Abstract
Cell fate decisions mediated by the Notch signalling pathway require direct cell–cell contact between adjacent cells. In Drosophila melanogaster, an external sensory organ (ESO) develops from a single sensory organ precursor (SOP) and its fate specification is governed by differential Notch activation. Here we show that mutations in actin-related protein-3 (Arp3) compromise Notch signalling, leading to a fate transformation of the ESO. Our data reveal that during ESO fate specification, most endocytosed vesicles containing the ligand Delta traffic to a prominent apical actin-rich structure (ARS) formed in the SOP daughter cells. Using immunohistochemistry and transmission electron microscopy (TEM) analyses, we show that the ARS contains numerous microvilli on the apical surface of SOP progeny. In Arp2/3 and WASp mutants, the surface area of the ARS is substantially reduced and there are significantly fewer microvilli. More importantly, trafficking of Delta-positive vesicles from the basal area to the apical portion of the ARS is severely compromised. Our data indicate that WASp-dependent Arp2/3 actin polymerization is crucial for apical presentation of Delta, providing a mechanistic link between actin polymerization and Notch signalling.
Notch signalling is an evolutionarily conserved pathway used by metazoans to control cell fate decisions1,2. The Notch receptor and its ligands Delta and Serrate (Jagged in vertebrates) are single-pass transmembrane proteins. Cell–cell communication begins when the extracellular domain of the ligand on the signal-sending cell interacts with the extracellular domain of the Notch receptor on the signal-receiving cell. This interaction triggers a series of proteolytic cleavages that releases the intracellular domain of Notch, which enters the nucleus and functions as a transcriptional regulator3.
Notch signalling mediates key decisions during nervous system develop-ment4, including patterning and fate specification of the ESOs5. Each ESO is composed of four cell types (shaft, socket, sheath and neuron) and is derived from a single cell, the SOP (also called the pI cell), which is selected through Notch-mediated lateral inhibition at about 8–12 h after puparium formation (APF; Fig. 1a). The stage when the SOP has not yet undergone cell division is referred to as the 1-cell stage (15–18 h APF). During the 2-cell stage (~18–18.30 h APF) the SOP undergoes asymmetric cell division to generate the anterior pIIb and posterior pIIa (Fig. 1a). Because of the asymmetric distribution of cell fate determinants such as Numb and Neuralized6,7, Notch signalling is differentially activated in pIIa and pIIb. The pIIa divides to create the external cells of the ESO, the shaft and socket cells. The pIIb divides twice to create the internal cells of the ESO, the neuron and sheath cell8. These four differentiated cells are collectively called the sensory cluster.
Figure 1.
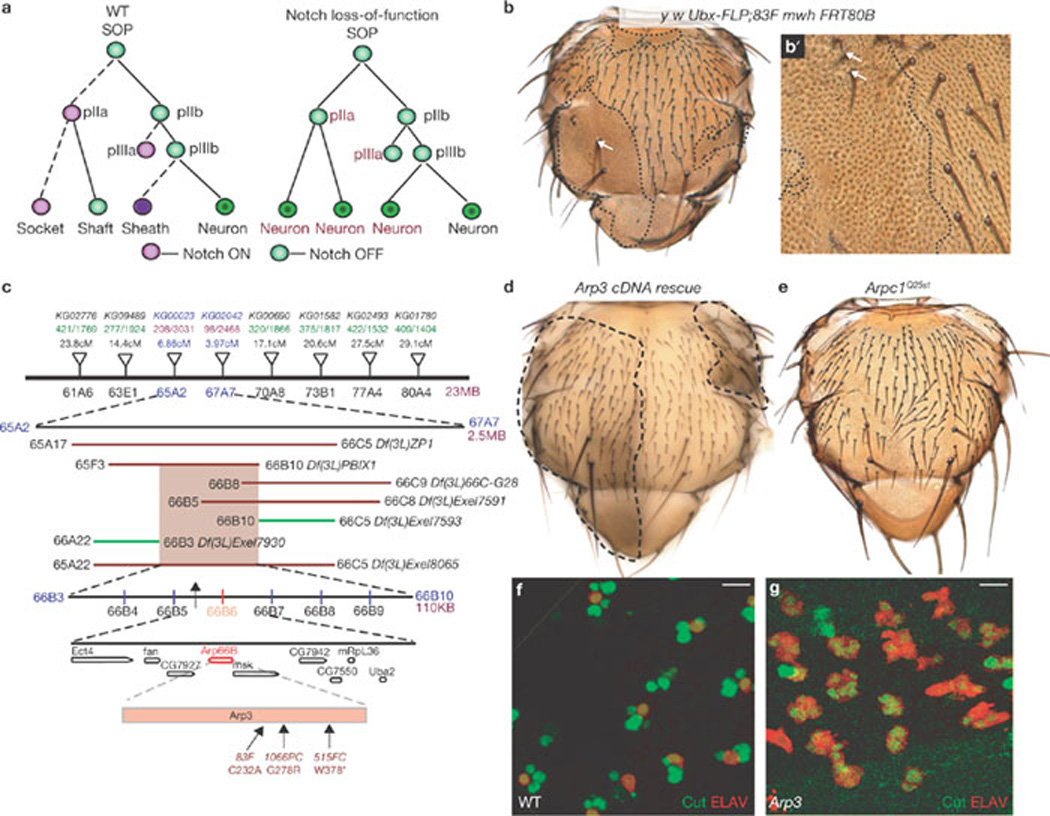
Arp3 mutations cause a pIIa-to-pIIb transformation in the ESO lineage (a) A diagram of the ESO lineage in wild-type (WT) and in Notch loss-of-function background. Each cell is represented by a circle; the cells in which Notch is activated are in purple and the signal-sending cells are in green. The dashed lines indicate daughter cells in which Notch is activated. (b) Homozygous clones of Arp383F on an adult thorax induced by Ubx–FLP. The clone (dashed lines) is identified by an epithelial cell marker multiple wing hair (mwh), which marks the trichomes (small hairlike structures) on epithelial cells. Mutant clones show loss of external structures, socket and shaft cells, of the microchaetae. Macrochaetae (arrow) sometimes show a double-shaft phenotype in Arp383F clones. (b´) Higher magnification of an Arp383F clone shows that rarely there are shaft and sockets (arrows) in the mutant clone. Most of the Arp383F clones show a balding phenotype. (c) Schematic representation of the mapping strategy. The inverted triangles represent P elements that were used for recombination mapping. Deficiencies represented by lines: those in red failed to complement the alleles, whereas those in green complement our alleles. (d) Rescue of the Arp3 phenotype by overexpression of an Arp3 cDNA construct in the mutant clones. An image of a pupal notum of an adult thorax which harbours Arp3 clones; the mutant clones (dashed lines) do not show bristle loss (compare with b). (e) Flies that harbour clones of Arpc1Q25st show bald patches. The clones were not generated in a Minute background and hence appear smaller than Arp3 clones (compare with b). We have not outlined the clones in Arpc1Q25st as they are unmarked clones. (f, g) A projection of confocal slices show part of the notum at 24–26 h APF stained for ELAV (red) and Cut (green). All cells in the wild-type (f) sensory clusters are positive for Cut and one of the cells is ELAV-positive. In Arp3 (g) mutant clones all of the cells in the sensory clusters are positive for Cut and ELAV, indicating the transformation of all SOP progeny to neurons. Scale bar, 10 µm.
Delta and Serrate act redundantly to activate Notch during specification of pIIa and pIIb9. Recent studies indicate that endocytosis of Delta in the signal-sending cell is crucial for its ability to activate Notch10. An alternative, but not mutually exclusive model, is that ligand endocytosis promotes trafficking of the ligand to an endocytic recycling compartment, resulting in its activation11,12. In addition, apical trafficking of Delta seems to be important for proper fate specification in the SOP lineage13. However, the nature of ligand activation or the requirement for apical trafficking of the ligand remains unclear.
Here, we report that there is an apical actin-enriched structure in the pIIa and pIIb cells that contains numerous microvilli. The surface area of the actin-rich region and the number of microvilli are markedly reduced in Arp2/3 complex and WASp mutants. More importantly, we found that the Arp2/3 complex and WASp have crucial roles in trafficking of endocytosed Delta vesicles to an apical ARS.
RESULTS
Mutations in Arp3 result in a pIIa-to-pIIb cell fate transformation in Drosophila ESO lineages
Notch loss-of-function results in a pIIa-to-pIIb transformation, leading to loss of bristles14. Previous genetic screens based on assaying mitotic clones on the adult Drosophila thorax for bristle abnormalities13,15,16 have identified components in the Notch pathway14. We performed a similar F1 mitotic recombination screen on chromosome arm 3L16 and isolated one complementation group consisting of three homozygous lethal alleles (83F, 515FC and 1066PC) that cause bristle loss in clones (Fig. 1b, b´). Using a recombination-based mapping strategy17, the lethality of these alleles was mapped to the 66B cytological region (Fig. 1c). We obtained a P element EP(3)3640 (ref. 18) inserted upstream of the Arp3 gene that failed to complement our alleles, and identified molecular lesions in Arp3 for the three alleles (Fig. 1c). Overexpression of the Arp3 cDNA in Arp3 mutant clones rescued the lethality and ESO phenotype (Fig. 1d), demonstrating that the observed phenotypes are caused by loss of Arp3.
Arp3 is part of the seven-protein Arp2/3 complex, which functions together for polymerization of branched actin filaments19. Another component of the Arp2/3 complex, Arpc1, was shown to be involved in ring canal formation during oogenesis in Drosophila18. As with Arp3 alleles, Arpc1Q25st clones also cause bristle loss (Fig. 1e)20. Bristle loss in Arp3 clones does not result from a failure to specify SOPs (Supplementary Information, Fig. S1a, a´). To examine whether bristle loss in Arp3 clones is associated with a Notch loss-of-function defect, SOP progeny at 24 h APF were labelled with differentiation markers. In wild-type sensory clusters, all four cells expressed the homeodomain protein Cut and one expressed the neuronal marker ELAV (Fig. 1f). In contrast, sensory clusters in both Arp3 and Arpc1Q25st mutant clones contained 4–6 ELAV-positive cells (Fig. 1g and data not shown), suggesting that there is a pIIa-to-pIIb fate transformation.
