Abstract
Atrial fibrillation (AF) is the most common cardiac arrhythmia in the clinic, and accounts for more than 15% of strokes. Mutations in cardiac sodium channel α, β1 and β2 subunit genes (SCN5A, SCN1B, and SCN2B) have been identified in AF patients. We hypothesize that mutations in the sodium channel β3 subunit gene SCN3B are also associated with AF. To test this hypothesis, we carried out a large scale sequencing analysis of all coding exons and exon-intron boundaries of SCN3B in 477 AF patients (28.5% lone AF) from the GeneID Chinese Han population. A novel A130V mutation was identified in a 46 year-old patient with lone AF, and the mutation was absent in 500 controls. Mutation A130V dramatically decreased the cardiac sodium current density when expressed in HEK293/Nav1.5 stable cell line, but did not have significant effect on kinetics of activation, inactivation, and channel recovery from inactivation. When co-expressed with wild type SCN3B, the A130V mutant SCN3B negated the function of wild type SCN3B, suggesting that A130V acts by a dominant negative mechanism. Western blot analysis with biotinylated plasma membrane protein extracts revealed that A130V did not affect cell surface expression of Nav1.5 or SCN3B, suggesting that mutant A130V SCN3B may not inhibit sodium channel trafficking, instead may affect conduction of sodium ions due to its malfunction as an integral component of the channel complex. This study identifies the first AF-associated mutation in SCN3B, and suggests that mutations in SCN3B may be a new pathogenic cause of AF.
Keywords: Atrial fibrillation, Cardiac sodium channel α subunit SCN5A (Nav1.5), Sodium channel β subunit SCN3B, Cardiac sodium current, Mutation, Ion channel
1. Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia at the clinical setting with a prevalence of 1% in the general population, which increases with aging and reaches >8% for people aged 80–89 years [4,11,17]. AF accounts for more than 15% of strokes and is associated with worsening heart failure and increased mortality [4,11,17]. AF can be associated with coronary artery disease (CAD), hypertension, valvular heart disease, hyperthyroidism, heart failure, and structural heart diseases, but more than 30% of AF cases are considered as lone AF without these complications.
Genetic factors play an important role in the pathogenesis of AF. AF-associated mutations have been identified in ion channel subunits including cardiac sodium channel α subunit gene SCN5A, β1 subunit gene SCN1B, β2 subunit gene SCN2B, potassium channel genes KCNQ1, KCNE2, KCNJ2, KCNA5, and KCNH2 [17,18]. Recently, we reported that mutations in NUP155 encoding one of nucleoporins, key components of the nuclear pore complex regulating exchange of macromolecules between the nucleus and cytoplasm, cause AF in humans and mice, indicating that non-ion channel genes are critical to the pathogenesis of AF [23]. Similarly, AF-associated mutations or variants were identified in the NPPA gene encoding atrial natriuretic peptide [6,12]. However, mutations or genes responsible for the majority of AF patients are unknown.
The cardiac sodium channel complex is critical for generation and propagation of the cardiac action potential. The complex contains multiple protein factors including the α subunit Nav1.5, β subunits (β1, β2, β3, or β4), and other accessory proteins such as MOG1, ankyrin-G, FHF1B, Fyn, PTPH1, and others [20]. Some of these core factors are involved in trafficking of sodium channels to plasma membranes, whereas others may be integral components required for conduction of sodium ions.
In this study, we used a candidate gene approach to identify a new gene for AF. SCN3B encodes the β3 subunit for sodium channels with 215 amino acid residues [10]. We hypothesized that mutations in the cardiac sodium channel β3 subunit gene SCN3B are associated with AF based on the following evidence. First, AF mutations were reported in the α subunit gene SCN5A, β1 subunit gene SCN1B, and β2 subunit gene SCN2B [8,9,18]. Second, SCN3B knockout (KO) mice developed atrial tachycardia and AF upon induction of atrial burst pacing protocols [5]. All coding exons and exon-intron boundaries of the SCN3B gene were sequenced in 477 AF patients to identify potential mutations associated with AF. A novel A130V mutation was identified in a 46 year-old patient with lone AF, and functionally characterized using biophysical and biochemical analyses.
