Abstract
The body of scientific literature linking Wnts and Wnt-associated proteins to human disease processes continues to grow in parallel with new discoveries from basic science laboratories that further characterize the elaborate cellular events following the binding of Wnts to their receptors. While Wnt-mediated signaling has long been known to play a major role in human carcinogenesis, accumulating evidence indicates that Wnts are also important mediators of inflammation and recovery from injury. The binding of secreted Wnt ligands to their receptors offers an attractive and accessible target for therapeutic regulation of these signaling pathways. Several promising preliminary studies have already addressed potential avenues for the manipulation of Wnt signaling in disease processes. This review will focus on disease processes involving the regulation of Wnt signaling at the level of Wnt binding to its target receptors. Wnt proteins, Wnt receptors, and secreted Wnt inhibitors are attractive as potential therapeutic agents and targets due to their extracellular location. In addition, since Wnt signaling results in a diverse array of downstream intracellular events, many of which are not fully understood, the targeting of this pathway at the most upstream site of pathway activation also provides a strategic advantage for therapy. As the list of Wnt-related diseases continues to grow, advances in our understanding of the biochemical and molecular mechanisms underlying Wnt signaling may ultimately translate into innovative ways to treat Wnt-related disease processes in patients.
Keywords: Disease, Wnt, Wnt signaling, beta-catenin, Wnt/Ca, Frizzled, Fzd, sFRPs, Dickkopf, DKK, LRP, RNAi, Blocking Antibodies, Translational Research, Therapy, Treatment, Therapeutic Strategies, Review
2. INTRODUCTION
2.1. From frogs and flies to human disease – a brief history of Wnts
The term ‘translational research’ was developed to connote the process whereby basic science bench research leads to breakthroughs that can be applied in clinical medicine. The development of the Wnt signaling field provides a classic example of how basic science research on fundamental cellular problems can ultimately provide ideas for therapeutic interventions in human disease. While the Wnt signaling pathway may not be familiar to many clinicians, it is well known in the field of developmental biology, where most of the initial studies characterizing this pathway were made. When the field of Wnt signaling first started, research focused on the embryonic development of organisms like Drosophila, Caenorhabditis elegans, the sea urchin, the chick embryo, and Xenopus. The subsequent progression of the Wnt signaling field has led to the understanding that these proteins play an important part in human development and human disease processes.
All of the important findings in the field of Wnt research have occurred within the last two decades, and would not have been possible without powerful models like Drosophila and Xenopus. In 1987, investigators sequenced a Drosophila gene known as “wingless”(Wg) and noted that it was identical to the murine mammary oncogene int-1, thus giving rise to the “Wnt” family of related proteins (1, 2). It was clear from the phenotype of Wg/Wnt flies that this family of genes played an important role in embryogenesis, determining the patterning and polarity of cells in the developing fly embryo. In 1989, evidence for the role of Wnts in vertebrate embryogenesis came from revealing studies showing that injection of Wnt mRNA into Xenopus embryos resulted in duplication of the embryonic axis, confirming a critical role for Wnts in the vertebrate embryonic patterning (3). A seminal paper published in 1990 demonstrated that in Drosophila, the Wg/Wnt gene product regulated the expression of another protein known as Armadillo, which is the Drosophila homolog of the mammalian protein beta-catenin (4). One year later, investigators characterized the Armadillo/beta-catenin protein as a component of adherens junctions (5). In 1993, two groups simultaneously made the observation that beta-catenin associated with the APC (adenomatous polyposis coli) protein (6, 7). Since APC was known to play an important role in familial adenomatous polyposis, the association with beta-catenin implied a role for cell adhesion in tumor formation.
In 1995, studies in Xenopus demonstrated that certain domains of beta-catenin could not only have signaling roles independent of cell adhesion, but also lead to duplication of the embryonic axis upon overexpression, similar to Wnts (8). These studies made the initial link between Wnts and beta-catenin, but the mechanism underlying this association was still unclear. It was suspected from genetic studies that Wnts could be acting as soluble factors that bound to receptors. This hypothesis was confirmed in 1996 with the finding that the Drosophila and rat Frizzled (FZD) proteins could act as a Wnt receptor (9, 10). In addition, these studies also demonstrated that Drosophila cells expressing FZD respond to Wnt by increasing levels of beta-catenin (9, 10). These findings from Drosophila and Xenopus development formed the cornerstone of what we now know to be a highly-conserved signaling pathway that plays a critical role in many cell processes.
Because the mammalian homolog of Wnt was oncogenic, most of the initial studies relating Wnt signaling to human disease focused on carcinogenesis. The subsequent identification of other components of the Wnt signaling pathway confirmed that perturbations in this pathway had important consequences for tumor formation. Recent studies have made it clear that Wnt signaling is also important for other processes including inflammation and healing. With the development of microarray and proteomic technology, the role of Wnts in different diseases is being continually updated and refined (11). The result has been intense scrutinization of this pathway as a target for therapeutic intervention. While there are already recent reviews highlighting the importance of Wnts in human disease and therapies, this review will focus on recent experimental findings demonstrating promise for the therapeutic targeting of Wnt signaling via modulation of Wnts, Wnt receptors, and the receptor-ligand interaction (11). A list of the diseases discussed in this review is included in Table 1.
Table 1.
