus America). To assess the histopathologic changes, we assigned a semi-quantitative grade of severity (0, 1, 2, or 3, indicating that the feature was absent, mild, moderate, or severe, respectively) to each of the following morphologic attributes: thickening, necrosis, thrombosis and hyalinosis of interstitial arteries; tuft necrosis, hyalinosis and thrombosis of glomerular capillaries; necrosis and thrombosis of glomerular arterioles and fibrosis. Thirty randomly selected non-overlapping fields of renal cortical interstitium and glomeruli were examined at 200x and 400x magnification, respectively, and the means of the values were calculated for each sample. Immunohistochemistry for CD3 and CD68: To evaluate the interstitial infiltration of T cells and macrophages, the appropriate software (DPController, Olympus Optical) was used to convert the image, and a 500 μm × 500 μm grid was superimposed onto the image at 400x magnification. Twenty grids from each slide were viewed, and positive cells were counted. For all studies, the examiner was unaware of the group designations during his/her evaluations.
Real-time Polymerase Chain Reaction (RT-PCR)
RNA was isolated from the renal cortex of control and Ang II-infused male and female SHR using the RNeasy Plus Mini kit (Qiagen, Valencia, CA; n=8/group). A blend of oligonucleotide and random hexanucleotide primers were used for the reverse transcription (RT) of equal amounts of total RNA (2 μg) using the iScript cDNA synthesis kit (Qiagen, Valencia, CA). RT-PCR was performed with QuantiTect SYBR Green RT-PCR Kit (Qiagen, Valencia, CA) with primer pairs for AT1, AT2, and the mas receptor (Qiagen, Valencia, CA). GAPDH was used as an internal standard and mRNA levels were expressed relative to male SHR. The amplification and quantification were performed using the iCycler iQ Real-Time detection system under the following conditions: RT PCR activation step 15 minutes at 95°C, denaturation for 15 seconds at 94°C, annealing 30 seconds at 55°C, extension 30 seconds at 72°C for 40 cycles (Applied Biosystems, Foster City, CA). Each sample was run in duplicate and the mean threshold cycle (Ct) was used to calculate relative mRNA expression (fold change) using the comparative Ct method (2−ΔΔCT).
Western blot analysis
Renal cortical samples (n=6/group) were homogenized as previously described, with minor modifications 1. Following homogenization, samples were centrifuged at 1,000 × g for 30 minutes at 4°C. The supernatant was collected and centrifuged at 30,000 × g for 45 minutes at 4°C. The resulting pellet fraction was resuspended in half of the original volume of homogenization buffer for use in Western blotting protocols. Protein concentrations were determined by standard Bradford assay (Bio-Rad, Hercules, CA) using bovine serum albumin as the standard. Western blotting was performed as previously described 1. Two-color immunoblots were performed using polyclonal primary antibodies to AT1 (~45K, polyclonal; Santa Cruz, Santa Cruz, CA), AT2 and mas receptor (~43K and 50K respectively, polyclonal; Alomone Labs, Jerusalem, Israel). Specific bands were detected using the Odyssey Infrared Imager in conjunction with the appropriate IRDye secondary antibodies (LI-COR Biosciences, Lincoln, NE). Actin (monoclonal; Sigma, St. Louis, MO) was used to verify equal protein loading and all densitometric results are reported normalized to actin.
Peptide analysis
Ang (1–7) levels were measured by enzyme immunoassay following methanol extraction of the renal cortex as previously described 1 via manufacturer’s protocol III (n=9–15; Bachem, San Carlos, CA). Ang II levels were measured by enzyme immunoassay directly in the plasma immediately following collection or following methanol extraction of the renal cortex as previously described 1 (n=6–10/group; Cayman Chemicals, Ann Arbor, MI).
Statistical analysis
All data are presented as mean ± SEM. MAP and urinary protein excretion data were analyzed using analysis of variance (ANOVA) for repeated measurements. Evaluation of renal lesions in Table 1, PCR, Western blot, and peptide data were compared using a 2-way ANOVA. Differences were considered statistically significant with p<0.05. Analyses were performed using GraphPad Prism Version 4.0 software (GraphPad Software Inc, La Jolla, CA).
Table 1.
Renal injury sores and interstitial infiltration of macrophages (CD68+) and T cells (CD3+) in male and female SHR treated with Ang II alone or in combination with A-779.
| Evaluation of Injury | Male | Male + Ang II | Male + A-779 + Ang II | Female | Female + Ang II | Female + A-779 + Ang II | |
|---|---|---|---|---|---|---|---|
| Interstitial Artery | Thickening | 0 | 1.0±0.01* | 1.0±0.1 | 0 | 0.9±0.1* | 0.6±0.4 |
| Necrosis | 0 | 2.0±0.01* | 0.8±0.4‡ | 0 | 0.7±0.3*† | 1.0±0.4 | |
| Thrombosis | 0 | 1.8±0.02* | 0.8±0.4 | 0 | 0.4±0.3*† | 0.4±0.2 | |
| Hyalinosis | 0 | 2.2±0.02* | 1.5±0.5 | 0 | 1.4±0.2*† | 1.4±0.4 | |
| Glomerulus | Tuft necrosis | 0 | 1.4±0.3* | 0‡ | 0 | 0.3±0.2*† | 0 |
| Tuft hyalinosis | 0 | 1.2 ±0.3* | 0‡ | 0 | 0.3±0.2*† | 0.2±0.2 | |
| Tuft thrombosis | 0 | 0.7±0.2* | 0‡ | 0 | 0.1±0.1*† | 0 | |
| Glomerular Arteriole | Necrosis | 0 | 1.3±0.3* | 1.0±0.01 | 0 | 0.3±0.2*† | 0.4±0.4 |
| Thrombosis | 0 | 1.7±0.2* | 1.0±0.01 | 0 | 0.4±0.2*† | 0.6±0.4 | |
| CD68+ cells/mm2 | 20±2 | 35±2* | 20±2 ‡ | 12±2 | 20±6*† | 24±3 | |
| CD3+ cells/mm2 | 30±4 | 43±2* | 32±2‡ | 30±1 | 38±2* | 22±6‡ |
Renal injury scores as assessed by an independent, blinded reviewer. Scores: 0, none; 1, mild; 2, moderate; 3, severe. Interstitial infiltration of CD68+ and CD3+ cells were counted in a blinded manner.
indicates significant difference from same sex control;
indicates significant difference between Female + Ang II and Male + Ang II;
indicates significant differences between Ang II alone and A-779 + Ang II in the same sex p<0.05. N=4–7.
