Abstract
We present a patient (male 26 years) with a short history of recurrent seizures induced by a largely intracerebrally located frontal lobe meningioma. The tumor displayed a heretofore unpublished combination of extensive metaplastic bone formation and prominent non-psammomatous calcifications with focal chicken-wire pattern.
Key words: meningioma, brain, ossification, metaplastic, calcification.
Introduction
Meningiomas are relatively common tumors derived from arachnoidal cells and most frequently occur in association with intra-cranial meninges. They make up about 30 per cent of primary brain and central nervous system tumors.1 Whilst psammomatous meningiomas may be associated with metaplastic bone formation, metaplastic meningiomas with significant ossification are distinctly uncommon. In this study, we present a metaplastic meningioma which in addition to widespread ossification also exhibited extensive calcifications which focally displayed a distinctive pattern of non-psammomatous (chicken-wire) calcification. This combination of histological features has, to the best of our knowledge, not been reported previously.
Case Report
The patient is a previously healthy 26-year-old man who developed recurrent generalized tonic-clonic seizures over a period of 3 months. A magnetic resonance imaging (MRI) of the brain showed a 4 cm large, rounded, heavily calcified tumor predominantly located in the brain parenchyma (right frontal region), causing underlying edema and left sided midline shift.
The tumor was attached to the dura by a very thin tail Figure 1. The tumor was excised.
Figure 1.
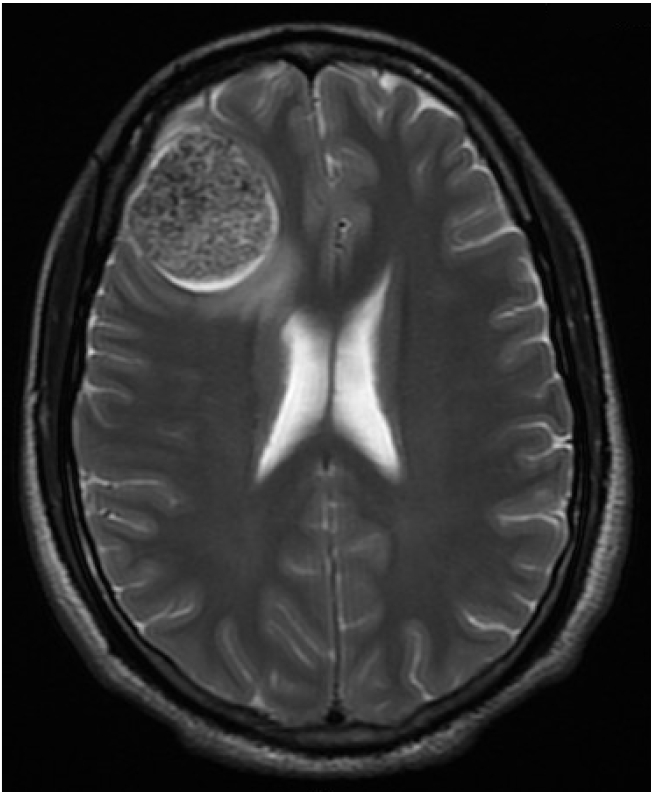
A right frontal lobe tumor. Magnetic resonance imaging scan of the brain was performed and revealed a 4.0− cm calcified tumor associated with edema of the adjacent brain parenchyma.
Materials and Methods
The tissue was fixed in formalin and embedded in paraffin. Four-micron thick sections were cut and stained with Hematoxylin and Eosin (H&E). An immunohistochemical study was performed with progesterone receptor antibody (Ventana) and the following primary antibodies from DAKO: epithelial membrane antigen (EMA [1:200]), CD34 (1:200) and S-100 protein (1:2000).
Results
The excision specimen revealed firm tissue with white, gritty and granular cut surface.
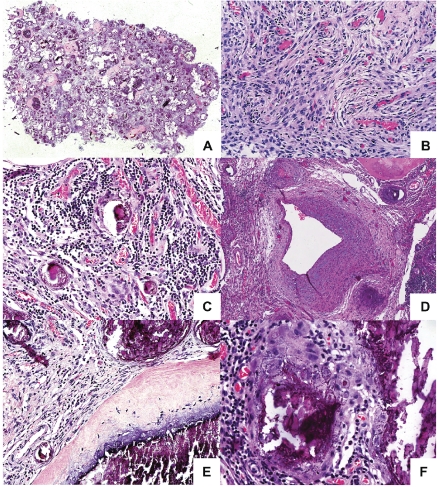
Routine histological examination disclosed a heterogeneous tumor composed of large areas of mature osseous tissue, conspicuous calcified nodules of varying sizes and intervening more cellular areas (Figure 2A).