Recent strategies targeting Wnts, Wnt receptors and the ligand-receptor interaction in human disease models
| PROTEIN | Disease condition | Effect |
|---|---|---|
| Wnt1 | Sarcoma | Blocking Ab, siRNA induce tumor cell apoptosis (42) |
| Wnt1 | Colon cancer | Blocking Ab, siRNA induce tumor cell apoptosis (44) |
| Wnt1 | Breast cancer | Blocking Ab, siRNA induce tumor cell apoptosis (43) |
| Wnt1 | Squamous cell carcinoma | Blocking Ab reduced proliferation, induced apoptosis (46) |
| Wnt2 | Mesothelioma | Blocking Ab potentiates cell death with adjunct chemotherapy in vitro (48) |
| Wnt2 | Malignant melanoma | Blocking Ab suppressed in vivo tumor growth (47) |
| Wnt2 | Non-small cell lung cancer | Blocking Ab, siRNA induce tumor cell death (45) |
| Wnt5a | Thyroid cancer | Overexpression reduces proliferation, invasion (49) |
| Wnt7a | Non-small cell lung cancer | Overexpression reversed transformation (50) |
| Wnt16 | Acute lymphoblastoid leukemia | Blocking Ab, siRNA increased tumor cell apoptosis (41) |
| FZD5 | Metastatic melanoma | Blocking Ab decreased motility (23) |
| FZD5 | Rheumatoid arthritis | Blocking Ab inhibited inflammatory cytokines (40) |
| FZD7 | hepatocellular carcinoma | Dominant-negative FZD7 reduced cell motility (61) |
| FZD7 | Colon cancer cell line | FZD7 ectodomain attenuated in vivo tumor growth (57) |
| FZD9 | Non-small cell lung cancer | Overexpression restored sensitivity to Wnt7a-mediated reversal of anchorage-independent growth (50) |
| FZD10 | Synovial sarcoma | Blocking Ab, siRNA inhibited tumor growth in vivo (55) |
| EPHB | Prostate cancer | Overexpression attenuated cell growth (63) |
| sFRP1 | Bone remodeling | Blocking Ab, siRNA enhanced osteoclast formation (68) |
| sFRP1,2,5 | Colon cancer | Overexpression attenuates beta-catenin signaling (69) |
| sFRP4 | Renal injury | Recombinant protein decreased fibrosis in vivo (65) |
| sFRP4 | Mesothelioma | Overexpression induces tumor cell apoptosis (71) |
| DKK1 | Mesothelioma | Overexpression induces tumor cell apoptosis (75) |
| DKK1 | Alzheimer’s disease | siRNA decreased tau phosphorylation (73) |
| DKK1 | Ischemic neuronal injury | in vivo siRNA protected neurons during ischemia (74) |
2.2. Interaction between Wnts and cell surface receptors
Despite over twenty years of intense investigation, it was only recently that Wnt proteins were purified and found to be not only highly glycosylated, but also palmitoylated (12). There have been 19 members of the Wnt family identified from mammals, many of which have distinct patterns of expression in both embryos and adults. Studies have also identified several cell surface proteins that can act as Wnt receptors. To this day, model organisms continue to play an integral role in the characterization of proteins involved in Wnt signaling.
2.2.1. Wnt receptors that activate beta-catenin signaling: FZD and LRP
In the Wnt/beta-catenin pathway, which is the best-studied model of Wnt signaling, secreted Wnts bind to proteins of the FZD family, which by sequence are predicted to be members of the seven transmembrane-spanning family of serpentine receptors that classically couple to G protein pathways (2). Both genetic and biochemical studies have led to the universally accepted view that FZD proteins function as the primary receptors for Wnt proteins. Binding of Wnts to FZD triggers a series of events resulting in activation of the intracellular phosphoprotein Dishevelled (DVL), which consequently inhibits the destruction of beta-catenin. As beta-catenin accumulates, it translocates to the nucleus where it interacts with members of the TCF and LEF family of transcription factors to regulate the expression of certain beta-catenin-responsive target genes. The downstream events that follow Wnt/FZD binding are reviewed extensively elsewhere in greater detail, and this review will focus primarily on events related to Wnts and their binding to cell surface receptors.
Activation of the Wnt/beta-catenin pathway by Wnt/FZD also involves a class of co-receptors from the LRP (low-density lipoprotein receptor-related protein) family of single transmembrane receptors (13). Both FZD and LRP are essential for Wnt/beta-catenin signaling. Recent evidence demonstrated that Wnt binds the extracellular domain of both FZD and LRP, suggesting that the cross-linking of these two receptors by Wnt may trigger the intracellular events that lead to beta-catenin signaling (14). Activation of the Wnt/beta-catenin pathway results in cellular responses related to cell proliferation, cell fate, and differentiation.
2.2.2. Wnt receptors that activate beta-catenin-independent signaling pathways
The binding of certain Wnt subtypes to certain receptors, including FZD, can also trigger less-understood pathways, commonly termed beta-catenin-independent pathways, that proceed via a variety of different signaling intermediaries (15, 16). One pathway, termed the Wnt/Ca pathway in vertebrates, is thought to involve increased intracellular calcium and activation of protein kinase C (PKC) (15). Another pathway regulates planar cell polarity (PCP) in Drosophila, and has been called the Wnt/PCP pathway (16). Evidence suggests that there is substantial overlap between the Wnt/Ca and the Wnt/PCP pathways, particularly since proteins involved in the Wnt/PCP pathway in Drosophila appear to be important components of the Wnt/Ca pathway in vertebrates (15). However, it is still unclear in vertebrates whether these pathways are truly distinct or whether they represent components of the same signaling cascade (16). In addition to FZD, several other receptors have been implicated in triggering beta-catenin-independent Wnt signaling, including ROR2 (receptor tyrosine kinase-like orphan receptor 2), MuSK (muscle, skeletal, receptor tyrosine kinase), RYK (RYK receptor-like tyrosine kinase) and EPHB (ELK-related tyrosine kinase) (17). The intracellular events triggered by the binding of Wnts to these receptors have not been clarified, although the activation of beta-catenin-independent pathways clearly plays pivotal roles in the regulation of cell polarity, cell motility, cell migration, and axonal development.
2.2.3. Regulation of Wnt signaling
The activation of both the Wnt/beta-catenin pathway as well as the Wnt/Ca pathway following Wnt binding led to three further interesting observations that have ramifications regarding therapeutic intervention. First, although specific Wnt isoforms seem to predominantly signal through one specific pathway in a specific context, it has been demonstrated that at least some Wnt isoforms are capable of coupling to both the Wnt/beta-catenin and the Wnt/Ca pathways, and thus Wnt isoforms cannot be readily assigned to a specific signaling pathway (18, 19). Second, it appears that activation of beta-catenin-independent Wnt signaling can actually antagonize activation of the Wnt/beta-catenin pathway, suggesting that activation of one pathway could potentially be utilized as a tool to manipulate the other (20). Third, despite this potential antagonism, the activation of both pathways can be seen within the context of certain biological processes. For example, in the case of melanoma, activation of the Wnt/beta-catenin pathway can be linked to tumorigenesis, transformation and cell proliferation, which is consistent with the role of this pathway in determination of cell fate and cell growth during embryonic development (21, 22). Activation of the Wnt/Ca pathway in melanoma, specifically via the Wnt5A isoform, has been linked to metastasis, potentially mirroring the observed role of the Wnt/Ca pathway in regulating cell motility during gastrulation (16, 23). The distinct roles for Wnts during different stages of cancer cell evolution could account for the observation that Wnt5A acts in some instances as a tumor suppressor, potentially by antagonizing Wnt/beta-catenin signaling in early tumorigenesis, while in other instances acting as a promoter of metastasis in later stages of tumorigenesis (23, 24).
