Abstract
The dimetallic endohedral heterofullerene (EHF), Gd2@C79N, was prepared and isolated in a relatively high yield when compared with the earlier reported heterofullerene, Y2@C79N. Computational (DFT), chemical reactivity, Raman, and electrochemical studies all suggest that the purified Gd2@C79N, with the heterofullerene cage, (C79N)5- has comparable stability with other better known isoelectronic metallofullerene (C80)6- cage species (e.g., Gd3N@C80). These results describe an exceptionally stable paramagnetic molecule with low chemical reactivity with the unpaired electron spin density localized on the internal diatomic gadolinium cluster and not on the heterofullerene cage. EPR studies confirm that the spin state of Gd2@C79N is characterized by a half-integer spin quantum number of S = 15/2. The spin (S = 1/2) on the N atom of the fullerene cage and two octet spins (S = 7/2) of two encapsulated gadoliniums are coupled with each other in a ferromagnetic manner with a small zero-field splitting parameter D. Because the central line of Gd2@C79N is due to the Kramer's doublet with a half-integer spin quantum number of S = 15/2, this relatively sharp line is prominent and the anisotropic nature of the line is weak. Interestingly, in contrast with most Gd3+ ion environments, the central EPR line (g=1.978) is observable even at room temperature in a toluene solution. Finally, we report the first EHF derivative, a diethyl bromomalonate monoadduct of Gd2@C79N, was prepared and isolated via a modified Bingel-Hirsch reaction.
Introduction
Shortly after the discovery and macroscopic production of fullerenes, the idea of modifying the all-carbon sp2 hybridized carbon surface of fullerenes has been an intriguing area of fullerene research. For example, fullerenes with icosahedral symmetry cages (e.g., C60 and C80) that have single atom single atom replacement (doping) with a trivalent heteroatom (N or B) leads to nearly spheroidal heterofullerene radicals which could have numerous applications.1-3 Wudl and coworkers first reported the preparation of the unstable aza(60) fullerene (C59N), which is easily dimerized as the corresponding dimer species (C59N)2.4
For the larger C80 cage system, it is well recognized that trimetallic nitride template (TNT) endohedral metallofullerenes (EMFs), such as, M3N@C80 and M2@C80 (M = metals) have highly stabilized icosahedral carbon (C80)6- cages. The stability of the aza(C80) fullerene (C79N) cage was first recognized by Akasaka and coworkers who reported evidence for (La2@C79N)+ by mass spectrometry.5 Other workers have reported computational studies supporting the stability of related heterofullerenes Sc3N@C79N, Sc3N@C79B, and Sc3N@C78BN.6 More recently, our laboratory has reported the isolation and characterization of the paramagnetic dimetallic endohedral heterofullerenes (EHFs), Y2@C79N and Tb2@C79N.7 The EPR studies of Y2@C79N indicate that the unpaired spin density is mainly localized between the two equivalent yttrium ions and not on the heterofullerene cage.7 Also, the trimetallic nitride cluster (La3N)6+ has been reported by Stevenson and coworkers in the same C79N cage, namely, La3N@C79N.8 Surprisingly, it was found that the large La3N cluster was preferentially encapsulated in a C79N cage, but not in a C80 cage (e.g., La2@C80).8 However, most studies of EHFs are based on computational studies because experimental isolation and detailed characterization is extremely difficult because of the small amount of sample usually available and the difficulty in the separation and characterization of EHFs.
Gadolinium based endohedral metallofullerenes (e.g., Gd@C82, Gd3N@C80) represent an important class of new nanomaterials since it is well recognized that these have important magnetic properties and are actively being explored as next generation magnetic resonance imaging (MRI) contrast agents.9-16 Herein, we report the preparation, purification, and spectroscopic studies of the first gadolinium based EHF, namely, Gd2@C79N. All theoretical and experimental studies confirm that this unique EHF displays an unusual high stability with a impressive yield compared with other EHFs reported to date.7,8 The magnetic properties for this paramagnetic molecule are also very interesting since the seven unpaired f electrons for each Gd3+ ion within the cage can interact with an additional unpaired electron formally provided by the nitrogen atom on the heterofullerene C79N cage. Additionally, the first successful functionalized gadolinium based EHF is also reported and isolated.
Experimental and Computational Methods
Preparation and Separation of Gd2@C79N
The preparation of Gd2@C79N was accomplished utilizing a Krätschmer-Huffman (K-H) generator as described previously.7,17 Specifically, soot containing Gd2@C79N and other fullerenes and endofullerenes were synthesized in a K-H generator by vaporizing composite graphite rods containing a mixture of gadolinium oxide, Gd2O3, graphite powder, and metallic Cu with a weight ratio of 2:1.0:2.1 in a dynamic flow of He and N2 (flow rate ratio of N2/He2=3:100). The resulting soot was then extracted with refluxing toluene in a Soxhlet extractor and the soluble extract was applied to a cyclopentadiene-functionalized Merrifield peptide resin (CPDE-MPR) column.18 As previously reported, most of the empty cages fullerenes and more reactive classical endofullerenes are retained on the CPDE-MPR column. There are seven distinct fractions obtained utilizing a pentabromobenzyl (PBB) HPLC column in similar fashion to results reported previously for other metal ions as shown in Figure 1. In order to estimate the overall yield, the relative yield ratio of Gd2@C79N/C60 is ∼0.05-0.1% using the current preparation and separation protocol; that is, for every gram of C60 formed we can isolate 0.5-1 mg of Gd2@C79N. Although this yield is relatively low, it is still ∼10 times greater than achieved for Y2@C79N (see Figure 1).
Figure 1.