Results
Ang II infusion in male and female SHR
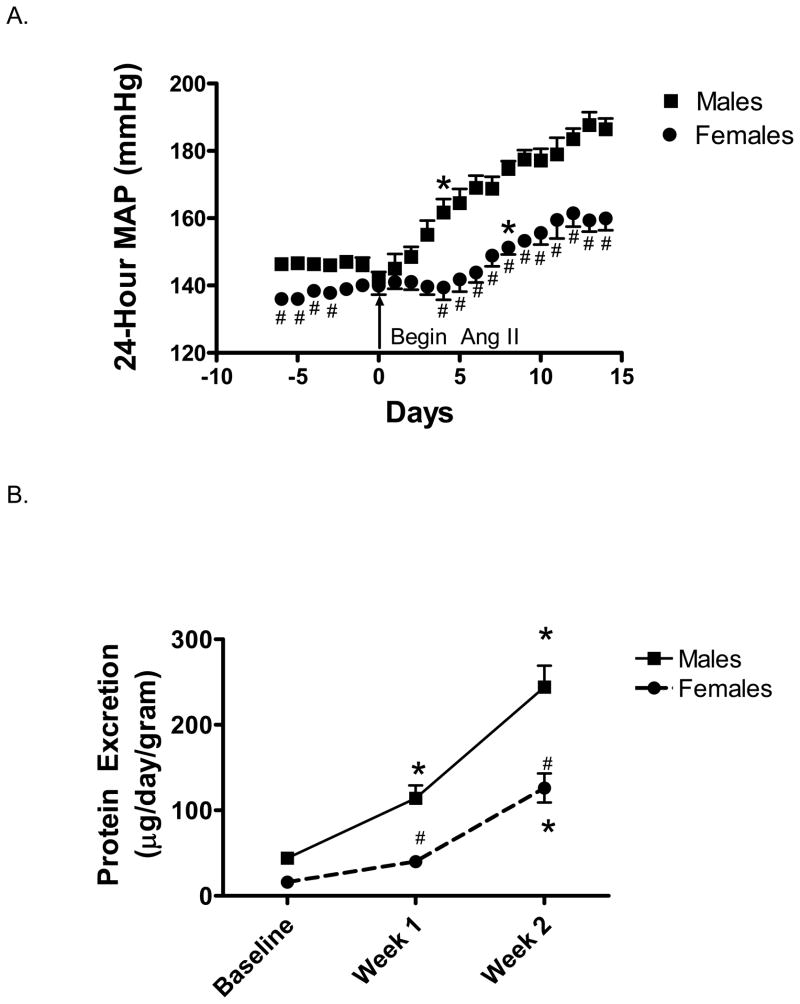
Blood pressure was measured in male and female SHR by telemetry. Baseline MAP was significantly higher in male SHR compared to female SHR (Figure 1A). Ang II infusion increased MAP in both male and female SHR, with the increase in MAP reaching significance in males after 4 days of Ang II infusion and in females after 8 days. Male SHR experienced a significantly greater increase in MAP compared to females at the end of 2 weeks (% increase in MAP from baseline: males 24±1 %; females 13±3 %, p<0.05).
Figure 1.
Effect of Ang II infusion on 24-hour mean arterial pressure as measured by telemetry (MAP; panel A) and proteinuria (panel B) in male and female SHR. Ang II infusion was initiated on day 0. # indicates significant difference from males, p<0.05. * indicates MAP has significantly increased from baseline in the same sex, p<0.05. N=6–7.
Indices of Renal Injury
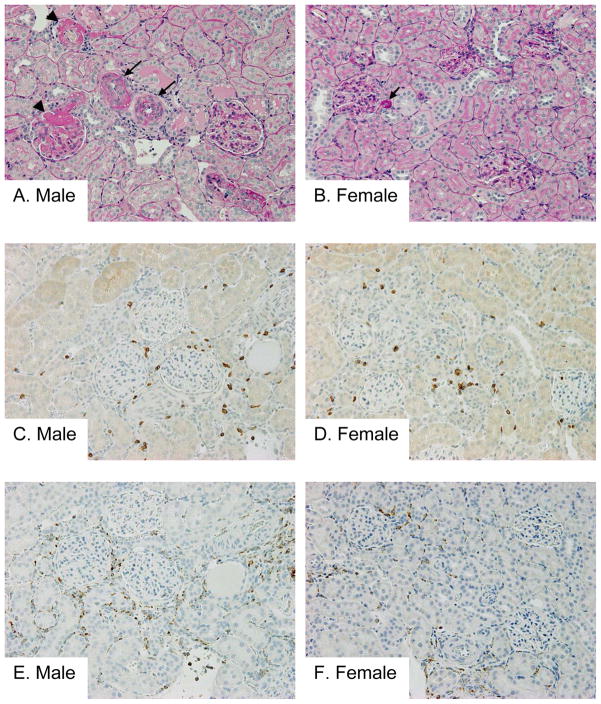
Male SHR had greater protein excretion than females at all time points (Figure 1B). Proteinuria was significantly increased in male SHR following 1 week of Ang II infusion compared to baseline values and further increased at week 2, however in female SHR a significant increase in proteinuria was not detected until week 2 of Ang II infusion. Kidneys were processed for histological analysis of renal morphology and immunohistochemical quantification of macrophage (CD68) and T-cell (CD3) infiltration. There were no overt structural alterations in normal renal morphology in control SHR (Table 1). Ang II infusion resulted in marked medial thickening of interstitial arteries, necrosis, thrombosis and hyalinosis of interstitial arteries, glomerular arterioles and glomerular tufts, and interstitial mononuclear cell infiltration in male SHR. Although mild medial thickening and hyalinosis were also noted in some interstitial arteries and glomerular arterioles in female SHR treated with Ang II, the degree of injury was much more severe in males compared to females (Table 1, Fig 2A–B). The amount of renal fibrosis evident was minimal as assessed by trichrome staining (tubulointerstitial fibrosis was scored at 0 in both the male and female SHR, data not shown). Ang II infusion also resulted in a significant increase in macrophage and T cell infiltration in the interstitial and periglomerular lesions of male and female SHR. While macrophage infiltration was significantly greater in male SHR compared to female SHR following Ang II infusion, the increase in T cell infiltration was comparable between the sexes (Figure 2C–F).