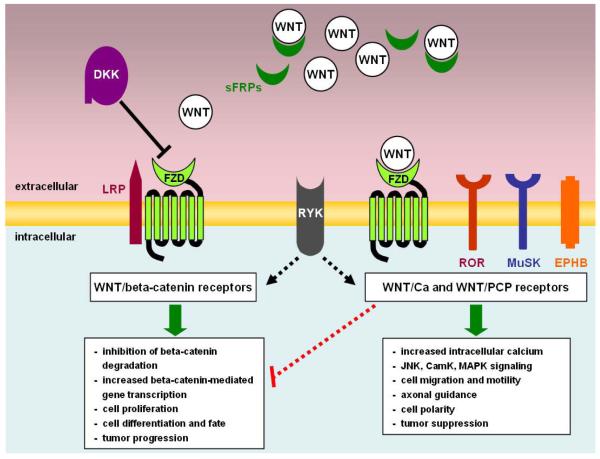
Wnts are also regulated by other extracellular proteins that act as Wnt antagonists. These antagonists, which include DKK (Dickkopf) and sFRPs (secreted FZD-related proteins), help to titrate secreted Wnts and prevent pathway activation below a certain threshold (see figure 1). DKK is a secreted protein that interferes with Wnt/beta-catenin signaling via binding to the Wnt co-receptor LRP6 (25). The sFRPs contain a cysteine-rich domain homologous to the Wnt-binding domain of FZD, and interfere with Wnt signaling by competing for binding to Wnt ligand (26, 27). The role of these secreted proteins in the regulation of Wnt-receptor interactions makes them another potential site for therapeutic intervention.
Figure 1.
A summary of the extracellular interactions between Wnts, Wnt receptors and Wnt regulatory proteins. This simplified representation of the known extracellular interactions governing Wnt signaling depicts how secreted Wnt ligands can interact with receptors that trigger either the Wnt/beta-catenin pathway or other pathways like the Wnt/Ca pathway that act via other signaling mediators. As indicated in the figure, activation of Wnt/Ca receptors can inhibit the Wnt/beta-catenin pathway. The portrayal of RYK reflects data suggesting that this receptor may be able to activate both the Wnt/beta-catenin as well as beta-catenin-independent Wnt signaling cascades (17). Extracellular levels of Wnt ligand are titrated by binding to sFRPs, which exhibit homology to the cysteine-rich Wnt-binding domains of the FZD receptor. The secreted protein DKK antagonizes Wnt signaling via binding to the canonical Wnt co-receptor LRP5/6.
3. POTENTIAL THERAPIES INVOLVING Wnt PROTEINS
Therapeutic intervention with Wnt proteins has been limited in part by two major factors. First, the purification of Wnts has proven particularly challenging, and it was only in 2003 that Wnt proteins were first isolated in an active form (12). Second, at least 19 Wnt isoforms have been identified in mammals, all sharing significant sequence homology, and the structural elements that determine each Wnt’s specificity remain unclear. In some instances, it is uncertain whether the effects of a Wnt are mediated by the expression of a specific Wnt isoform, the expression of a specific receptor isoform, or both. Many of the Wnts are now available as purified proteins, and as new innovations allow for advances in protein purification, the prospect of obtaining enough Wnt protein for therapeutic purposes may become possible (11). Biochemical studies on Wnts may also reveal potential ways in which recombinant engineering of Wnt proteins could result in enhanced activity and improved specificity of targeting.
3.1. Wnts as tools in stem cell manipulation, organ development, and recovery from injury
The possibility of using Wnt proteins (or small molecule modulators) as therapeutic tools holds particular promise in the field of stem cell biology (28). The importance of Wnts in bone marrow function is highlighted by the variety of hematologic malignancies resulting from alterations in Wnt signaling. Studies of mouse and human Wnt proteins have revealed a role for several Wnt isoforms in hematopoeisis (28). Several groups have demonstrated the effects of Wnt expression on the survival, proliferation and self-renewal of hematopoetic stem cells (HSCs). These findings highlight the potential utility of Wnt proteins in the ex vivo manipulation of stem cells from patients with hematologic disorders, including malignancies.
Other important processes regulated by Wnt proteins also have potential medical therapeutic importance. Wnts have demonstrated involvement in the regulation of stem cell differentiation into mature neuronal cells, hepatic cells, cardiomyocytes and skeletal muscle myocytes (29-33). Wnt signaling occupies a pivotal role in determining the fate of stem cells in the skin, and will likely be important for any future efforts to generate ex vivo organoid skin cultures (34). With the rise of obesity as a health care problem in industrialized countries, the inhibitory role of Wnt in adipogenesis and the recent association of polymorphisms of Wnt5 with type 2 diabetes are worth mentioning as a potential avenues for exploring Wnt pathways as therapeutic agents (35, 36). Wnts are also involved in the development of many other cell types and organs. Wnt4 plays an important role in renal development, and the upregulation of Wnt4 expression following acute ischemic renal injury or acute tubular injury in rat and mouse models, respectively, may reflect similarities between development and recovery from injury in the kidney (37-39). These similarities could potentially be exploited to encourage or facilitate kidney repair and recovery from injury in other conditions. While all of these areas show tremendous promise, our understanding of the specifics underlying Wnt signaling in these cell types currently limits the options for therapy.
3.2. Altering Wnt expression in disease processes
Although much of the therapies outlined above speculate on the use of purified Wnt proteins, there are other potential approaches for manipulating Wnt signaling. Recent studies have shown promising preliminary results using siRNA directed against Wnt proteins. In synovial cells from patients with rheumatoid arthritis, Wnt5A is expressed at high levels, along with pro-inflammatory cytokines; these cytokines are decreased following inhibition of Wnt5A using anti-sense strategies (40). In acute lymphoblastoid leukemia, where Wnt16 is upregulated in a subset of cancers containing a chromosomal translocation, the use of siRNA against Wnt16 resulted in increased apoptosis of tumor cell lines in vitro (41). The use of anti-Wnt1 siRNA was effective in inducing tumor cell apoptosis in cell lines from sarcomas, colon cancer, and breast cancers that expressed Wnt1 (42-44). In a model of non-small cell lung cancer, anti-Wnt2 siRNA similarly induced tumor cell death (45). The strategy of using RNA inhibition in vivo will likely remain an exciting approach as the technology for delivery of siRNAs continues to improve.
