Abstract
A 75-year-old female, who had an abnormal stomach x-ray finding, was admitted to the hospital for further examination and therapy. Upper GI endoscopy showed reddish and swollen folds on the greater curvature of the gastric body and a biopsy was of this lesion revealed malignant lymphoma (small cell type or mucosa-associated lymphoid tissue (MALT) lymphoma suspected). The patient was infected with Helicobacter pylori (H. pylori), however, in response to the patient's wishes, a total gastrectomy, omentectomy and splenectomy were performed and the histological diagnosis was gastric MALT lymphoma. Two courses of CHOP therapy (cyclophosphamide (CPM) 750 mg/m2/day, day 1, adriamycin (ADM) 50 mg/m2/day, day 1, vincristine sulfate (VCR) 1.4 mg/m2/day, day 1, prednisolone 100 mg/body, day 1–5) were administered as adjuvant chemotherapy. A colonoscopic examination performed about 4.5 yr after the operation revealed rectal submucosal tumors and the biopsied specimens were diagnosed as malignant lymphoma. A transanal focal resection was performed and the histological diagnosis was metachronous and ectopic development of MALT lymphoma. The histological finding was similar to the gastric lesion. About 4 and 7 yr after the first development of rectal MALT lymphoma, MALT lymphomas developed repeatedly in the rectal lesion, however, these were resected repeatedly and no developmenthas occurred during the past two years. This report presents a very rare case of metachronous and ectopic MALT lymphoma development in the gastric and rectal lesions.
Key words: mucosa-associated lymphoid tissue lymphoma, metachronous development, ectopic development, CHOP therapy, eradication of H. pylori.
Introduction
Mucosa-associated lymphoid tissue (MALT) lymphoma is a lymphoma derived from B-cells originating from the marginal zone of the MALT in an extra-nodal organ in the presence of chronic inflammations in the gastrointestinal tract, thyroid gland, salivary gland, lung and so on.1,2 Many cases of MALT lymphoma of the stomach have been reported to date in association with an Helicobacter pylori (H. pylori) infection, however, the development of MALT lymphoma in the colon and rectum without the apparent etiology such as an H. pylori infection is comparably rare. Anecdotal reports in which MALT lymphomas develop metachronously in the same or different organs have been reported to date.3–9 In the present case, a focal resection was performed for MALT lymphoma developed metachronously and ectopically in the rectum 3 years after a total gastrectomy for gastric MALT lymphoma followed by 2 courses of adjuvant chemotherapy. MALT lymphomas developed repeatedly in the rectal lesion, however, these were focally resected repeatedly and no recurrence has occurred over the past two years. This report presents a very rare case of successfully treated MALT lymphomas that developed metachronously and ectopically in gastric and rectal lesions.
Case Report
A 75-year-old woman showed an abnormality in the findings of barium X-ray screening for gastric cancer and was transferred to our hospital (Medical Hospital, The Nippon Dental University School of life Dentistry at Niigata) for further examination and therapy in October 1995. Upper GI endoscopy revealed swollen giant folds of the body (Figure 1A, 1B). A biopsy was performed and malignant lymphoma (small cell type or MALT lymphoma) was suspected. The background mucosa of the stomach was positive for an H. pylori infection in a retrospective pathological examination. We did not perform any chromosomic study at that time. Very recently, we performed the FISH assay to evaluate the translocation of t(11;18) in formalin fixed tissues of gastric and colonic tumors, however, we could not perform any proper examination on this