HPLC Chromatography of soot extracts containing Gd1 (top) and Y1 fractions (bottom) utilizing a PBB column; λ=390 nm; flow rate 2.0 mL/min; toluene as eluent; 25 °C; upper right insert HPLC chromatographic Gd2@C79N and Y2@C79N separation with a PYE column; λ=390 nm; flow rate 2.0 mL/min; toluene as eluent; 25 °C
Synthesis of the diethyl bromomalonate monoadduct of Gd2@C79N
Approximately 100 μg of Gd2@C79N was dissolved in 2 mL toluene, and ∼20 equivalent of diethyl bromomalonate (Aldrich Chemical Co.) and ∼10 equivalents of 1,8-Diazabicycloundec-7-ene (DBU, Aldrich Chemical Co.) was added for the synthetic preparation and reactivity comparison study, respectively. Finally, a drop of DMF was added with syringe as a catalyst. For the synthetic reaction mixture and the comparison study the reaction mixtures were degassed and subsequently stirred at room temperature for 40 and 60 minutes, respectively. The product was isolated by HPLC.
Characterization
Mass spectrometry was performed on a Kratos Analytical Kompact SEQ LD-TOF mass spectrometer. Cyclic voltammetric measurements were conducted using a CH Instruments model 600A potentiostat (Austin, TX) and a single compartment microelectrochemical cell. A 2 mm glassy carbon disk electrode along with a Pt auxiliary was applied as working electrode using Ag/AgCl as a reference. Measurements were performed using o-dichlorobenzene solutions containing 0.100 M tetra-n-butylamonium tetrafluoroborate. Potentials were reported relative to the reversible ferrocene oxidation couple. The x-band EPR spectrum of Gd2@C79N at 4K was obtained by using a Bruker E500 spectrometer. The w-band EPR spectrum of Gd2@C79N at 30K was obtained by using a Bruker E680 spectrometer. Other EPR spectra were recorded with a Bruker D200 ER IBM-Bruker spectrometer.
Computational Study
Density functional theory (DFT) computations were performed using the spin-unrestricted B3LYP functional as provided in the Gaussian 03 program package.19 All of the molecules were geometry optimized at the UB3LYP level with a 6-31G* basis set for carbon and nitrogen atoms and the CEP-121G basis set for gadolinium atoms. DFT-optimized energy values were obtained starting from the X-ray crystallographic structures of the corresponding Tb2@C79N.7 The DFT computations employed the PBE0 functional20 as described in the Gaussian 0919 program package were utilized for Gd2@C79N in an effort to properly treat the unpaired electrons on the gadolinium atoms, while the B3LYP functional set21-23 was used for the empty cage calculations. The 6-31G basis set24 was used for all carbon and nitrogen atoms in all computations.
Results and Discussions
Preparation and separation of Gd2@C79N
A sample of Gd2@C79N was synthesized by utilizing a Krätschmer-Huffman (K-H) generator by vaporizing graphite rods containing a mixture of Gd2O3 and graphite power and Cu as a catalyst. The toluene extract from the raw soot was applied to a cyclopentadiene-functionalized Merrifield peptide resin (CPDE-MPR). The eluent was further separated by two-stage HPLC.17 The first stage was carried out on a 5PBB column and there are seven distinct fractions obtained utilizing PBB HPLC column in similar fashion to results reported previously for other metal ions as shown in Figure 1.17 The first fraction Gd1 obtained from the PBB column contains C84 and Gd2@C79N, which was further purified by a 5PYE column as illustrated in the top inset of Figure 1. An unusual feature of the preparation and separation process is the significantly higher yield of Gd2@C79N in comparison with the previously reported Y2@C79N as illustrated in Figure 1. The higher yield at this stage in the separation is either due to a higher inherent yield in the K-H generator and/or lower chemical Diels-Alder reactivity of Gd2@C79N in comparison with Y2@C79N on the CPDRE-MPR column and this point is discussed vita infra.
The purity of Gd2@C79N was confirmed by laser-desorption time-of-light (LD-TOF) mass spectrometry. The HPLC chromatogram, LD-TOF mass spectrum, and the UV-vis absorption spectrum of the purified Gd2@C79N is shown in Figure 2. When compared with Y2@C79N and Tb2@C79N, the similarity of UV-vis spectra and chromatographic retention behavior of Gd2@C79N indicate a close correspondence of these cage structures (See SI and Figure 3). 7
Figure 2.

(a) HPLC Chromatography of Gd2@C79N (a 10 × 250 mm 5PYE column; λ=390 nm; flow rate 2.0 mL/min; toluene as eluent; 25 °C); (b) positive ionization LD-TOF mass spectra of Gd2@C79N, Inset: theoretical and experimental isotopic distribution of Gd2@C79N comparision; (C) Uv-vis spectrum of Gd2@C79N in toluene.
Figure 3.
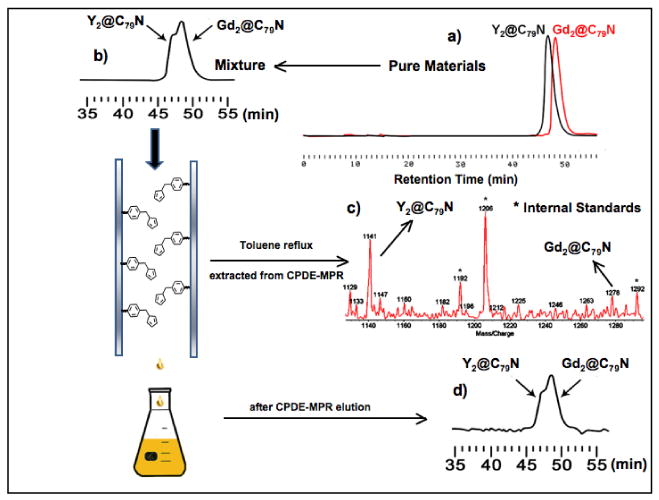
(a) HPLC Chromatography of pure Gd2@C79N and Y2@C79N samples (a) and the mixture (b) before and after separation and reaction on a CPDE-MPR column (a 10 × 250 mm 5PYE column; λ=390 nm; flow rate 2.0 mL/min; toluene as eluent; 25 °C); (c) Negative ion ionization LD-TOF mass spectra of recovered Gd2@C79N and Y2@C79N mixture from CPDE-MPR column with internal standard C23H15O6N3P3F36.18 (d) HPLC Chromatography of recovered Gd2@C79N and Y2@C79N mixture.