Figure 2.
Representative pictures of the renal lesions (A and B; PAS staining), intrarenal infiltration of T cells (C and D; CD3 staining) and macrophages (E and F; CD68 staining) in male (A, C and E) and female (B, D and F) SHR treated with Ang II. Marked medial thickening of interstitial arteries (arrows in A), fibrinoid necrosis (arrowheads in A), thrombosis and hyalinosis of interstitial arteries, glomerular arterioles, and glomerular tufts, and focal interstitial mononuclear cell infiltration were noted in male SHR rats treated with Ang II. Although mild medial thickening and hyalinosis (arrow in B) were also noted in some interstitial arteries and glomerular arterioles in female SHR rats treated with Ang II, the degree of injury was much more severe in males than females. Increased interstitial and periglomerular infiltration of T cells (C and D) and macrophages (E and F) were noted in male and female SHR rats treated with Ang II. While the increase in T cell infiltration was almost comparable between the sexes (C and D), macrophage infiltration was greater in male (E) compared with female (F). Original magnification, 200x, n=6–8.
Assessment of classical and non-classical RAS components
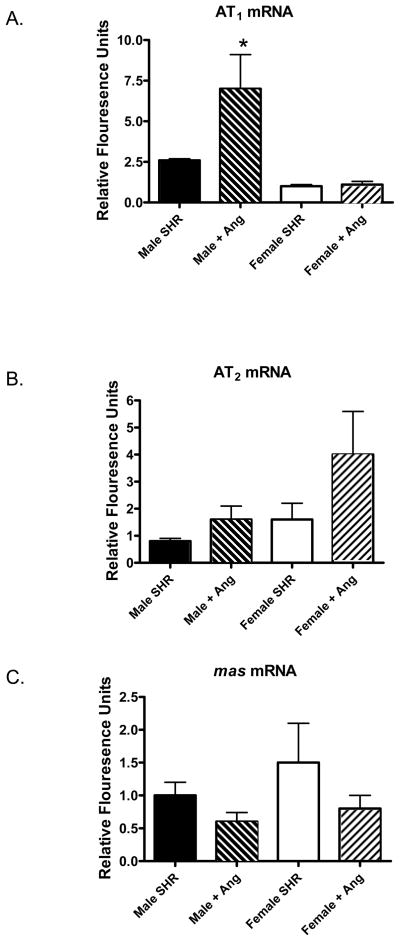
We next assessed mRNA and protein expression levels of RAS components in the renal cortex of male and female SHR. Under basal conditions, there were no significant differences in AT1, AT2, or mas receptor mRNA expression in the renal cortex of male and female SHR (Figure 3). Ang II infusion resulted in a significant increase in AT1 mRNA expression in male SHR with no change in females. In contrast, AT2 mRNA expression was not changed in male SHR following Ang II infusion (p=0.14), however expression tended to increase in the renal cortex of female SHR (p=0.06). Mas receptor mRNA expression was comparable among all groups.
Figure 3.
mRNA expression levels of AT1 (panel A), AT2 (panel B), and the mas receptor (panel C) in the renal cortex of control and Ang II-infused male and female SHR. * indicates significant difference from same sex control, p<0.05. N=8.