translocation because the tumor tissues with formalin fixation were in bad condition. A computed tomography (CT) scan of the chest, abdomen and pelvic lesion demonstrated no swollen lymph nodes. Although there were several options for treatment, the patient and her family requested that the lesion be treated surgically. A total gastrectomy, omentectomy and splenectomy (D2 resection of regional lymph nodes and Roux-en-Y gastrojejunostomy) were thus performed in November 1995 (Figure 2A, B). The histopathological diagnosis was MALT lymphoma (Figure 3 A,B) with a slight invasion into the spleen. Lymphoepithelial lesion (LEL), positive immunoreactivity for CD20 (L26) and CD79a, and negative immunoreactivity for cyclin D1 and CD5 were observed (figure not shown). A Positive IgG-rearrangement and monoclonal proliferation were confirmed (figure not shown). The depth of greatest invasion of the tumor was the muscularis propria. No metastatic lymph nodes and no vestigial remnant of the tumor were seen. Therefore, the patient was diagnosed as stage∣∣E by Ann Arbor classification,10 as stage∣∣E1 by Lugano International classification11 and as stage∣∣1 by Blackledge Staging System.12 Two courses of CHOP therapy (cyclophosphamide (CPM) 750 mg/m2/day, day 1, adriamycin (ADM) 50 mg/m2/day, day 1, vincristine sulfate (VCR) 1.4 mg/m2/day, day 1, prednisolone 100 mg/body, day 1–5) were administered as an adjuvant chemotherapy, beginningDecember 11, 1995. Afterward, the patient was followed as an outpatient. A colonoscopic examination in May, 1999 showed two rectal submucosal tumors (SMT), 2 cm or less in diameter (Figure 4A, B). A biopsy was performed and malignant lymphoma was suspected. A trans-anal focal resection of the tumor was performed in May, 1999. The histopathological diagnosis was MALT lymphoma which was similar to the gastric lesion which had been resected previously (Figure 3C, D). Lymphoepithelial lesion (LEL), positive immunoreactivity for CD20 (L26) and CD79a, and negative one for cyclin D1 and CD5 were observed (figure not shown). Colonoscopy in February, 2003 showed a rectal SMT (figure not shown) and the biopsy specimens were diagnosed as lymphoid hyperplasia. Trans-anal focal resection of the tumor was performed in March, 2003. The histopathological diagnosis also suggested lymphoid hyperplasia but could not be diagnosed as an apparent lymphoma. Afterward, colonoscopy performed in May, 2006 again revealed SMT-like lesions (figure not shown). A biopsy was performed and the pathological diagnosis was malignant lymphoma as with the previously resected tumors. A trans-anal focal resection was again performed in June, 2006. The pathological diagnosis was MALT lymphoma, which strongly correlated with the pathological findings of the tumor resected in May, 1999 and in March, 2003, however, the malignant cells of the tumor resected in June, 2006 were characterized by well differentiated plasma cells (figure not shown). The patient is currently being followed as an outpatient without additional adjuvant chemotherapy, however, the patient shows no sign of recurrence of MALT lymphoma and her serum IL2-recepter levels remain within the normal limits.
Figure 1.
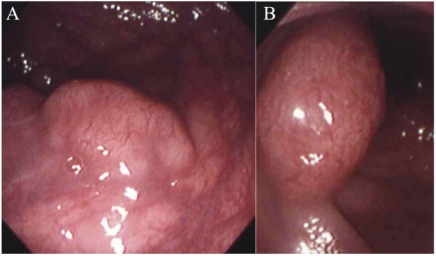
(A) Upper gastrointestinal endoscopic findings on 31 October 1994 showed swollen and reddish folds with erosions. (B) Upper gastrointestinal endoscopic findings on 31 October 1994 stained with indigotindisulfo nate sodium (indigo carmine).
Figure 2.
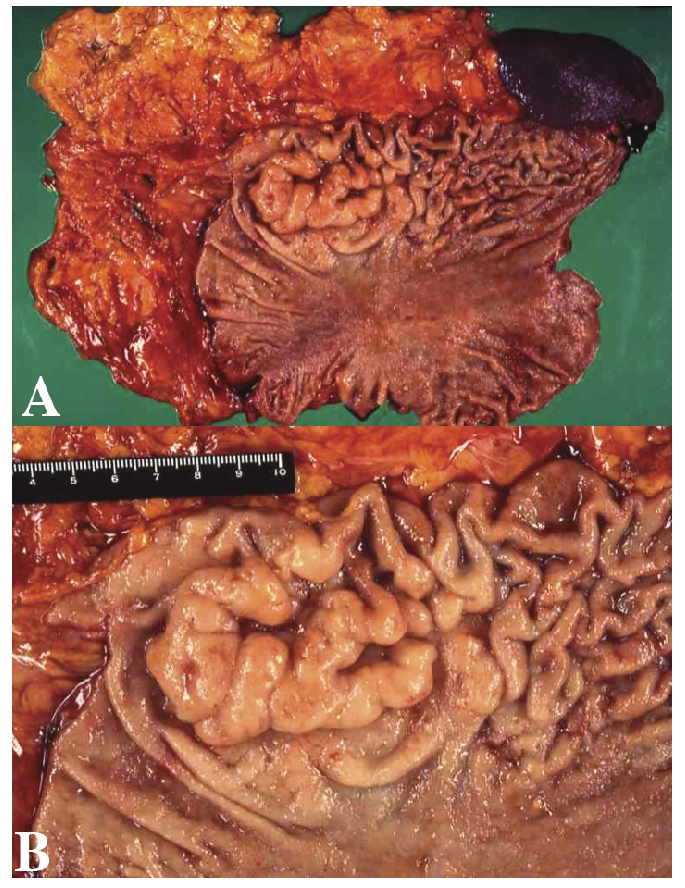
(A) Gross appearance of the entire resected stomach, greater omentum and spleen. (B) Gross appearance of the resected gastric lesion of MALT lymphoma. Swollen folds, so-called giant folds with erosions were observed on the greater curvature of the gastric body.