In addition, another strategy that has demonstrated promising potential from recent studies is the use of blocking antibodies directed against Wnt proteins. Antibodies directed against Wnt1 exhibited anti-tumor activity against sarcomas, colon cancer, breast cancer, and head-and-neck squamous cell carcinomas (42-44, 46). Blocking antibodies against Wnt2 display anti-tumor activity in models of non-small cell lung cancer in vitro, and excitingly also display inhibition of melanoma growth in an in vivo model (45, 47). The aforementioned study on acute lymphoblastoid leukemia also demonstrated the induction of tumor cell apoptosis with the use of anti-Wnt16 antibodies (41). Although blocking antibodies against Wnt2 had a modest effect on their own in a model of mesothelioma, the use of an anti-Wnt2 antibody appeared to augment the effects of a conventional chemotherapeutic agent when given together, again demonstrating that this strategy could have benefit even as an adjunct to currently available treatments (48). While the use of targeted RNA inhibition and Wnt-specific antibodies may be technically feasible, more information is needed regarding the role of Wnts in adults in order to predict and minimize potential adverse effects of therapy.
Recent studies revealed that heterologous overexpression of Wnts can also have tumor suppressor activity. Transfection of a thyroid tumor cell line with Wnt5A was able to reduce proliferative and invasive characteristics in vitro, which is consistent with the finding that more aggressive forms of thyroid carcinoma express lower amounts of Wnt5A (49). Similarly, transfection of some cell lines from non-small cell lung cancer (NSCLC) with Wnt7A was also able to inhibit more aggressive tumor phenotypes, again paralleling the observation that lung tumors express lower levels of Wnt7A than normal lung tissue (50). In the future, the use of Wnts or small peptides with Wnt-like activity as adjunct therapy in certain subsets of cancers may prove an attractive area for further research. Retinoic acid can regulate the expression of some Wnts, raising the possibility that Wnt signaling might be manipulated with other types of pharmaceutical agents (51, 52).
4. POTENTIAL THERAPIES INVOLVING Wnt RECEPTORS
Wnt receptors provide another target for therapeutic intervention in the Wnt signaling pathway. The issue of whether FZD is a true seven transmembrane-spanning G protein-coupled receptor, as suggested by its sequence, remains the topic of ongoing investigation. In some instances, Wnt signaling does indeed appear to involve G proteins, which can be targeted by pertussis toxin as well as by inhibitory intracellular G-beta-gamma binding peptides (53). Knowledge regarding the mechanisms of signaling of other Wnt receptors, including ROR, MuSK, EPHB and RYK, remains limited (17). As extracellular targets, they are readily accessible for binding by either small molecule drugs or designer antibodies. The strategy of targeting these receptors also holds tremendous promise given the scope of biological processes under the control of Wnt signaling.
4.1. Blockade at the level of Wnt/FZD binding
Microarray studies identified Wnt5A as a gene that was upregulated in a subset of metastatic melanomas exhibiting high motility in vitro (54). In a melanoma cell line expressing increased levels of Wnt5A, the activation of the Wnt/Ca pathway along with the increased motility seen in vitro were both inhibited by blocking antibodies to FZD, suggesting that targeting of FZD may be a potential therapeutic option for certain subsets of metastatic cancers with upregulated Wnt signaling (23). In the previously mentioned study on Wnt5A signaling in rheumatoid arthritis, investigators were also able to inhibit levels of pro-inflammatory cytokines using blocking antibodies to FZD (40). Likewise, the use of antibodies against FZD10 demonstrated promising activity in attenuating the growth of synovial sarcoma cells both in vitro and also in vivo on mouse xenografts (55).
Another interesting strategy that has been successful in vitro involves expressing the ectodomain of FZD, which presumably acts as an antagonist to WNT signaling through competition for WNT ligand (56). Expression of the ectodomain of FZD7 in a colon cancer cell line resulted in impressive attenuation of tumor size in a SCID mouse xenograft model (57). While the current therapeutic targeting of FZD receptors in the clinical setting is limited by incomplete knowledge regarding the specificities of Wnt-FZD pairings throughout the adult, the preliminary success of blocking antibodies and extracellular antagonists like the FZD ectodomain in models of cancer and inflammation makes FZD an attractive target for future studies.
4.2. Therapies addressing receptor expression and targeting
Therapies targeting FZD and other Wnt receptors will not necessarily be limited to blocking antibodies. In the inherited condition known as familial exudative vitreoretinopathy (FEVR), certain mutations of FZD may act in a dominant-negative manner to sequester the receptor in the endoplasmic reticulum, and therapy in this instance would focus on increasing the amount of functional FZD receptor at the cell surface (58). A protein called ‘Shisa’ was recently identified as an important regulator of FZD trafficking and maturation, potentially providing another target for future intervention in this pathway (59). Likewise, a new class of drugs termed ‘molecular chaperones’ are already exhibiting promise as therapeutic tools that can rescue the function of mutations that lead to misfolded proteins in other diseases (60).
In other disease processes, therapeutic benefit may require the inactivation of FZD. In synovial sarcoma cells, the use of siRNA directed against FZD10 decreased tumor growth in vitro, suggesting that this technique could be used as a tool to modulate FZD expression (55). Another strategy that has been utilized in vitro is the use of dominant-negative FZD proteins. In human hepatocellular carcinoma, FZD7 expression is detected in a large number of tumor specimens with correlation between increased FZD7 levels and increased cell motility (61). Overexpression of a dominant-negative FZD7 led to reduced cell motility in vitro, highlighting both the importance of FZDs in regulating cancer processes like motility along with the possibility of using dominant-negative proteins to modulate FZD signaling (61).
In some diseases, therapeutic efforts may involve overexpression of Wnt receptors. Studies on certain human lung cancers noted that while expression of Wnt7a was able to decrease transformation and growth in soft agar, certain cell lines were insensitive to Wnt7a (62). The overexpression of FZD9 in these Wnt7a-insensitive cell lines restored sensitivity to Wnt7a, along with subsequent induction of differentiation and inhibition of anchorage-independent growth (62). After finding that EPHB receptor levels were decreased in prostate cancers, investigators found that transfection of EPHB into a metastatic prostate cell line was able to attenuate growth in vitro (63).
The list of disease conditions involving Wnt receptors continues to expand at a rapid pace. The results of these preliminary studies show promise for the potential regulation of human disease processes through the modulation of FZD. Given recent advances RNA inhibition and in the targeting of lentiviral vectors to tumor cells in mice, it may not be long before expression of FZD proteins and FZD siRNAs can be directed in a targeted manner in vivo (64).
