Abstract
One conundrum of binge eating is that women are more likely to suffer from binge-related disorders, even though estradiol decreases food intake. 2-hydroxyestradiol (2OHE2), an estrogen metabolite, may account for the contradiction, due to possible interference with DA signaling. We hypothesized that 2OHE2 would enhance bingeing in a rodent model. Two cohorts (1 male, 1 female) of 34 non-food-deprived rats were separated into daily control (D) (received an optional source of dietary fat for 20 minutes every day) or bingeing (INT) groups (received fat intermittently, i.e. 20 minutes on Mon, Weds, Fri). During the 5-wk binge induction period, shortening intakes escalated significantly faster in females than in males, such that males consumed significantly less fat/kg body mass than did females after 5 weeks. This result is consistent with the idea that biological differences contribute to sex differences in bingeing. Rats were then injected with 2OHE2 (1.0, 3.0, and 10.0 μg/kg intraperitoneally), vehicle, or 2-methoxyestradiol (2ME2) immediately prior to fat access. Fat intake was significantly stimulated by 2OHE2 only in the INT rats (p<0.03). Furthermore, this effect seemed to be more subtle in females than in males. Thus, 2OHE2 appears to exacerbate binge size. These data suggest a novel biological mechanism for sex differences in the risk of eating disorders.
Keywords: binge eating, dietary fat, 2-hydroxyestradiol, dopamine, sex differences
Introduction
Binge eating is defined as the consumption of more food in relatively brief periods of time than most individuals would eat under similar circumstances accompanied by a sense of loss of control. Bingeing characterizes eating disorders such as bulimia nervosa (BN) and binge eating disorder (BED) [1]. Additionally, even if individuals do not meet DSM-IV criteria for these disorders, they may still engage in subthreshold bingeing. The total lifetime risk of any form of bingeing is almost 1 in 20 [2], meaning that approximately 14 million Americans will suffer the emotional, personal, and social consequences associated with binge eating.
An important consideration in the study of binge eating is the presence of sex differences. While it is true that both men and women binge, women are more than twice as likely to develop binge-related eating disorders such as BN and BED [2]. A conundrum exists, however, in that women have higher levels of estradiol (E2), which is known to decrease meal size in rats [3,4] and binge frequency in humans [5], and is therefore thought to be protective. The explanation for this apparent contradiction may involve one of the metabolites of E2, 2-hydroxyestradiol (2OHE2) and its effects on and interactions with dopamine (DA). Specifically, 2OHE2 may competitively inhibit degradation of DA [6] and/or mimic or enhance D2 receptor actions [7-10]. Previous literature has suggested that disruptions in DA signaling are present in bingeing-related eating disorders such as bulimia [11,12].
The mechanism accounting for increased risk of developing binge-related eating disorders in females is not presently understood. The goal of the current study was to investigate possible causes of sex differences in binge-type eating by administration of the estrogen metabolite 2OHE2 using a non-food-deprived rat bingeing model that demonstrates face, construct, and predictive validity [13-15].
Materials and Methods
Animals
Male and female rats were used in separate cohorts. For each cohort, 34 Sprague Dawley rats (Harlan, Indianapolis, IN), 60 days of age, were individually housed in hanging stainless steel wire cages in a temperature- and humidity-controlled environment placed on a 12:12 light: dark cycle. All rats had ad libitum access to a nutritionally complete commercial laboratory rodent diet at all times during the study (Laboratory Rodent Diet 5001, PMI Feeds, Richmond IN; percent of calories as protein: 28.05%, fat: 12.14%, carbohydrate: 59.81%; 3.3 kcal/g) placed in hanging metal food hoppers at the front of the cage. Tap water also was freely available. All procedures were approved by the Pennsylvania State University Institutional Animal Care and Use Committee.
