Abstract
Gastrointestinal stromal tumors (GISTs) are rare, but represent the most common mesenchymal neoplasms of the gastrointestinal tract. Tumor resection is the treatment of choice for localized disease. Tyrosine kinase inhibitors (imatinib, sunitinib) are the standard therapy for metastatic or unresectable GISTs. GISTs usually metastasize to the liver and peritoneum. Bone metastases are uncommon. We describe three cases of bone metastases in patients with advanced GISTs: two women (82 and 54 years of age), and one man (62 years of age). Bones metastases involved the spine, pelvis and ribs in one patient, multiple vertebral bodies and pelvis in one, and the spine and iliac wings in the third case. The lesions presented a lytic pattern in all cases. Two patients presented with multiple bone metastases at the time of initial diagnosis and one patient after seven years during the follow-up period. This report describes the diagnosis and treatment of the lesions and may help clinicians to manage bones metastases in GIST patients.
Key words: gastrointestinal stromal tumors, computerezed tomografy, scan, bone metastases, Imatinib.
Introduction
Gastrointestinal stromal tumors (GISTs) are rare, but represent the most common mesenchymal neoplasms of the gastrointestinal tract. The most frequent site of occurrence is the stomach (60% of cases), followed by the small bowel (35%) and other sites (colon, rectum, oesophagus <5%).1 GISTs may also develop as primary tumors of the omentum, mesentery or retroperitoneum. They are classified as spindle cell, epithelioid or pleomorphic mesenchymal tumors of the GI tract. Tumor resection is the treatment of choice for localized disease. The risk of recurrence is identified evaluating the mitotic index, dimension and site of the tumor.2,3 Selective tyrosine kinase inhibitors (imatinib, sunitinib) are the standard therapy for metastatic or unresectable GISTs.4,5 Evaluation of tumor response to this molecular target therapy, is very important because a decrease in tumor size (RECIST criteria) is not adequate since target therapies do not always affect tumor dimensions but usually lead to metabolic and densitometric changes.6 Other CT scan parameters and PET imaging have been established to assess tumor response more accurately (CHOI criteria).7
GISTs usually metastasize to the liver and peritoneum. Rare sites of metastasis are lymph-nodes, lung and subcutaneous tissue, and intracranial localizations have only been described in a case report.8–10 Bone metastases are also uncommon.11–14
We describe three cases of bone metastases in patients with advanced GISTs. Two of them showed bone metastases at disease presentation, whereas the third patient was diagnosed after several years of follow-up.
Case #1
A 62-year-old man presented to the Emergency Room in April 2004 with acute lumbar back pain. Multiple hepatic and skeletal metastases were detected involving the spine, pelvis and ribs with a lytic pattern (Figure 1A,B,C). A CT scan disclosed the primary tumor as an abdominal mass of 8 cm, contiguous to ileal loops. Liver biopsy revealed a GIST. The immunoistochemical analysis revealed the tumor was positive CD 117 and CD 34, negative S100, mitotic index was not available. One month later, the patient started imatinib 400 mg/die, and zoledronic acid concurrent with radiotherapy on the spine (from T12 to L2 with a total dose of 3000 cGy). The patient had a clinical benefit and disease stability for two years. In 2006, when the patient was referred to us, a disease progression was documented. Zoledronic acid was stopped due to mandibular osteonecrosis. The patient was then enrolled in a clinical protocol with sunitinib and the disease was stable until January 2007 when the patient’s clinical conditions worsened. He died in February 2007 from pulmonary edema and acute renal failure.
Figure 1.