5. POTENTIAL THERAPIES INVOLVING SECRETED Wnt INHIBITORS
5.1. Disease processes involving secreted Frizzled-related proteins
The secreted nature of sFRPs makes them potential agents that can be used to manipulate the Wnt signaling pathway by inhibition of Wnt-mediated triggering of signaling events. Manipulation of these regulatory proteins has already shown promise in the recovery of tissue from inflammatory injury. In a model of renal injury based on unilateral ureteral obstruction, the administration of recombinant sFRP4 protein not only decreased the beta-catenin activation usually seen after renal injury, but also reduced the amount of myofibroblasts and subsequent fibrosis (65). Overexpression of sFRP in mice appears to confer a protective effect following induced myocardial infarction, suggesting that these proteins might prove useful in conditions involving acute tissue injury (66). Secreted Wnt inhibitors like the sFRPs could potentially be used to rescue the loss of function of endogenous sFRPs, as is seen with some polymorphisms that result in decreased sFRP3 antagonism of Wnt and increased osteoarthritis in females (67). The potential importance of sFRPs in bone metabolism is also highlighted by the finding that blocking antibodies against sFRP1, as well as siRNA knockdown of sFRP1 expression, enhanced osteoclast formation in vitro (68).
The use of sFRP may also be important in tumor biology. In the case of colon cancer, inactivation of sFRP occurs early on in tumor progression (69, 70). Surprisingly, the restoration of sFRP expression in colon cancer is able to attenuate Wnt signaling even when there are mutations in proteins downstream of Wnt and FZD that constitutively activate the Wnt/beta-catenin pathway (69). This unexpected and promising finding suggests that manipulation of the Wnt signaling pathway can play an important role in shutting down Wnt signaling despite the presence of downstream oncogenic mutations. Inhibition of tumor growth has also been seen with expression of sFRPs in mesothelioma (71). However, in the case of human malignant glioma cells, sFRP-2 appears to cause increased growth, indicating that the actions of sFRPs are likely dependent on tumor type (72).
5.2. Potential therapies involving Dickkopf
As a soluble molecule, DKK would be readily accessible by both small molecules as well as engineered antibodies. In addition, siRNA will likely be another strategy that will be useful for manipulating DKK levels in disease. One study found increased levels of DKK1 in neurofibrillary tangles of Alzheimer’s disease, where Wnt/beta-catenin signaling has been implicated in disease pathogenesis (73). In the same study, in vitro knockdown of DKK expression with siRNA was able to attenuate events such as tau phosphorylation that are thought to play an important role in Alzheimer’s pathogenesis (73). A more recent report from the same group implicated DKK in neuronal death following ischemic injury, and had promising results demonstrating protection of neurons from ischemic injury with the in vivo use of anti-DKK siRNA (74).
In other cases, therapeutic strategy might focus on expression of DKK rather than inhibition of DKK. In mesothelioma cell lines, exogenous overexpression of DKK has a tumor suppressor effect, leading to suppression of growth and induction of apoptosis (75). These studies nicely complement previously described results from a mesothelioma model demonstrating that another secreted inhibitor of Wnt signaling, sFRP4, had similar effects (71). Since DKK is heavily upregulated by UV radiation and can facilitate apoptosis under certain conditions, it might be conceivable that this property of DKK could be exploited for therapeutic advantage (76). DKK plays a role in regulating the entry of human bone marrow stroma stem cells into the cell cycle, and in the context of both stem cell biology and human cancers, the role of DKK in regulation of cell cycle entry may again be an important opportunity for beneficial manipulation of Wnt signaling (77, 78). Given the soluble nature of DKK, it will be an inviting target for future studies involving manipulation of the Wnt pathway.
6. PERSPECTIVES
It is becoming increasingly clear that Wnt signaling is involved in a broad spectrum of human pathophysiology, much of which would be amenable to therapeutic intervention. As the underlying molecular and cellular mechanisms are continually uncovered at the basic science benchtop, these findings will eventually translate into therapies that target Wnt signaling pathways. The responses controlled by the Wnts, including cell proliferation, differentiation, fate specification, motility/migration, and polarity, are all promising avenues for therapeutic management of human disease processes. The interaction between Wnt and its receptors, along with the interactions between Wnts and Wnt-inhibitory factors, have the added benefit of being extracellular, and potentially more accessible to pharmaceutical agents than intracellular targets of the Wnt signaling pathway. Since data has shown that Wnt binding can result in many different downstream signaling events, manipulation of the pathway at the most proximal point, namely receptor activation, could have clear advantages over isolated targeting of intracellular effector proteins.
The rapid pace of technological and scientific advancement has led to many recent breakthroughs in the field of Wnt signaling. As the list of conditions related to Wnts continues to expand, more efforts will be made to explore the feasibility of treatment using Wnt-specific drugs. The knowledge gained at the benchtop will undoubtedly lead to new innovations in therapy that may one day overcome some of the obstacles that currently limit the use of Wnts and their receptors as targets for clinical management.
7. ACKNOWLEDGEMENTS
R.T.M. is supported as an investigator of the Howard Hughes Medical Institute. A.J.C. is supported by a training grant from the National Institutes of Health (2T32 AR07019) as well as a 2005 Dermatologist Investigator Research Fellowship from the Dermatology Foundation. We are indebted to these agencies for their continued support of our ongoing research. As with any review of the Wnt field, this effort is an attempt to give a current overview of a field that continues to advance at a rapid and prolific pace. We would therefore like to apologize for any unintended oversights or omissions.