Figure 3.
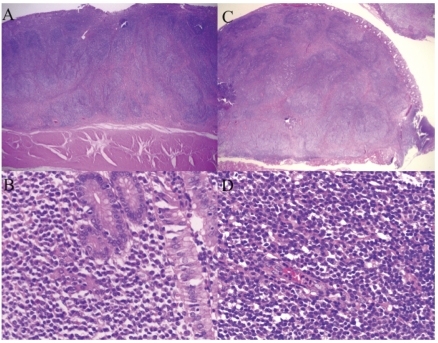
(A) A low-power histological picture of the gastric MALT lymphoma lesion resected at the first operation on 21 November 1995 showing distinct, poorly circumscribed lymphoid follicles and the infiltration of lymphoma cells into the muscularis propria of the gastric wall. The lesions were covered with normal mucosa (H & E stain). (B) High-power view showing the diffuse proliferation of many small centrocyte-like cells (H & E stain). (C) The histopathological diagnosis of the rectal submucosal tumors focally resected on 10 June 1999 was MALT lymphoma which was similar to the gastric lesion which had been resected on 21 November 1995. (D) A low-power histological picture of the rectal MALT lymphoma lesion focally resected on 10 June 1999 showing distinct, poorly circumscribed lymphoid follicles and the infiltration of lymphoma cells into the muscularis propria of the gastric wall. The lesions were covered with normal mucosa (H & E stain).
Figure 4.
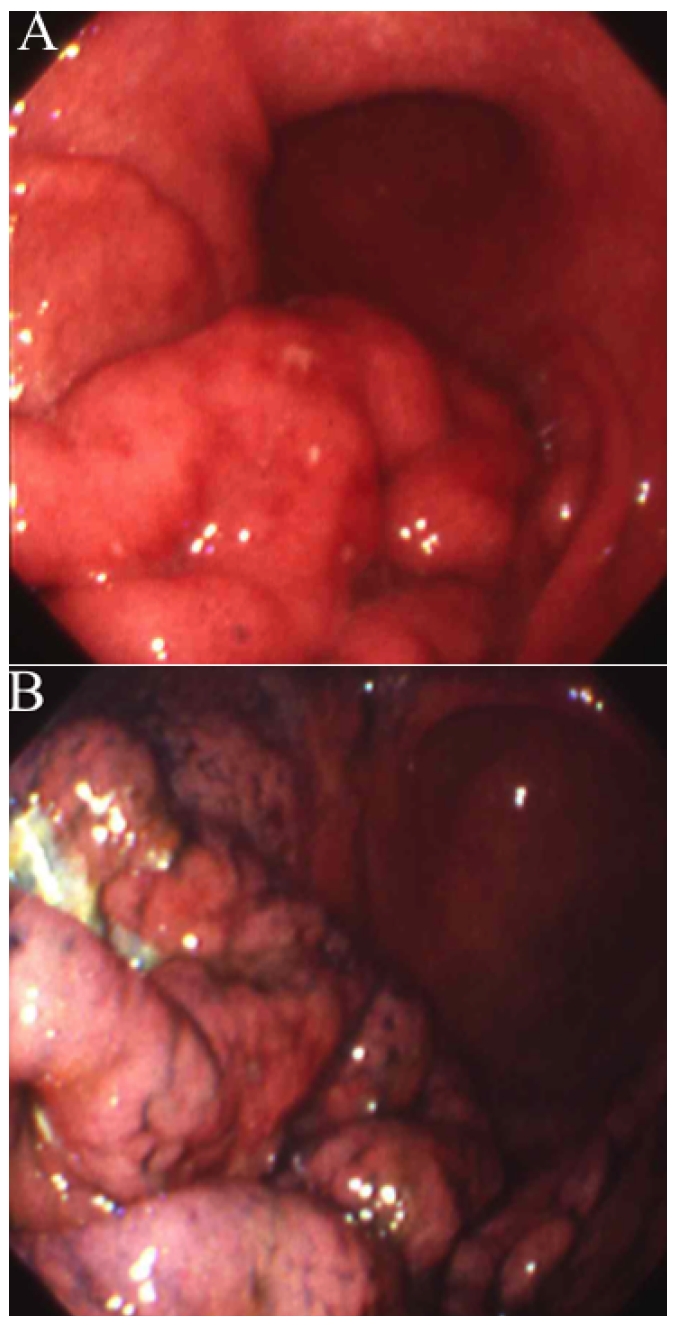
(A)(B) Colonoscopic findings on 13 May 1999, about 3 and a half years after the first operation for gastric MALT lymphoma, showed two SMT-like lesions in the rectum. The biopsy specimens indicated that the lesions were suspected to be malignant lymphomas.