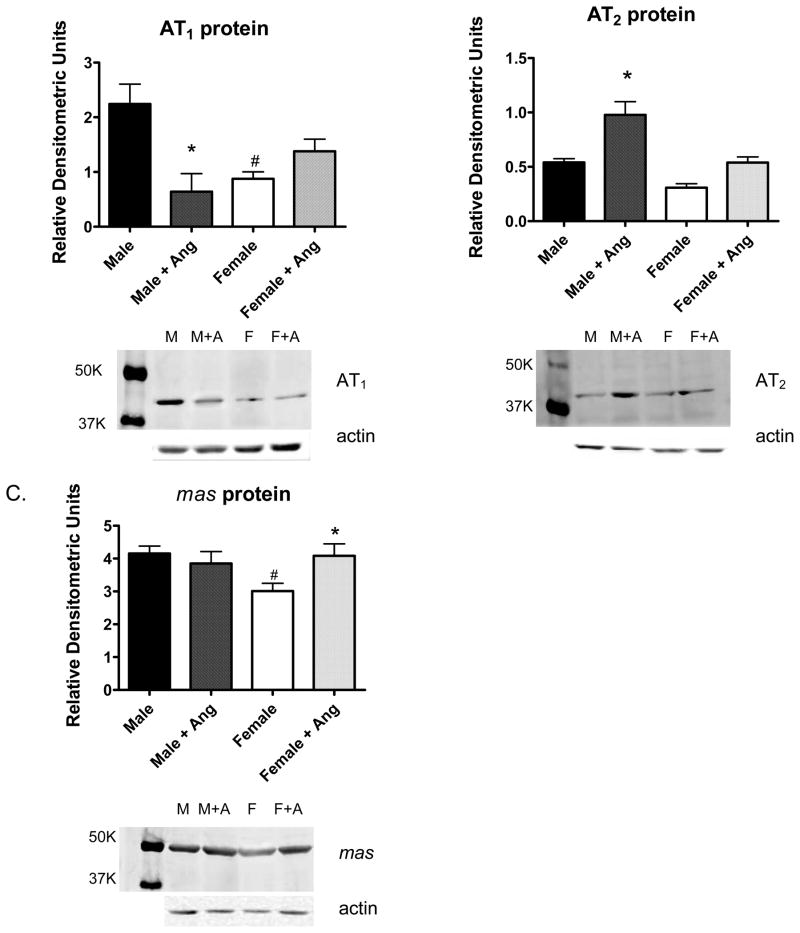
Additional experiments assessed AT1, AT2, and mas receptor protein expression in the renal cortex of male and female SHR. AT1 and mas receptor protein expression were greater in the renal cortex of male SHR compared to female SHR under basal conditions (Figure 4). AT2 protein expression was comparable between males and females. 2 weeks of Ang II infusion significantly decreased AT1 protein expression in males and significantly increased AT2 protein expression. In contrast, chronic Ang II infusion resulted in a significant increase in mas receptor expression in the renal cortex of female SHR.
Figure 4.
Protein expression levels of AT1 (panel A), AT2 (panel B), and the mas receptor (panel C) in the renal cortex of control and Ang II-infused male and female SHR. # indicates significant difference from males, p<0.05. * indicates significant difference from same sex control, p<0.05. N=6.
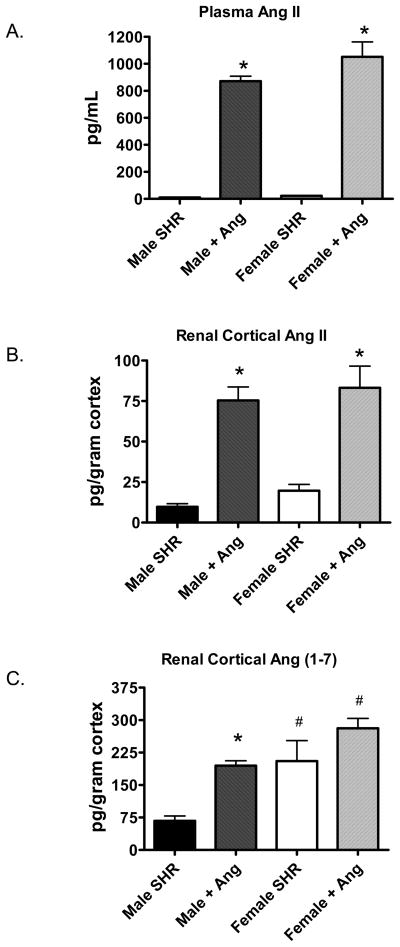
In separate animals, plasma and renal cortical Ang II levels and renal cortical Ang (1–7) levels were measured. Basal Ang II levels in plasma were less in male SHR (11±1 pg/mL) compared to female SHR (23±2 pg/mL, p<0.05). Chronic Ang II infusion increased plasma Ang II to comparable levels in male and female SHR (Figure 5A). Cortical Ang II levels were comparable in male and female SHR both under basal conditions and following Ang II infusion (Figure 5B). In contrast, Ang (1–7) levels were significantly less in male SHR compared to female SHR both under basal conditions and following chronic Ang II infusion (Figure 5C). Ang II infusion increased Ang (1–7) levels in the renal cortex of both male (~3-fold) and female SHR (1.3-fold); however, this increase was only significant in the males.
Figure 5.
Plasma Ang II (panel A, n=6–8), renal cortical Ang II (panel B, n=6–10) and renal cortical Ang (1–7) levels (panel C, n=9–15) in control and Ang II-infused male and female SHR. # indicates significant difference from males, p<0.05. * indicates significant difference from same sex control, p<0.05.
Ang (1–7) receptor antagonism alters Ang II-induced hypertension
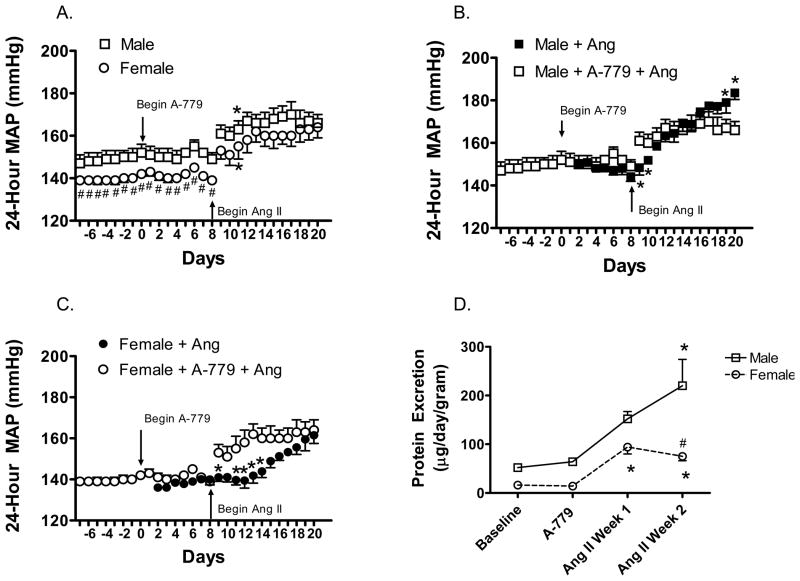
Based on the findings that Ang II infusion increases mas receptor expression in female SHR and female SHR have higher levels of Ang (1–7) in the renal cortex, we examined the ability of Ang (1–7) antagonism to influence the blood pressure response and renal injury to chronic Ang II infusion. Infusion of A-779 did not alter basal blood pressure in either male or female SHR (Figure 6). However, there was no sex difference in the blood pressure response to Ang II in the presence of A-779 (Figure 6A). This was associated with an initial increase in sensitivity to Ang II in both males and females and an attenuation of the Ang II-induced increase in blood pressure in male SHR (Figures 6B–C).