2. Material and methods
2.1. Study subjects and isolation of human genomic DNA
This study was approved by local institutional review boards on human subject research and carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Written informed consent was obtained from the participants who enrolled in our GeneID studies or their guardians. The GeneID project aims to identify disease-causing and susceptibility genes for various cardiovascular diseases in the Chinese Han population. The study subjects have been enrolled from multiple hospitals in Central and Northern China. The study subjects for the present study were selected from the GeneID database. AF was diagnosed using the standards based on the ACC/AHA/ESC AF guidelines by expert cardiologists using data from electrocardiograms (ECG) and/or Holter ECG recordings [4]. Lone AF was defined as AF without coronary artery disease, essential hypertension, ischemic stroke, congestive heart failure or diabetes. The 500 controls were normal healthy individuals without AF or any other cardiac disorders. Subjects with other types of arrhythmias, congenital heart disease, vulvular heart disease or cardiomyopathies were excluded.
Genomic DNA was isolated from peripheral blood samples using standard protocols with the Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA).
2.2. Mutational analysis
Direct DNA sequence analysis was used for identifying mutations as described previously [2,14,23]. The five coding exons of SCN3B were amplified by polymerase chain reactions (PCR) from AF patient DNA samples and sequenced. The sequences for PCR primers are (5′ to 3′):
Exon 1: 1aF: GAGCGCGCGAGCAAAGATATC, 1aR: TGCGCCCAGGGTAAGCTCAG; 1bF: TAGGGCGGACGAAGCAGGAG, 1bR: GGGCGGAAGAACCACCAAAG
Exon 2: F:GCGCAGGTGAAGGTGTAGACATG, R: GGAAGAGAAGGAGCCAGTGTTTG
Exon 3: F: GGTGGCATTGTCCCCTCTCT, R: TTGCACTCTTTAAGGGCCTCAC
Exon 4: F: GGCGGGAGAGTCAGGATTTG, R: GGGTGGAGGATGAATGTAAACTG
Exon 5: F: GCTCCTTCCCCATCTTGTGTT, R: TCCGAAGCGCTGACATCATAC
2.3. Construction of expression plasmids for SCN5A and SCN3B
The mammalian expression plasmid for cardiac sodium channel α subunit gene SCN5A was described previously [1–3,14,23]. A HEK293 cell line that stably overexpresses SCN5A (HEK293/Nav1.5) was a kind gift from Drs. Glenn E. Kirsch and Xiaoping Wan, and was described previously [20].
We purchased SCN3B cDNA from Thermo Scientific Company (Rockford, IL). The SCN3B cDNA was amplified by PCR using forward primer 5′-CCA AGC TGC TCG AGC AGA AGA TGC CTG CCT TCA A-3′ and reverse primer 5′-GTT TAA ACG GAT CCT TCC TCC ACT GGT ACC GCA GA-3′, digested with restriction enzymes Xho I and BamH I, and subcloned into the eukaryotic expression vector pEGFP-N3 (Clontech, Mountain View, CA) (pEGFP-N3-SCN3B). The SCN3B protein is tagged with EGFP after expression. The A130V mutation was introduced into pEGFP-N3-SCN3B using a PCR-based mutagenesis method (pEGFP-N3-SCN3B A130V).
2.4. Assay for plasma membrane localization of SCN5A (Nav1.5)
Wild type SCN3B expression plasmid pEGFP-N3-SCN3B (2.5 μg), mutant pEGFP-N3-SCN3B A130V (2.5 μg), or a mixture of wild type and mutant plasmids in a 1:1 ratio (1.25 μg each) were transiently transfected into HEK299/Nav1.5 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). After 48 hours, cells were rinsed twice with cold PBS, and proteins on the plasma membranes were biotinylated at 4°C for 30 min using 0.25 mg/mL EZ-Link Sulfo-NHS-SS-Biotin in PBS (Pierce, Rockford, IL). After quenching with 25 mM Tris-HCl, pH 7.3, the cells were scraped and collected by centrifugation at 500 × g for 3 min, rinsed with PBS, and lysed with 50 mM tris-HCl (pH 8.0) containing 1% (v/v) NP-40, 150 mM NaCl, and 1x protease inhibitors prepared from the complete protease inhibitor cocktail tablets (Roche Applied Sience, Indinapolis, IN). An aliquot of the lysate was used to determine the protein concentration. Equal amounts of cell lysates containing the biotinylated proteins from each treatment group were incubated with UltraLink Immobized NeutrAvidin Protein Plus beads (Pierce, Rockford, IL) to immobilize and precipitate the biotinylated proteins. The precipitated biotinylated proteins from the plasma membranes were eluted with SDS-PAGE sample buffer containing 50 mM DTT and subjected to Western blot analysis using a polyclonal anti-Nav1.5 antibody [19]. The same blot was probed with an anti-GFP antibody to detect the level of SCN3B on plasma membrane and with an anti-integrin α5 antibody to calibrate for loading.