Although a pIIa-to-pIIb transformation might result from disruption of asymmetric localization of cell fate determinants6,7, both Neuralized and Numb were asymmetrically localized in Arp3 mutant SOPs (Supplementary Information, Fig. S1c, e). One of the activators of the Arp2/3 complex, Wiskott-Aldrich syndrome protein (WASp)21, is also involved in a similar fate specification process in Drosophila22. Together these observations suggest a specific requirement for WASp-regulated Arp2/3-complex function in Notch signalling.
Arp3 functions in the signal-sending cell during Notch signalling
Is Arp2/3 function required in the signal-sending or the signal-receiving cell during Notch signalling? We first determined the epistatic relationship between Notch and Arp3 with a constitutively active Notch that is independent of ligand activation (NECN)23. Expression of NECN in the ESO lineage causes a Notch gain-of-function phenotype, which results in generation of extra socket cells13. Overexpression of NECN in Arp3 clones, as in wild-type cells, resulted in a Notch gain-of-function phenotype, indicating that a ligand-independent form of Notch is epistatic to Arp3 (Fig. 2a). This places the function of Arp3 upstream of Notch activation, possibly in the signal-sending cell.
Figure 2.
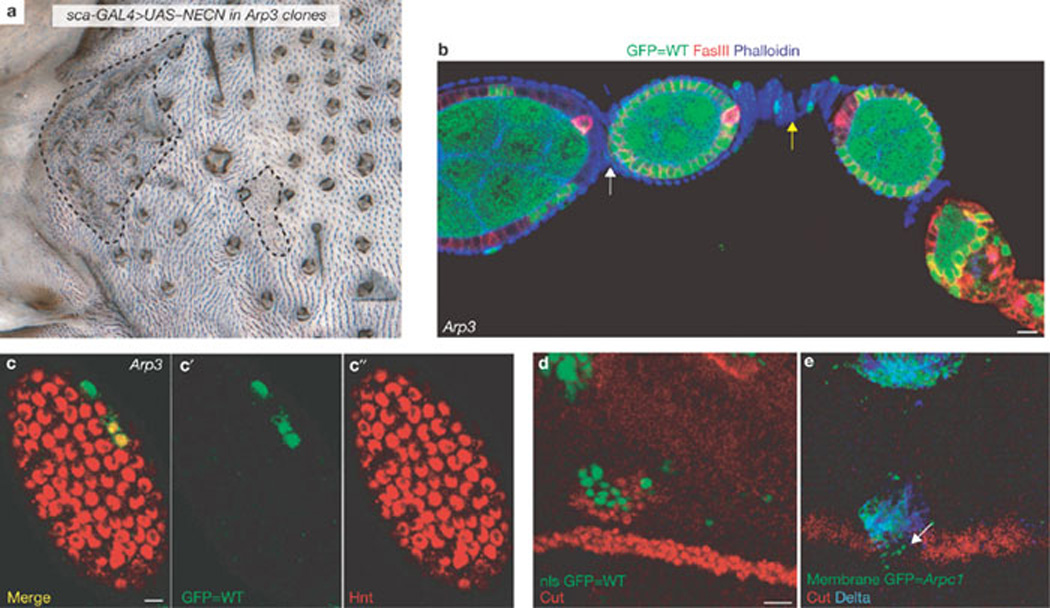
Arp3 is required in the signal sending cells during Notch signalling (a) Overexpression of NECN in wild-type SOPs using the sca109-68-GAL4 driver results in a multiple socket phenotype in the majority of the sensory clusters. We generated Arp3 clones (dashed line) using Ubx–FLP in this NECN overexpression background. We did not observe a region of bald cuticle in the Arp3 clones. (b) Clones of Arp3515FC induced by hs-FLP in follicle cells are marked by the absence of GFP (green). FasciclinIII (red) marks the follicle cells and is upregulated in polar follicle cells. Phalloidin (blue) marks the membrane of all cells. When polar follicle cells are wild-type (WT), stalk cells (yellow arrow) are formed normally, separating two cysts, whereas, when the polar follicle cells are mutant for Arp3, we found a loss of stalk cells between the cysts, resulting in a partial fusion of cysts (white arrow). (c–c´´) The follicle cells of the cyst harbour mutant clones of Arp3 induced by hs-FLP at stage 7 of oogenesis. Arp3 mutant clones are marked by the absence of nuclear GFP (green). The cyst was immunostained for Hnt (red), a Notch downstream target gene in the follicle cells. Note that Hnt is still expressed in the Arp3 mutant follicle cell clones (non-green cells). (d) Overexpression of Delta in WT cells (green) near the dorsal-ventral boundary of the wing can induce Cut expression (red) in the adjacent cells near the dorsal-ventral boundary at the dorsal compartment. (e) Overexpression of Delta (blue) in Arpc1 mutant cells (green) cannot induce Cut expression (red) in the adjacent cells near the dorsal-ventral boundary at the dorsal compartment. Note the loss of Cut expression when the clone crosses the dorsal-ventral boundary (arrow). Scale bars, 10 µm (b, d) and 5 µm (c).
To gather evidence for a requirement of Arp3 in the signal-sending cell, we examined its function in oogenesis. Egg chambers are individual units, consisting of germline cells surrounded by somatic follicle cells. The follicle cells can be further divided into three distinct populations: main body follicle cells (phalloidin-positive cells, Fig. 2b), which encapsulate the germline cyst; polar cells, which function as signalling centres (FasIII-positive cells, Fig. 2b); and stalk cells that connect neighbouring cysts (yellow arrow, Fig. 2b). The role of Notch signalling is well-documented in oogenesis24,25, and signal-sending and receiving cells are spatially well-segregated. Notch loss-of-function causes the inability of the follicle cells to encapsulate germline cysts and leads to the formation of giant compound egg chambers25. However, Delta loss-of-function in follicle cells does not result in an encapsulation defect25 but rather, loss of stalk cells and partial fusion of the cysts. Delta is required in the anterior polar follicle cells of the posterior egg chamber to specify stalk cells25,26. Generating follicle cell clones of Notch and Delta, therefore, results in distinct phenotypes. We found that loss of Arp3 phenocopied loss-of-function of Delta. Mutant clones of Arp3 (n = 14) in anterior polar follicle cells resulted in loss of stalk cells and partial fusion of adjacent cysts (white arrow, Fig. 2b). At later stages of oogenesis, Delta signals from the germ cells (signal-sending cells) activate Notch in the overlying somatic follicle cells (signal-receiving cells), resulting in expression of a Notch downstream target, Hindsight (Hnt)27. Arp3 does not seem to be required in the signal-receiving cell for Notch function, as expression of Hnt was normal in Arp3 mutant follicle cell clones (Fig. 2c, c´).
To further examine whether Arp2/3 function is required in the signal-sending cell during wing formation, a Delta overexpression assay was performed. During wing development, pre-patterning signals, including Notch, are required to compartmentalize the immature wing imaginal disc at the third-instar larva28. Notch signalling is required to activate Cut expression at the dorsal-ventral boundary29,30. Previous studies have shown that overexpression of Delta in wild-type clones near the dorsal-ventral boundary results in ectopic Cut expression in the neighbouring cells (Fig. 2d)11,16,29,30. However, similar overexpression of Delta in Arpc1 clones failed to activate Cut expression and resulted in loss of endogenous Cut expression when the clone crossed the dorsal-ventral boundary (Fig. 2e). These data suggest that Arp2/3 complex function is required for the normal function of Delta in the signal-sending cell.
The Arp2/3 complex is not required for Delta endocytosis
Delta must be endocytosed in the signal-sending cell to activate Notch on the receiving cell6,31. As Arp2/3 and WASp have been shown to be required for clathrin-mediated endocytosis in yeast32,33, Arp2/3 might be required for Delta endocytosis during fate specification. However, by performing a Delta endocytosis assay6 at the 2-cell stage, we found that Delta is endocytosed similarly to wild-type cells (Fig. 3a) in Arpc1 and Arp3 mutant tissue (Fig. 3c, d). By contrast, in shibire (Dynamin) mutant cells kept at the restrictive temperature (Fig. 3b), Delta is not endocytosed34,35. This indicates that the Arp2/3 complex is not required for ligand endocytosis during Notch signalling.
Figure 3.
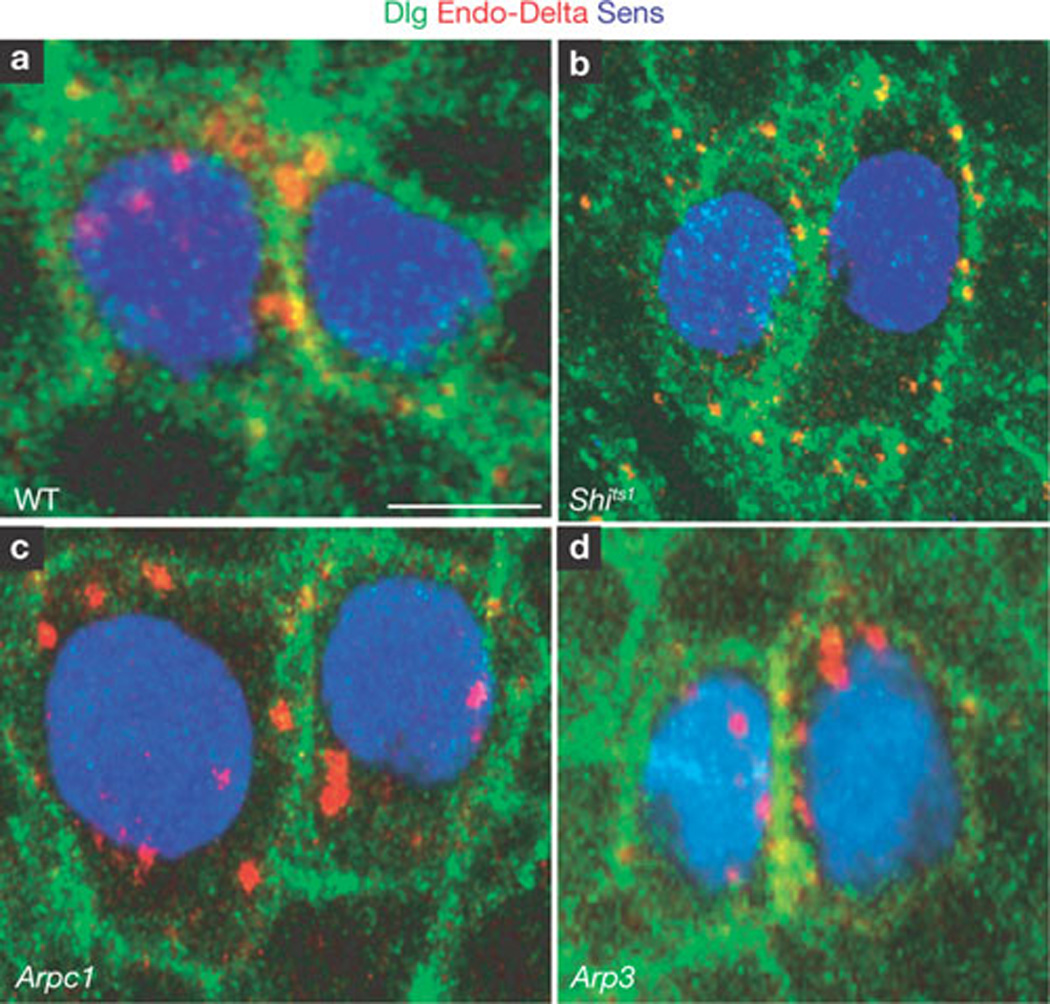
Delta is normally endocytosed in Arp3 and Arpc1 mutant pIIa-pIIb. (a–d) Endocytosis assay for Delta ligand (red) performed at the 2-cell stage in pIIa-pIIb. Sens (blue) labels the nucleus and Dlg (green) marks the sub-apical membrane. A projection of optical slices shows that in the negative control (shits1 (b), Delta (red) is found only on the membrane and not in cytoplasmic vesicles between the nucleus and membrane. However, in the wild-type (WT, a), Arpc1 (c) and Arp3 (d) pIIa-pIIb, endocytosed Delta vesicles (red) are present in the cytoplasm, indicating that Arp2/3 function is not required for Delta endocytosis. Note small punctae in b when Delta is not endocytosed. Scale bar, 5 µm.