Figure 6.
Effect of A-779 on 24-hour mean arterial pressure as measured by telemetry (panels A-C) and protein excretion (panel D) in male and female SHR. A-779 infusion was begun on Day 0 (panel A). Ang II infusion was started on Day 8 and continued until Day 21. Panels B and C are a direct comparison of the blood pressure response to Ang II in the absence and presence of A-779 in male and female SHR, respectively. # indicates significant difference from males, p<0.05* indicates significant difference from same sex baseline blood pressure, p<0.05. N=6–7.
To assess renal injury, urinary protein excretion was measured at baseline and weekly thereafter (Figure 6D, Table 2). A-779 infusion did not significantly alter protein excretion in male SHR compared to infusion with Ang II alone (Table 2). However, 1 week of Ang II infusion in female SHR resulted in a ~7-fold increase in proteinuria in the presence of A-779 as compared to a ~2.5-fold increase with Ang II alone (p<0.05). There was not a significant change in proteinuria from week 1 of Ang II infusion to week 2 of Ang II infusion in females that had been treated with A-779 (p=0.2), in contrast, females treated with Ang II alone displayed a 3-fold increase in protein excretion from weeks 1 to 2 (p<0.05). Kidneys were also processed for histological analysis of renal morphology and immunohistochemical quantification of macrophage and T-cell infiltration. As compared to rats infused with Ang II alone, A-779 normalized glomerular arteriole morphology, glomerular tufts and interstitial mononuclear cell infiltration in male SHR, however there was still evidence of medial thickening of interstitial arteries, necrosis, thrombosis and hyalinosis of interstitial arteries. In contrast, A-779 did not alter Ang II induced morphological changes in the kidneys of female SHR, although T-cell infiltration was normalized (Table 1).
Table 2.
Urinary protein excretion (μg/day/gram body weight) in male and female SHR treated with Ang II alone or in combination with A-779.
| Treatment | Male SHR | Female SHR |
|---|---|---|
| Ang II Study | ||
| Baseline | 44±4 | 16±1 |
| Ang II Week 1 | 114±44* | 41±3# |
| Ang II Week 2 | 244±25* | 127±18 †* |
| A-779 Study | ||
| Baseline | 52±3 | 16±2 |
| A-779 | 64±4 | 14±1 |
| Ang II Week 1 | 152±15 | 94±14 †*‡ |
| Ang II Week 2 | 220±54* | 75±9*‡ |
indicates significant difference from same sex at baseline;
indicates significant difference between Female + Ang II and Male + Ang II;
indicates significant differences between Ang II alone and A-779 + Ang II in the same sex p<0.05. N=4–7.
Renal cortical Ang II levels and Ang (1–7) levels were measured in rats treated with A-779 following 2 weeks of Ang II infusion. Ang II levels were comparable in male and female SHR (64±10 pg/g cortex vs. 74±14 pg/g cortex, respectively) and not significantly altered relative to SHR treated with Ang II alone. Ang (1–7) levels tended to be less in male SHR (212±26 pg/g cortex) compared to female SHR (269±66 pg/g cortex), however, again there was no significant difference in Ang (1–7) levels in rats treated with A-779 compared to rats treated with Ang II alone.
Discussion
The RAS is a critical system in controlling blood pressure and renal health under physiological conditions, and a contributing factor to the dysregulation of blood pressure control under numerous pathological conditions. Females have been shown both clinically and experimentally to be less sensitive to the hypertensive effects of Ang II compared to males, although the molecular mechanisms responsible are unknown. In this study, we verified that similar to other species and strains, male SHR have a larger increase in blood pressure in response to exogenous Ang II infusion compared to females. The primary novel findings of this study are (1) Ang II infusion differentially regulates the expression of the primary RAS receptors in the renal cortex of male and female SHR, (2) Ang (1–7) levels are greater in female SHR under basal conditions and following Ang II infusion compared to male SHR, and (3) Ang (1–7) receptor antagonism abolishes the sex difference in the blood pressure response to Ang II infusion. A-779 resulted in an initial increase in blood pressure sensitivity and proteinuria to Ang II especially in females, suggesting that Ang (1–7) antagonizes the immediate Ang II-induced increases in blood pressure and renal injury. In contrast, male SHR treated with A-779 had a significantly lower blood pressure following 2 weeks of Ang II infusion, suggesting that Ang (1–7) contributes to the elevation in blood pressure with Ang II infusion in males.
Infusion of Ang II increased MAP in both sexes, however, the increase in MAP was more pronounced in males. These data agree with studies in Sprague-Dawley rats and C57/Bl6 mice in which females have an attenuated increase in MAP to Ang II infusion 13–16. In contrast, in the presence of an ACE1 inhibitor, female Sprague-Dawley rats are more sensitive to Ang II-induced hypertension compared to males 17, however in C57/Bl6 mice even in the presence of an ACE inhibitor males had a greater increase in blood pressure with Ang II infusion than females 13. Sex differences in response to RAS activation are also apparent in humans. Increases in Ang II in men correlates with increases in blood pressure and renal injury 18. However, in women, increases in Ang II levels have been shown to decrease MAP and attenuate vasoconstrictor responses to RAS activation 19, 20.