2.5. Electrophysiological studies
HEK293/Nav1.5 cells were transfected with wild type or mutant SCN3B plasmids (0.366 μg) or a mixture of both plasmids (0.183 μg each) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Cells were cultured for 48 hours and used for electrophyiological studies. Recordings of cardiac sodium currents and follow up analyses were carried out using the Multipatch 700A amplifier(Axon Instruments, Sunnyvale, CA) under the control of a desktop computer with pCLAMP software (9.0; Axon Instruments, Sunnyvale, CA) as described previously [1,15,20–22]. EGFP-positive cells, which achieved successful transfection of SCN3B, were selected for recording of sodium currents. The pipette solution contains the following composition: 20 mM NaCl, 150 mM CsCl, 10 mM HEPES, 10 mM EGTA, pH 7.2. The bath solution contains 70 mM NaCl, 80 mM CsCl,5.4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 10 mM glucose, pH 7.3.
3. Results
A total of 477 patients with a definitive diagnosis of AF were screened for mutations in the cardiac sodium channel β3 subunit gene, SCN3B (Table 1). Among the AF patients, 28.5% had lone AF. The ratios of paroxysmal AF, persistent AF, and permanent AF were 65.4%, 30%, and 4.6%, respectively. 15.1% of AF patients had strokes, a finding that is consistent with previous epidemiological studies [4]. Other characteristics of the study population are shown in Table 1.
Table 1.
Clinical characteristics of the study population with AF
| Demographic and clinical feature | Number (%) |
|---|---|
| Total AF | 477 |
| Gender, female | 181 (37.9%) |
| Age (mean ± SD years) | 60.3±14.7 |
| Long AF | 136 (28.5%) |
| Paroxysmal AF | 312 (65.4%) |
| Persistent AF | 143 (30.0%) |
| Permanent AF | 22 (4.6%) |
| Hypertension | 174 (36.4%) |
| CAD | 80 (16.7) |
| Stroke | 72 (15.1%) |
SD, standard deviation.
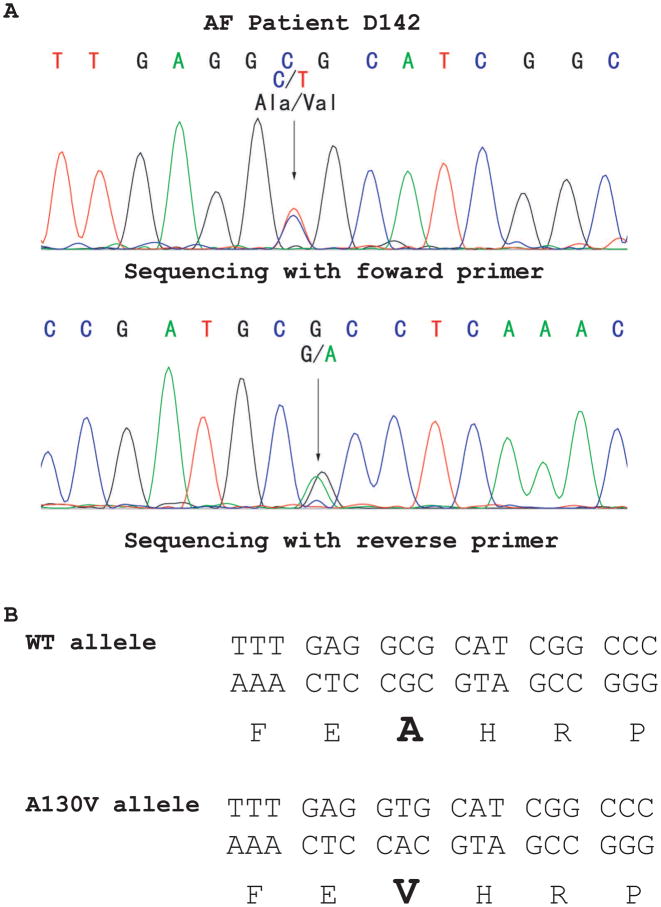
All 5 coding exons and exon-intron boundaries of SCN3B were amplified by PCR from 477 AF patients and sequenced. An interesting missense mutation that substitutes an alanine residue for a valine residue at codon 130 was identified in AF patient D142 (Fig. 1). The A130V mutation was not identified in 500 control Chinese Han individuals, consistent with the possibility that A130V is a mutation associated with a case of AF. Patient D142 was a 46-year-old male patient affected with paroxysmal AF and lone AF without any other cardiac or systemic abnormalities. Other family members declined genetic analysis. No other coding variants were identified (data not shown).
Fig. 1.