8. REFERENCES
- 1.Rijsewijk F, Schuermann M, Wagenaar E, Parren P, Weigel D, Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987;50:649–57. doi: 10.1016/0092-8674(87)90038-9. [DOI] [PubMed] [Google Scholar]
- 2.Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–6. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- 3.McMahon AP, Moon RT. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell. 1989;58:1075–84. doi: 10.1016/0092-8674(89)90506-0. [DOI] [PubMed] [Google Scholar]
- 4.Riggleman B, Schedl P, Wieschaus E. Spatial expression of the Drosophila segment polarity gene armadillo is posttranscriptionally regulated by wingless. Cell. 1990;63:549–60. doi: 10.1016/0092-8674(90)90451-j. [DOI] [PubMed] [Google Scholar]
- 5.McCrea PD, Turck CW, Gumbiner B. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science. 1991;254:1359–61. doi: 10.1126/science.1962194. [DOI] [PubMed] [Google Scholar]
- 6.Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–4. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- 7.Su LK, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science. 1993;262:1734–7. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- 8.Funayama N, Fagotto F, McCrea P, Gumbiner BM. Embryonic axis induction by the armadillo repeat domain of beta-catenin: evidence for intracellular signaling. J Cell Biol. 1995;128:959–68. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–30. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- 10.Yang-Snyder J, Miller JR, Brown JD, Lai CJ, Moon RT. A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr Biol. 1996;6:1302–6. doi: 10.1016/s0960-9822(02)70716-1. [DOI] [PubMed] [Google Scholar]
- 11.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 12.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–52. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 13.Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–30. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- 14.Cong F, Schweizer L, Varmus H. Wnt signals across the plasma membrane to activate the beta-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development. 2004;131:5103–15. doi: 10.1242/dev.01318. [DOI] [PubMed] [Google Scholar]
- 15.Kohn AD, Moon RT. Wnt and calcium signaling: beta-Catenin-independent pathways. Cell Calcium. 2005;38:439–46. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–77. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 17.Cheyette BN. Ryk: another heretical Wnt receptor defies the canon. Sci STKE. 2004;2004:pe54. doi: 10.1126/stke.2632004pe54. [DOI] [PubMed] [Google Scholar]
- 18.Maye P, Zheng J, Li L, Wu D. Multiple mechanisms for Wnt11-mediated repression of the canonical Wnt signaling pathway. J Biol Chem. 2004;279:24659–65. doi: 10.1074/jbc.M311724200. [DOI] [PubMed] [Google Scholar]
- 19.Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, Heasman J. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–71. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Weidinger G, Moon RT. When Wnts antagonize Wnts. J Cell Biol. 2003;162:753–5. doi: 10.1083/jcb.200307181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen D, Xu W, Bales E, Colmenares C, Conacci-Sorrell M, Ishii S, Stavnezer E, Campisi J, Fisher DE, Ben-Ze’ev A, Medrano EE. SKI activates Wnt/beta-catenin signaling in human melanoma. Cancer Res. 2003;63:6626–34. [PubMed] [Google Scholar]
- 22.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–2. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 23.Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279–88. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 24.Liang H, Chen Q, Coles AH, Anderson SJ, Pihan G, Bradley A, Gerstein R, Jurecic R, Jones SN. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell. 2003;4:349–60. doi: 10.1016/s1535-6108(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 25.Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–5. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 26.Finch PW, He X, Kelley MJ, Uren A, Schaudies RP, Popescu NC, Rudikoff S, Aaronson SA, Varmus HE, Rubin JS. Purification and molecular cloning of a secreted, Frizzled-related antagonist of Wnt action. Proc Natl Acad Sci U S A. 1997;94:6770–5. doi: 10.1073/pnas.94.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rattner A, Hsieh JC, Smallwood PM, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci U S A. 1997;94:2859–63. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleber M, Sommer L. Wnt signaling and the regulation of stem cell function. Curr Opin Cell Biol. 2004;16:681–7. doi: 10.1016/j.ceb.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Hussain SZ, Sneddon T, Tan X, Micsenyi A, Michalopoulos GK, Monga SP. Wnt impacts growth and differentiation in ex vivo liver development. Exp Cell Res. 2004;292:157–69. doi: 10.1016/j.yexcr.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 30.Morin PJ, Weeraratna AT. Wnt signaling in human cancer. Cancer Treat Res. 2003;115:169–87. doi: 10.1007/0-306-48158-8_7. [DOI] [PubMed] [Google Scholar]
- 31.Pandur P, Lasche M, Eisenberg LM, Kuhl M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature. 2002;418:636–41. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- 32.Seale P, Polesskaya A, Rudnicki MA. Adult stem cell specification by Wnt signaling in muscle regeneration. Cell Cycle. 2003;2:418–9. [PubMed] [Google Scholar]
- 33.Terami H, Hidaka K, Katsumata T, Iio A, Morisaki T. Wnt11 facilitates embryonic stem cell differentiation to Nkx2.5-positive cardiomyocytes. Biochem Biophys Res Commun. 2004;325:968–75. doi: 10.1016/j.bbrc.2004.10.103. [DOI] [PubMed] [Google Scholar]
- 34.Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev. 2003;17:1189–200. doi: 10.1101/gad.1086903. [DOI] [PubMed] [Google Scholar]