Comparative Diels-Alder Reactivity Study of Gd2@C79N and Y2@C79N
Although the enhanced stability of the icosahedral (C80)6- cage is well documented, the stability of the isolectronic (C79N)5- cage is not as well recognized. As noted above, a surprising feature during the purification of Gd2@C79N was the significantly higher yield of Gd2@C79N obtained in the separation process in comparison with Y2@C79N (Figure 1). This enhanced yield is either due to a higher inherent yield in the K-H electric-arc generator process and/or lower chemical Diels-Alder reactivity of Gd2@C79N in comparison with Y2@C79N during the chemical CPDE-MPR separation process. To test between these alternatives, we prepared a two component mixture (in toluene) containing nearly equal quantities of pure Gd2@C79N and Y2@C79N samples and the overall process is outlined in Figure 3. As expected, these two heterofullerenes have very similar chromatographic retention times and are only slightly resolved on a PYE column. A two component mixture of Gd2@C79N and Y2@C79N was applied a second time to the cyclopentadiene-functionalized Merrifield peptide resin (CPDE-MPR) column as summarized in Figure 3. Greater than 70 % of the mixture was recovered from the (CPDE-MPR) column and the resulting mixture exhibits a slight enhancement (∼5-10 %) in the concentration of the Gd2@C79N species. This preferential reaction of the Y2@C79N species was confirmed by heating the recovered cyclopentadiene-functionalized Merrifield peptide resin under reflux conditions in toluene for 12 hrs. The resulting toluene solution was concentrated and mass spectral analysis on the recovered sample indicates a preponderance of the Y2@C79N species (Figure 3c). These results confirm very low chemical reactivity of both Gd2@C79N and Y2@C79N toward the cyclopentadiene moiety on the Merrifield peptide supported column.18 Thus, we observe a slightly enhanced Diels-Alder chemical reactivity of the Y2@C79N species in comparison with Gd2@C79N toward the supported cyclopentadiene moiety. These results are consistent with an enhanced yield of the Gd2@C79N species in the K-H electric-arc process and a slightly lower Diels-Alder chemical reactivity relative to Y2@C79N. These results suggest that an optimum size of the (M2)5+ cluster is necessary for optimize metal-carbon cage bonding in the (C79N)5- cage. This is also consistent with the optimized formation of the (La3N)5+ cluster in the (C79N)5- cage (La3N@C79N), but not in the isoelectronic (C80)6-cage.8
Computational Studies of the Heterofullerenes [C79N]5- and Gd2@C79N
Because of the recent experimental success in preparing stable heterofullerenes with (C79N)5- cages with different endohedral clusters (M2)5+ and (La3N)5+.7,8 We have explored the stability of the heterofullerene cage alone and with two Gd+3 encapsulated ions by density functional theory (DFT) computations using the spin-unrestricted B3LYP functional in the Gaussian 03 program.19 For the Ih-C80 cage there are two types of carbon atoms: 60 carbon atoms reside at pentagon sites at a 665 junction, while the remaining 20 carbons are not at pentagon sites but reside at the junctions of three hexagons (a 666 junction). Our studies have confirmed that the nitrogen atom in the Ih-C80 cage is significantly more stable at the 665 junction (pentagon) position than the 666 junction and this cage with a (Gd2)5+ cluster yields Gd2@C79N.6 Encapsulation of dimetallic cluster into a C79N cage with a N at 665 junction.
As shown in Figure 4, the heterofullerene (C79N)5- and isoelectronic Ih-(C80)6- cage both have relatively large HOMO-LUMO gaps of 2.67 and 3.16 eV, respectively. These values are similar to the HOMO-LUMO gaps for other well known stable M3N@Ih-C80 molecules.25-28It is also significant that the HOMO level and total energy of the heterofullerene cage (C79N)5- is even lower than isolectronic (C80)6-. However, when an extra electron is added (Figure 4c) to the cage surface, the LUMO-HOMO gap of the resulting (C79N)6- is relatively small (1.47 eV) and is destabilized relative to (C79N)5-. This result is consistent with the notion that both (M2)5+ and (La3N)5+ endohedral clusters would be suitable for stabilization of a heterofullerene (C79N)5- cage and the internal cluster can accept the unpaired electron formally from the N atom of the fullerene cage.
Figure 4.
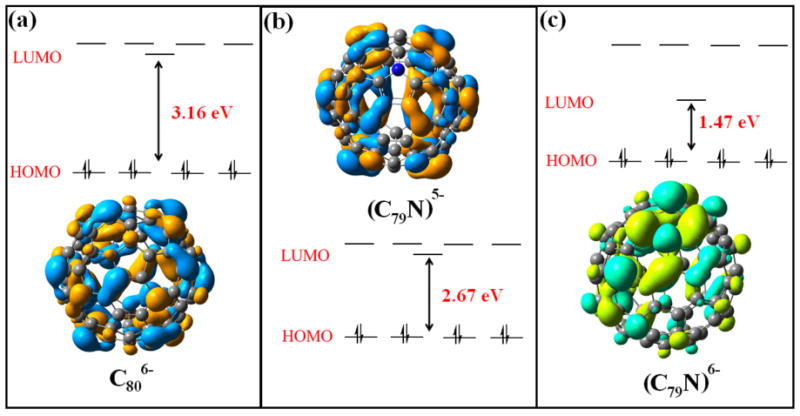
DFT HOMO-LUMO levels of (C80)6-, (C79N)5-, (C79N)6- cages.