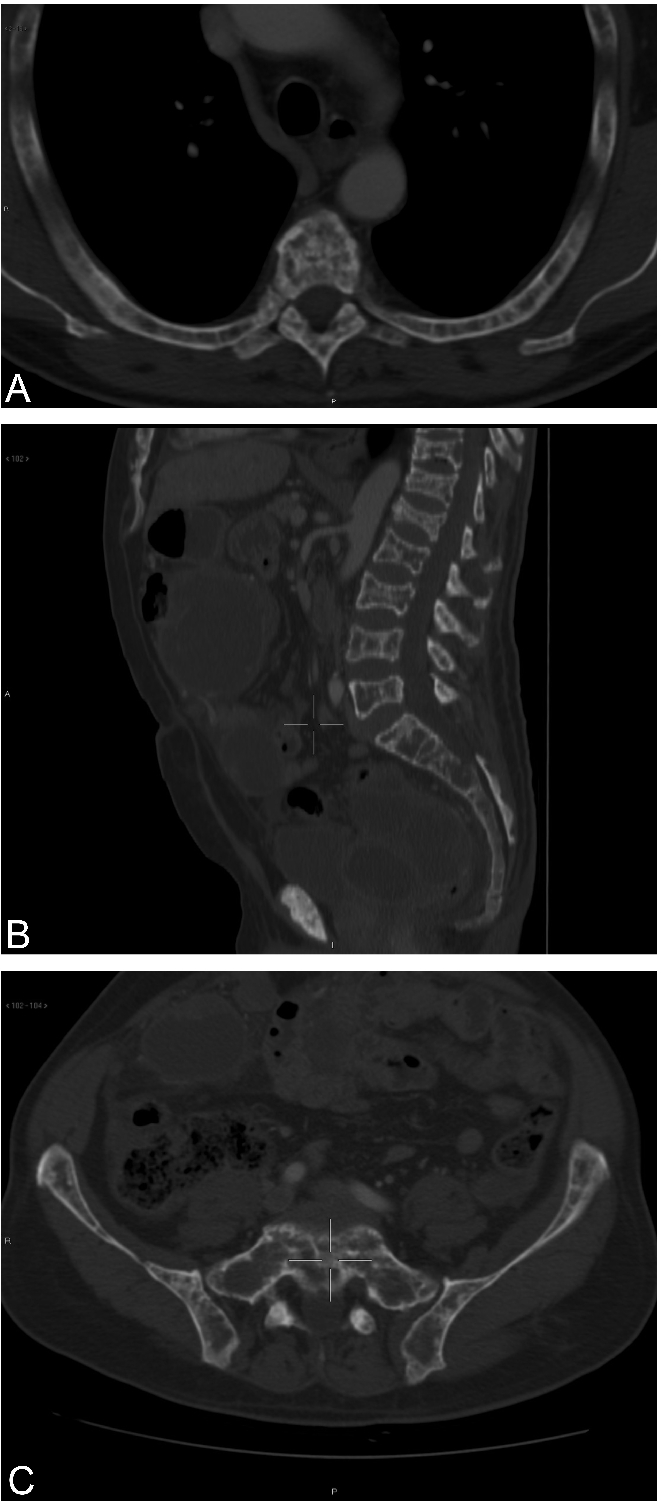
(A) Multiple lytic lesions of the ribs and vertebral body. (B) Multiple lytic lesions of the spine with vertebral collapse. (C) Multiple lytic lesions of the pelvis.
Case #2
An 82-year-old woman underwent several examinations in October 2006 for acute anemia. A CT scan disclosed a gastric lesion with hepatic and bone metastases. Many vertebral bodies and the pelvis were involved and the lesions had a lytic pattern (Figure 2). She underwent a partial gastric resection. The tumor size was 8.5 cm, the mitotic index was 16/50 High Power Field (HPF) and the immunoistochemical analysis revealed the tumor was positive CD 117 and CD 34, negative S100. The tumor presented KIT exon 11 mutation (KIT exon 11 c.1696_1718del (p.N566_P573delinsA). She started imatinib 400 mg/die and zoledronic acid with disease stability until April 2009 when an increase in size of a lesion in the right iliac fossa was documented in spite of stable hepatic and bone metastases. The patient started imatinib 800 mg/die, but she had to suspend the drug in July 2009 due to intolerance. Since June 2010 she has been taking sunitinib 37.5 mg/ die and is well.
Figure 2.