- 35.Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati N, Johnson KW, Harrison SD, MacDougald OA. Regulation of Wnt signaling during adipogenesis. J Biol Chem. 2002;277:30998–1004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- 36.Kanazawa A, Tsukada S, Sekine A, Tsunoda T, Takahashi A, Kashiwagi A, Tanaka Y, Babazono T, Matsuda M, Kaku K, Iwamoto Y, Kawamori R, Kikkawa R, Nakamura Y, Maeda S. Association of the gene encoding wingless-type mammary tumor virus integration-site family member 5B (WNT5B) with type 2 diabetes. Am J Hum Genet. 2004;75:832–43. doi: 10.1086/425340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perantoni AO. Renal development: perspectives on a Wnt-dependent process. Semin Cell Dev Biol. 2003;14:201–8. doi: 10.1016/s1084-9521(03)00022-3. [DOI] [PubMed] [Google Scholar]
- 38.Surendran K, Simon TC. CNP gene expression is activated by Wnt signaling and correlates with Wnt4 expression during renal injury. Am J Physiol Renal Physiol. 2003;284:F653–62. doi: 10.1152/ajprenal.00343.2002. [DOI] [PubMed] [Google Scholar]
- 39.Terada Y, Tanaka H, Okado T, Shimamura H, Inoshita S, Kuwahara M, Sasaki S. Expression and function of the developmental gene Wnt-4 during experimental acute renal failure in rats. J Am Soc Nephrol. 2003;14:1223–33. doi: 10.1097/01.asn.0000060577.94532.06. [DOI] [PubMed] [Google Scholar]
- 40.Sen M, Chamorro M, Reifert J, Corr M, Carson DA. Blockade of Wnt-5A/frizzled 5 signaling inhibits rheumatoid synoviocyte activation. Arthritis Rheum. 2001;44:772–81. doi: 10.1002/1529-0131(200104)44:4<772::AID-ANR133>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 41.Mazieres J, You L, He B, Xu Z, Lee AY, Mikami I, McCormick F, Jablons DM. Inhibition of Wnt16 in human acute lymphoblastoid leukemia cells containing the t (1;19) translocation induces apoptosis. Oncogene. 2005;24:5396–400. doi: 10.1038/sj.onc.1208568. [DOI] [PubMed] [Google Scholar]
- 42.Mikami I, You L, He B, Xu Z, Batra S, Lee AY, Mazieres J, Reguart N, Uematsu K, Koizumi K, Jablons DM. Efficacy of Wnt-1 monoclonal antibody in sarcoma cells. BMC Cancer. 2005;5:53. doi: 10.1186/1471-2407-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He B, You L, Uematsu K, Xu Z, Lee AY, Matsangou M, McCormick F, Jablons DM. A monoclonal antibody against Wnt-1 induces apoptosis in human cancer cells. Neoplasia. 2004;6:7–14. doi: 10.1016/s1476-5586(04)80048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He B, Reguart N, You L, Mazieres J, Xu Z, Lee AY, Mikami I, McCormick F, Jablons DM. Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene. 2005;24:3054–8. doi: 10.1038/sj.onc.1208511. [DOI] [PubMed] [Google Scholar]
- 45.You L, He B, Xu Z, Uematsu K, Mazieres J, Mikami I, Reguart N, Moody TW, Kitajewski J, McCormick F, Jablons DM. Inhibition of Wnt-2-mediated signaling induces programmed cell death in non-small-cell lung cancer cells. Oncogene. 2004;23:6170–4. doi: 10.1038/sj.onc.1207844. [DOI] [PubMed] [Google Scholar]
- 46.Rhee CS, Sen M, Lu D, Wu C, Leoni L, Rubin J, Corr M, Carson DA. Wnt and frizzled receptors as potential targets for immunotherapy in head and neck squamous cell carcinomas. Oncogene. 2002;21:6598–605. doi: 10.1038/sj.onc.1205920. [DOI] [PubMed] [Google Scholar]
- 47.You L, He B, Xu Z, Uematsu K, Mazieres J, Fujii N, Mikami I, Reguart N, McIntosh JK, Kashani-Sabet M, McCormick F, Jablons DM. An anti-Wnt-2 monoclonal antibody induces apoptosis in malignant melanoma cells and inhibits tumor growth. Cancer Res. 2004;64:5385–9. doi: 10.1158/0008-5472.CAN-04-1227. [DOI] [PubMed] [Google Scholar]
- 48.Mazieres J, You L, He B, Xu Z, Twogood S, Lee AY, Reguart N, Batra S, Mikami I, Jablons DM. Wnt2 as a new therapeutic target in malignant pleural mesothelioma. Int J Cancer. 2005;117:326–332. doi: 10.1002/ijc.21160. [DOI] [PubMed] [Google Scholar]
- 49.Kremenevskaja N, von Wasielewski R, Rao AS, Schofl C, Andersson T, Brabant G. Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene. 2005;24:2144–54. doi: 10.1038/sj.onc.1208370. [DOI] [PubMed] [Google Scholar]
- 50.Winn RA, Marek L, Han SY, Rodriguez K, Rodriguez N, Hammond M, Van Scoyk M, Acosta H, Mirus J, Barry N, Bren-Mattison Y, Van Raay TJ, Nemenoff RA, Heasley LE. Restoration of Wnt-7a expression reverses non-small cell lung cancer cell transformation through frizzled-9 mediated growth inhibition and promotion of cellular differentiation. J Biol Chem. 2005 doi: 10.1074/jbc.M409392200. [DOI] [PubMed] [Google Scholar]
- 51.Bouillet P, Oulad-Abdelghani M, Ward SJ, Bronner S, Chambon P, Dolle P. A new mouse member of the Wnt gene family, mWnt-8, is expressed during early embryogenesis and is ectopically induced by retinoic acid. Mech Dev. 1996;58:141–52. doi: 10.1016/s0925-4773(96)00569-2. [DOI] [PubMed] [Google Scholar]
- 52.Shum AS, Poon LL, Tang WW, Koide T, Chan BW, Leung YC, Shiroishi T, Copp AJ. Retinoic acid induces down-regulation of Wnt-3a, apoptosis and diversion of tail bud cells to a neural fate in the mouse embryo. Mech Dev. 1999;84:17–30. doi: 10.1016/s0925-4773(99)00059-3. [DOI] [PubMed] [Google Scholar]
- 53.Malbon CC, Wang H, Moon RT. Wnt signaling and heterotrimeric G-proteins: strange bedfellows or a classic romance? Biochem Biophys Res Commun. 2001;287:589–93. doi: 10.1006/bbrc.2001.5630. [DOI] [PubMed] [Google Scholar]
- 54.Carr KM, Bittner M, Trent JM. Gene-expression profiling in human cutaneous melanoma. Oncogene. 2003;22:3076–80. doi: 10.1038/sj.onc.1206448. [DOI] [PubMed] [Google Scholar]
- 55.Nagayama S, Fukukawa C, Katagiri T, Okamoto T, Aoyama T, Oyaizu N, Imamura M, Toguchida J, Nakamura Y. Therapeutic potential of antibodies against FZD10, a cell-surface protein, for synovial sarcomas. Oncogene. 2005 doi: 10.1038/sj.onc.1208780. [DOI] [PubMed] [Google Scholar]
- 56.He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, Varmus H. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science. 1997;275:1652–4. doi: 10.1126/science.275.5306.1652. [DOI] [PubMed] [Google Scholar]
- 57.Vincan E, Darcy PK, Smyth MJ, Thompson EW, Thomas RJ, Phillips WA, Ramsay RG. Frizzled-7 receptor ectodomain expression in a colon cancer cell line induces morphological change and attenuates tumor growth. Differentiation. 2005;73:142–53. doi: 10.1111/j.1432-0436.2005.00015.x. [DOI] [PubMed] [Google Scholar]