Identification of a novel SCN3B mutation, A130V, in an AF patient (D142). (A) DNA sequence analysis revealed a heterozygous mutation of C to T at codon A130 (G to A on reverse sequence) in exon 3 of SCN3B. (B) Schematic representation of mutation A130V (substitution of an alanine residue by a valine residue).
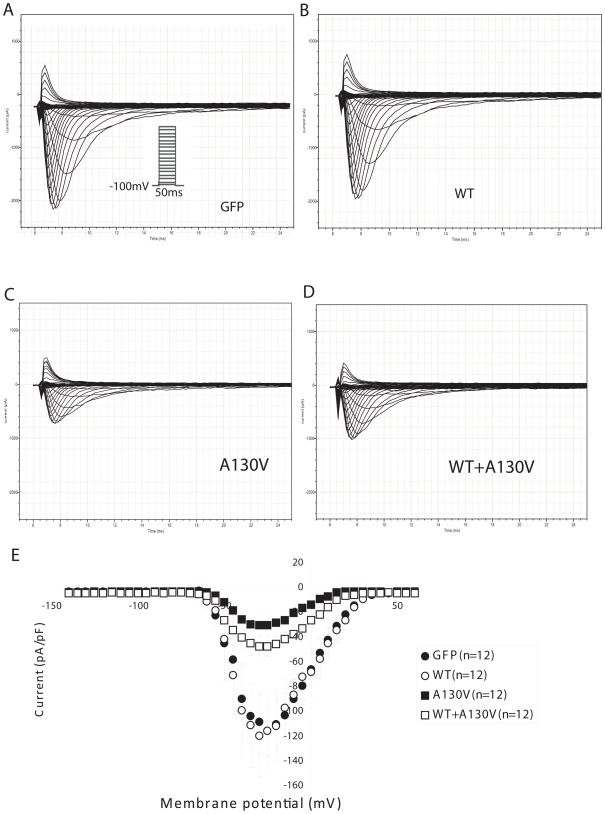
To determine whether A130V is a functional mutation associated with AF, we assessed the effect of this mutation on the cardiac sodium current generated from SCN5A (Nav1.5). Wild type or mutant SCN3B with A130V was over-expressed in HEK293/Nav1.5 stable cells and sodium currents were recorded (Fig. 2, Fig. 3). Consistent with a recent report [16], the sodium current density from cells with wild type SCN3B was comparable to cells transfected with an empty vector (Fig. 2), which may suggest that endogenous SCN3B was sufficient for normal cardiac sodium channel function. However, cells with over-expression of mutant SCN3B showed a dramatic decrease of the cardiac sodium current density compared to cells with wild type SCN3B or with an empty vector (Fig. 2). These results indicate that the A130V mutation in SCN3B has a deleterious effect on cardiac sodium channel function.
Fig. 2.
SCN3B mutation A130V decreased peak sodium current density. Representative whole-cell current traces are shown for HEK293/Nav1.5 cells transfected with an empty vector (A), wild type SCN3B expression plasmid (B), mutant SCN3B-A130V expression plasmid (C), and a mixture of wild type and mutant SCN3B expression plasmids (1:1 ratio) (D). The current protocol was depicted in the inset. (E) The current-voltage relationship for all fours groups is summarized with current amplitudes normalized to cell capacitance (pA/pF). In all groups, 12 cells were studied. Empty vector, 114.10 +/− 20.57 mV; WT SCN3B, 118.03 +/− 33.34 mV; mutant A130V SCN3B, 29.15 +/− 3.29 mV; WT+A130V, 46.33 +/− 9.21 mV.
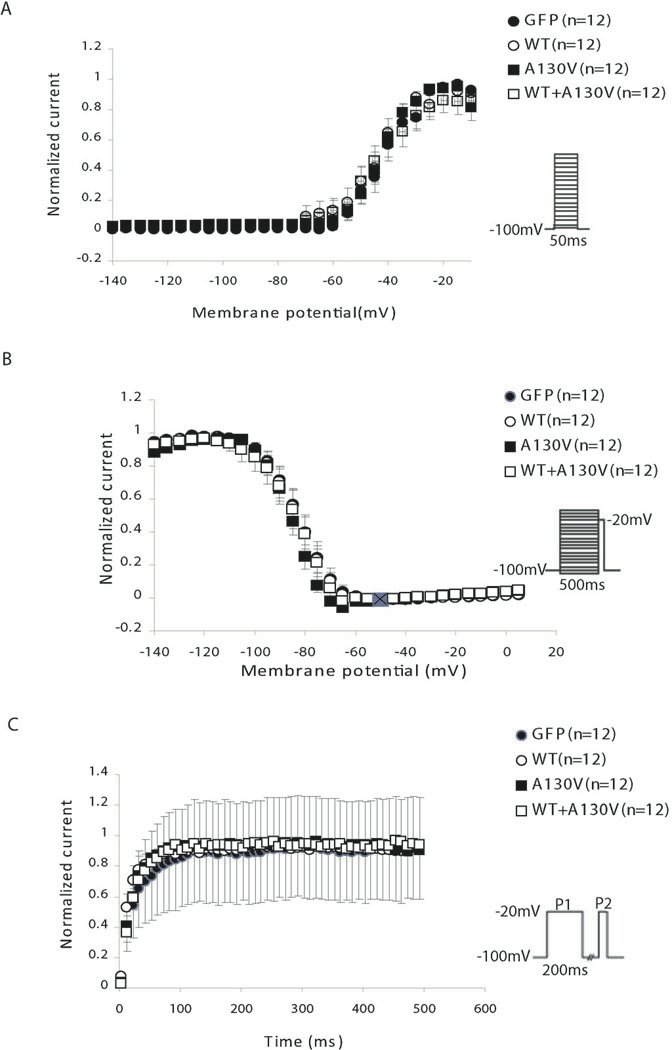
Fig. 3.