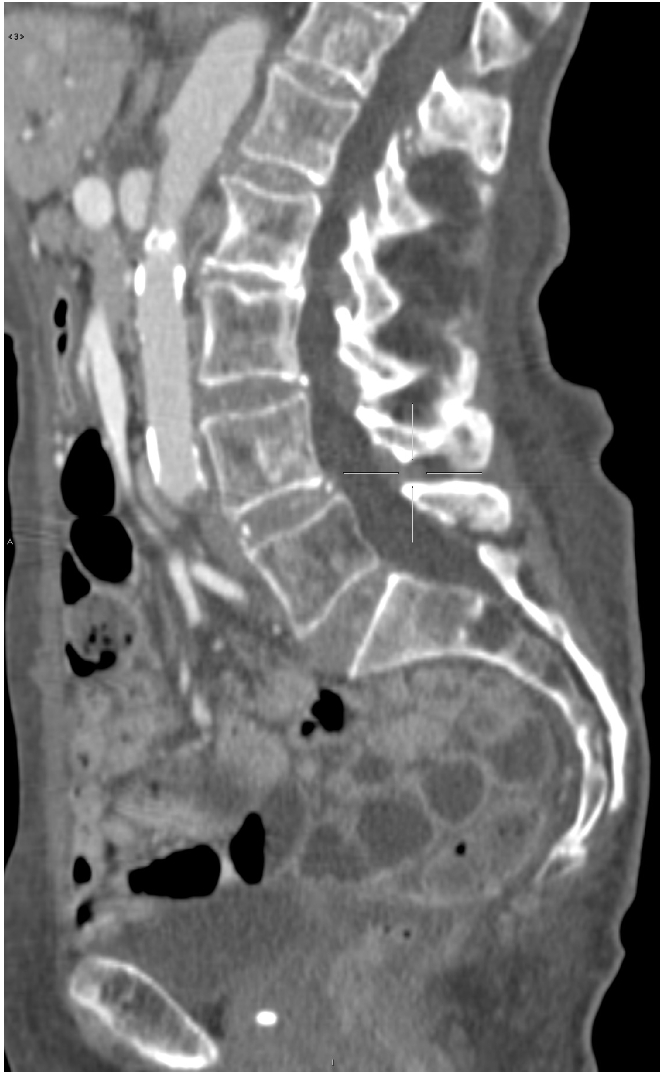
Sacral lytic lesion.
Case #3
A 54-year-old woman came to our attention for a duodenal mass. In 2001 she had undergone a segmentary resection of the small intestine. The tumor size was 4 cm, mitotic index was >5/50 HPF and the immunoistochemical analysis revealed the tumor was positive CD 117 and CD 34. The tumor presented KIT exon 11 mutation (KIT exon 11 T>A 69429 (p.V559d). In 2002, because of tumor recurrence with multiple hepatic lesions, she started a treatment with imatinib 400 mg/die with disease stability until February 2005. At that time, a CT scan documented disease progression in the liver, so a treatment with imatinib 800 mg/die was started. In November 2005, a new progression led to a second-line protocol with sunitinib 37.5 mg/die. This new therapy stabilized the multiple hepatic lesions until March 2007 when an increase in size and vascularisation of the lesions was diagnosed. In July she started a new treatment with nilotinib. In April 2008 the patient complained of severe pain in the right intercostal site, not controlled by analgesic therapy. A CT scan disclosed stable abdominal disease but multiple osteolytic bone metastases had appeared in her ribs (with pathologic fractures), spine and iliac wings. In particular, the osteolytic metastasis of the left iliac wing was induced by a solid pathologic tissue eroding the bone cortex and invading adjacent tissues (Figure 3A). The patient again received sunitinib but had to suspend the drug because of intolerance. A CT scan in January 2009 showed an enlargement of the iliac bone metastasis (Figure 3B) with the appearance of new lesions. The patient died in August 2009 from progressive disease.
Figure 3.
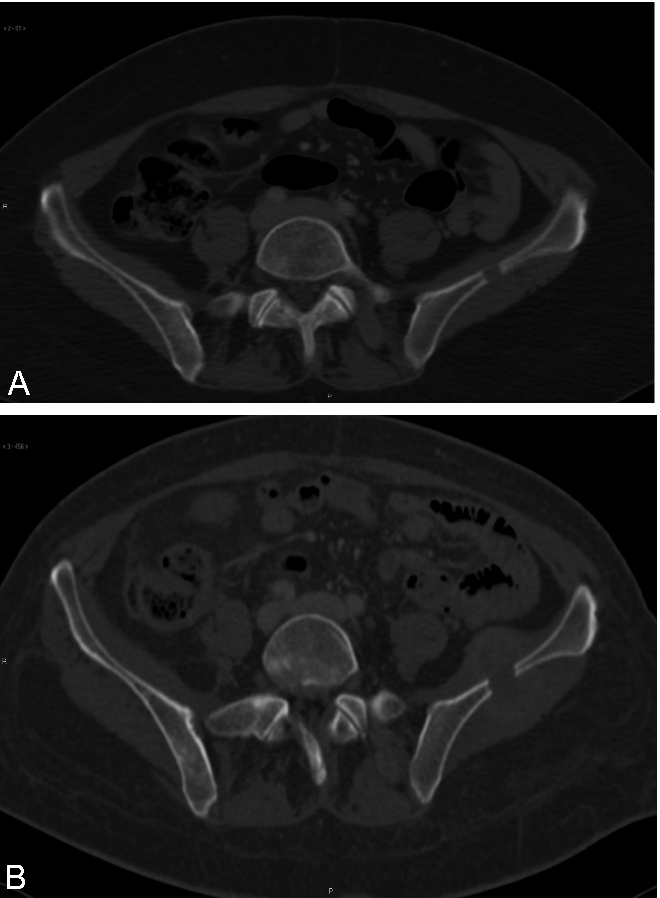
(A) Lytic lesion of the left iliac wing. (B) Enlargement of the iliac lesion.