Discussion
MALT lymphoma is a low grade malignant B-cell lymphoma that develops from mucosa-associated lymphoid tissue (MALT). The concept of MALT lymphoma was first introduced by Isaacson et al.13 The pathological features of MALT lymphoma are as follows: i) lymphoma cells with faint, bright and abundant cell body (clear cells), and mildly pinched-off nuclei (Centrocyte-like cells: CCLCs); ii) a diffuse proliferation in the marginal zone of monocytic B-cells and into the follicles; iii) tumor cells likely to be differentiated into the plasma cells (lymphoplasmacytoid cells); iv) lymphoepithelial lesions (LEL) and so on.13–15 Centrocyte-like cells, diffuse proliferation in the marginal zone of monocytic B-cell lymphoma cells, LEL, positive IgG-reconstruction and monoclonal proliferation were observed in the current case. In addition, the histopathological examinations revealed positive immunoreactivity for CD20 (L26) and CD79a and negative immunoreactivity for cyclin D1 and CD5. Therefore, with the case herein presented many of the above-mentioned features of MALT lymphoma. The surgical specimen in the last (third) focal resection showed that the tumor cells appeared to be well differentiated plasma cells, partly because a lymphoma cell characterized as a MALT lymphoma may develop and proliferate monoclonally over an extended time through metachronous development. Since many of metachronous and multiple MALT lymphoma cases can arise from identical cells, the MALT lymphoma which developed metachronously in the gastric and rectal lesions may have arisen from identical lymphoma cells and was more likely to be differentiated into plasma cells.
MALT lymphoma is likely to develop from inflammatory lesions after a prior infection, not only an H. pylori infection of the stomach, but also autoimmune diseases, such as, Torsten Sjogren's syndrome of salivary gland and thymus and Hashimoto's disease (hypothyroidism) of thyroid have been associated with the development of MALT lymphoma.2 While MALT lymphomas in the gastrointestinal tract are likely to develop from gastric lesions associated with H. pylori infection; MALT lymphoma development in the colon and rectum in which H. pylori infection and other apparent inflammation are not seen, is comparatively rare. Patients with MALT lymphoma have a higher incidence of colonic involvement than previously reported.
Furthermore, Yoshino et al.16 and Thieblemont et al.17 reported that synchronous and multiple MALT lymphoma cases occur in 7 (2.3%) of 304 (Yoshino et al.), 54 (35.1%) of 154 (Thieblemont et al.) MALT lymphoma cases, respectively. These incidences are different, but low in general. Metachronous MALT lymphoma cases (including the cases with metachronous development of MALT lymphoma and other lymphoma types) are very rare. A literature search of Japana Centra Revuo Medicina identified only 3 cases7–9 of metachronous MALT lymphomas (Key words: MALT lymphoma, metachronous, case reports excluding the minutes of oral presentations, retrieval period: 1983–2008) and a search of MEDLINE found only 4 cases3–6 of metachronous MALT lymphomas (Key words: MALT lymphoma, metachronous). No cases of MALT lymphomas that develop metachronously and ectopically in gastric and rectal lesions have been reported and this present case seems to be the first reported to date.
There are various macroscopic forms of MALT lymphoma. Most gastric MALT lymphoma show surface-accretive lesions with groveling progress in the mucosa because the mucosal invasive type accounts for many of the gastric MALT lymphoma cases. In contrast, because colonic MALT lymphomas are likely to progress mainly in the submucosal layers, most colonic MALT lymphomas appear to be a macroscopic form of submucosal tumor (SMT).18,19 In the present case, the gastric lesion showed swollen and reddish folds on the greater curvature and the colonic lesion showed a flat elevation like a SMT. These macroscopic forms were characteristic of MALT lymphoma as described above.