After seven days of adaptation to the vivarium, body weights were recorded and solid vegetable shortening (Crisco® All-Vegetable shortening, J.M Smucker Co., Orrville, OH) was provided during a single overnight period, in addition to the continuously available chow. In each cohort, two groups of 17 rats each were then matched by body weight and the amount of shortening consumed [t-test; p>0.05 for both measures] (Supplemental Table 1). All bingeing and drug administration procedures were consistent across the two cohorts.
Bingeing Procedure
After grouping, rats were given limited access to shortening in a glass jar clipped to the front of the cage 2-3 hours prior to the start of the dark cycle. Chow was available during the shortening access period and at all other times. One group was given shortening every day (D) and the other group was given shortening intermittently, i.e. only on Mondays, Wednesdays, and Fridays (INT). To maximize exposure early in the experiment and decrease the effects of neophobia, fat was available for longer periods in the first week and availability was decreased in following weeks. That is, in week 1, shortening was available to the rats for one hour; in week 2 it was available for 40 minutes, and for all subsequent weeks it was available for 20 minutes. The shortening availability was limited to 20 minutes in this study due to the relatively short half-life of 2OHE2 (t1/2(1)=0.54 min, t1/2(2)=10 min) [16].
Drug Administration
After 5 weeks, rats were injected intraperitoneally with vehicle (saline with 0.25% ethanol and 2-3 drops of TWEEN-80), 2-methoxyestradiol (2ME2), or 2OHE2 (Sigma Aldrich, St. Louis, MO, USA) with doses assigned to each rat using a Latin Square. Injections were given on Mondays and Fridays. 2OHE2 doses were 1.0, 3.0, and 10.0 μg/kg body weight. Since 2OHE2 is considered a pro-drug for 2ME2, a 3.0 μg/kg body weight injection of 2ME2 was included in the Latin Square to rule out its possible effects. Rats were given 20 minutes of access to shortening in their home cages immediately after injection.
Assessment of Chow Intake
In order to determine if 2OHE2 would stimulate intake of chow as well as shortening, all rats were injected with either the highest effective 2OHE2 dose (3.0 μg/kg body weight) or vehicle in a crossover design on the Monday and Friday of the week immediately following the full dose effect study. Rats were then given 20-minute access to shortening, and both shortening and chow intake were assessed.
Statistics
Data were analyzed using SAS v.9.1 (SAS Institute, Cary, NC). Intakes were assessed in several ways. Terminal normalized intake in week five was analyzed via 2-way ANOVA (sex X access schedule). To determine differences in intakes between INT and D groups within the male and female cohorts, between-group t-tests (INT vs. D) were used. Linear regression analyses equivalent to analysis of covariance were conducted using GraphPad Prism 4 (GraphPad Software, Inc.) to compare escalations in normalized shortening intakes between males and females. Drug effects were assessed using a 3-way repeated measures analysis of variance (ANOVA; drug X sex X group) with drug dose as the repeated measure. Follow-up analyses were conducted using 1-way repeated measures ANOVAs. Tukey's HSD post-hoc test was used for comparisons among more than two doses (full dose effect function assessment); within-group t-tests were used for post-hoc comparisons between 3ug/kg 2OHE2 and vehicle in the chow intake assessment study. Shortening intakes were normalized to body weight using the formula: kcal/body mass2/3 [17] to account for sex differences.
Results
Escalation and Onset of Bingeing
At the end of the bingeing procedure (week 5), normalized shortening intake was significantly greater in the females than in the males [main effect of sex: F(1, 64) = 12.65, p 0.0007], and also significantly greater in INT than in D rats [main effect of access schedule: F(1, 64) = 10.38, p 0.0020]. There was no interaction between sex and access schedule for the terminal week 5 intakes. Shortening intake in the INT rats was significantly higher during the 20-minute access period than in the D rats in both the male (0.82 ± 0.07 v. 0.53 ± 0.06 kcal/g body mass2/3; t-test p<0.01) and female (1.20 ± 0.12 v. 0.85 ± 0.08 kcal/g body mass2/3; t-test p<0.02) cohorts. Therefore, the INT rats in both cohorts were bingeing as operationally defined by previous studies [18-20], which also is consistent with the DSM-IV [1].