- 58.Kaykas A, Yang-Snyder J, Heroux M, Shah KV, Bouvier M, Moon RT. Mutant Frizzled 4 associated with vitreoretinopathy traps wild-type Frizzled in the endoplasmic reticulum by oligomerization. Nat Cell Biol. 2004;6:52–8. doi: 10.1038/ncb1081. [DOI] [PubMed] [Google Scholar]
- 59.Yamamoto A, Nagano T, Takehara S, Hibi M, Aizawa S. Shisa promotes head formation through the inhibition of receptor protein maturation for the caudalizing factors, Wnt and FGF. Cell. 2005;120:223–35. doi: 10.1016/j.cell.2004.11.051. akihito@cdb.riken.jp. [DOI] [PubMed] [Google Scholar]
- 60.Bernier V, Lagace M, Bichet DG, Bouvier M. Pharmacological chaperones: potential treatment for conformational diseases. Trends Endocrinol Metab. 2004;15:222–8. doi: 10.1016/j.tem.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Merle P, de la Monte S, Kim M, Herrmann M, Tanaka S, Von Dem Bussche A, Kew MC, Trepo C, Wands JR. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology. 2004;127:1110–22. doi: 10.1053/j.gastro.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 62.Winn RA, Marek L, Han SY, Rodriguez K, Rodriguez N, Hammond M, Van Scoyk M, Acosta H, Mirus J, Barry N, Bren-Mattison Y, Van Raay TJ, Nemenoff RA, Heasley LE. Restoration of Wnt-7a expression reverses non-small cell lung cancer cellular transformation through frizzled-9-mediated growth inhibition and promotion of cell differentiation. J Biol Chem. 2005;280:19625–34. doi: 10.1074/jbc.M409392200. [DOI] [PubMed] [Google Scholar]
- 63.Huusko P, Ponciano-Jackson D, Wolf M, Kiefer JA, Azorsa DO, Tuzmen S, Weaver D, Robbins C, Moses T, Allinen M, Hautaniemi S, Chen Y, Elkahloun A, Basik M, Bova GS, Bubendorf L, Lugli A, Sauter G, Schleutker J, Ozcelik H, Elowe S, Pawson T, Trent JM, Carpten JD, Kallioniemi OP, Mousses S. Nonsense-mediated decay microarray analysis identifies mutations of EPHB2 in human prostate cancer. Nat Genet. 2004;36:979–83. doi: 10.1038/ng1408. [DOI] [PubMed] [Google Scholar]
- 64.Morizono K, Xie Y, Ringpis GE, Johnson M, Nassanian H, Lee B, Wu L, Chen IS. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat Med. 2005 doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- 65.Surendran K, Schiavi S, Hruska KA. Wnt-Dependent {beta}-Catenin Signaling Is Activated after Unilateral Ureteral Obstruction, and Recombinant Secreted Frizzled-Related Protein 4 Alters the Progression of Renal Fibrosis. J Am Soc Nephrol. 2005;16:2373–84. doi: 10.1681/ASN.2004110949. [DOI] [PubMed] [Google Scholar]
- 66.van Gijn ME, Daemen MJ, Smits JF, Blankesteijn WM. The wnt-frizzled cascade in cardiovascular disease. Cardiovasc Res. 2002;55:16–24. doi: 10.1016/s0008-6363(02)00221-3. [DOI] [PubMed] [Google Scholar]
- 67.Loughlin J, Dowling B, Chapman K, Marcelline L, Mustafa Z, Southam L, Ferreira A, Ciesielski C, Carson DA, Corr M. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc Natl Acad Sci U S A. 2004;101:9757–62. doi: 10.1073/pnas.0403456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hausler KD, Horwood NJ, Chuman Y, Fisher JL, Ellis J, Martin TJ, Rubin JS, Gillespie MT. Secreted frizzled-related protein-1 inhibits RANKL-dependent osteoclast formation. J Bone Miner Res. 2004;19:1873–81. doi: 10.1359/JBMR.040807. [DOI] [PubMed] [Google Scholar]
- 69.Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Dong Chen W, Pretlow TP, Yang B, Akiyama Y, Van Engeland M, Toyota M, Tokino T, Hinoda Y, Imai K, Herman JG, Baylin SB. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36:417–22. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- 70.Taketo MM. Shutting down Wnt signal-activated cancer. Nat Genet. 2004;36:320–2. doi: 10.1038/ng0404-320. [DOI] [PubMed] [Google Scholar]
- 71.He B, Lee AY, Dadfarmay S, You L, Xu Z, Reguart N, Mazieres J, Mikami I, McCormick F, Jablons DM. Secreted frizzled-related protein 4 is silenced by hypermethylation and induces apoptosis in beta-catenin-deficient human mesothelioma cells. Cancer Res. 2005;65:743–8. [PubMed] [Google Scholar]
- 72.Roth W, Wild-Bode C, Platten M, Grimmel C, Melkonyan HS, Dichgans J, Weller M. Secreted Frizzled-related proteins inhibit motility and promote growth of human malignant glioma cells. Oncogene. 2000;19:4210–20. doi: 10.1038/sj.onc.1203783. [DOI] [PubMed] [Google Scholar]
- 73.Caricasole A, Copani A, Caraci F, Aronica E, Rozemuller AJ, Caruso A, Storto M, Gaviraghi G, Terstappen GC, Nicoletti F. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer’s brain. J Neurosci. 2004;24:6021–7. doi: 10.1523/JNEUROSCI.1381-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cappuccio I, Calderone A, Busceti CL, Biagioni F, Pontarelli F, Bruno V, Storto M, Terstappen GT, Gaviraghi G, Fornai F, Battaglia G, Melchiorri D, Zukin S, Nicoletti F, Caricasole A. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is required for the development of ischemic neuronal death. J Neurosci. 2005;25:2647–57. doi: 10.1523/JNEUROSCI.5230-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee AY, He B, You L, Xu Z, Mazieres J, Reguart N, Mikami I, Batra S, Jablons DM. Dickkopf-1 antagonizes Wnt signaling independent of beta-catenin in human mesothelioma. Biochem Biophys Res Commun. 2004;323:1246–50. doi: 10.1016/j.bbrc.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 76.Grotewold L, Ruther U. The Wnt antagonist Dickkopf-1 is regulated by Bmp signaling and c-Jun and modulates programmed cell death. Embo J. 2002;21:966–75. doi: 10.1093/emboj/21.5.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gregory CA, Perry AS, Reyes E, Conley A, Gunn WG, Prockop DJ. Dkk-1-derived synthetic peptides and lithium chloride for the control and recovery of adult stem cells from bone marrow. J Biol Chem. 2005;280:2309–23. doi: 10.1074/jbc.M406275200. [DOI] [PubMed] [Google Scholar]
- 78.Gregory CA, Singh H, Perry AS, Prockop DJ. The Wnt signaling inhibitor dickkopf-1 is required for reentry into the cell cycle of human adult stem cells from bone marrow. J Biol Chem. 2003;278:28067–78. doi: 10.1074/jbc.M300373200. [DOI] [PubMed] [Google Scholar]