Extension of the DFT computational approach to Gd2@C79N (PBEO functional) and the CEP-121G basis set for gadolinium atoms provides energy levels that are comparable with the previously reported Y2@C79N system (Figure 5).7 The computed HOMO-LUMO gap for Gd2@C79N (2.74 eV) is slightly larger than the previously computed HOMO-LUMO gap for Y2@C79N (2.39 eV).7 Careful examination of each HOMO level indicates no significant Gd-Gd bonding overlap until the HOMO −7 to HOMO-22 levels. We assign the former HOMO-7 level as the highest of the fifteen singly-occupied molecular orbitals (SOMO) on Gd2@C79N. The HOMO-7 SOMO orbital can be described in terms of a natural bonding order (NBO) as a Gd(s1p0.95d0.11f0.06)-Gd(s1p0.95d0.11f0.06) bond. In analogous fashion with Y2@C79N, Gd2@C79N represents another example of spin-polarized orbitals that occur at energy levels below the HOMO.7 The large degree of s character on the Gd2 cluster as well as the α spin densities illustrated in Figure 6 for both Gd2@C79N and Y2@C79N clearly indicate unpaired electron spin density localized between the two Gd atoms and not delocalized on the heterofullerene cage. Although we previously reported the spin density for Y2@C79N, a significant difference is the higher degree of p and d orbital hybridization in the Gd2@C79N case.7 The significant s-orbital Fermi contact is also an important feature in understanding the EPR spectra vide infra. The computed Gd– Gd bond length for Gd2@C79N is 3.808 Å. This is another example of a long M-M bond, but is close to the Tb –Tb distance X-ray crystallographic value for Tb2@C79N (3.902 Å) and the previously computed Y – Y distance in Y2@C79N (3.994 Å).7 The electrostatic metal ion repulsion within these M25+ units and fullerene cage restrictions is clearly an important factor in determining the M – M bond distances.
Figure 5.

DFT MO energy level diagrams for optimized Y2@C79N (from ref. 3) and Gd2@C79N (LUMO, HOMO and HOMO-7 orbitals shown)
Figure 6.
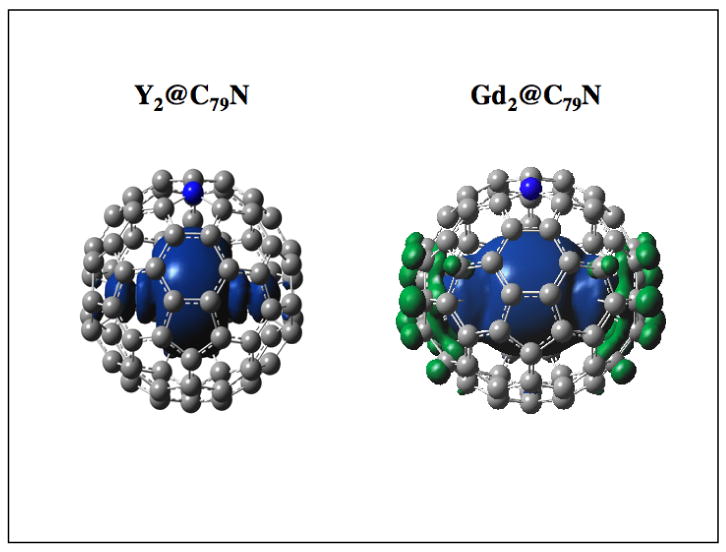
Unpaired Spin Density (α) Distribution for Y2@C79N and Gd2@C79N
Electrochemical Studies of Gd2@C79N
Electrochemical studies provide an excellent approach to further understand the redox chemistry of metallofullerenes and the unusual electronic and magnetic properties of Gd2@C79N. Specifically, cyclic voltammetry electrochemical data for Gd2@C79N and directly compared with Gd3N@C80 under the same conditions (Figure 7). In addition, this data is compared with other related endohedral metallofullerenes and summarized in Table 1. The electrochemical response for Gd2@C79N exhibits an electrochemically reversible oxidation accompanied by two prominent reduction potentials; the first reduction peak is reversible with a half-wave potential (E1/2red1 = -0.96 V) and the second reduction is irreversible at -1.98 V. The order of the first reduction potentials for La2@C80, Gd2@C79N, and Gd3N@C80 are E1/2red1 = -0.31 V E1/2red1 = -0.96 V, and E1/2red1 = -1.44 V, respectively. It has previously been reported that the low 1st reduction potential of La2@C80 is associated with a lower level LUMO level in comparison with other trimetallic nitride endohedral metallofullerenes (e.g., Gd3N@C80 and Y3N@C80). 40 Whereas, the 1st reversible reduction of Gd2@C79N is significantly different and can be assigned to the low-lying SOMO vide supra. The oxidation of [Gd2@C79N]-/[Gd2@C79N]0 is not as kinetically favored as the forward reversible reduction reaction as shown by scan rate dependence (SI). One explanation of this is a possible structural rearrangement when the SOMO is filled due to the change in bond order of the Gd-Gd complex. The question of the reversibility of the 1st oxidation and reduction steps for Gd2@C79N was explored in greater detail with additional cyclic voltammetry experiments as well as independent samples (see SI).
Figure 7.

Cyclic voltammogram of (a) Gd3N@C80 and (b) Gd2@C79N in o-dichlorobenzene, 0.1 M TBABF4, 100 mV/s scan rate.
Table 1.
The redox potentials of Gd2@C79N and related EMFs
| Epcred1 | Epcred2 | E1/2oxl | ΔE | |
|---|---|---|---|---|
| *La2@C80[37] | -0.31 | -1.72 | 0.56 | 0.87 |
| Gd2@C79N | -0.96 | -1.98 | 0.51 | 1.47 |
| Gd3N@(Ih)-C80 | -1.44 | -1.86 | 0.58 | 2.02 |
| Y3N@(Ih)-C80 | -1.41 | -1.83 | 0.64 | 2.05 |
denotes reversible electrochemical redox potentials. Values vs. ferrocene.