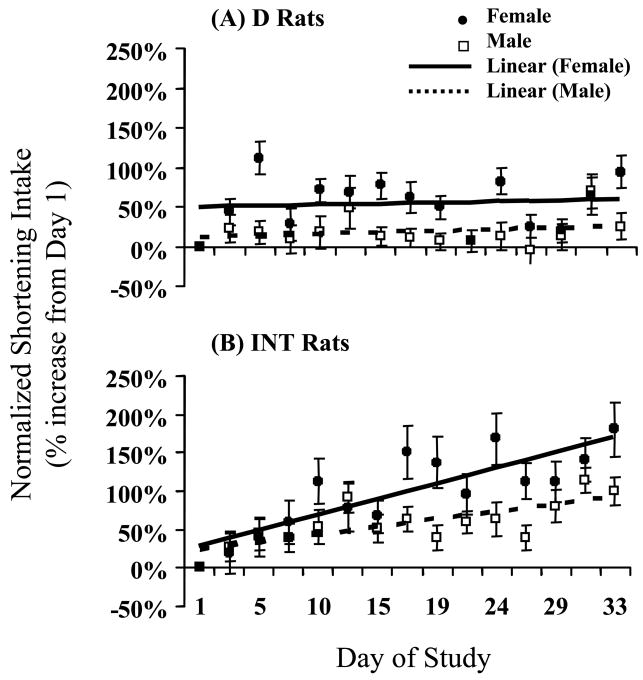
Normalized intake data were also analyzed and compared as a percentage of the mean shortening intake on Day 1 of the bingeing procedure (Figure 1). Regression analyses revealed a slope of 0.048 for the male INT rats and 0.101 for the female INT rats indicating that shortening intake in the INT females escalated significantly faster than it did in the INT males [F(1,506)=6.79, p<0.01] (Figure 1B). The slopes of the D rats' intakes did not differ from zero for either cohort (Figure 1A).
Figure 1.
Escalation of normalized shortening intake across the 5-week binge induction period. (A) The intakes of both male D and female D groups did not escalate across time, i.e. neither slope was different from zero. (B) The female INT rats (slope=0.101) escalated significantly faster than the male INT rats (slope=0.048; n=17, p<0.01).
Effect of 2OHE2
Normalized shortening intake during the 20-minute access period was evaluated after administration of 2OHE2, 2ME2, or vehicle. There was a main effect of sex [F(1,64)=12.54, p<0.0008], in that the female rats had significantly higher normalized intakes than did males across all doses. This is consistent with the differences in escalation between males and females for the first 5 weeks. There was also a main effect of access protocol [F(1,64)=13.18, p<0.006] due to higher intakes in INT rats of both sexes compared to D rats (i.e. bingeing in INT rats).