Discussion
In the past seven years, we have followed-up 71 patients with GISTs. Among them, we discovered three cases of bone metastases. Clinically, bone metastases were symptomatic in two patients and presenting with a pathological fracture in a single case. In only one patient bone lesions were asymptomatic and diagnosed as occasional finding. Two patients had bone metastases at disease presentation and in one of them bone pain led to diagnosis of the primary tumor. In the third patient, bone metastases were found many years after diagnosis of the primary tumor. Medical therapy with zoledronic acid in association with TK inhibitors yielded long-term disease stabilization in two cases, whereas the disease progressed rapidly in the third patient. Only one patient was also treated with radiotherapy for palliative purpose.
Bone metastases in GISTs are rare, but they are encountered more frequently than in the past. This is probably due to advances in imaging techniques and to an improvement in overall patient survival following the introduction of tyrosine kinase inhibitors.
Diagnosis of bone metastases in GISTs is often based on clinical findings alone (i.e. bone fractures or bone pain) or is occasionally made during imaging evaluation. We think more attention should be paid to the diagnosis of bone metastases in clinical practice despite the dearth of available data on the sensitivity and specificity of bone scintigraphy and PET.
CT scan in our three patients disclosed unknown bone metastases. The lesions were mostly lytic, with a complete rearrangement of bone structure, cortex erosion and, in one case, a solid mass invading adjacent soft tissues. The most frequent sites of bone metastases were spine and pelvis.
Few data can be found in literature on the treatment of bone metastases in GISTs. The effect of imatinib on bone lesions is unknown as is the activity of zoledronic acid, even though it may be recommended. Zoledronic acid is a bisphosphonate administered intravenously. It is the current standard therapy for osteoporosis and is used to combat hypercalcemia and bone metastases from solid tumors in the colon, breast, lung, prostate and renal cell carcinoma.15,16 Zoledronic acid has also proved effective in the prevention and treatment of bone localizations in patients with multiple myeloma. Zoledronic acid penetrates osteoclast cells selectively and promotes their apoptosis, thereby reducing bone resorption.17 The drug also reduces the frequency of bone events and bone pain and improves quality of life.16–18 In vitro studies have demonstrated the antitumor properties of zoledronic acid,19–22 and the role of this targeted therapy in the management of patients with GISTs merits investigation. Radiotherapy and orthopedic surgery can also be adopted in the treatment of bone metastases in GISTs, but they have a palliative role, reducing bone pain or preventing pathologic fractures and other skeletal-related events. There are no standard criteria for imaging or metabolic assessment of tumor response, and few data are available on density changes in bone metastases after therapy.
Likewise, the clinical and molecular risk factors for bone metastases have yet to be defined. The primary tumours in our patients were localized in three different sites (small intestine, stomach and duodenum) and measured 3 cm in case 1, 8.5 cm in case #2 and 4 cm in case 3. The mitotic index was not available for case 1, was 16/50 HPF for case #2, and 5–10 /50 for case #3. Molecular analysis was performed in two patients (cases #2 and #3) and both presented a KIT exon 11 mutation (KIT exon 11 c.1696_1718del (p.N566_P573 delinsA) case 1; KIT exon 11 T>A 69429 (p.V559d) case #2). Hence, there was no correlation in our small cohort between GISTs clinical and pathological presentation, GISTs molecular status and bone metastases. A molecular analysis of bone metastases may serve to disclose any secondary KIT and PDGFRA mutations associated with bone metastatic spread.