MALT lymphomas also show characteristic chromosomal and genetic features. Most MALT lymphomas have API2/MALT1 chimeric DNA resulting from a translocation of t(11;18).20,21 These chromosomal changes are recognized in about 50% of all MALT lymphomas and in 10–20% of all gastric MALT lymphomas.22 Additional chromosomal changes include trisomy 323 and t(1;14) with a translocation of the Bcl10-gene.24 Many of the MALT lymphoma cases with the t(11;18) translocation are resistant to eradication of H. pylori and show multiorgan involvement,25,26 although, there is little or no concern of their developing malignant potential. However, several previous reports indicate that, in most MALT lymphoma patients with multiple organ involvement, the multiple lesions consisted of an identical clone.27–29 Iwano et al. reported that most API2-MALT1-positive gastric MALT lymphomas with multiple lesions or multiple organ involvement appear to originate from a single lesion and disseminate to other lesions or organs.30 In the present case, the presence of t(11;18), API2-MALT1 fusion transcripts and the monoclonality of CDR3 was not determined, partly because there was no frozen sample of the tumor lesions. Therefore, it is impossible to determine whether the primary lesion metastasized to the rectum or whether the MALT lymphomas developed metachronously from the background so common in the gastric and rectal lesions. In the current case, the rectal MALT lymphoma developed about 3 and a half years after the operation for the gastric lesion. The incidence of metastasis from a gastric lesion to the rectum is very low, with respect to the metastatic pathway. The background factors common to gastric and rectal lesions are not known nor is the association between the infection and the inflammation caused from the infection or other factors. However, anecdotal reports have indicated that antibiotics were effective for treating colonic MALT lymphoma regardless of the presence of an H. pylori infection in the gastric lesion.18,19,31 These reports suggested the possibility of a correlation between colonic MALT lymphoma development and colonic bacteria.
In contrast, Grünberger et al. reported that 16 patients with extra-gastric MALT lymphoma and positive H. pylori infection in the gastric lesion underwent eradication before the initiation of definitive treatment with extra-gastric MALT lymphoma and none of these 16 patients showed regression of the lymphoma.32 This report suggested that H. pylori-eradication or antibiotic treatment targeting H. pylori was ineffective for treatment of extra-gastric MALT lymphomas.32
In the past, the first line of therapy for the limited type of malignant lymphoma has been a resection of the tumor including the gastric lesion. Adjuvant chemotherapy has been administered in Japan and adjuvant radiotherapy in Western countries and the United States for cases with positive lymph node metastasis and focal progression. The concept of MALT lymphoma was introduced in the 1980s1,13 and the correlation with H. pylori infections was initially suggested in 1995.33–35 Eradication of H. pylori is now considered to be an internationally accepted standard therapy for the limited type of gastric MALT lymphoma. In particular, in those cases with positive H. pylori infections, eradication of the infection has been shown to improve the cure rate from 80% to 90%. Even if the patients are negative for H. pylori infections, eradication is advantageous in the cases positive for H. heil-mani or false-negative for H. pylori infections, however, most cases negative for H. pylori infections have the API2/MALT1 transcripts produced by the translocation of t(11;18),22 reveal multiorgan involvements and show no effect of H. pylori eradication.
A salvage operation is the standard therapy for patients who do not respond to eradication, however, radiation therapy,36,37 administration of CHOP therapy,38 single drug therapy with cyclophosphamide,39 cladribine,40 etc. and Rituximab (anti-CD20 antibody)41–43 are other selected forms of treatment.
No therapy has been established for colonic MALT lymphoma. Anecdotal reports have described effective eradication for colonic MALT lymphoma as with gastric lesions, regardless of H. pylori infection status in the stomach,19,31,44,45 and it would seem appropriate to perform eradication therapy because it involves simple oral therapy for 7 days. However, it has been reported that eradication therapy was not effective for extra-gastric MALT lymphoma in one study32 and there are a number of questions of its therapeutic effect. Surgical resection is the most frequent therapeutic procedure for rectal MALT lymphoma in Japan. Further more, CHOP therapy and oral cyclophosphamide,39 etc., or Rituximab etc. are used as they are with gastric MALT lymphoma.