Figure 2.
A heterogeneous tumor with areas of calcification and bone formation. (A) Tumor seen at low power showing cellular areas with areas of calcification and ossification. (B) The cellular areas are composed of plump, elongated cells with meningothelial features. (C) Prominent lymphoplasmacytic infiltrate is present within the cellular areas. (D) The highly vascularized tumour also contain dysplastic blood vessels. (E) The areas of calcification show crystalline deposits of calcium merge seamlessly with woven bone. No osteoblastic rimming is seen. (F) An interlacing chicken-wire-like calcification is seen in several areas.
The cellular areas of the tumor were composed of plump to spindly elongated cells forming a fascicular pattern in some areas, and syncytial sheets and whorls in others. The cells had indistinct cell borders and oval nuclei containing delicate chromatin and small nucleoli (Figure 2B). In the non-mineralized areas, a significant lymphoplasmacytic infiltrate was present (Figure 2C). The tumor was highly vascularized. In addition to numerous small caliber vessels, scattered large blood vessels with a dysplastic appearance, including walls showing very irregular thickness and focally myxoid features (Figure 2D).
A striking feature of the neoplasm was prominent calcified nodules of varying sizes that were seen throughout the tumor. The calcifications formed crystalline deposits with no (psammomatous) concentrically laminated rings, and merged with spicules of woven bone which displayed no definite osteoblastic rimming (Figures 2E and 3). In several calcified areas, wire-like streaks of calcification could be seen surrounding ghost cell outlines, forming patterns reminiscent of chicken wires Figure 2F). Focally, the osseous islands showed myxoid-edematous degenerative changes. In the immunohistochemical study, the cells uniformly showed cytoplasmic expression of EMA and some of the cells displayed nuclear expression of progesterone receptor (Figure 4A and B). Only scattered cells expressed S-100. No expression of CD34 was detected.
Figure 3.
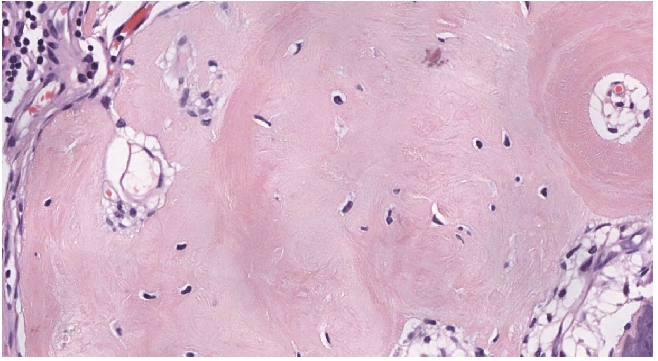
Bone formation within the tumour. Bony trabeculae with no osteoblastic rimming is seen within the tumour.
Figure 4.
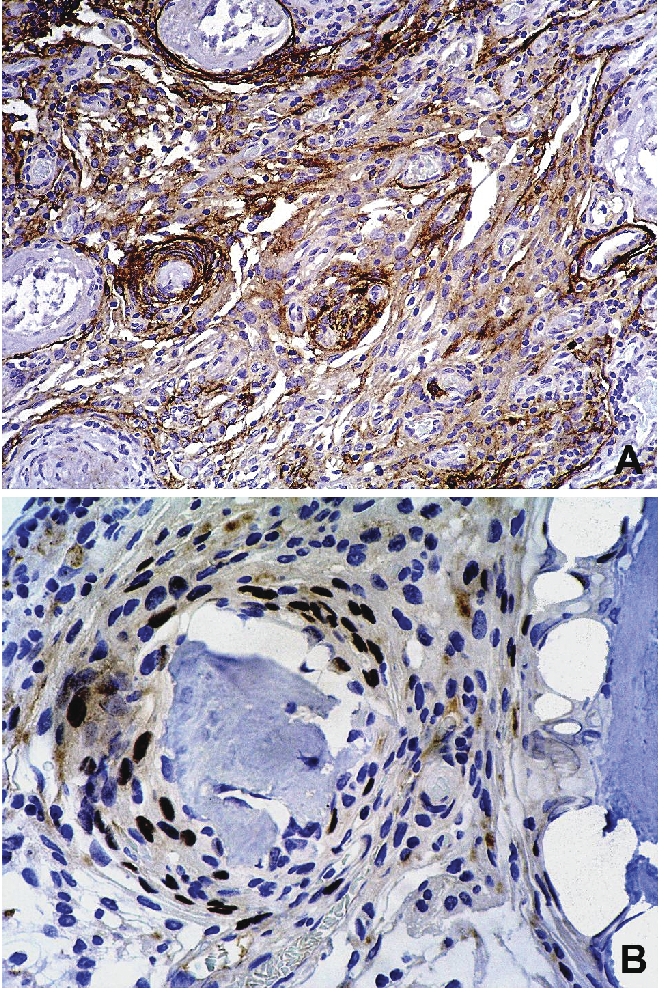
The tumor expresses epithelial membrane antigen and progesterone receptor. Sections of the tumor were stained with anti-EMA and anti-progesterone receptor primary antibodies and couterstained with hematoxylin. (A) Diffuse expression of cytoplasmic EMA is seen. (B) Scattered cells show nuclear expression of progesterone receptor.