Although the highest sensitivity to the vascular effects of Ang II are found in the kidney, little is known regarding how sex of the animals influences Ang II-induced renal injury 21. Proteinuria, morphology and inflammatory cell infiltration were measured to assess renal injury in this study and Ang II-induced renal injury was more pronounced in males compared to females. While sex differences in renal injury are likely related to sex differences in blood pressure, we have previously shown blood pressure is not the sole factor responsible for sex differences in albuminuria 1. This was verified in the present study where males had greater protein excretion following Ang II treatment in the presence of A-779 relative to female SHR despite comparable blood pressures. In addition, Sartori-Valinotti et al. showed that treatment with an ACE1 inhibitor in conjunction with Ang II infusion increased albuminuria in male Sprague-Dawley rats, with no effect in females despite the finding that MAP was higher in the females 17. Therefore, males are more sensitive to Ang II-induced renal injury compared to females.
To determine the molecular mechanisms responsible for sex differences in response to Ang II infusion, we examined how Ang II infusion altered the balance of the primary components of the classical (AT1 and Ang II) and non-classical RAS (AT2, mas, Ang (1–7)). The majority of the studies in the literature that have examined these RAS receptors have looked at mRNA expression. However, since protein levels are potentially of greater physiological relevance, we examined both mRNA and protein expression in the renal cortex of male and female SHR. Consistent with our mRNA results, renal cortical and left ventricular AT1a receptor mRNA expression is increased in male Sprague-Dawley rats with Ang II infusion but not in females 12, 22, and infusion of Ang II increased AT1 mRNA and protein in the paraventricular nucleus of the hypothalamus 22. In contrast, Sampson et al. published that in the whole kidney high-dose Ang II infusion had no effect on AT1 or AT2 mRNA expression in male and female Sprague-Dawley rats 12. However, this finding may be related to looking at the whole kidney level as opposed to the different regions of the kidney. In the present study, we found that Ang II decreases AT1 protein expression only in males, increases AT2 expression only in males and increases mas receptor expression only in females, thereby underscoring the importance of determining protein expression. The fact that AT1 receptor protein expression is not down-regulated in the renal cortex of female SHR may suggest an inability of the female to compensate to the increase in Ang II levels relative to the males. This may explain the comparable levels of tissue Ang II measured in male and female SHR assuming cortical levels of Ang II arise primarily from uptake of the high content of the circulating peptide.
While AT2 mRNA expression has been reported to be greater in kidneys from females compared to males 10, of more potential relevance are reports that normotensive females have greater AT2-dependent regulation of the vasculature and blood pressure. Low-dose (50 ng/kg/min) Ang II results in an AT2 receptor-dependent decrease in MAP in female, but not male, Sprague-Dawley rats 12. Greater AT2 activity has also been shown in female mice where treatment with an AT1 receptor blocker attenuates vascular injury to a greater extent in arteries from female mice due to increased AT2 receptor expression in females 23. We report in this study that male SHR following chronic Ang II infusion have an increase in AT2 receptor expression which is not evident in female SHR. However, we did not assess AT2 receptor activity. A difference in receptor expression alone is insufficient to conclude that a parallel sex difference exists in the physiological contribution of the receptor to blood pressure regulation. Alternatively, this increase in AT2 expression in males may be a compensatory response in conjunction with a decrease in AT1 receptor expression to limit the rise in blood pressure with Ang II. There is recent evidence in the literature supporting a functional role for the AT2 receptor in male SHR to offer neuroprotection against ischemic stroke24, and lower blood pressure when the AT1 receptor is blocked25. Therefore, it is possible that under certain conditions, such as in the presence of high levels of circulating and tissue Ang II, the AT2 receptor contributes to blood pressure regulation in male SHR, however, additional work is needed to determine the role of the AT2 receptor in Ang II–mediated hypertension in SHR. Since the mas receptor was the only RAS receptor regulated by Ang II in females, the remainder of this study focuses on the effect of Ang II infusion on Ang (1–7) to test the hypothesis that greater Ang (1–7) and mas receptor activation attenuates Ang II-induced hypertension in female SHR accounting for the sex difference in response to Ang II infusion.
We previously published that plasma levels of Ang II are greater in female SHR compared to males, and renal cortical Ang II levels under basal conditions are comparable in males and females 1. We verified our previous finding; however, there were no sex differences in Ang II levels following Ang II infusion. We next measured Ang (1–7) levels in the renal cortex and we found female SHR to have significantly higher levels of Ang (1–7) in the renal cortex following Ang II infusion. This is consistent with reports in the literature in congenic mRen(2). Lewis rats in which plasma Ang (1–7) levels are higher in females than males 11. Previous findings also reported that female Dahl rats are more sensitive to the hypotensive effects of Ang (1–7) 26. It is interesting to note that while Ang II infusion increased Ang (1–7) levels in the renal cortex of both male and female SHR, in female SHR there was a 1.3-fold increase in Ang (1–7) levels following chronic Ang II infusion, while in males there was a 2.7-fold increase. These data raise the possibility that Ang (1–7) may play an important role as a compensatory inhibitor of increases in blood pressure in males, however, only in female SHR was there also an increase in mas receptor expression with Ang II infusion.