In the present case, eradication seems appropriate to the initially selected therapeutic procedure, because the patient was positive for H. pylori infection. Ho wever, in 1995, when the correlations between MALT lymphoma and H. pylori were initially reported, the patient and her family strongly requested a surgical resection. Therefore, a total gastrectomy was performed. The pathological examination suggested the involvement of the regional lymph nodes and the invasion into the spleen, thus two courses of CHOP therapy were added as adjuvant chemotherapy, however, rectal MALT lymphomas thereafter developed metachronously and repeatedly. Anecdotal reports on the effectiveness of eradication for colonic MALT lymphomas have been published19,31,44,45 and eradication therapy could have been administered, however, because the patient and her family requested a surgical operation, we judged that the advantages due to a preservation of a quality of life by not performing a colostomy were therefore greater than the disadvantages due to a relapse after a focal resection and thus selected a trans-anal focal resection of the tumor. It was strongly suggested that MALT lymphomas are likely to develop due to some background factor common in the stomach and rectum.
These common factors in the stomach and rectum are unknown, however, because several cases in which antibiotics for eradication were effective, have been reported to date,19,31,44,45 such factors may be an infection of some kind other than H. pylori.
Eradication therapy was administered in August 2006, to prevent further metachronous developmentof MALT lymphoma. In the current case, careful observations, including periodical endoscopic examinations, computed tomography (CT) scans, measurement of the levels of serum interleukin-2 (s-IL-2) receptor, are needed. Further experimental and clinical research on the most appropriate treatments for colonic MALT lymphoma is awaited with great interest.
References
- 1.Isaacson PG, Spencer J. Malignant lymphoma of mucosa-associated lymphoid tissue. Histopathology. 1987;11:445–62. doi: 10.1111/j.1365-2559.1987.tb02654.x. [DOI] [PubMed] [Google Scholar]
- 2.Takeshita M, Iwashita A, Yao T, et al. [Clinicopathological features of colonic malignant B cell lymphomas] I To Cho. 1998;33:405–14. [Google Scholar]
- 3.Kuhnt T, Wollschläger B, Bloching M, et al. [Extranodal non-Hodgkin's lymphoma of MALT-type stage I A case report] Strahlenther Onkol. 2003;179:396–400. doi: 10.1007/s00066-003-1020-5. [DOI] [PubMed] [Google Scholar]
- 4.Kröber SM, Aepinus C, Ruck P, et al. Extranodal marginal zone B cell lymphoma of MALT type involving the mucosa of both the urinary bladder and stomach. J Clin Pathol. 2002;55:554–7. doi: 10.1136/jcp.55.7.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright M, Maclean H, Ironside J. Metachronous lymphoma of the breast and conjunctiva. Br J Ophthalmol. 1996;80:574–574. doi: 10.1136/bjo.80.6.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimizu H, Takiguchi Y, Kobayashi H, et al. [Case of metachronous mucosa-associated lymphoid tissue (MALT) lymphoma originated from a distinct B-cell clone] Nihon Kokyuki Gakkai Zasshi. 2008;46:759–63. [PubMed] [Google Scholar]
- 7.Fukami S, Hirabayashi H, Tanigaito Y, et al. [A Case of MALT Lymphoma of the Larynx Occurring Asynchronously in the Parotid Gland] Jibi-inkoka Rinsho (Practica Oto-Rhino-Laryngologica) 2005;98:315–21. [Google Scholar]
- 8.Sagawa T, Tsuji Y, Takayanagi N, et al. [Metachronous and Multiple Mucosa-Associated Lymphoid Tissue Lymphomas of the Cecum and Rectum] Gastroenterol Endoscopy. 2003;45:2111–7. [Google Scholar]
- 9.Iwai H, Okamura M, Sakaida N, et al. [A synchronous Low-Grade Malignant B-cell MALT Lymphoma of Parotid Glands; A Case Report] Jibi-inkoka Rinsho (Practica Oto-Rhino-Laryngologica) 1995;88:1439–45. [Google Scholar]
- 10.Carbone PP, Kaplan HS, Musshoff K, et al. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res. 1971;31:1860–1. [PubMed] [Google Scholar]