Discussion
Whilst all meningiomas show characteristic ultrastructural features of well-formed desmosomes and interdigitating processes on electron microscopy, there is a great diversity in morphological presentation on a light microscopical level. Metaplastic meningioma (MM) constitute an uncommon subset of low grade meningiomas that contain a significant component of fat, bone, cartilage or myxoid tissue. Reportedly, the most commonly encountered mesenchymal tissue component in metaplastic meningioma is mature adipose tissue.2 Extensive ossification in MM is distinctly uncommon. To date, it is not known how these metaplastic changes occur. Bone morphogenetic protein-2 (BMP2), a cytokine involved in osteogenesis, was found to be expressed in a case of ossified en plaque psammomatous meningioma of the thoracic spine.3 However, it has not been shown if BMP2 plays a direct role in the ossification process or if it is also expressed in the more common meningothelial meningiomas. It is also not clear if the ossification process in psammomatous meningiomas and metaplastic meningiomas are significantly different, although they are classified as distinct histologic subtypes. To the best of our knowledge, the vast majority of reported cases of extensively ossified meningiomas have contained psammoma bodies.3–6 However, one case of a meningioma (in the lacrimal fossa) which showed osseous metaplasia without significant non-osseous calcifications is on record.7 In our case, the extensive calcium deposits formed crystalline structures rather than the characteristic concentric laminations of psammoma bodies and the basophilic calcifications transitioned seamlessly into wove bone in many areas. An interesting finding in the tumor presented herein was the chicken-wire-type calcifications. This pattern of calcification is very similar to what is commonly encountered in chondroblastomas (CB) and has not previously been reported in a metaplastic meningioma. Chondroblastomas are rare primary bone tumors (1% of all primary bone neoplasms) which most commonly occur in the epiphysis of long bones with only 1% seen in the skull (Kobayashi Y 2001). Especially on small biopsy specimens with a paucity of lesional cells, the rare examples of the latter category may pose differential diagnostic difficulties to a MM where this type of calcification pattern is encountered. However, paying close attention to the morphological features of CB should help to resolve this. CB has a characteristic matrix (pink cartilage) and the lesional cells are rounded-epithelioid with round to ovoid grooved nuclei and the presence of multinucleated giant cells in CB are in contrast to the whorls and spindly morphology of most meningothelial cells which also frequently display nuclear inclusions. Of note is that immunohistochemically, in addition to expressing S-100 protein, chondroblastomas have been shown to express EMA.8 Osteoblastomas may arise both in the periosteum of the calvarium (calvarial periosteal osteoblastomas9,10 and in an extra-osseous and intracranial locations with similar radiologic features as a meningioma.11 This is especially important since in one case located in the calvarium, EMA expression was detected.9 Another differential diagnosis that should be contemplated is the calcifying pseudotumor of the neural axis. This rare lesion presents as a granular mass located in the meninges and is characterized by amorphous calcifying material surrounded by palisading epithelioid cells. Importantly, published histological features of the calcifying pseudotumor of the neural axis that overlap with those seen in our case are: an interlacing linear pattern of calcification, occasional ossification and EMA expression of the epithelioid cells.12,13 Ossifying fibroma (OF), especially the juvenile psammomatoid (JPOF) variant14 of the skull is also a differential diagnostic possibility. OF is composed of a fibroblastic stroma admixed with woven and lamellar bone and basophilic cementum-like material. The proportions of the various components may vary significantly. Furthermore (which adds to the potential differential diagnostic difficulties), JPOF may contain thread-like calcifications in hyaline deposits of collagenous tissue. However, the osseous tissue in all variants of OF characteristically shows osteoblastic rimming that was conspicuously absent in our case. In addition, bone-invading meningioma could mimic a metaplastic meningioma especially in small biopsies and close correlation with biopsy site will be necessary.