In conclusion, bone metastases from GISTs are rare, but they may become more prevalent due to increased patient life expectancy as well as the improvement in imaging techniques and they should always be sought. Data on risk factors, molecular background, treatment response and prognostic significance are not defined yet and should be collected in a larger series.
References
- 1.Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–65. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 2.Miettinen M, Lasota J. Gastronitestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466–78. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 3.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastronitestinal stromal tumors. N Engl J Med. 2002;347:472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 5.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastronitestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–38. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 6.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 7.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–9. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 8.Casali PG, Jost L, Reichardt P, et al. ESMO guidelines working group. Gastrointestinal stromal tumours: ESMO Clinical Recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20:ii64–7. doi: 10.1093/annonc/mdp131. [DOI] [PubMed] [Google Scholar]
- 9.Nannini M, Biasco G, Di Scioscio V, et al. Clinical, radiological and biological features of lung metastases in gastrointestinal stromal tumors. Oncol Rep. 2011;25:113–20. [PubMed] [Google Scholar]
- 10.Barrière J, Thariat J, Vandenbos F, et al. Diplopia as the first symptom of an aggressive metastatic rectal stromal tumor. Onkologie. 2009;32:345–7. doi: 10.1159/000215712. [DOI] [PubMed] [Google Scholar]
- 11.Kaku S, Tanaka T, Ohtuka T, et al. Perisacral gastrointestinal stromal tumor with intracranial metastasis. Case report. Neurol Med Chir. 2006;46:254–7. doi: 10.2176/nmc.46.254. [DOI] [PubMed] [Google Scholar]
- 12.Ozan E, Oztekin O, Alacacioğlu A, et al. Esophageal gastrointestinal stromal tumor with pulmonary and bone metastases. Diagn Interv Radiol. 2010;16:217–20. doi: 10.4261/1305-3825.DIR.1861-08.2. [DOI] [PubMed] [Google Scholar]
- 13.Tezcan Y, Koc M. Gastrointestinal stromal tumor of the rectum with bone and liver metastasis: a case study. Med Oncol. 2010 Oct 17; doi: 10.1007/s12032-010-9697-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Tariq Z, Ghose A, et al. Gastrointestinal stromal tumor with primary resistance to imatinib and extensive bone metastases. Am J Ther. 2010 Jun 25; doi: 10.1097/MJT.0b013e3181d41eef. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Saad F. New research findings on zoledronic acid: survival, pain, and antitumor effects. Cancer Treat Rev. 2008;34:183–92. doi: 10.1016/j.ctrv.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Machado M, Cruz LSC, Tannus G, Fonseca M. Efficacy of clodronate, pamidronate, and zoledronate in reducing morbidity and mortality in cancer patients with bone metastasis: a meta-analysis of randomized clinical trials. Clin Therap. 2009;31:962–79. doi: 10.1016/j.clinthera.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Mhaskar R, Redzepovic J, Wheatley K, et al. Bisphosphonates in multiple myeloma. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD003188.pub2.D003188 [DOI] [PubMed] [Google Scholar]
- 18.Berenson JR. Therapeutic options in the management of myeloma bone disease. Semin Oncol. 2010;37:S20–9. doi: 10.1053/j.seminoncol.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Green JR. Antitumor effects of bisphosponates. Cancer. 2003 Feb;97(suppl 3):840–847. doi: 10.1002/cncr.11128. [DOI] [PubMed] [Google Scholar]
- 20.Morgan G, Lipton A. Antitumor effects and anticancer applications of bisphosphonates. Semin Oncol. 2010;37:530–40. doi: 10.1053/j.seminoncol.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Koto K, Murata H, Kimura S, et al. Zoledronic acid inhibits proliferation of human fibrosarcoma cells with induction of apoptosis, and shows combined effects with other anticancer agents. Oncol Rep. 2010;24:233–9. doi: 10.3892/or_00000851. [DOI] [PubMed] [Google Scholar]
- 22.Di Salvatore M, Orlandi A, Bagalà C, et al. Anti-tumour and anti-angiogenetic effects of zolederonic acid on human non small-cell lung cancer cell line. Cell Prolif. 2011;44:139–46. doi: 10.1111/j.1365-2184.2011.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