EPR Studies of Gd2@C79N
For most lanthanide ions, It is well recognized that the corresponding EPR signals are not readily observed at room temperature because of relatively short electron spin-lattice relaxation times (T1e<10-8-10-9 s) with large zero-field splitting parameters (D) and corresponding broad EPR line widths for these f-orbital ions.29 In contrast, the X-band EPR spectrum (Figure 8) for a dilute solution of Gd2@C79N in toluene exhibits a broadened, but observable, symmetric spectral line with a g-factor 1.978 similar to Y2@C79N (g=1.9740) at 298 K. A solid sample of Gd2@C79N at room temperature exhibits increased broadening due to increased Heisenberg exchange. This effect was confirmed by a solid-state dilution experiment where the Gd2@C79N sample was mixed with an empty cage fullerene C60 (ratio of C60: Gd2@C79N ∼9:1, See SI). There is a small sharp peak with the observed g-factor (g0=2.001), which has been previously reported as defect sites in C60 cages 30 (See SI). The ambient temperature EPR spectrum of the solution and mixed solid sample suggests considerable motional averaging motion of the Gd2@C79N molecule and/or Gd-Gd cluster motional averaging in the C60 solid matrix without an observable nuclear hyperfine interaction.
Figure 8.

EPR spectra of Gd2@C79N samples at 298 K in toluene solution, as a solid, and as a solid solution with C60.
In contrast, low temperature X-band and W-band EPR spectra of Gd2@C79N were also obtained, as shown in Figure 9. Both spectra are featured by the fine structure of a high spin state. The w-band measurement gives a more simplified spectrum, which is almost symmetric around the intense central line, as shown in Figure 9b. Seven peaks on both side of the central line at equidistant intervals can be recognized (inset of the Figure 9b), and a total of 15 peaks can be counted. The 15 peaks suggest the spin quantum number of 15/2 for the spin state of Gd2@C79N. As previously reported,31 the ground spin state of Gd@C82-I is characterized by an integer spin quantum number of S = 3. The spin (S = 1/2) on the π orbital of the fullerene cage is coupled with the octet spin (S = 7/2) of the encapsulated gadolinium in an antiferromagnetic manner with the exchange coupling constant of J = -1.8 cm-1. In the case of Gd2@C79N, however, the spin state is characterized by a half-integer spin quantum number of S = 15/2. The spin (S = 1/2) on the N atom of the fullerene cage and two octet spins (S = 7/2) of two encapsulated gadoliniums are coupled with each other in a ferromagnetic manner. The difference of the electronic state of Gd2@C79N also reflects its small zero-field splitting parameter D. The value of D is estimated about 70mT for Gd2@C79N, which is much smaller than the D = 420mT of Gd@C82-I.
Figure 9.

(a) X-band EPR spectrum of Gd2@C79N sample at 4 K in CS2; (b) W-band EPR spectrum of Gd2@C79N sample at 30 K; vertical expansion, horizontal axis, 34-44 × 102 gauss.
Because the central line of Gd2@C79N is due to the Kramer's doublet in the state with a half-integer spin quantum number of S = 15/2, the sharpness of the line is prominent and the anisotropic nature of the line is weak. As a result the sharp central line is observable even at room temperature in solution vide supra. The other surprising feature of Gd2@C79N is a long spin relaxation time, which enables the detection of electron spin echo (ESE) signal by a pulsed EPR spectrometer even at 20 K. It is rather exceptional that a pulsed EPR observation is applicable to the high spin system of S=15/2 (See SI).
Raman Spectroscopy
As described above, Gd2@C79N has a similar structure to Gd3N@C80 32 and Sc3N@C80 33, which have previously been studied by Raman spectroscopy, except that a nitrogen atom, donating a free electron, has replaced one carbon atom in the cage and the endohedral cluster is a (Gd2)+5 dimer. The full Raman spectrum of Gd2@C79N is shown in Figure 10 and can be divided into four parts: low intensity modes above 1700 cm-1, tangential C79N modes are found between 1000 and 1700 cm-1, radial C79N modes between 200 and 1000 cm-1, and a fourth group from 200 cm-1 and below which has a counterpart only in the spectra of endohedral fullerenes which exhibit low energetic Gd-fullerene cage, intermolecular, and center-of-mass (COM) modes. Low-energy Raman modes of endohedral fullerenes (Gd3N@C80 and Sc3N@C80) have been observed previously, confirming the coupling between the core and the cage by hindered rotation modes 29,30. We observe similar hindered rotation modes for Gd2@C79N at 156.1 and 170.4 cm-1.
Figure 10.

Analysis of full Raman spectra for Gd2@C79N taken at 90 K. Tangential and radial C79N modes are observed, as well as hindered rotation and COM modes.
The vibrational modes of Gd2@C79N and Gd3N@C80 were calculated by hybrid DFT (B3LYP) and UFF in the GAUSSIAN 03 package34 and compared with the experimental data. We identify tangential and radial C79N cage modes, which are similar to the C80 cage modes. A comparison of the Gd2@C79N and Gd3N@C80 experimental data is shown in Figure 11. In particular, we identify the Hg(1) squashing mode at 226 cm-1 for Gd2@C79N and at 234 cm-1 for Gd3N@C80.
Figure 11.
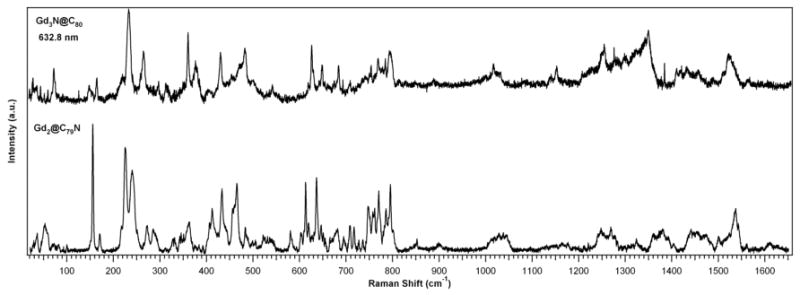
Comparison of Gd2@C79N and Gd3N@C80 Raman spectra taken at 90 K. The C79N and C80 cage modes are comparable, and both samples exhibit hindered rotation modes below 200 cm-1.