A specific ARS forms during fate specification in the ESO lineage
As Arp2/3 is required for polymerization of branched actin filaments19, we visualized filamentous actin (F-actin) in the ESO lineage with phalloidin. In the wild-type, a prominent apical ARS was present in the pIIa and pIIb (pIIa-pIIb) cells (Fig. 4a, a´´). Co-staining of phalloidin and E-cadherin (DE-Cad), which highlights the apical-most stalk region of the pIIb cell that is engulfed by the pIIa cell36, indicates that the ARS is present in both pIIa-pIIb cells apically (Supplementary Information, Fig. S1f, f´). However, no specialized apical actin enrichment was observed at the earlier 1-cell stage (Supplementary Information, Fig. S1g, g´). In Arpc1 (yellow arrows, Fig. 4a, a´´), Arp3 and WASp (data not shown) pIIa-pIIb cells, the ARS was formed. However, the apical area of the ARS was markedly reduced in Arp3 (9.57 ± 5.32 µm2; mean ± s.e.m, n = 22), Arpc1 (12.25 ± 6.89 µm2; n = 19) and WASp (21.86 ± 7.74 µm2; n = 19) pIIa-pIIb cells when compared with the wild-type (43.48 ± 13.79 µm2; Fig. 4b; n = 18). The ARS in wild-type pIIa-pIIb cells formed an umbrella shape along the xy axis, whereas in about 50% of the mutant ARS, the stalk of the umbrella was not formed properly (Fig. 4a´´, d).
Figure 4.
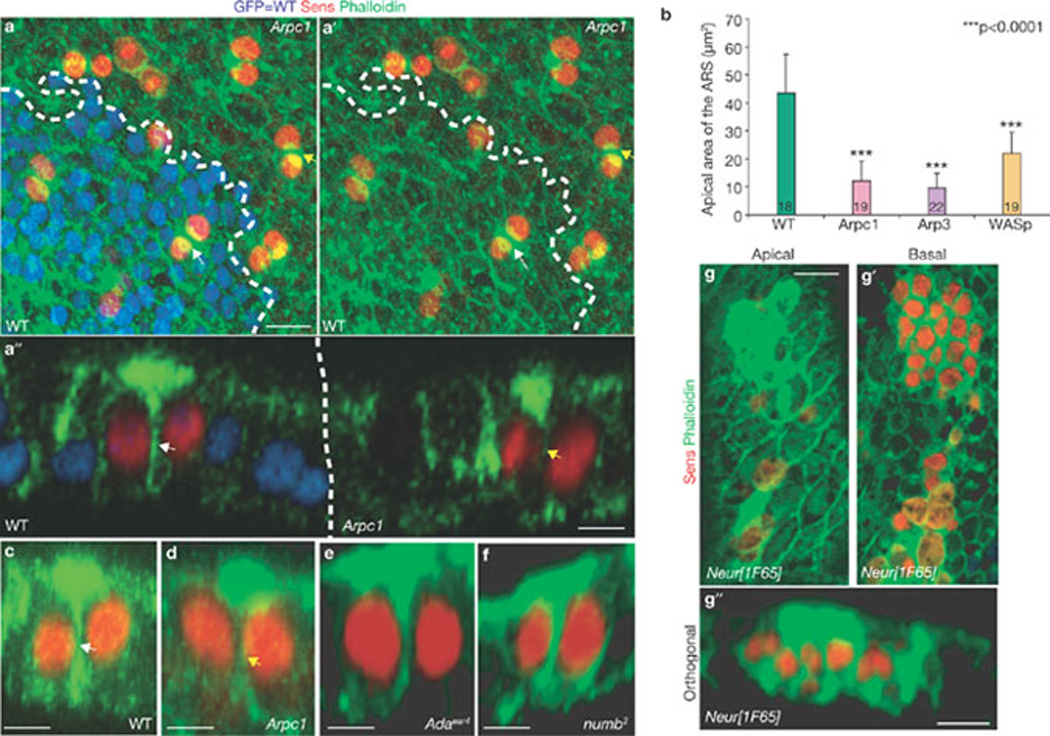
The ARS forms specifically in the pIIa-pIIb progeny and is reduced in Arp3, Arpc1 and WASp mutant SOP progeny. (a, a´) A projection of confocal sections shows that the ARS identified by phalloidin (green) staining is present in both wild-type (WT, white arrow) pIIa-pIIb and Arpc1 (yellow arrow) mutant pIIa-pIIb cells marked by Sens (red). Arpc1 homozygous mutant clones (dotted lines) are marked by the absence of nuclear GFP (blue). (a´´) An orthogonal confocal section shows that the ARS is quite broad in the WT pIIa-pIIb (white arrow) and has an umbrella-shaped structure, whereas the ARS in the Arpc1 homozygous clones (yellow arrow) seems compressed and the lateral ‘stalk’ of the ARS is malformed. (b) Quantification of the apical area of the ARS in different genotypes. The ARS area was quantified using the Measure function of ImageJ software. The measurements were analysed using a Student’s t-test (***P <0.0001). Data are mean ± s.e.m. and the number of SOP progeny pairs used for quantification per genotype is indicated in the bars. (c–g´´) Pupal nota stained with Sens (red) and phalloidin (green) reveal ARS in pIIa-pIIb. Projections of orthogonal slices show the ARS in WT (c, white arrow), Arpc1 (d, yellow arrow), α-adaptin (e), numb (f) and neuralized (g–g´´) pIIa-pIIb. An apical section (g) reveals apical (0.5 µm) actin enrichment whereas a basal section (g´) of the sample (~6 µm) shows the nuclei of the SOP progeny. Scale bars, 10 µm (a, a´´, g, g´´) and 5 µm (c–f).
To test whether the ARS is affected in other mutants, α-Adaptin15 and numb7, which regulate Notch signalling during pIIa-pIIb specification, were examined. In mutant clones of α-Adaptin (Fig. 4e) and numb (Fig. 4f) the ARS was formed normally, suggesting that the ARS defect is specific to Arpc1, Arp3 and WASp. In neuralized clones, where both lateral inhibition and fate specification37 are affected, the ARS was clearly observed in all SOP progeny (Fig. 4 g, g´´). This suggests that most, if not all, SOP progeny at the 2-cell stage are instructed to form an ARS.
To examine whether the Arp2/3 complex colocalizes with the ARS, we overexpressed a GFP-tagged Arp3 cDNA construct (UAS–Arp3-GFP) by neuralized-GAL4. We observed that much of the GFP-tagged Arp3 protein colocalized with the ARS (Supplementary Information, Fig. S1h, h´´). The presence of the ARS in the pIIa-pIIb cells during fate specification and the fact that the ARS is morphologically affected in the Arp3, Arpc1 and WASp mutants indicate that it has a role in Notch signal transduction.
Abundant actin-rich microvilli are present at the apical surface of pIIa-pIIb
The ARS was further analysed using TEM to visualize the actin cytoskeleton at the ultracellular level38. To distinguish the pIIa-pIIb cell-membrane from that of epithelial cells, HRP was overexpressed in the pIIa-pIIb cells using neuralized–GAL4 and UAS-CD2::HRP (Fig. 5a). On DAB staining, HRP labelling was visualized as a darker cell membrane outline in the SOPs. The serial apical cross-sections (0–2520 nm) of the pIIa-pIIb cells revealed numerous membrane protrusions (Fig. 5b; Supplementary Information, Fig. S2). At high magnification (× 10,000), we clearly observed actin bundles within these membranous extensions (Fig. 5c), which was confirmed by immuno-electron microscopy with phalloidin (Fig. 6a, a´). TEM analysis of Arp3 pIIa-pIIb cells (Fig. 5d – f) revealed fewer finger-like projections than in wild-type cells (Fig. 5g), consistent with the marked reduction in apical surface area of the ARS in Arp3, Arpc1 and WASp mutants (Fig. 4b). Finger-like projections were present on the epithelial cells, but there were fewer and they were markedly shorter (only about 60 nm in length), compared with those of pIIa-pIIb (Supplementary Information, Fig. S3a, c).
Figure 5.