- 11.Rohatiner A, d'Amore F, Coiffier B, et al. Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Ann Oncol. 1994;5:397–400. doi: 10.1093/oxfordjournals.annonc.a058869. [DOI] [PubMed] [Google Scholar]
- 12.Blackledge G, Best JK, Crowther D. Role of computed tomography in staging and management of gastrointestinal lymphoma. J R Soc Med. 1979;72:818–22. doi: 10.1177/014107687907201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983;52:1410–6. doi: 10.1002/1097-0142(19831015)52:8<1410::aid-cncr2820520813>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Harris NL, Isaacson PG. What are the criteria for distinguishing MALT from non-MALT ltmphoma at extranodal sites? Am J Clin Pathol. 1999;111:S126–32. [PubMed] [Google Scholar]
- 15.Nakamura H, Nakamura T, Yatabe Y, et al. [Pathology of MALT lymphoma] I To Cho. 1998;33:271–80. [Google Scholar]
- 16.Yoshino T, Ichimura K, Mannami T, et al. Multiple organ mucosa-associated lymphoid tissue lymphomats often involve the intestine. Cancer. 2001;91:346–53. doi: 10.1002/1097-0142(20010115)91:2<346::aid-cncr1008>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Thieblemont C, Berger F, Dumontet C, et al. Mucosa-associated lymphoid tissue lymphoma is a disseminated disease in one third of 158 patients analyzed. Blood. 2000;95:802–6. [PubMed] [Google Scholar]
- 18.Inoue F, Chiba T. Regression of MALT lymphoma of the rectum after anti-H. pylori therapy in a patient negative for H. pylori. Gastroenterology. 1999;117:514–5. doi: 10.1053/gast.1999.0029900514b. [DOI] [PubMed] [Google Scholar]
- 19.Nakase H, Okazaki K, Ohana M, et al. The possible involvement of micro-organisms other than Helicobacter pylori in the development of rectal MALT lymphoma in H. pylori-negative patients. Endoscopy. 2002;34:343–6. doi: 10.1055/s-2002-23643. [DOI] [PubMed] [Google Scholar]
- 20.Akagi T, Motegi M, Tamura A, et al. A novel gene, MALT1 at 18q21, is involved in t(11;18)(q21;q21) found in low-grade B-cell lymphoma of mucosa-associated lymphoid tissue. Oncogene. 1999;18:5785–94. doi: 10.1038/sj.onc.1203018. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura T, Nakamura S, Yonezumi M, et al. Helicobacter pylori and the t(11;18)(q21;q21) translocation in gastric low-grade B-cell lymphoma of mucosa-associated lymphoid tissue type. Jpn J Cancer Res. 2000;91:301–9. doi: 10.1111/j.1349-7006.2000.tb00945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura T, Nakamura S, Yokoi T, et al. Clinicopathologic comparison between the API2-MALT1 chimeric transcript-positive and –negative gastric low-grade B-cell lymphoma of mucosa-associated lymphoid tissue type. Jpn J Cancer Res. 2002;93:677–84. doi: 10.1111/j.1349-7006.2002.tb01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wotherspoon AC, Finn TM, Isaacson PG. Trisomy 3 in low-grade B-cell lymphomas of mucosa-associated lymphoid tissue. Blood. 1995;85:2000–4. [PubMed] [Google Scholar]
- 24.Willis TG, Jadayel DM, Du MQ, et al. Bcl10 is involved in t(1;14)(p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell. 1999;96:35–45. doi: 10.1016/s0092-8674(00)80957-5. [DOI] [PubMed] [Google Scholar]
- 25.Inagaki H, Okabe M, Seto M, et al. API2-MALT1 fusion transcripts involved in mucosa-associated lymphoid tissue lymphoma: multiplex RT-PCR detection using formalin-fixed paraffin-embedded specimens. Am J Pathol. 2001;158:699–706. doi: 10.1016/S0002-9440(10)64012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenwald A, Ott G, Stilgenbauer S, et al. Exclusive detection of the t(11;18)(Q21;q21) in extranodal marginal zone B cell lymphomas (MZBL) of MALT type in contrast to other MZBL and extranodal large B cell lymphomas. Am J Pathol. 1999;155:1817–21. doi: 10.1016/S0002-9440(10)65499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du MQ, Xu CF, Diss TC, et al. Intestinal Dissemination of gastric mucosa-associated lymphoid tissue lymphoma. Blood. 1996;88:4445–51. [PubMed] [Google Scholar]
- 28.Wotherspoon AC, Doglioni C, Isaacson PG. Low-grade gastric B-cell lymphoma of mucosa-associated lymphoid tissue (MALT): a multifocal disease. Histopathology. 1992;20:29–34. doi: 10.1111/j.1365-2559.1992.tb00912.x. [DOI] [PubMed] [Google Scholar]