Additionally, upon further investigation of low-energy Raman modes (Figure 12) and comparison with the theoretical model, Gd2@C79N hindered rotation and COM modes are observed below 200 cm-1. A Gd–Gd stretching mode is observed at 52.4 cm-1 and confirmed by simulations. The Gd–cage mode at 156.1 cm-1 has a very narrow peak, which is attributed to a Gd–Gd stretch mode interacting with the C79N cage and due to the Gd2 confinement in the smaller C79N cage, when compared to similar molecules with larger cages, such as Gd2@C90 35.
Figure 12.

Analysis of low-energy Raman spectra of Gd2@C79N taken at 90 K, indicating hindered rotation due to the coupling of the core complex to the cage. There is a strong Gd–Gd stretching mode at 52.4 cm-1 and a narrow Gd–cage stretching mode at 156.1 cm-1.
Bingel-Hirsch Gd2@C79N Diethyl Bromomalonate Monoadduct
We have previously described the exceptional chemical stability of Gd2@C79N and corresponding low Diels-Alder reactivity (Figure 3) and relatively large HOMO-LUMO gap (Figures 4 and 5) from the computational studies. Although purified sample quanities were limited to several mgs, other unsuccessful chemical reactions were explored and are summarized (Figure 13). For example, in similar fashion with Y2@C79N, we found no evidence of dimerization of Gd2@C79N, which readily occurs with C59N.1 In an attempt to explore known heterofullerene reactions of C59N, the arylation in the presence of p-toluenesulfonic acid with toluene of anisole36, does not lead to any product in observable quantities. Alternatively, we attempted a chemical reaction of 5,5-dimethyl-1-pyrroline-1-oxide (DMPO), a well known spin trap37 with Gd2@C79N in toluene, but the resulting EPR spectrum provides no evidence of a chemical reaction between DMPO and Gd2@C79N. This low chemical reactivity of Gd2@C79N is consistent with an exceptionally stable molecule with the unpaired electron spin density mainly localized between the Gd metal atoms and is not easily localized on the heterofullerene cage surface.
Figure 13.

Chemical Reactivity Summary of Gd2@C79N
In spite of this low chemical reactivity, the first successful functionalization of Gd2@C79N was achieved via a Bingel-Hirsch reaction by cyclopropanation with diethyl bromomalonate, but only in the presence of DMF (Figure 13). 38 To directly compare the reactivity of Gd3N@C80, Sc3N@C80 and Gd2@C79N, we prepared toluene solutions of these three purified metallofullerene respectively in nearly equal molarities and performed Bingel-Hirsch reactions under the same conditions. Approximately 20 equivalence of diethyl bromomalonate, 10 equivalence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and 1 drop of DMF were added to the solutions under argon at room temperature. All three reaction mixtures were stirred for 1 hour and then subject to HPLC analysis (see SI for the Sc3N@C80 results). As summarized in Figure 14, the Bingel-Hirsch chemical reactivity of Gd3N@C80 in the presence of DMF when directly compared with Gd2@C79N indicates higher reactivity of Gd3N@C80 with a higher overall yield of mono and especially multiadduct formation (Figure 14a). This reaction was previously reported for Gd3N@C80 in the absence of DMF.14 In contrast, a lower yield of the Gd2@C79N monoadduct was obtained after DMF was added to the reaction mixture, (Figure 14c). The isolated monoadduct was isolated and characterized by mass spectrometry. Assuming the diethyl bromomalonate anion is the actual intermediate for attack at the carbon sites on the heterofullerene carbon surface, the DFT positive charge density (green) sites adjacent to the nitrogen atom are likely sites as illustrated in Figure 15.
Figure 14.
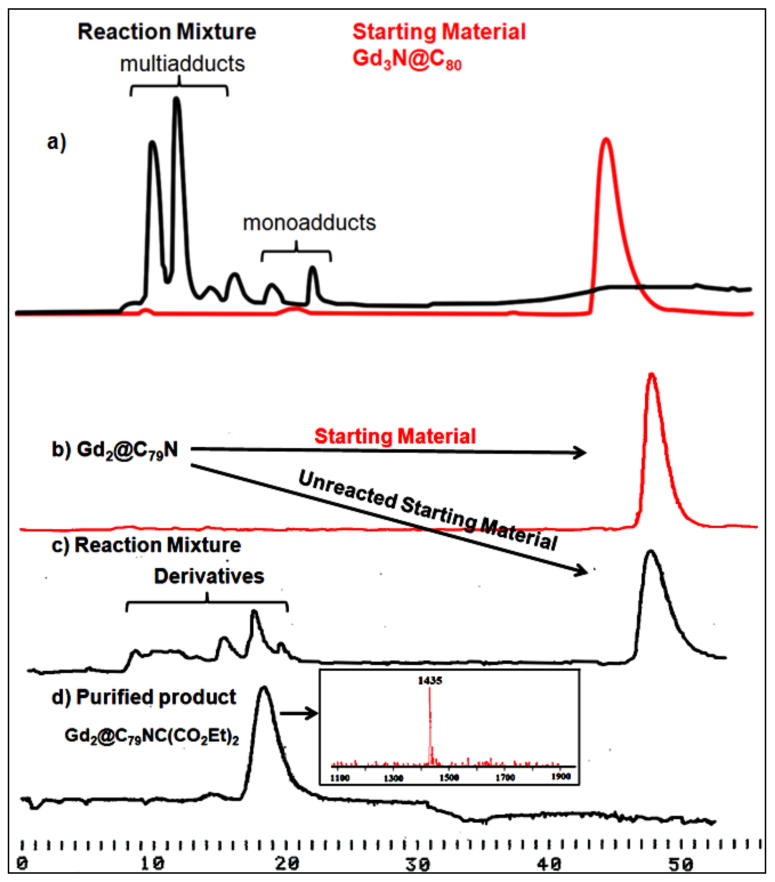
(a) Chromatogram of the Bingel-Hirsch reaction of Gd3N@C80 (a) and Gd2@C79N (c) with diethyl bromomalonate (10 × 250 mm 5PYE column; λ=390 nm; flow rate 2.0 mL/min; toluene as eluent; 25 °C) (d) Purified Gd2@C79NC(CO2Et)2 monoadduct, laser desorption time-of-flight mass spectrum of the isolated monoadduct.