Effects of SCN3B mutation A130V on sodium current kinetics in HEK293/Nav1.5 cells. (A) Steady-state activation curves. Cells were held at −100 mV and depolarized in 5-mV increments. Steady-state activation was plotted over the indicated voltage range and expressed as the current at the test potential over the maximum current (I/Imax). (B) Voltage dependence of inactivation curves. A two-pulse protocol was used to estimate the membrane potential dependence of inactivation. Cells were stepped to conditioning potentials for 500 ms before depolarization to −20 mV (50-ms step), and the peak sodium current from the test potentials was normalized to peak sodium current in the absence of a conditioning step. (C) Steady-state time dependence of recovery curves from inactivation. Recovery from inactivation was assessed for all groups utilizing a two-pulse protocol, and the fractional current (P2/P1) was plotted against interpulse duration between P1 and P2. The fraction of channels that had recovered following various time intervals was calculated by dividing the peak current measured during a test pulse to −20 mV.
To distinguish whether the A130V mutation acts by a loss of function or a dominant negative mechanism, we co-expressed both wild type and mutant SCN3B together in HEK293/Nav1.5 cells. Combination of wild type and mutant SCN3B had a similar effect on the sodium current as the mutant SCN3B alone (Fig. 2). These results indicate that mutation A130V acts by a dominant negative mechanism in which the mutant protein negates or counteracts with the function of the wild type SCN3B.
The effects of the A130V mutation on steady-state activation and inactivation of sodium currents as well as recovery from inactivation were also studied. No significant effect was found, although the mutation slightly shifted the voltage-dependent inactivation to more negative potentials only by 2 mV (statistically not significant) (Fig. 3).
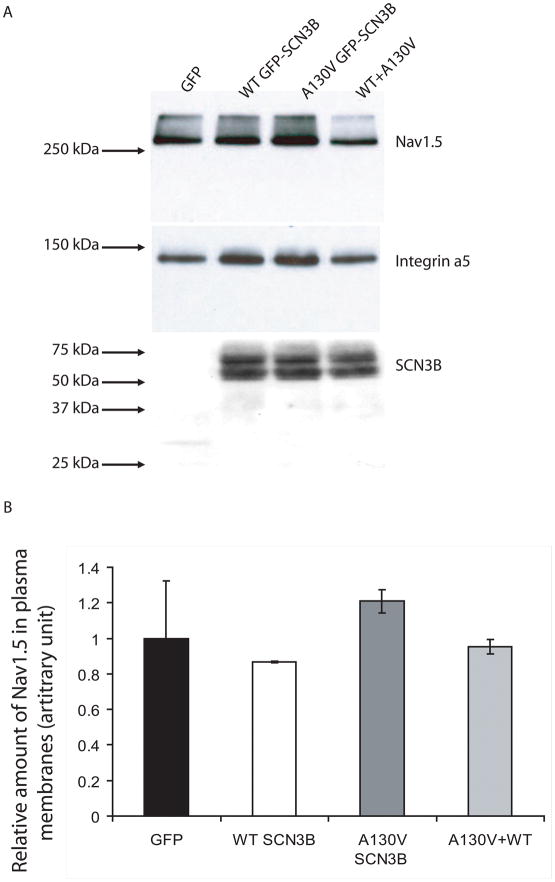
To determine whether the A130V mutation reduces sodium current density by decreasing trafficking of sodium channels to plasma membrane, we carried out Western blot analysis with isolated plasma membrane protein extracts by the biotinylation method to measure the level of Nav1.5 on plasma membranes. As shown in Fig. 4, no significant difference on the expression levels of Nav1.5 or SCN3B on plasma membranes was found in cells over-expressing wild type SCN3B, mutant SCN3B, or a combination of both wild type and mutant SCN3B (Fig. 4). These results suggest that the A130V mutation may not affect the expression level of Nav1.5 on plasma membranes under our experimental condition.