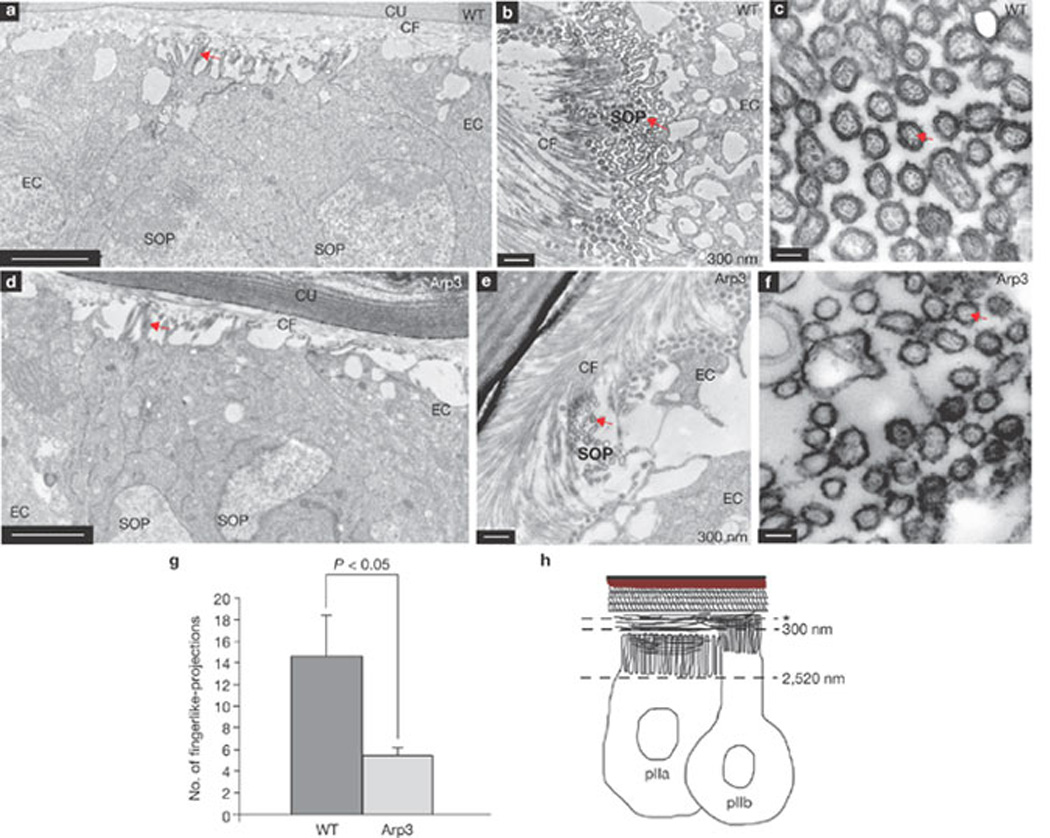
TEM analysis reveals enrichment of actin-filled finger-like projections in pIIa-pIIb cells at 18 h APF. (a, d) Orthogonal sections of wild-type (WT, a) and Arp3 (d) pIIa-pIIb cells show finger-like projections (arrows) at the apical domain of the cells. (b–f) Cross-section of WT (b) and Arp3 (e) pIIa-pIIb cells show finger-like projections (arrows). (c, f) Higher magnification of the apical surface of WT (c) and Arp3 (f) pIIa-pIIb cells shows actin bundles (arrows) inside the finger-like projections. (g) Quantification of the number of finger-like projections at the 2-cell stage in WT and Arp3. The total number of microvilli in SOP and epithelial cells were quantified using ImageJ. The data are mean ± s.e.m and measurements were analysed using Student’s t-test. Three SOP progeny pairs were used for this quantification per genotype. (h) Schematic representation of pIIa-pIIb in the prepupal thorax epithelium. The asterisk represents the level of the first electron microscopy section at 60 nm. Abbreviations: cuticle (Cu), chitin fibre (CF), epithelial cell (EC), sensory organ precursor cell (SOP). Scale bars, 0.5 µm (a, b, d, e) and 0.1 µm (c, f).
Figure 6.
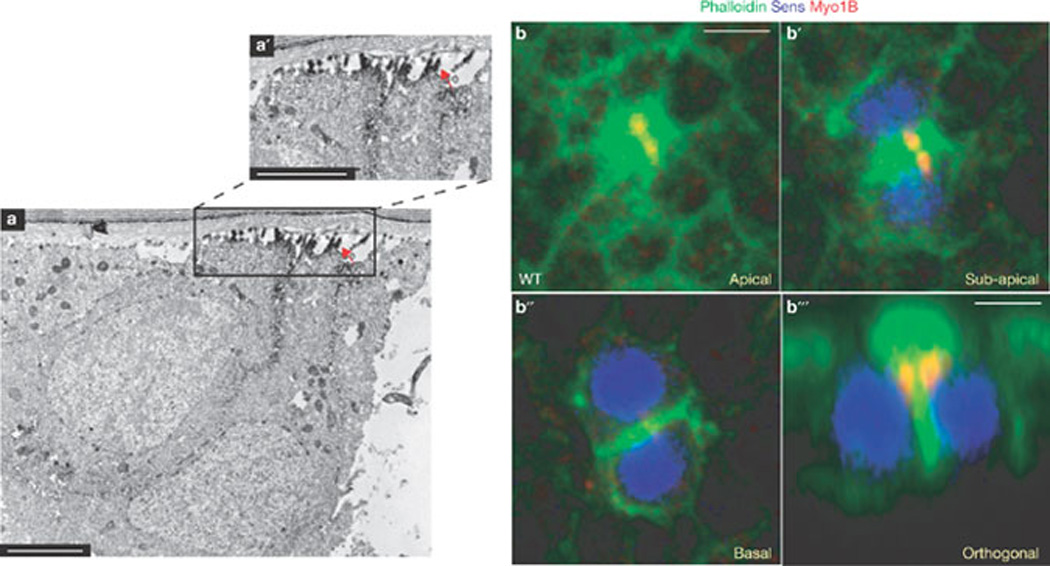
Finger-like projections in pIIa-pIIb cells are enriched with F-actin bundles and are marked by a microvillar marker Myo1B. (a, a´) Immuno-electron microscopy image of an orthogonal section through the wild-type pIIa-pIIb of a pupal notum shows an enrichment of phalloidin (electron-dense material) in the finger-like projections along the apical region of the ARS. (a´) A higher magnification view of the boxed region in a is shown in a´. The arrow points to the enrichment of phalloidin in the finger-like projections (b–b´´´) Confocal images of single optical (xy axis) sections (b–b´´) and orthogonal section (b´´´) of wild-type (WT) pIIa-pIIb cells immunostained for Myo1B (red), phalloidin (green) and Sens (blue). Scale bars, 0.5 µm (a, a´) and 5 µm (b, b´´´).
The finger-like actin projections on the pIIa-pIIb cells resemble micro-villi, which are typically observed to be densely packed in intestinal and kidney epithelial cells39, and circulating leukocytes40. Microvilli on the intestinal and kidney epithelial cells are thought to increase the surface area for absorption, whereas in leukocytes they have been implicated in receptor presentation, which enables leukocyte adhesion41,42. To examine whether the finger-like projections are microvilli, the ARS was immunostained with a microvilli marker myosin 1B (Myo1B), which forms lateral tethers between the microvillar membrane and underlying actin filament core43. We found that Myo1B is indeed enriched in the apical region of pIIa-pIIb cells (Fig. 6b, b´), specifically at the base of the ‘umbrella’ region of the ARS (Fig. 6b´´´). This localization of Myo1B was unaffected in Arp3 mutant pIIa-pIIb cells (Supplementary Information, Fig. S3e, e´). These data indicate that microvilli are present on the apical region of pIIa-pIIb cells.
Delta traffics to the ARS
Intracellular vesicular trafficking of Delta is emerging as a key regulatory step in the activation of Notch44,45. We investigated Delta trafficking by co-staining of phalloidin and Delta. In wild-type pIIa-pIIb cells, Delta vesicles colocalized with the apical microvillar region of the ARS (Fig. 7a and transverse section in 7a´). In Arpc1 (Fig. 7b and transverse section in Fig. 7b´) and Arp3 (data not shown) pIIa-pIIb, fewer Delta vesicles were colocalized with the ARS. Furthermore, when serial sections were projected to visualize the whole cell (Fig. 7c, c´´), the Delta vesicles were clustered close to the wild-type ARS, whereas the vesicles were widely distributed in the cytoplasm of Arpc1 pIIa-pIIb cells. The marked reduction of Delta vesicles colocalizing with the ARS in the mutant pIIa-pIIb cells suggests that Arp2/3 has a role in Delta trafficking to the ARS.
Figure 7.
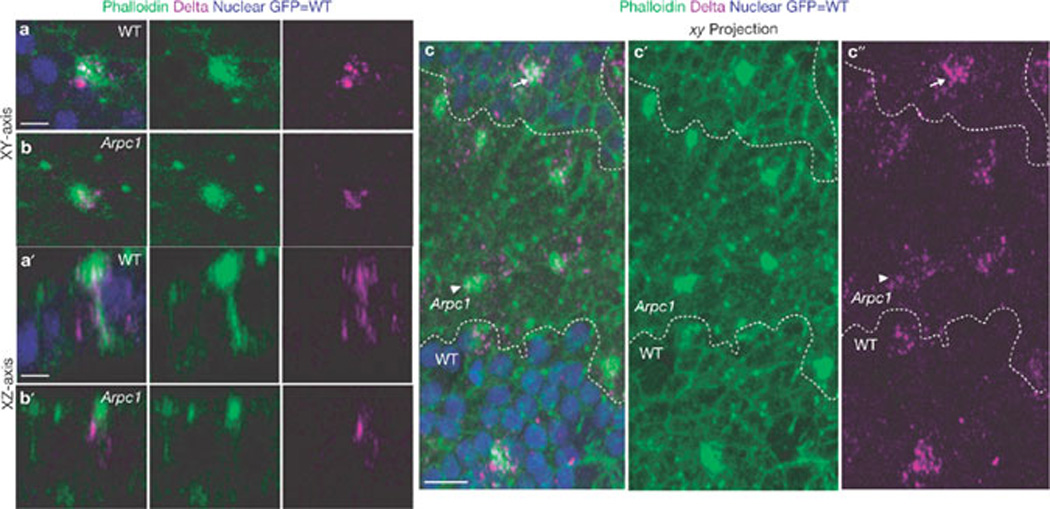
Delta localization to the ARS is reduced in Arpc1 mutants. (a–c´´) Pupal wing nota at the 2-cell SOP stage (18.30 h APF) were immunostained with phalloidin (green) and Delta (magenta). Arpc1 homozygous mutant cells are marked by the absence of GFP (blue). (a, b) A single section along the xy axis through pIIa-pIIb cells shows an enrichment of Delta on the ARS in wild-type (WT, a) and this enrichment is much reduced in Arpc1 (b). (a´, b´) A single section along the xy axis of pIIa-pIIb shows that the Delta vesicles colocalize along the lateral stalk of the ARS in WT and in the basal portion of the umbrella region of the ARS (a´). In Arpc1 (b´), the lateral stalk of the ARS is malformed and there is a reduction in the number of Delta vesicles that colocalize on the apical portion of the ARS. (c–c´´) A projection of confocal sections of a pupal notum harbouring an Arpc1 mutant clone (dashed line). In the WT region, a high density of Delta vesicles are clustered on and around the ARS, whereas in the mutant clones, the Delta vesicles are more widely distributed and do not cluster around the ARS; compare arrowheads (Arpc1) with arrows (WT). Scale bars, 5 µm (a, a´) and 10 µm (c).