Figure 15.

DFT MO charge densities for Gd2@C79N view from vertical C2 axis (left), from orthogonal C2 axis (middle), and carbon surface motif about the N atom (right)
The HPLC chromatograms also show that Gd3N@C80 was completely consumed, while about 73% Sc3N@C80 and 83% Gd2@C79N was retained based on respective peak areas. This suggests Gd3N@C80 has much higher reactivity towards this Bingel reaction than Sc3N@C80 and Gd2@C79N, which react in a similar fashion, but limited extent. The reason for the observation that Gd2@C79N is less reactive than Gd3N@C80 despite a somewhat lower computed HOMO-LUMO band gap and electrochemically derived band gap vide supra is not completely understood, but this observation is consistent with previously reported reactivity comparisons between Gd3N@C80, Gd3N@C84 and Gd3N@C88, in which the actual reactivity of the gadofullerenes in the Bingel reaction showed an inverse dependence of predicted stability based on band gap values.39
Conclusion
In summary, we have prepared, separated and characterized a new dimetallic EHF, Gd2@C79N in high yield for the first time. Theoretical and experimental results confirm that this molecule has an unusually high chemical stability and the unpaired electron spin density is centered between the encapsulated Gd2 cluster. In addition, the spin (S = 1/2) on the π orbital of the fullerene cage is coupled with the octet spin (S = 7/2) of the encapsulated gadolinium in an antiferromagnetic manner with a small exchange coupling constant. This EPR result suggest the magnetic properties of Gd2@C79N can be described by a spin quantum number of S = 15/2 resulting from spin (formally associated with the N atom of the fullerene cage) and two octet spins of the two encapsulated gadolinium ions. Although the heterofullerene Gd2@C79N is surprisingly unreactive, we have successfully prepared and isolated the first monoadduct of Gd2@C79N. We believe this unique EHF could be very useful in future biomedicine, magnetic, and electronic application areas.
Supplementary Material
Acknowledgments
We are grateful for support of this work by the National Science Foundation [CHE-0443850 (H.C.D.), DMR-0507083 (H.C.D.)] and the National Institutes of Health [1R01-CA119371-01 (H.C.D.)].
Footnotes
Supporting Information Available
References
- 1.Prassides K, Keshavarz KM, Hummelens JC, Andreoni W, Giannozzi P, Beer E, Bellavia C, Cristofolini L, Gonzalez R, Lappas A, Murata Y, Malecki N, Srdanov V, Wudl F. Science. 1996;271(5257):1833–1835. [Google Scholar]
- 2.Poblet JM, Munoz J, Winkler K, Cancilla M, Hayashi A, Lebrilla CB, Balch AL. Chemical Communications. 1999;(6):493–494. [Google Scholar]
- 3.Vostrowsky O, Hirsch A. Chemical Reviews. 2006;106(12):5191–5207. doi: 10.1021/cr050561e. [DOI] [PubMed] [Google Scholar]
- 4.Hummelen JC, Knight B, Pavlovich J, Gonzalez R, Wudl F. Science. 1995;269(5230):1554–1556. doi: 10.1126/science.269.5230.1554. [DOI] [PubMed] [Google Scholar]
- 5.Akasaka T, Kato T, Kobayashi K, Nagase S, Yamamoto K, Funasaka H, Takahashi T. Nature. 1995;374(6523):600–601. [Google Scholar]
- 6.Hou JQ, Kang HS. Chemical Physics. 2007;334(1-3):29–35. [Google Scholar]
- 7.Zuo T, Xu L, Beavers CM, Olmstead MM, Fu W, Crawford DT, Balch AL, Dorn HC. Journal of the American Chemical Society. 2008;130(39):12992–12997. doi: 10.1021/ja802417d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevenson S, Ling Y, Coumbe CE, Mackey MA, Confait BS, Phillips JP, Dorn HC, Zhang Y. Journal of the American Chemical Society. 2009;131(49):17780–17782. doi: 10.1021/ja908370t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson LJ, Cagle DW, Thrash TP, Kennel SJ, Mirzadeh S, Alford JM, Ehrhardt GJ. Coordination Chemistry Reviews. 1999;190-192:199–207. [Google Scholar]
- 10.Bolskar RD, Benedetto AF, Husebo LO, Price RE, Jackson EF, Wallace S, Wilson LJ, Alford JM. Journal of the American Chemical Society. 2003;125(18):5471–5478. doi: 10.1021/ja0340984. [DOI] [PubMed] [Google Scholar]
- 11.Kato H, Kanazawa Y, Okumura M, Taninaka A, Yokawa T, Shinohara H. Journal of the American Chemical Society. 2003;125(14):4391–4397. doi: 10.1021/ja027555+. [DOI] [PubMed] [Google Scholar]
- 12.Fatouros PP, Corwin FD, Chen ZJ, Broaddus WC, Tatum JL, Kettenmann B, Ge Z, Gibson HW, Russ JL, Leonard AP, Duchamp JC, Dorn HC. Radiology. 2006;240(3):756–764. doi: 10.1148/radiol.2403051341. [DOI] [PubMed] [Google Scholar]