Fig. 4.
Assessment of the effect of SCN3B mutation A130V on plasma membrane expression of Nav1.5. (A) Stable cell line HEK293/Nav1.5 was transfected with an empty vector (GFP), or expression plasmids for GFP-tagged wild type (WT GFP-SCN3B) or mutant A130V GFP-SCN3B, or combination of both (WT+A130V). Plasma membranes were isolated by the biotinylation method, separated by SDS-PAGE, and probed with an anti-Nav1.5 antibody to measure the amount of Nav1.5 in plasma membranes. The same membrane was probed with an anti-GFP antibody to measure the amount of SCN3B in plasma membranes or an anti-integrin α5 antibody to calibrate for loading of samples. (B) Western blot images were scanned and the intensity of each band for Nav1.5 or SCN3B was quantified and calibrated to loading control integrin α5. No significant differences were observed among experimental groups (P>0.05). All studies were repeated twice, and similar results were obtained (data not shown).
4. Discussion
In this study, we carried out a large scale sequencing analysis of the voltage-gated sodium channel β3 subunit gene SCN3B in 477 AF patients. One novel, non-conservative missense variant of an alanine residue to a valine residue, A130V, was identified in one patient with lone AF. The A130V variant was not found in 500 control individuals. Electrophysiological studies demonstrated that A130V dramatically decreased the density of cardiac sodium currents, indicating that it is a functional mutation. Together with a previous report showing that atrial burst pacing protocols could induce atrial tachycardia and AF in SCN3B KO mice [5], we propose SCN3B as an important candidate pathogenic gene for human AF. To the best of our knowledge, this is the first time that a SCN3B mutation has been formally reported in AF. Interestingly, SCN3B mutation A130V acts by a dominant negative mechanism, and interferes with the function of the wild type allele. Many dominant negative mutations have been identified in potassium channel α or β subunits, for example, KCNQ1, KCNH2, and KCNE1, but to date no dominant negative mutations have been reported in the cardiac sodium channel subunits. Our results demonstrate that dominant negative mutations can also exist in sodium channel subunits.
The detailed molecular mechanism by which mutation A130V decreases cardiac sedum current density is not known. A most logical hypothesis is that SCN3B is required for trafficking of sodium channel Nav1.5 to plasma membranes, and mutation A130V inhibits Nav1.5 trafficking. However, our Western blot analysis for Nav1.5 with isolated plasma membrane protein extracts by biotinylation failed to verify the trafficking hypothesis under our experimental condition. The other hypothesis is that SCN3B is an integral structural component of the cardiac sodium channel complex required for conduction of sodium ions across the membranes. Extensive future studies are needed to test this alternative hypothesis.
One functional mutation L10P in SCN3B was reported in a 64-year-old Caucasian male with BrS [7]. Another functional mutation V54G in SCN3B was found in a 20 year old patient with idiopathic VF [16] and in a 6 month old male infant died suddenly [13]. In a 6 week old female infant who died suddenly, a V36M mutation was identified in SCN3B [13]. The A130V mutation identified in this study is the 3rd mutation identified in SCN3B, but it is associated with a distinctly different cardiac disorder, AF. There are many examples in which different mutations in the same gene cause AF and VT/VF [17]. For example, different mutations in cardiac sodium channel α subunit gene can cause long QT syndrome (LQTS), Brugada syndrome (BrS), sudden infant death, cardiac conduction disease, and AF [17]. Other examples are KCNQ1 and KCNH2 [17]. Therefore, there is no surprise that different mutations in SCN3B can cause AF, BrS, and VT/VF, however, detailed molecular mechanisms by which different SCN3B mutations cause different cardiac arrhythmias remain to be established in the future.
5. Conclusions
The results in this study identify a novel mutation in SCN3B associated with AF, which expands the spectrum of mutations in SCN3B associated with various forms of cardiac arrhythmias. Together with reported results showing induced AF from SCN3B KO mice [5], we propose that SCN3B is a new pathogenic gene for AF. This study identifies a new genetic and molecular determinant for AF and shows that reduction of sodium currents by SCN3B mutations may be new molecular mechanism for the pathogenesis of AF.