Arp2/3 and WASp are required for trafficking of endocytosed Delta to the apical ARS
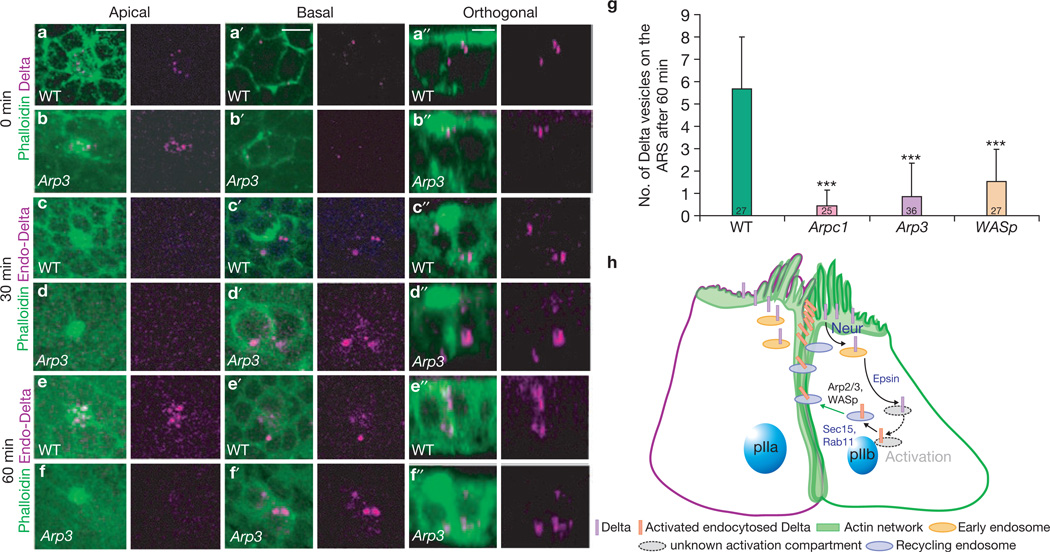
To investigate Delta trafficking in Arp2/3 and WASp mutants, we performed pulse-chase labelling experiments12 to monitor the internalization of Delta in living pupae. Internalization of Delta vesicles with respect to ARS was examined at three different time-points (0, 30 and 60 min). At 0 min Delta vesicles were present apically (~0.5 µm into the sample) and colocalized with ARS in wild-type (Fig. 8a, a´´), Arp3 (Fig. 8b, b´´), Arpc1 and WASp (data not shown) SOP progeny. At 30 min post-internalization, Delta vesicles were localized basally (~6 µm) in wild-type (Fig. 8c, c´´) and Arp3 (Fig. 8d, d´´) SOP progeny, indicating that the Delta vesicles had trafficked intracellularly at this time-point. However, 60 min after internalization, localization of Delta vesicles in mutants differed from the wild-type. In the wild-type, about 6–10 Delta-positive vesicles colocalized apically on the ARS (Fig. 8e, e ´´), suggesting that endocytosed Delta traffics back to the apical microvilli. In Arp3 (Fig. 8f, f´´), Arpc1 (Supplementary Information, Fig. S4a, a´´) and WASp (Supplementary Information, Fig. S4b, b´´) mutants, Delta vesicles were not localized apically on the ARS. Instead, they were found basally in the cytoplasm (~6 µm into the cell; Fig. 8f´, f´´; Supplementary Information, Fig. S4a´−b´´), suggesting a defect in Delta trafficking. Indeed, the number of Delta vesicles that traffic to the microvillar region of the ARS at 60 min post-chase was significantly lower in the Arpc1, Arp3 and WASp pIIa-pIIb than in wild-type cells (Fig. 8g). However, the total number of internalized Delta vesicles and the intensity of Delta signal in the SOP progeny at 60 min post-chase were very similar in wild-type and mutants (Supplementary Information, Fig. S4c, d). In summary, initially Delta is properly targeted apically at the ARS and endocytosed (Fig. 8a–b ´´). Delta traffics basally in both wild-type and mutants (Fig. 8c–d´´) 30 min after internalization. However, endocytosed Delta is not targeted back to the microvillar region in Arp3, Arpc1 and WASp SOP progeny 60 min post-chase.
Figure 8.
Arp2/3 and WASp are required for trafficking of endocytosed Delta to the apical ARS 1 h post-endocytosis. (a–f´´) A pulse-chase assay for the trafficking of endocytosed Delta (magenta) at different time-points with respect to the ARS (green) was performed in live pupal nota of wild-type (WT) and Arp3 mutants. Confocal images show apical (0.5 µm), basal (6 µm) and orthogonal sections of the pIIa-pIIb cells of the WT notum (a–a´´, c–c´´, e–e´´), and Arp3 mutant clones (b–b´´, d–d´´, f–f´´). The pulse-chase assays for three different time-points, 0 min, 30 min and 60 min, are shown. (g) Quantification of the number of internalized Delta vesicles that are present apically and colocalize with the ARS. Measurements of total number of Delta vesicles that traffic to the ARS 1 h after chase were analysed using a Student’s t-test (***P < 0.0001). Data are mean ± s.e.m. and the number of SOP progeny pairs quantified per genotype is indicated in the bars. Note that fewer vesicles that colocalize in mutants when compared with the WT control and the difference is statistically significant. (h) Proposed model. In the pIIb cell, Delta is endocytosed by Neuralized (Neur)6 and trafficked by Epsin11 to an endocytic compartment where it undergoes activation, probably by a proteolytic cleavage event. It is trafficked back to the membrane in a compartment positive for Rab11 (ref. 12) and the exocyst complex member Sec15 (ref. 13). Arp2/3 and WASp are required for the formation of branched actin networks to form the ‘stalk’ of the ARS and enables endocytosed vesicles containing activated Delta to traffic back to the dense actin-rich microvilli at the apical membrane of the pIIb cell, where it can signal. Scale bars, 5 µm.
It has been proposed that Delta must be endocytosed and targeted to a specific endosomal compartment to become activated11, possibly through Rab11-positive recycling endosomes12,13. By examining the distribution of the vesicular compartments, we found that the early endosome and the recycling endosome were enriched on the ARS (Supplementary Information, Fig. S4e–h´). Pulse-chase of endocytosed Delta through these compartments (Supplementary Information, Fig S5, Fig S6), showed no significant defects in the localization and abundance of these endosomal compartments or the ability of Delta to traffic through these endosomal compartments in Arpc1 mutant SOP progeny. The internalized Delta is thought to be proteolytically cleaved in an unknown compartment11. We found that Delta processing in Arp3 mutants is similar to that in the wild-type (Supplementary Information, Fig. S7).
In summary, we surmise that a defect in trafficking of endocytosed Delta to the apical microvillar portion of the ARS leads to a failure in Delta signalling. We conclude that this defect underlies the pIIa-to-pIIb fate transformation phenotype in Arp3, Arpc1 and WASp mutants.
DISCUSSION
Previous reports have suggested that trafficking of a subset of endocytosed Delta to the apical membrane in the pIIb cell is required for its ability to activate Notch in the pIIa cell12,13. We have uncovered a highly stereotyped ARS that consists of apical microvilli and a lateral ‘stalk’ region. In Arp2/3 and WASp pIIa-pIIb cells, the apical surface area of the ARS was significantly reduced and the number of microvilli on the apical region was also reduced. In addition, trafficking of endocytosed Delta to the apical microvilli-rich region of the ARS was severely impaired in Arp3 mutants. Although numerous studies have focused on the SOP daughter cells, the ARS and the microvilli have not been described previously. These microvillar structures are very different from filopodia46, which have been reported to have a role in lateral inhibition47 at an earlier stage. Our data indicate that apical trafficking of Delta to the ARS is required for its ability to signal.
Given the role of Arp2/3 in forming branched actin filaments, one of the primary roles of the Arp2/3 complex and WASp during Notch signalling is probably to form actin networks48, and to enable and/or to promote the trafficking of Delta vesicles to the ARS (Fig. 8h). This requirement for endocytosed Delta localization to the microvilli during Notch signalling is akin to findings showing that localization of Smoothened to primary cilia is important for its activation during Hedgehog (Hh) signal transduction49,50. An interesting study performed in circulating lymphocytes has demonstrated a crucial requirement for microvillar receptor presentation in leukocyte adhesion to the endothelial membrane41. In an analogous manner to findings in leukocytes, microvillar presentation of Delta might enhance its ability to contact Notch on the surface of the adjacent cell. As Notch signalling is a major contact-dependent signalling pathway, microvilli might therefore increase the surface area of contact between the signal-sending and receiving cells, enhancing the ability of the ligand to interact with the receptor.
On the basis of the well-characterized role for WASp and Arp2/3 in clathrin-mediated endocytosis32, it was speculated that Arp2/3 and WASp might be required for endocytosis of Delta and/or Notch during signalling51. However, our data indicate that the Arp2/3 complex is not required for Notch in the signal-receiving cell. Our data also indicate that the Arp2/3 complex is not required to endocytose Delta. It is possible that endocytosis of Delta occurs in a clathrin-independent manner52,53.