- 13.Sitharaman B, Wilson LJ. Journal of Biomedical Nanotechnology. 2007;3(4):342–352. [Google Scholar]
- 14.Chaur MN, Melin F, Athans AJ, Elliott B, Walker K, Holloway BC, Echegoyen L. Chemical Communications. 2008;(23):2665–2667. doi: 10.1039/b804847a. [DOI] [PubMed] [Google Scholar]
- 15.Shu CY, Ma XY, Zhang J, Corwin FD, Sim JH, Zhang EY, Dorn HC, Gibson HW, Fatouros PP, Wang CR, Fang XH. Bioconjugate Chemistry. 2008;19(3):651–655. doi: 10.1021/bc7002742. [DOI] [PubMed] [Google Scholar]
- 16.Shu C, Corwin FD, Zhang J, Chen Z, Reid JE, Sun M, Xu W, Sim JH, Wang C, Fatouros PP, Esker AR, Gibson HW, Dorn HC. Bioconjugate Chemistry. 2009;20(6):1186–1193. doi: 10.1021/bc900051d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu W, Xu L, Azurmendi H, Ge J, Fuhrer T, Zuo T, Reid JE, Shu C, Harich K, Dorn HC. Journal of the American Chemical Society. 2009;131(33):11762–11769. doi: 10.1021/ja902286v. [DOI] [PubMed] [Google Scholar]
- 18.Ge Z, Duchamp JC, Cai T, Gibson HW, Dorn HC. Journal of the American Chemical Society. 2005;127(46):16292–16298. doi: 10.1021/ja055089t. [DOI] [PubMed] [Google Scholar]
- 19.Frisch MJ, et al. GAUSSIAN 09, Revision A.1ed. Gaussian, Inc.; Wallingford, CT: 2009. [Google Scholar]
- 20.Adamo C, Barone V. Journal of Chemical Physics. 1999;110:6158–6170. [Google Scholar]
- 21.Becke AD. Journal of Chemical Physics. 1993;98:5648. [Google Scholar]
- 22.Lee C, Yang W, Parr RG. Physical Review B. 1988;37:785. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 23.Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ. Journal of Physical Chemistry. 1994;98:11623. [Google Scholar]
- 24.Hehre WJ, Ditchfield R, Pople JA. Journal of Chemical Physics. 1972;56:2257. [Google Scholar]
- 25.Krause M, Dunsch L. ChemPhysChem. 2004;5(9):1445–1449. doi: 10.1002/cphc.200400085. [DOI] [PubMed] [Google Scholar]
- 26.Elliott B, Yu L, Echegoyen L. Journal of the American Chemical Society. 2005;127(31):10885–10888. doi: 10.1021/ja052446r. [DOI] [PubMed] [Google Scholar]
- 27.Iiduka Y, Ikenaga O, Sakuraba A, Wakahara T, Tsuchiya T, Maeda Y, Nakahodo T, Akasaka T, Kako M, Mizorogi N, Nagase S. Journal of the American Chemical Society. 2005;127(28):9956–9957. doi: 10.1021/ja052534b. [DOI] [PubMed] [Google Scholar]
- 28.Cai T, Xu L, Anderson MR, Ge Z, Zuo T, Wang X, Olmstead MM, Balch AL, Gibson HW, Dorn HC. Journal of the American Chemical Society. 2006;128(26):8581–8589. doi: 10.1021/ja0615573. [DOI] [PubMed] [Google Scholar]
- 29.Atsarkin VA, Demidov VV, Vasneva GA, Odintsov BM, Belford RL, Raduechel B, Clarkson RB. Journal of Physical Chemistry A. 2001;105(41):9323–9327. [Google Scholar]
- 30.Kempinski W, Piekara-Sady L, Katz EA, Shames AI, Shtutina S. Solid State Communications. 2000;114(3):173–176. [Google Scholar]
- 31.Furukawa K, Okubo S, Kato H, Shinohara H, Kato T. Journal of Physical Chemistry. 2003;107(50):10933–10937. [Google Scholar]
- 32.Burke BG, Chan J, Williams JA, Ge J, Shu CY, Fu W, Dorn HC, Kushmerick JG, Puretzky AA, Geohegan DB. Physical Review B: Condensed Matter and Materials. 2010;81(11):115423/1–115423/7. [Google Scholar]
- 33.Krause M, Kuzmany H, Georgi P, Dunsch L, Vietze K, Seifert G. Journal of Chemical Physics. 2001;115(14):6596–6605. [Google Scholar]
- 34.Frisch MJ, et al. GAUSSIAN 03, Revision C.02. Gaussian Inc.; Willingford, CT: 2004. [Google Scholar]
- 35.Burke BG, Chan TW, Williams KA, Ge J, Shu CY, Fu W, Dorn HC, Puretzky AA, Geohegan DB. Materials Research Society Symposium Proceedings. 2009;1204(Nanotubes and Related Nanostructures) No pp. given, Paper #: 1204-K10-20. [Google Scholar]
- 36.Nuber B, Hirsch A. Chemical Communications. 1998;(3):405–406. [Google Scholar]
- 37.Reszka KJ, McCormick ML, Buettner GR, Hart CM, Britigan BE. Nitric Oxide-Biology and ChemistryNitric Oxide-Biology and Chemistry. 2006;15(2):133–141. doi: 10.1016/j.niox.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Pinzon JR, Zuo T, Echegoyen L. Chemistry – A European Journal. 2010;16(16):4864–4869. S4864/1–S4864/15. doi: 10.1002/chem.200903155. [DOI] [PubMed] [Google Scholar]
- 39.Chaur MN, Melin F, Elliott B, Athans AJ, Walker K, Holloway BC, Echegoyen L. Journal of the American Chemical Society. 2007;129(47):14826–14829. doi: 10.1021/ja075930y. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki T, Maruyama Y, Kato T, Kikuchi K, Nakao Y, Achiba Y, Kobayashi K, Nagase S. Angewandte Chemie International Edition. 1995;107:1228–1230. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