Acknowledgments
We thank the study participants for their support of this study, Susmita Chakravarti and other members of Wang laboratory for help. This work was supported by the China National Basic Research Program (973 program 2007CB512001, 2007CB512002), fellowships from the China Scholarship Council, a Hubei Province Natural Science Key Program (2008CDA047), China National 863 Scientific Program (2006AA02Z476), a Key Academic Program Leader Award of Wuhan City (200951830560), and an NIH R01 grant (HL094498).
Abbreviations
- AF
atrial fibrillation
- VT
ventricular tachycardia
- VF
ventricular fibrillation
- BrS
Brugada syndrome
- LQTS
long QT syndrome
- CAD
coronary artery disease
- KO
knockout
- PCR
polymerase chain reaction
- ECG
electrocardiogram
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Qiuyun Chen, Email: chenq3@ccf.org.
Xin Tu, Email: xtu@hust.edu.cn.
Qing K. Wang, Email: qkwang@hust.edu.cn.
References
- 1.Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, Potenza D, Moya A, Borggrefe M, Breithardt G, Ortiz-Lopez R, Wang Z, Antzelevitch C, O’Brien RE, Schulze-Bahr E, Keating MT, Towbin JA, Wang Q. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392:293. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- 2.Du W, Bautista JF, Yang H, ez-Sampedro A, You SA, Wang L, Kotagal P, Luders HO, Shi J, Cui J, Richerson GB, Wang QK. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet. 2005;37:733. doi: 10.1038/ng1585. [DOI] [PubMed] [Google Scholar]
- 3.Dumaine R, Towbin JA, Brugada P, Vatta M, Nesterenko DV, Nesterenko VV, Brugada J, Brugada R, Antzelevitch C. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent [see comments] Circ Res. 1999;85:803. doi: 10.1161/01.res.85.9.803. [DOI] [PubMed] [Google Scholar]
- 4.Fuster V, Ryden LE, Asinger RW, Cannom DS, Crijns HJ, Frye RL, Halperin JL, Kay GN, Klein WW, Levy S, McNamara RL, Prystowsky EN, Wann LS, Wyse DG, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC, Klein WW, Alonso-Garcia A, Blomstrom-Lundqvist C, De Backer G, Flather M, Hradec J, Oto A, Parkhomenko A, Silber S, Torbicki A. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation): developed in Collaboration With the North American Society of Pacing and Electrophysiology. J Am Coll Cardiol. 2001;38:1231. doi: 10.1016/s0735-1097(01)01587-x. [DOI] [PubMed] [Google Scholar]
- 5.Hakim P, Brice N, Thresher R, Lawrence J, Zhang Y, Jackson AP, Grace AA, Huang CL. Scn3b knockout mice exhibit abnormal sino-atrial and cardiac conduction properties. Acta Physiol (Oxf) 2010;198:47. doi: 10.1111/j.1748-1716.2009.02048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodgson-Zingman DM, Karst ML, Zingman LV, Heublein DM, Darbar D, Herron KJ, Ballew JD, de AM, Burnett JC, Jr, Olson TM. Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation. N Engl J Med. 2008;359:158. doi: 10.1056/NEJMoa0706300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu D, Barajas-Martinez H, Burashnikov E, Springer M, Wu Y, Varro A, Pfeiffer R, Koopmann TT, Cordeiro JM, Guerchicoff A, Pollevick GD, Antzelevitch C. A mutation in the beta 3 subunit of the cardiac sodium channel associated with Brugada ECG phenotype. Circ Cardiovasc Genet. 2009;2:270. doi: 10.1161/CIRCGENETICS.108.829192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Huang H, Liu G, Lam K, Rutberg J, Green MS, Birnie DH, Lemery R, Chahine M, Gollob MH. Gain-of-function mutation of Nav1.5 in atrial fibrillation enhances cellular excitability and lowers the threshold for action potential firing. Biochem Biophys Res Commun. 2009;380:132. doi: 10.1016/j.bbrc.2009.01.052. [DOI] [PubMed] [Google Scholar]