The prominent lymphoplasmacytic infiltration present in our case gave a vague association to the recently described IgG4-related sclerosing pachymeningitis.15 In our case, the absence of areas of sclerosis and obstructive phlebitis and the observation that the majority of the cells were lymphocytes (although with focally significant numbers of plasma cells), the predominantly intracerebral location and the results of the immunohistochemical study, strongly militate against this diagnostic possibility. In addition, the patient did not show any clinical features to suggest the possibility of IgG4-related sclerotic disease elsewhere.
In summary, we present a case of a metaplastic meningioma with widespread ossification and extensive non-psammomatous calcification with focal areas forming linear chicken-wire patterns. The unique pattern of calcification and bone formation has, to the best of our knowledge, not been reported previously and may give rise to a number of interesting, some of which probably just as rarely occurring, differential diagnostic possibilities.
References
- 1.Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99:307–14. doi: 10.1007/s11060-010-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roncaroli F, Scheithauer BW, Laeng RH, et al. Lipomatous meningioma: a clinicopathologic study of 18 cases with special reference to the issue of metaplasia. Am J Surg Pathol. 2001;25:769–75. doi: 10.1097/00000478-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Uchida K, Nakajima H, Yayama T, et al. Immunohistochemical findings of multiple ossified en plaque meningiomas in the thoracic spine. J Clin Neurosci. 2009;16:1660–2. doi: 10.1016/j.jocn.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Kato K, Chernov M, Urino T, et al. Ossified frontosphenoorbital meningioma en plaque, mimicking extensive hyperostosis. Minim Invasive Neurosurg. 2008;51:237–9. doi: 10.1055/s-2008-1080906. [DOI] [PubMed] [Google Scholar]
- 5.Liu CL, Lai PL, Jung SM, Liao CC. Thoracic ossified meningioma and osteoporotic burst fracture: treatment with combined vertebroplasty and laminectomy without instrumentation: case report. J Neurosurg Spine. 2006;4:256–9. doi: 10.3171/spi.2006.4.3.256. [DOI] [PubMed] [Google Scholar]
- 6.Naderi S, Yilmaz M, Canda T, Acar U. Ossified thoracic spinal meningioma in childhood: a case report and review of the literature. Clin Neurol Neurosurg. 2001;103:247–9. doi: 10.1016/s0303-8467(01)00157-3. [DOI] [PubMed] [Google Scholar]
- 7.Decock CE, Kataria S, Breusegem CM, et al. Ectopic meningioma anterior to the lacrimal gland fossa. Ophthal Plast Reconstr Surg. 2009;25:57–9. doi: 10.1097/IOP.0b013e3181936811. [DOI] [PubMed] [Google Scholar]
- 8.Semmelink HJ, Pruszczynski M, Wiersmavan Tilburg A, et al. Cytokeratin expression in chondroblastomas. Histopathology. 1990;16:257–63. doi: 10.1111/j.1365-2559.1990.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 9.Tawil A, Comair Y, Nasser H, et al. Periosteal osteoblastoma of the calvaria mimicking a meningioma. Pathol Res Pract. 2008;204:413–22. doi: 10.1016/j.prp.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Lin YC, Commins DL, Fedenko AN, Pinsky GS. A rare case of periosteal osteoblastoma located in the frontal cranial bone. Arch Pathol Lab Med. 2005;129:787–9. doi: 10.5858/2005-129-787-ARCOPO. [DOI] [PubMed] [Google Scholar]
- 11.Al Mestady RM, Alorainy IA, El Watidy SM, Arafah MM. Intracranial extraosseous chondroblastoma simulating meningioma. AJNR Am J Neuroradiol. 2007;28:1880–1. doi: 10.3174/ajnr.A0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian J, Rubio A, Powers JM, et al. Fibroosseous lesions of the central nervous system: report of four cases and literature review. Am J Surg Pathol. 1999;23:1270–5. doi: 10.1097/00000478-199910000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Tsugu H, Fukushima T, Takeno Y. Calcifying pseudotumor of the neural axis-- case report. Neurol Med Chir. 1999;39:762–5. doi: 10.2176/nmc.39.762. [DOI] [PubMed] [Google Scholar]
- 14.Bohn OL, Kalmar JR, Allen CM, et al. Trabecular and Psammomatoid Juvenile Ossifying Fibroma of the Skull Base Mimicking Psammomatoid Meningioma. Head Neck Pathol. 2011;5:71–5. doi: 10.1007/s12105-010-0212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan SK, Cheuk W, Chan KT, Chan JK. IgG4-related sclerosing pachymeningitis: a previously unrecognized form of central nervous system involvement in IgG4-related sclerosing disease. Am J Surg Pathol. 2009;33:1249–52. doi: 10.1097/PAS.0b013e3181abdfc2. [DOI] [PubMed] [Google Scholar]