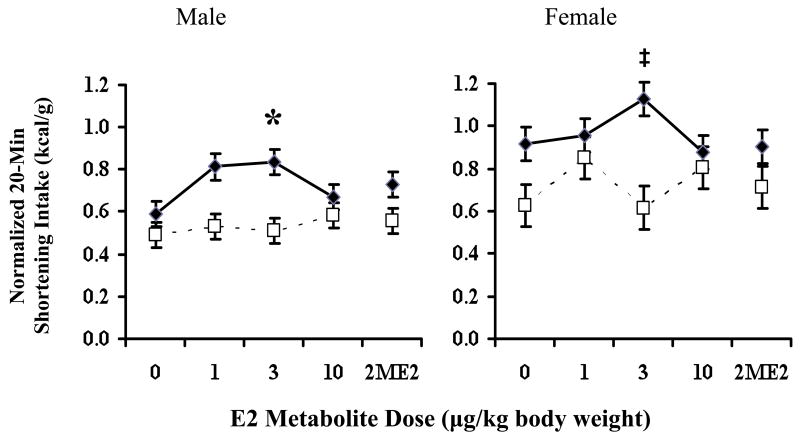
There was a main effect of drug [F(4,256)=2.86, p<0.024]. Follow-up analyses revealed that the 3.0 μg/kg dose of 2OHE2 significantly stimulated shortening intake relative to vehicle in the INT rats [F(4,33)=5.29, p 0.0006 (Figure 2)]; there was no effect in the D rats. Although there was no sex X schedule X drug interaction [F(4,256)=1.61, p 0.171], it is important to note that we were not able to find a significant effect at any dose relative to vehicle in the female rats when assessed with 1-way repeated measures ANOVA. The male INT rats, however, did show significant stimulation at the 3.0 μg/kg dose when assessed in this manner. Additionally, there was a drug by schedule interaction [F(4,256)=4.21, p<0.003], since 2OHE2 only significantly affected intake in the bingeing rats.
Figure 2.
The effect of 2OHE2 on 20-minute shortening intake in male and female rats. 2OHE2 (3 μg/kg) increased shortening intake in the INT rats relative to vehicle [F(4,256)=2.86, p<0.024]. *When assessed with follow-up one-way ANOVA, 3 μg/kg was significantly different from vehicle [F(4,16)=6.15, p=0.0003]. ‡There was no significant difference at any dose when assessed via follow-up one-way ANOVA testing [F(4,16)=2.08, p NS]. 2OHE2 did not have a significant effect on intake in D animals.
Assessment of Chow Intake
The effect of 2OHE2 on fat intake was reproduced, i.e. 3 μg/kg 2OHE2 stimulated shortening intake relative to vehicle [main effect of dose F(1,64) = 7.42, p 0.0083]. In contrast, 2OHE2 had no effect on chow consumption (Supplemental Table 2).
Discussion
As with previous studies where rats were given 1-hour access to shortening [4,18-20], both male and female rats on the INT schedule of access consumed significantly more shortening than rats given daily access. Therefore, both INT males and INT females met our operational criterion for bingeing, i.e. both groups consumed more than their respective D controls during week 5 of the study. Importantly, when consumption was normalized to body weight, shortening intakes in both the male and female INT groups escalated across time. In contrast, neither D group showed an escalation in intake during the 5-week period, i.e. the slopes of the two D groups were not different from zero. Thus, the INT schedule caused 20-min shortening intake of male and female INT rats to escalate over time, a finding consistent with other studies from our group when longer shortening access periods were used [21-23].
Although intakes of the male and female INT rats both escalated across the initial 5 weeks of the study, there were differences in the rates of escalation between the two groups. Specifically, the intakes of the INT females escalated faster than did the intakes of the INT males. Furthermore, normalized shortening intakes were not different between male and female INT groups on the first binge day (Student's t-test, p NS), indicating that a baseline effect cannot explain the differences in escalation. This finding is consistent with previous reports that bingeing in female humans is more likely to progress to binge-related eating disorders than is bingeing in males [2]. It is currently unclear why this sex difference exists, though it is possible that the effect is the result of hormonal differences between sexes.
There were subtle differences present between male and female INT rats during the administration of acute injections of 2OHE2. For instance, intakes in the males after the effective dose (3.0 μg/kg) were comparable to those of females after administration of vehicle (Student's t-test, p NS). In addition, while the female INT rats seemed to have similar trends in intake as a result of 2OHE2 (Figure 2 and Supplemental Table 2), there were no significant differences at any dose relative to vehicle when we assessed with a 1-way repeated measures ANOVA (Figure 2) or paired t-test (Supplemental Table 2), however effects were significant in the INT males. This seems to suggest that the stimulation effect of 2OHE2 is much more subtle in female rats, and the overall schedule X drug interaction is driven predominantly by the male rats. This idea is further supported by the fact that the male INT rats had a much larger overall stimulation at the effective dose than did the female INT rats (42.3% compared to 23.2%, respectively).