- 29.Kawamata N, Miki T, Fukuda T, et al. Determination of a common clonal origin of gastric and pulmonary mucosa-associated ltmphoid tissue lymphomas presenting 5 years apart. Intern Med. 1995;34:220–3. doi: 10.2169/internalmedicine.34.220. [DOI] [PubMed] [Google Scholar]
- 30.Iwano M, Okazaki K, Uchida K, et al. Characteristics of gastric B-cell lymphoma of mucosa-associated lymphoid tissue type involving multiple organs. J Gastroenterol. 2004;39:739–46. doi: 10.1007/s00535-004-1382-1. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto T, Iida M, Shimizu M. Regression of mucosa-associated lymphoid-tissue lymphoma of rectum after eradication of Helicobacter pylori. Lancet. 1997;350:115–6. doi: 10.1016/S0140-6736(05)61818-1. [DOI] [PubMed] [Google Scholar]
- 32.Grünberger B, Wöhrer S, Streubel B, et al. Antibiotic Treatment Is Not Effective in Patients Infected With Helicobacter pylori Suffering From Extragastric MALT lymphoma. J Clin Oncol. 2006;24:1370–5. doi: 10.1200/JCO.2005.02.9025. [DOI] [PubMed] [Google Scholar]
- 33.Isaacson PG. Gastric lymphoma and Helicobacter pylori. N Engl J Med. 1994;330:1310–1. doi: 10.1056/NEJM199405053301812. [DOI] [PubMed] [Google Scholar]
- 34.Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, et al. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338:1175–6. doi: 10.1016/0140-6736(91)92035-z. [DOI] [PubMed] [Google Scholar]
- 35.Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of primary low-grade B-cell lymphoma of mucosa-associated lymphoid tissue. Lancet. 1993;342:575–7. doi: 10.1016/0140-6736(93)91409-f. [DOI] [PubMed] [Google Scholar]
- 36.Miller TP, Dahlberg S, Cassady JR, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate and high-grade non-Hodgkin's lymphoma. N Engl J Med. 1998;339:21–6. doi: 10.1056/NEJM199807023390104. [DOI] [PubMed] [Google Scholar]
- 37.Schechter NR, Portlock CS, Yahalom J. Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone. J Clin Oncol. 1998;16:1916–21. doi: 10.1200/JCO.1998.16.5.1916. [DOI] [PubMed] [Google Scholar]
- 38.Raderer M, Valencak J, Osterreicher C, et al. Chemotherapy for the treatment of patients with primary high grade gastric B-cell lymphoma of modified Ann Arbor Stages ∣E and ∣∣E. Cancer. 2000;88:1979–85. doi: 10.1002/(sici)1097-0142(20000501)88:9<1979::aid-cncr1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 39.Hammel P, Haioun C, Chaumette MT, et al. Efficacy of single-agent chemotherapy in low-grade B-cell mucosa-associated lymphoid tissue lymphoma with prominent gastric expression. J Clin Oncol. 1995;13:2524–9. doi: 10.1200/JCO.1995.13.10.2524. [DOI] [PubMed] [Google Scholar]
- 40.Jager G, Neumeister P, Brezinschek R, et al. Treatment of extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type with cladribine: a phase ∣∣study. J Clin Oncol. 2002;20:3872–7. doi: 10.1200/JCO.2002.05.117. [DOI] [PubMed] [Google Scholar]
- 41.Martinelli G, Laszlo D, Ferreri AJM, et al. Clinical activity of rituximab in gastric marginal zone non-Hodgkin's lymphoma to or not elegible for anti-Helicobacter pylori therapy. J Clin Oncol. 2005;23:1979–83. doi: 10.1200/JCO.2005.08.128. [DOI] [PubMed] [Google Scholar]
- 42.Chaudhary N, Ozer H, Huard D, et al. Successful treatment of Helicobacter pylori-negative gastric MALT lymphoma with rituximab. Dig Dis Sci. 2006;51:775–8. doi: 10.1007/s10620-006-3205-0. [DOI] [PubMed] [Google Scholar]
- 43.Kato T, Sasaki S, Funakoshi K, et al. [Clinical Findings of Gastric MALT Lymphoma Not Eligible for Helicobacter pylori, Eradication with Rituximab as second-line Treatment.] I To Cho. 2007;42:1217–23. [Google Scholar]
- 44.Fischbach W, Tacke W, Greiner A, et al. Regression of immunoproliferative small intestinal disease after eradication of Helicobacter pylori. Lancet. 1997;349:31–2. doi: 10.1016/s0140-6736(05)62165-4. [DOI] [PubMed] [Google Scholar]
- 45.Oiya H, Okawa K, Noguchi A, et al. [Regression of MALT Lymphoma of the Rectum after Helicobacter pylori Eradication in a Patient Negative for Helicobacter pylori] Gastroenterol Endosc. 2003;45:965–9. [Google Scholar]