- 9.Makiyama T, Akao M, Shizuta S, Doi T, Nishiyama K, Oka Y, Ohno S, Nishio Y, Tsuji K, Itoh H, Kimura T, Kita T, Horie M. A novel SCN5A gain-of-function mutation M1875T associated with familial atrial fibrillation. J Am Coll Cardiol. 2008;52:1326. doi: 10.1016/j.jacc.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Morgan K, Stevens EB, Shah B, Cox PJ, Dixon AK, Lee K, Pinnock RD, Hughes J, Richardson PJ, Mizuguchi K, Jackson AP. beta 3: an additional auxiliary subunit of the voltage-sensitive sodium channel that modulates channel gating with distinct kinetics. Proc Natl Acad Sci U S A. 2000;97:2308. doi: 10.1073/pnas.030362197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters NS, Schilling RJ, Kanagaratnam P, Markides V. Atrial fibrillation: strategies to control, combat, and cure. Lancet. 2002;359:593. doi: 10.1016/S0140-6736(02)07748-6. [DOI] [PubMed] [Google Scholar]
- 12.Ren X, Xu C, Zhan C, Yang Y, Shi L, Wang F, Wang C, Xia Y, Yang B, Wu G, Wang P, Li X, Wang D, Xiong X, Liu J, Liu Y, Liu M, Liu J, Tu X, Wang QK. Identification of NPPA variants associated with atrial fibrillation in a Chinese GeneID population. Clin Chim Acta. 2010;411:481. doi: 10.1016/j.cca.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Tan BH, Pundi KN, Van Norstrand DW, Valdivia CR, Tester DJ, Medeiros-Domingo A, Makielski JC, Ackerman MJ. Sudden infant death syndrome-associated mutations in the sodium channel beta subunits. Heart Rhythm. 2010 doi: 10.1016/j.hrthm.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian XL, Kadaba R, You SA, Liu M, Timur AA, Yang L, Chen Q, Szafranski P, Rao S, Wu L, Housman DE, DiCorleto PE, Driscoll DJ, Borrow J, Wang Q. Identification of an angiogenic factor that when mutated causes susceptibility to Klippel-Trenaunay syndrome. Nature. 2004;427:640. doi: 10.1038/nature02320.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian XL, Yong SL, Wan X, Wu L, Chung MK, Tchou PJ, Rosenbaum DS, Van Wagoner DR, Kirsch GE, Wang Q. Mechanisms by which SCN5A mutation N(1325)S causes cardiac arrhythmias and sudden death in vivo. Cardiovasc Res. 2004;61:256. doi: 10.1016/j.cardiores.2003.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valdivia CR, Medeiros-Domingo A, Ye B, Shen WK, Algiers TJ, Ackerman MJ, Makielski JC. Loss-of-function mutation of the SCN3B-encoded sodium channel {beta}3 subunit associated with a case of idiopathic ventricular fibrillation. Cardiovasc Res. 2010;86:392. doi: 10.1093/cvr/cvp417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang QK. Atrial Fibrillation: Genetic Considerations: The Basic Scientist’s Perspective. In: Natale Andrea, Jalife Jose., editors. Atrial Fibrillation: From Bench to Bedside. Humana Press; Totowa, NJ: 2008. pp. 133–144. [Google Scholar]
- 18.Watanabe H, Darbar D, Kaiser DW, Jiramongkolchai K, Chopra S, Donahue BS, Kannankeril PJ, Roden DM. Mutations in sodium channel beta1- and beta2-subunits associated with atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2:268. doi: 10.1161/CIRCEP.108.779181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu L, Nishiyama K, Hollyfield JG, Wang Q. Localization of Nav1.5 sodium channel protein in the mouse brain. NeuroReport. 2002;13:2547. doi: 10.1097/01.wnr.0000052322.62862.a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu L, Yong SL, Fan C, Ni Y, Yoo S, Zhang T, Zhang X, Obejero-Paz CA, Rho HJ, Ke T, Szafranski P, Jones SW, Chen Q, Wang QK. Identification of a new co-factor, MOG1, required for the full function of cardiac sodium channel Nav 1.5. J Biol Chem. 2008;283:6968. doi: 10.1074/jbc.M709721200. [DOI] [PubMed] [Google Scholar]
- 21.Yong SL, Ni Y, Zhang T, Tester DJ, Ackerman MJ, Wang QK. Characterization of the cardiac sodium channel SCN5A mutation, N(1325)S, in single murine ventricular myocytes. Biochem Biophys Res Commun. 2007;352:378. doi: 10.1016/j.bbrc.2006.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang T, Yong SL, Tian XL, Wang QK. Cardiac-specific overexpression of SCN5A gene leads to shorter P wave duration and PR interval in transgenic mice. Biochem Biophys Res Commun. 2007;355:444. doi: 10.1016/j.bbrc.2007.01.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Chen S, Yoo S, Chakrabarti S, Zhang T, Ke T, Oberti C, Yong SL, Fang F, Li L, de la FR, Wang L, Chen Q, Wang QK. Mutation in nuclear pore component NUP155 leads to atrial fibrillation and early sudden cardiac death. Cell. 2008;135:1017. doi: 10.1016/j.cell.2008.10.022. [DOI] [PubMed] [Google Scholar]