The involvement of WASp during Notch-mediated fate decisions might have implications for its mammalian homologue in Wiskott-Aldrich syndrome, an X-linked immunodeficiency54. Given that Notch signalling is required for proper T-cell development55 and differentiation of peripheral T-cells56, defects in Delta trafficking caused by WASp-mediated actin polymerization might underlie the loss and aberrant function of T cells in patients with Wiskott-Aldrich syndrome. Interestingly, microvilli on the surface of lymphocytes might also have a central role in receptor presentation in macrophages and T cells41,42. It will be interesting to investigate whether WASp has a role in Notch signalling during T-cell development and activation.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/naturecellbiology/
Drosophila genetics
Stocks used in this study were: 1) y w; FRT80B (isogenized), 2) y w Ubx–FLP; RpS174 Ubi–GFP.nls FRT80B/TM3 Ser, 3) y w hs-FLP; UAS–NECN(NEXT)/CyO; MKRS/TM2 (ref. 57), 4) y w; UAS-Arp3::GFP58, 5) w; Wsp3/TM6B Tb59, 6) Df(3R)3450/TM6B Tb, 7) y w; Arpc1Q25St FRT40A /CyO Kr-GAL4, UAS–GFP60, 8) y w Ubx–FLP; Ubi–GFP.nls FRT40A/CyO, 9) y w hs-FLP; RpS174 Ubi–GFP FRT80B/ TM6B Tb, 10) y hs-FLP tubα1–GAL4 UAS–GFP. nls-6xMyc; tub–GAL80 RpS174 FRT80B/TM6B Tb, 11) w*; UAS–CD2::HRP/ CyO (Bloomington Stock Center)61, 12) w1118; neurA101–GAL4 KgV/TM3 Sb1 (Bloomington Stock Center)62, 13) y w; numb2 ck FRT40A/CyO63, 14) y w ey-FLP; Adaear4 FRT40A/CyO y+ (ref. 64), 15) w; FRT82B neur1F65/TMB6B Tb65 16) y w; sca109–68-GAL4 (ref. 66).
Rescue experiments were performed using the MARCM technique. Flies of genotype y hs-FLP tubα1–GAL4 UAS–GFP.nls-6×.Myc; UAS–Arp3::GFP/+; tub–GAL80 RpS174 FRT80B/ Arp3515FC FRT80B were examined. The homozygous mutant bristles with longer and thicker appearance were differentiated from the short and thin RpS174 (Minute phenotype) bristles.
Epistasis analysis of Arp3 with the ligand-independent form of Notch57, NECN was performed by examining flies of the genotype y w Ubx–FLP; sca109–68-GAL4/ UAS-NECN; y+ w+ FRT80B/mwh Arp383F FRT80B. Arp3 follicle cell clones in egg chambers were generated by heat-shocking virgin females of genotype y w hs-FLP/+; FRT80B Arp3515FC/ RpS174 Ubi–GFP FRT80B for 90 min at 38 °C for 3 consecutive days. Ovaries of heat-shocked females were dissected after 2–3 days of mating on medium supplemented with yeast.
The wing-disc signal-sending cell assay was performed as described previously67,68 and flies of the genotype y w hs-FLP UAS–GFP.CD8; tub–GAL80 FRT40A/ Arpc1 FRT40A; tub–GAL4/ UAS-Dl were examined.
Immunohistochemistry
For conventional immunostaining, ovaries, wing discs from third instar larvae or pupal nota were dissected in PBS and fixed with 4% formaldehyde for 20 min. The samples were then permeabilized in PBS + 0.2% Triton X-100 (PBST) for 20 min and blocked with 5% normal donkey serum in PBST for 1 h. Samples were incubated with primary antibodies at 4 °C overnight. The following primary antibodies were used: chicken anti-GFP (1:2,000, Abcam), mouse anti-Cut (1:500; 2B10; Developmental Studies Hybridoma Bank, University of Iowa (DSHB))69, rat anti-ELAV (1:200; 7E8A10; DSHB)70, guinea pig anti-Sens (1:1,000; ref 71), mouse anti-DlECD (1:1,000; C594.9B; DSHB)72, guinea pig anti-Delta (1:3,000; M. Muskavitch and A. L. Parks)73, mouse anti-Fasciclin III (1:10; 7G10; DSHB)74, mouse anti-Hnt (1:10; 1G9; DSHB)75, Alexa Fluor 488- and 546-conjugated phalloidin (1 unit per reaction, Invitrogen), rabbit anti-Dlg (1:1,000; P. Bryant)76, rat anti-Myo1B (1:500; M.S. Mooseker)77. The following antibodies were used in the experiments included in the Supplementary Information: rabbit anti-Numb (1:1,000; Y. N. Jan)78, rabbit anti-Neuralized (1:600; E. C. Lai)65, rat anti-DE-Cadherin (1:1,000, DCAD2, DSHB)79, rabbit anti-Rab5 (1:200; M. González Gaitán)80, rabbit anti-Rab11 (1:1,000, D. F. Ready)81, guinea pig anti-Spinster/Benchwarmer (1:100; G. W. Davis)82, guinea pig anti-Hrs-FL (1:600; ref. 83).The samples were then incubated with Cy3- and/ or Cy5-conjugated affinity purified donkey secondary antibodies (1:500; Jackson ImmunoResearch Laboratories). Images were captured using an LSM510 confocal microscope and Leica TCS SP5 confocal microscope. Images were processed with Amira 5.0.1 and Adobe PhotoShop 7.0.
Transmission electron microscopy (TEM)
To identify the pIIa-pIIb cells, we used flies of the following genotype: UAS–CD2::HRP; neurA101-GAL4 (ref. 61). In this genotype the HRP-labelled cell membranes correspond to pIIa-pIIb at the 16–18 h APF time-point, as neurA101-GAL4 drives expression of the CD2::HRP in the SOP and its progeny. To identify the SOP progeny in Arp3 mutant clones for TEM analysis, we examined the flies with the genotype y w Ubx–FLP;UAS–-CD2::HRP; Arp3515FC FRT80B neurA101-GAL4/ arm-lacZ M(3) tub–GAL80 FRT80B in which the CD2::HRP is activated only in Arp3 mutant SOP progeny.
HRP label was visualized by TEM as described previously84 except for the following modifications: the pupal thorax was dissected at 18 h APF. After amplification and visualization of the HRP signal under a dissecting microscope, the tissues were fixed85 to preserve the actin filament structures. The tissues were then processed for TEM using microwave irradiation with PELCO BioWave equipped with PELCO Cold Spot and Vacuum System. Serial sections (60 nm) were cut and post-stained with Reynold’s lead citrate, and examined with a JEOL transmission electron microscope (JEOL 1010). The serial sections were labelled on the basis of their depth from the first electron micrograph that shows the most apical portion of HRP labelled SOP microvilli.
Immunoelectron microscopy of phalloidin
To label actin, the pupal thorax was dissected at 18 h APF, fixed in 1% glutaraldehyde in 0.1M PB pH 7.2 for 1.5 h, permeabilized in 0.1% Triton PBS for 5 min, labelled with biotin-XX phalloidin (3 units; Invitrogen) in PBS for 30–35 min. Samples were then incubated in streptavidin-HRP in TNT buffer (1:100; Sigma). To develop enzyme activity, we used a procedure described previously84.
Delta endocytosis and pulse-chase assay
The endocytosis and pulse-chase assays were modified from previous reports86,87. Pupae were partially dissected in Schneider’s medium at 18 h APF by making an incision along the dorsal side, and the internal tissues were washed out. The ‘empty’ pupal case was incubated with the supernatant of monoclonal antibody mouse anti-DeltaECD (1:10; C594.9B; DSHB)72 for 15–20 min on ice in Schneider’s medium supplemented with 25 µg ml−1 of 20-hydroxy-ecdysone (Sigma). The tissue was washed three times by medium changes. For the Delta pulse-chase assay the pupal cases were incubated at 25 °C for different time periods (0, 30 and 60 min) in Schneider’s medium supplemented with 5 µg ml−1 of 20-hydroxy-ecdysone. For the endocytosis assay, the pupal cases were incubated in pre-warmed Schneider’s medium supplemented with 5 µg ml−1 of 20-hydroxy-ecdysone at 34 °C in a water bath to inactivate the shibire gene in the negative control shits1. After incubation at 25 °C (pulse-chase assay) or 34 °C (endocytosis assay), the pupal cases were fixed for 20 min with 4% formaldehyde in Schneider’s medium supplemented with 5 µg ml−1 of 20-hydroxy-ecdysone. The normal immunostaining protocol was then followed.
The following antibodies were used in the experiments in the pulse-chase co-labelling experiments in the Supplementary Information: rabbit anti-Rab5 (1:200; M. González Gaitán)80, rabbit anti-Rab11 (1:1,000, D. F. Ready)81, guinea pig anti-Spinster/Benchwarmer (1:100; G. W. Davis)82, guinea pig anti-Hrs-FL (1:600; ref. 83).
Statistical analysis
Measurements of total number of Delta vesicles that traffic to the ARS 1 h after chase, and measurements of the total number of Delta vesicles endocytosed were analysed using a Student’s t-test (***P <0.0001. Measurements of the ARS area were quantified using the Measure function of the ImageJ software. The measurements were analysed using a Student’s t-test (***P <0.0001). For TEM, measurements of total number of microvilli in SOP and epithelial cells were quantified using ImageJ. The measurements were analysed using a Student’s t-test (P <0.05).
The measurement of Delta colocalization with Rab5 and Rab11 as well as the determination of Delta, Rab11 and Rab5 signal intensities were quantified using the labelvoxel and materialstatistics functions in Amira 5.0.1. The measurements were analysed using a Student’s t-test (*P =0.01).
Western blotting
For the Delta western blots, 50 embryos of the appropriate genotypes were collected at 0–13 h AEL and 13–19 hAEL and lysed in ice-cold filtered RIPA buffer (150 mM NaCl, 1.0% NP-40, 0.5% deoxycholic acid, 0.1% SDS, and 50 mM Tris, pH 8.0) with complete protease inhibitor cocktail (Roche). Lysates were suspended in equal volume of 3× Laemmli sample buffer in the absence of reducing agents, and proteins were resolved by SDS–PAGE. Delta was detected on a western blot using anti-Delta (mAb C594.9B) ascites fluid at 1:10,000. HRP-conjugated goat anti-mouse IgG (Jackson ImmunoResearch) was used at 1:10,000 and the blots were developed using Western Lightning chemilu-minescent substrate (PerkinElmer LAS).