To examine the functional implications of the increase in mas receptor expression with Ang II-infusion and higher Ang (1–7) levels in female SHR, rats were treated with the Ang (1–7) receptor antagonist A-779. A-779 had no effect on baseline blood pressure in either male or female SHR which is consistent with previous reports in male control and diabetic SHR, WKY, and 2K1C Goldblatt hypertensive rats 27–29. However, with the initiation of Ang II infusion female SHR experienced a much more robust increase in MAP as compared to infusion of Ang II alone. Similarly, female SHR had a significantly larger increase in proteinuria following the first week of Ang II infusion when pretreated with A-779 as opposed to Ang II infusion alone. These data suggest that Ang (1–7) normally acts to buffer the immediate increase in MAP and renal injury with Ang II in female SHR. However, at the end of the 2 week Ang II infusion there was no significant difference in blood pressure in females treated with A-779 compared to those infused with Ang II alone. In male SHR, although there was an increase in sensitivity to Ang II initially, over time the males treated with Ang (1–7) maintained a lower blood pressure compared to males infused with Ang II alone. Although A-779 had no effect on Ang II-induced proteinuria in male SHR, there was an improvement in structural damage to the kidney. These data suggest that Ang (1–7) has opposite effects in males and females, and that in males Ang (1–7) may contribute to Ang II mediated hypertension. Ang (1–7) effects are thought to be primarily mediated by the G-protein-coupled receptor mas 8, 9. However, there are also reports that Ang (1–7) binds and activates the AT2 receptor in male SHR 30 and there was an increase in AT2 receptor expression in the renal cortex of male SHR following Ang II infusion. If the lower blood pressure in male SHR treated with A-779 is due to loss of Ang (1–7) activation of the mas receptor or increased AT2 receptor activation is not known. It is also possible that there is a sex difference in the time course of the increase in Ang (1–7) in males and females such that it took males longer to increase Ang (1–7) levels. Alternatively, additional vasoactive angiotensin peptides may be present and contribute to Ang II-induced hypertension. Additional studies are planned to further investigate the mechanism by which A-779 differentially influences the blood pressure responses to Ang II in male and female SHR. Our study demonstrates that Ang (1–7) contributes to sex differences in the physiological responses to Ang II infusion and adds to our knowledge of how sex of the animal influences the balance of the classical and non-classical RAS.
Perspectives
ACE inhibitors and ARBs are among the most commonly prescribed drugs to help control blood pressure in hypertensive patients, regardless of sex of the patient. There is accumulating evidence in the literature, at both the clinical and basic science level, to support the idea that the RAS of males is not the same as the RAS of females. Our studies support this notion and further show that not only may males and females respond differently to Ang II but it is also likely that they respond differently to Ang (1–7). Better understanding of the components of the RAS that are being inappropriately activated or suppressed in hypertension and following activation of the RAS may lead to the development of more targeted therapies for more efficient blood pressure regulation.
Acknowledgments
The authors are grateful to Dr. David Pollock for assistance with telemetry studies. The authors would like to acknowledge the technical assistance of Heather Walker Smith.
Source of Funding: This study was funded by 1R01 HL093271-01A1 to JCS.
Footnotes
Disclosures: None.
References
- 1.Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM, Pollock JS. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. American Journal of Physiology. 2007;293:R1573–1579. doi: 10.1152/ajpregu.00429.2007. [DOI] [PubMed] [Google Scholar]
- 2.Reckelhoff JF, Zhang H, Granger JP. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension. 1998;31:435–439. doi: 10.1161/01.hyp.31.1.435. [DOI] [PubMed] [Google Scholar]
- 3.Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension. 2000;35:480–483. doi: 10.1161/01.hyp.35.1.480. [DOI] [PubMed] [Google Scholar]
- 4.Brewster UC, Perazella MA. The renin-angiotensin-aldosterone system and the kidney: effects on kidney disease. Am J Med. 2004;116:263–272. doi: 10.1016/j.amjmed.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 5.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- 6.Carey RM, Wang ZQ, Siragy HM. Novel actions of angiotensin II via its renal type-2 (AT(2)) receptor. Current Hypertension Reports. 1999;1:151–157. doi: 10.1007/s11906-999-0012-y. [DOI] [PubMed] [Google Scholar]
- 7.Iyer SN, Ferrario CM, Chappell MC. Angiotensin-(1–7) contributes to the antihypertensive effects of blockade of the renin-angiotensin system. Hypertension. 1998;31:356–361. doi: 10.1161/01.hyp.31.1.356. [DOI] [PubMed] [Google Scholar]
- 8.Ferrario CM, Averill DB, Brosnihan KB, Chappell MC, Iskandar SS, Dean RH, Diz DI. Vasopeptidase inhibition and Ang-(1–7) in the spontaneously hypertensive rat. Kidney Int. 2002;62:1349–1357. doi: 10.1111/j.1523-1755.2002.kid559.x. [DOI] [PubMed] [Google Scholar]