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to W. Theurkauf, L. Cooley, E. Schejter, J. Skeath, D. F. Ready, P. Badenhorst, Y. N. Jan, P. Bryant, M. González Gaitán, G. Struhl, E. C. Lai, M. Muskavitch, A. L. Parks, F. B. Gertler, L. M. Lanier, J. Knoblich, F. Schweisguth, R. Dubreuil, W. Sullivan, M. S. Mooseker, G.M. Guild, the Bloomington Stock Center and the Developmental Studies Hybridoma Bank for reagents. We thank G. Emery for advice regarding the Delta endocytosis assay. We would like to thank H. Jafar-Nejad for suggestions and advice during the screen and comments on the manuscript. We thank P. Verstreken and C. V. Ly for their help with the screen, and R. Atkinson for advice on imaging. Confocal microscopy was supported by the BCM Mental Retardation and Developmental Disabilities Research Center. H.J.B. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Note: Supplementary Information is available on the Nature Cell Biology website.
AUTHOR CONTRIBUTIONS
A.R., A.T. and H.B. conceived the project. A.R. and A.T. carried out the screen, mapped the genes and executed the project. K.S. was involved in the screen and mapping of the genes. C.M.H. in collaboration with A.R. and A.T. designed the TEM experiments and C.M.H. carried out the TEM experiments.
COMPETING INTERESTS
The authors declare that they have no competing financial interest.
References
- 1.Tien AC, Rajan A, Bellen HJ. A Notch updated. J. Cell Biol. 2009;184(5):621–629. doi: 10.1083/jcb.200811141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray SJ. Notch signalling: a simple pathway becomes complex. Nature Rev. Mol. Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 3.Schweisguth F. Regulation of Notch signaling activity. Curr. Biol. 2004;14:R129–R138. [PubMed] [Google Scholar]
- 4.Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nature Rev. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- 5.Bray S. Notch signalling in Drosophila: three ways to use a pathway. Sem. Cell Dev. Biol. 1998;9:591–597. doi: 10.1006/scdb.1998.0262. [DOI] [PubMed] [Google Scholar]
- 6.Le Borgne R, Schweisguth F. Unequal segregation of Neuralized biases Notch activation during asymmetric cell division. Dev. Cell. 2003;5:139–148. doi: 10.1016/s1534-5807(03)00187-4. [DOI] [PubMed] [Google Scholar]
- 7.Rhyu MS, Jan LY, Jan YN. Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell. 1994;76:477–491. doi: 10.1016/0092-8674(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 8.Gho M, Bellaiche Y, Schweisguth F. Revisiting the Drosophila microchaete lineage: a novel intrinsically asymmetric cell division generates a glial cell. Development. 1999;126:3573–3584. doi: 10.1242/dev.126.16.3573. [DOI] [PubMed] [Google Scholar]
- 9.Zeng C, Younger-Shepherd S, Jan LY, Jan YN. Delta and Serrate are redundant Notch ligands required for asymmetric cell divisions within the Drosophila sensory organ lineage. Genes Dev. 1998;12:1086–1091. doi: 10.1101/gad.12.8.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parks AL, Klueg KM, Stout JR, Muskavitch MA. Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development. 2000;127:1373–1385. doi: 10.1242/dev.127.7.1373. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Struhl G. Drosophila Epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development. 2004;131:5367–5380. doi: 10.1242/dev.01413. [DOI] [PubMed] [Google Scholar]
- 12.Emery G, et al. Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell. 2005;122:763–773. doi: 10.1016/j.cell.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Jafar-Nejad H, et al. Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev. Cell. 2005;9:351–363. doi: 10.1016/j.devcel.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Hartenstein V, Posakony JW. A dual function of the Notch gene in Drosophila sensillum development. Dev. Biol. 1990;142:13–30. doi: 10.1016/0012-1606(90)90147-b. [DOI] [PubMed] [Google Scholar]
- 15.Berdnik D, Torok T, Gonzalez-Gaitan M, Knoblich JA. The endocytic protein α-Adaptin is required for numb-mediated asymmetric cell division in Drosophila. Dev. Cell. 2002;3:221–231. doi: 10.1016/s1534-5807(02)00215-0. [DOI] [PubMed] [Google Scholar]
- 16.Tien AC, et al. Ero1L, a thiol oxidase, is required for Notch signaling through cysteine bridge formation of the Lin12-Notch repeats in Drosophila melanogaster. J. Cell Biol. 2008;182:1113–1125. doi: 10.1083/jcb.200805001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhai RG, et al. Mapping Drosophila mutations with molecularly defined P element insertions. Proc. Natl Acad. Sci. USA. 2003;100:10860–10865. doi: 10.1073/pnas.1832753100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hudson AM, Cooley L. A subset of dynamic actin rearrangements in Drosophila requires the Arp2/3 complex. J. Cell Biol. 2002;156:677–687. doi: 10.1083/jcb.200109065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machesky LM, Gould KL. The Arp2/3 complex: a multifunctional actin organizer. Curr. Opin. Cell Biol. 1999;11:117–121. doi: 10.1016/s0955-0674(99)80014-3. [DOI] [PubMed] [Google Scholar]
- 20.Tal T, Vaizel-Ohayon D, Schejter ED. Conserved interactions with cytoskeletal but not signaling elements are an essential aspect of Drosophila WASp function. Dev. Biol. 2002;243:260–271. doi: 10.1006/dbio.2002.0571. [DOI] [PubMed] [Google Scholar]
- 21.Machesky LM, Insall RH. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr. Biol. 1998;8:1347–1356. doi: 10.1016/s0960-9822(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Yaacov S, Le Borgne R, Abramson I, Schweisguth F, Schejter ED. Wasp, the Drosophila Wiskott-Aldrich syndrome gene homologue, is required for cell fate decisions mediated by Notch signaling. J. Cell Biol. 2001;152:1–13. doi: 10.1083/jcb.152.1.1-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Struhl G, Fitzgerald K, Greenwald I. Intrinsic activity of the Lin-12 and Notch intracellular domains in vivo. Cell. 1993;74:331–345. doi: 10.1016/0092-8674(93)90424-o. [DOI] [PubMed] [Google Scholar]
- 24.Ruohola H, et al. Role of neurogenic genes in establishment of follicle cell fate and oocyte polarity during oogenesis in Drosophila. Cell. 1991;66:433–449. doi: 10.1016/0092-8674(81)90008-8. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Schier H, St Johnston D. Delta signaling from the germ line controls the proliferation and differentiation of the somatic follicle cells during Drosophila oogenesis. Genes Dev. 2001;15:1393–1405. doi: 10.1101/gad.200901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Assa-Kunik E, Torres IL, Schejter ED, Johnston DS, Shilo BZ. Drosophila follicle cells are patterned by multiple levels of Notch signaling and antagonism between the Notch and JAK/STAT pathways. Development. 2007;134:1161–1169. doi: 10.1242/dev.02800. [DOI] [PubMed] [Google Scholar]
- 27.Sun J, Deng WM. Hindsight mediates the role of notch in suppressing hedgehog signaling and cell proliferation. Dev. Cell. 2007;12:431–442. doi: 10.1016/j.devcel.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Celis JF, Garcia-Bellido A, Bray SJ. Activation and function of Notch at the dorsal-ventral boundary of the wing imaginal disc. Development. 1996;122:359–369. doi: 10.1242/dev.122.1.359. [DOI] [PubMed] [Google Scholar]
- 29.de Celis JF, Bray S. Feed-back mechanisms affecting Notch activation at the dor-soventral boundary in the Drosophila wing. Development. 1997;124:3241–3251. doi: 10.1242/dev.124.17.3241. [DOI] [PubMed] [Google Scholar]
- 30.Micchelli CA, Rulifson EJ, Blair SS. The function and regulation of cut expression on the wing margin of Drosophila: Notch, Wingless and a dominant negative role for Delta and Serrate. Development. 1997;124:1485–1495. doi: 10.1242/dev.124.8.1485. [DOI] [PubMed] [Google Scholar]
- 31.Seugnet L, Simpson P, Haenlin M. Requirement for dynamin during Notch signaling in Drosophila neurogenesis. Dev. Biol. 1997;192:585–598. doi: 10.1006/dbio.1997.8723. [DOI] [PubMed] [Google Scholar]
- 32.Kaksonen M, Toret CP, Drubin DG. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell. 2005;123:305–320. doi: 10.1016/j.cell.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 33.Galletta BJ, Chuang DY, Cooper JA. Distinct roles for Arp2/3 regulators in actin assembly and endocytosis. PLoS Biol. 2008;6:e1. doi: 10.1371/journal.pbio.0060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen MS, et al. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature. 1991;351:583–586. doi: 10.1038/351583a0. [DOI] [PubMed] [Google Scholar]
- 35.van der Bliek AM, Meyerowitz EM. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature. 1991;351:411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]
- 36.Le Borgne R, Bellaiche Y, Schweisguth F. Drosophila E-cadherin regulates the orientation of asymmetric cell division in the sensory organ lineage. Curr. Biol. 2002;12:95–104. doi: 10.1016/s0960-9822(01)00648-0. [DOI] [PubMed] [Google Scholar]
- 37.Lai EC, Rubin GM. neuralized functions cell-autonomously to regulate a subset of notch-dependent processes during adult Drosophila development. Dev. Biol. 2001;231:217–233. doi: 10.1006/dbio.2000.0124. [DOI] [PubMed] [Google Scholar]
- 38.Maupin P, Pollard TD. Improved preservation and staining of HeLa cell actin filaments, clathrin-coated membranes, and other cytoplasmic structures by tannic acid-glutaraldehyde-saponin fixation. J. Cell Biol. 1983;96:51–62. doi: 10.1083/jcb.96.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heintzelman MB, Mooseker MS. Assembly of the intestinal brush border cytoskel-eton. Curr. Top. Dev. Biol. 1992;26:93–122. doi: 10.1016/s0070-2153(08)60442-1. [DOI] [PubMed] [Google Scholar]
- 40.Majstoravich S, et al. Lymphocyte microvilli are dynamic, actin-dependent structures that do not require Wiskott-Aldrich syndrome protein (WASp) for their morphology. Blood. 2004;104:1396–1403. doi: 10.1182/blood-2004-02-0437. [DOI] [PubMed] [Google Scholar]
- 41.von Andrian UH, Hasslen SR, Nelson RD, Erlandsen SL, Butcher EC. A central role for microvillous receptor presentation in leukocyte adhesion under flow. Cell. 1995;82:989–999. doi: 10.1016/0092-8674(95)90278-3. [DOI] [PubMed] [Google Scholar]
- 42.Singer II, et al. CCR5, CXCR4, and CD4 are clustered and closely apposed on microvilli of human macrophages and T cells. J. Virol. 2001;75:3779–3790. doi: 10.1128/JVI.75.8.3779-3790.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgan NS, Heintzelman MB, Mooseker MS. Characterization of myosin-IA and myosin-IB, two unconventional myosins associated with the Drosophila brush border cytoskeleton. Dev. Biol. 1995;172:51–71. doi: 10.1006/dbio.1995.0005. [DOI] [PubMed] [Google Scholar]
- 44.Le Borgne R. Regulation of Notch signalling by endocytosis and endosomal sorting. Curr. Opin. Cell Biol. 2006;18:213–222. doi: 10.1016/j.ceb.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 45.Emery G, Knoblich JA. Endosome dynamics during development. Curr. Opin. Cell Biol. 2006;18:407–415. doi: 10.1016/j.ceb.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 46.Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nature Cell Biol. 2007;9:1110–1121. doi: 10.1038/ncb1007-1110. [DOI] [PubMed] [Google Scholar]
- 47.De Joussineau C, et al. Delta-promoted filopodia mediate long-range lateral inhibition in Drosophila. Nature. 2003;426:555–559. doi: 10.1038/nature02157. [DOI] [PubMed] [Google Scholar]
- 48.Mullins RD, Heuser JA, Pollard TD. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl Acad. Sci. USA. 1998;95:6181–6186. doi: 10.1073/pnas.95.11.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 50.Kiprilov EN, et al. Human embryonic stem cells in culture possess primary cilia with hedgehog signaling machinery. J. Cell Biol. 2008;180:897–904. doi: 10.1083/jcb.200706028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ilagan MX, Kopan R. SnapShot: notch signaling pathway. Cell. 2007;128:1246. doi: 10.1016/j.cell.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 52.Chen H, De Camilli P. The association of epsin with ubiquitinated cargo along the endocytic pathway is negatively regulated by its interaction with clathrin. Proc. Natl Acad. Sci. USA. 2005;102:2766–2771. doi: 10.1073/pnas.0409719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sigismund S, et al. Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl Acad. Sci. USA. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Imai K, Nonoyama S, Ochs HD. WASP (Wiskott-Aldrich syndrome protein) gene mutations and phenotype. Curr. Opin. Allergy Clin. Immunol. 2003;3:427–436. doi: 10.1097/00130832-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Tanigaki K, Honjo T. Regulation of lymphocyte development by Notch signaling. Nature Immunol. 2007;8:451–456. doi: 10.1038/ni1453. [DOI] [PubMed] [Google Scholar]
- 56.Osborne BA, Minter LM. Notch signalling during peripheral T-cell activation and differentiation. Nature Rev. Immunol. 2007;7:64–75. doi: 10.1038/nri1998. [DOI] [PubMed] [Google Scholar]
- 57.Struhl G, Greenwald I. Presenilin-mediated transmembrane cleavage is required for Notch signal transduction in Drosophila. Proc. Natl Acad. Sci.USA. 2001;98:229–234. doi: 10.1073/pnas.011530298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hudson AM, Cooley L. A subset of dynamic actin rearrangements in Drosophila requires the Arp2/3 complex. J. Cell Biol. 2002;156:677–687. doi: 10.1083/jcb.200109065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ben-Yaacov S, Le Borgne R, Abramson I, Schweisguth F, Schejter ED. Wasp, the Drosophila Wiskott-Aldrich syndrome gene homologue, is required for cell fate decisions mediated by Notch signaling. J. Cell Biol. 2001;152:1–13. doi: 10.1083/jcb.152.1.1-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zallen JA, et al. SCAR is a primary regulator of Arp2/3-dependent morphological events in Drosophila. J. Cell Biol. 2002;156:689–701. doi: 10.1083/jcb.200109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watts RJ, Schuldiner O, Perrino J, Larsen C, Luo L. Glia engulf degenerating axons during developmental axon pruning. Curr. Biol. 2004;14:678–684. doi: 10.1016/j.cub.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 62.Lai EC, Rubin GM. neuralized functions cell-autonomously to regulate a subset of notch-dependent processes during adult Drosophila development. Dev. Biol. 2001;231:217–233. doi: 10.1006/dbio.2000.0124. [DOI] [PubMed] [Google Scholar]
- 63.Frise E, Knoblich JA, Younger-Shepherd S, Jan LY, Jan YN. The Drosophila Numb protein inhibits signaling of the Notch receptor during cell-cell interaction in sensory organ lineage. Proc. Natl Acad. Sci. USA. 1996;93:11925–11932. doi: 10.1073/pnas.93.21.11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berdnik D, Torok T, Gonzalez-Gaitan M, Knoblich JA. The endocytic protein α-Adaptin is required for numb-mediated asymmetric cell division in Drosophila. Dev. Cell. 2002;3:221–231. doi: 10.1016/s1534-5807(02)00215-0. [DOI] [PubMed] [Google Scholar]
- 65.Lai EC, Deblandre GA, Kintner C, Rubin GM. Drosophila neuralized is a ubiquitin ligase that promotes the internalization and degradation of Delta. Dev. Cell. 2001;1:783–794. doi: 10.1016/s1534-5807(01)00092-2. [DOI] [PubMed] [Google Scholar]
- 66.Manning L, Doe CQ. Prospero distinguishes sibling cell fate without asymmetric localization in the Drosophila adult external sense organ lineage. Development. 1999;126:2063–2071. doi: 10.1242/dev.126.10.2063. [DOI] [PubMed] [Google Scholar]
- 67.Wang W, Struhl G. Drosophila Epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development. 2004;131:5367–5380. doi: 10.1242/dev.01413. [DOI] [PubMed] [Google Scholar]
- 68.Tien AC, et al. Ero1L, a thiol oxidase, is required for Notch signaling through cysteine bridge formation of the Lin12-Notch repeats in Drosophila melanogaster. J. Cell Biol. 2008;182:1113–1125. doi: 10.1083/jcb.200805001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blochlinger K, Bodmer R, Jan LY, Jan YN. Patterns of expression of cut, a protein required for external sensory organ development in wild-type and cut mutant Drosophila embryos. Genes Dev. 1990;4:1322–1331. doi: 10.1101/gad.4.8.1322. [DOI] [PubMed] [Google Scholar]
- 70.Robinow S, White K. Characterization and spatial distribution of the ELAV protein during Drosophila melanogaster development. J. Neurobiol. 1991;22:443–461. doi: 10.1002/neu.480220503. [DOI] [PubMed] [Google Scholar]
- 71.Nolo R, Abbott LA, Bellen HJ. Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell. 2000;102:349–362. doi: 10.1016/s0092-8674(00)00040-4. [DOI] [PubMed] [Google Scholar]
- 72.Fehon RG, et al. Molecular interactions between the protein products of the neu-rogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell. 1990;61:523–534. doi: 10.1016/0092-8674(90)90534-l. [DOI] [PubMed] [Google Scholar]
- 73.Parks AL, Klueg KM, Stout JR, Muskavitch MA. Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development. 2000;127:1373–1385. doi: 10.1242/dev.127.7.1373. [DOI] [PubMed] [Google Scholar]
- 74.Patel NH, Snow PM, Goodman CS. Characterization and cloning of fasciclin III: a glycoprotein expressed on a subset of neurons and axon pathways in Drosophila. Cell. 1987;48:975–988. doi: 10.1016/0092-8674(87)90706-9. [DOI] [PubMed] [Google Scholar]
- 75.Yip ML, Lamka ML, Lipshitz HD. Control of germ-band retraction in Drosophila by the zinc-finger protein HINDSIGHT. Development. 1997;124:2129–2141. doi: 10.1242/dev.124.11.2129. [DOI] [PubMed] [Google Scholar]
- 76.Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- 77.Morgan NS, Heintzelman MB, Mooseker MS. Characterization of myosin-IA and myosin-IB, two unconventional myosins associated with the Drosophila brush border cytoskeleton. Dev. Biol. 1995;172:51–71. doi: 10.1006/dbio.1995.0005. [DOI] [PubMed] [Google Scholar]
- 78.Rhyu MS, Jan LY, Jan YN. Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell. 1994;76:477–491. doi: 10.1016/0092-8674(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 79.Oda H, Uemura T, Harada Y, Iwai Y, Takeichi M. A Drosophila homolog of cadherin associated with armadillo and essential for embryonic cell-cell adhesion. Dev. Biol. 1994;165:716–726. doi: 10.1006/dbio.1994.1287. [DOI] [PubMed] [Google Scholar]
- 80.Wucherpfennig T, Wilsch-Brauninger M, Gonzalez-Gaitan M. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J. Cell Biol. 2003;161:609–624. doi: 10.1083/jcb.200211087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dollar G, Struckhoff E, Michaud J, Cohen RS. Rab11 polarization of the Drosophila oocyte: a novel link between membrane trafficking, microtubule organization, and oskar mRNA localization and translation. Development. 2002;129:517–526. doi: 10.1242/dev.129.2.517. [DOI] [PubMed] [Google Scholar]
- 82.Sweeney ST, Davis GW. Unrestricted synaptic growth in spinster-a late endosomal protein implicated in TGF-β-mediated synaptic growth regulation. Neuron. 2002;36:403–416. doi: 10.1016/s0896-6273(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 83.Lloyd TE, et al. Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell. 2002;108:261–269. doi: 10.1016/s0092-8674(02)00611-6. [DOI] [PubMed] [Google Scholar]
- 84.Larsen CW, Hirst E, Alexandre C, Vincent JP. Segment boundary formation in Drosophila embryos. Development. 2003;130:5625–5635. doi: 10.1242/dev.00867. [DOI] [PubMed] [Google Scholar]
- 85.Maupin P, Pollard TD. Improved preservation and staining of HeLa cell actin filaments, clathrin-coated membranes, and other cytoplasmic structures by tannic acid-glutaraldehyde-saponin fixation. J. Cell Biol. 1983;96:51–62. doi: 10.1083/jcb.96.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Le Borgne R, Schweisguth F. Unequal segregation of Neuralized biases Notch activation during asymmetric cell division. Dev. Cell. 2003;5:139–148. doi: 10.1016/s1534-5807(03)00187-4. [DOI] [PubMed] [Google Scholar]
- 87.Emery G, et al. Asymmetric Rab 11 endosomes regulate Delta recycling and specify cell fate in the Drosophila nervous system. Cell. 2005;122:763–773. doi: 10.1016/j.cell.2005.08.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.