- 9.Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva-Antonialli MM, Tostes RC, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MH, Fortes ZB, Nigro D. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res. 2004;62:587–593. doi: 10.1016/j.cardiores.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Pendergrass KD, Pirro NT, Westwood BM, Ferrario CM, Brosnihan KB, Chappell MC. Sex differences in circulating and renal angiotensins of hypertensive mRen(2). Lewis but not normotensive Lewis rats. Am J Physiol Heart Circ Physiol. 2008;295:H10–20. doi: 10.1152/ajpheart.01277.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sampson AK, Moritz KM, Jones ES, Flower RL, Widdop RE, Denton KM. Enhanced angiotensin II type 2 receptor mechanisms mediate decreases in arterial pressure attributable to chronic low-dose angiotensin II in female rats. Hypertension. 2008;52:666–671. doi: 10.1161/HYPERTENSIONAHA.108.114058. [DOI] [PubMed] [Google Scholar]
- 13.Venegas-Pont M, Sartori-Valinotti JC, Glover PH, Reckelhoff JF, Ryan MJ. Sexual dimorphism in the blood pressure response to angiotensin II in mice after angiotensin-converting enzyme blockade. American Journal of Hypertension. 23:92–96. doi: 10.1038/ajh.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatchum-Talom R, Eyster KM, Martin DS. Sexual dimorphism in angiotensin II-induced hypertension and vascular alterations. Canadian Journal of Physiology and Pharmacology. 2005;83:413–422. doi: 10.1139/y05-012. [DOI] [PubMed] [Google Scholar]
- 15.Xue B, Pamidimukkala J, Lubahn DB, Hay M. Estrogen receptor-alpha mediates estrogen protection from angiotensin II-induced hypertension in conscious female mice. American Journal of Physiology. 2007;292:H1770–1776. doi: 10.1152/ajpheart.01011.2005. [DOI] [PubMed] [Google Scholar]
- 16.Xue B, Johnson AK, Hay M. Sex differences in angiotensin II- induced hypertension. Brazilian Journal of Medical and Biological Researchl. 2007;40:727–734. doi: 10.1590/s0100-879x2007000500018. [DOI] [PubMed] [Google Scholar]
- 17.Sartori-Valinotti JC, Iliescu R, Yanes LL, Dorsett-Martin W, Reckelhoff JF. Sex differences in the pressor response to angiotensin II when the endogenous renin-angiotensin system is blocked. Hypertension. 2008;51:1170–1176. doi: 10.1161/HYPERTENSIONAHA.107.106922. [DOI] [PubMed] [Google Scholar]
- 18.Miller JA, Anacta LA, Cattran DC. Impact of gender on the renal response to angiotensin II. Kidney Int. 1999;55:278–285. doi: 10.1046/j.1523-1755.1999.00260.x. [DOI] [PubMed] [Google Scholar]
- 19.Chidambaram M, Duncan JA, Lai VS, Cattran DC, Floras JS, Scholey JW, Miller JA. Variation in the renin angiotensin system throughout the normal menstrual cycle. J Am Soc Nephrol. 2002;13:446–452. doi: 10.1681/ASN.V132446. [DOI] [PubMed] [Google Scholar]
- 20.Harvey PJ, Morris BL, Miller JA, Floras JS. Estradiol induces discordant angiotensin and blood pressure responses to orthostasis in healthy postmenopausal women. Hypertension. 2005;45:399–405. doi: 10.1161/01.HYP.0000157161.78721.5c. [DOI] [PubMed] [Google Scholar]
- 21.Bjorck S. The renin angiotensin system in diabetes mellitus. A physiological and therapeutic study. Scand J Urol Nephrol Suppl. 1990;126:1–51. [PubMed] [Google Scholar]
- 22.Wei SG, Yu Y, Zhang ZH, Felder RB. Angiotensin II upregulates hypothalamic AT1 receptor expression in rats via the mitogen-activated protein kinase pathway. Am J Physiol Heart Circ Physiol. 2009;296:H1425–1433. doi: 10.1152/ajpheart.00942.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okumura M, Iwai M, Ide A, Mogi M, Ito M, Horiuchi M. Sex difference in vascular injury and the vasoprotective effect of valsartan are related to differential AT2 receptor expression. Hypertension. 2005;46:577–583. doi: 10.1161/01.HYP.0000178564.14464.80. [DOI] [PubMed] [Google Scholar]
- 24.McCarthy CA, Vinh A, Callaway JK, Widdop RE. Angiotensin AT2 receptor stimulation causes neuroprotection in a conscious rat model of stroke. Stroke. 2009;40:1482–1489. doi: 10.1161/STROKEAHA.108.531509. [DOI] [PubMed] [Google Scholar]
- 25.Bosnyak S, Welungoda IK, Hallberg A, Alterman M, Widdop RE, Jones ES. Stimulation of angiotensin AT2 receptors by the non-peptide agonist, Compound 21, evokes vasodepressor effects in conscious spontaneously hypertensive rats. Br J Pharmacol. 159:709–716. doi: 10.1111/j.1476-5381.2009.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eatman D, Wang M, Socci RR, Thierry-Palmer M, Emmett N, Bayorh MA. Gender differences in the attenuation of salt-induced hypertension by angiotensin (1–7) Peptides. 2001;22:927–933. doi: 10.1016/s0196-9781(01)00404-1. [DOI] [PubMed] [Google Scholar]
- 27.Al-Maghrebi M, Benter IF, Diz DI. Endogenous angiotensin-(1–7) reduces cardiac ischemia-induced dysfunction in diabetic hypertensive rats. Pharmacol Res. 2009;59:263–268. doi: 10.1016/j.phrs.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgelova M, Vanourkova Z, Thumova M, Dvorak P, Opocensky M, Kramer HJ, Zelizko M, Maly J, Bader M, Cervenka L. Impairment of the angiotensin-converting enzyme 2-angiotensin-(1–7)-Mas axis contributes to the acceleration of two-kidney, one-clip Goldblatt hypertension. Journal of Hypertension. 2009;27:1988–2000. doi: 10.1097/HJH.0b013e32832f0d06. [DOI] [PubMed] [Google Scholar]
- 29.Widdop RE, Sampey DB, Jarrott B. Cardiovascular effects of angiotensin-(1–7) in conscious spontaneously hypertensive rats. Hypertension. 1999;34:964–968. doi: 10.1161/01.hyp.34.4.964. [DOI] [PubMed] [Google Scholar]
- 30.Walters PE, Gaspari TA, Widdop RE. Angiotensin-(1–7) acts as a vasodepressor agent via angiotensin II type 2 receptors in conscious rats. Hypertension. 2005;45:960–966. doi: 10.1161/01.HYP.0000160325.59323.b8. [DOI] [PubMed] [Google Scholar]