These results cannot be explained by order of dose administration, since all doses of drug and vehicle were assigned using a Latin Square. Furthermore, effects of 2OHE2 cannot be explained by generalized increases in food intake, since chow intake was unaffected by doses that stimulated fat intake in the male rats. Effects cannot be explained by the metabolite 2ME2, since 2ME2 at the same dose (3.0 μg/kg or ∼1 × 10-5 M) had no effect on fat intake. The decision to test the same dose of both compounds was based upon reports that 2OHE2 is considered to be a pro-drug of 2ME2 [16]. Finally, the more subtle stimulation in female rats was not due to ceiling effects, since all rats were observed to have consumed larger quantities of shortening during the escalation period than they ate after drug administration. That is, they could have eaten more than they did on drug administration days.
Since females already have higher circulating levels of 2OHE2 and other estrogens than males, it is plausible that the females were under greater influence of these hormones than the males prior to drug administration, and were therefore less sensitive to the acute injections of 2OHE2. This chronic exposure to 2OHE2 could also help to explain the elevated intakes and greater degree of escalation that occurred during the 5-week bingeing period in female INT rats compared to males. Acute exogenous administration of 2OHE2 to the females would result in higher overall levels, essentially shifting the females toward the descending limb of the concentration effect function. Conversely, exogenous administration of 2OHE2 to the males likely produced serum concentrations comparable to those of the females at baseline [16], and stimulated intake to that seen in the females after administration of vehicle. Importantly, there were no differences in either D group, which supports the idea that acute exposure to 2OHE2 exacerbated intake in bingeing rats, but did not affect intake in the D rats that is presumably driven primarily by palatability.
The mechanism by which 2OHE2 enhances binge intake is unknown, though there is evidence that the metabolite may be affecting neuronal signaling by DA. DA signaling in various brain regions has been heavily implicated in neural mechanisms of reward, including food reward [24,25]. Among these regions are two terminal areas of ventral tegmental area (VTA) dopamine projections, the prefrontal cortex (PFC) and the nucleus accumbens (NA). Others have shown that PFC DA increases in response to food anticipation [26], as well as to repeated presentation of sugary palatable foods [27]. In addition, accumbens DA is repeatedly stimulated by sucrose consumption in sucrose bingeing rats, but not in sucrose controls [28], suggesting that binge behavior affects DA signaling in a manner that is different from intake induced simply by palatability. Finally, others have reported increases in accumbens DA in rats sham-feeding corn oil [29]. Though differences are known to exist, it is possible that bingeing on fatty foods may affect DA signaling in a manner similar to that of sucrose [30].
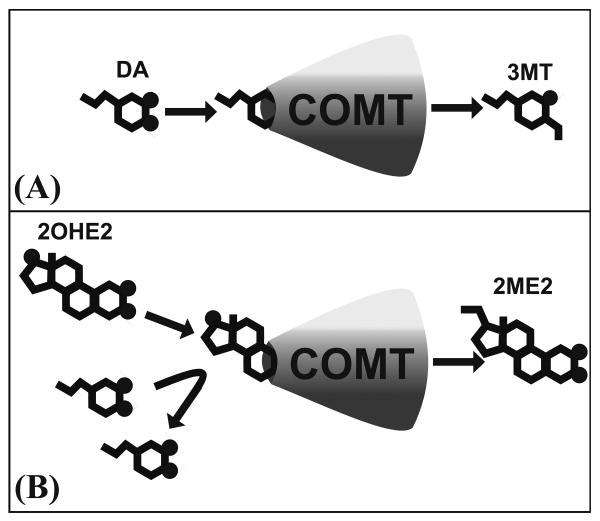
2OHE2 may affect reward-associated DA signaling, specifically in cortical regions. In the PFC, where DA transporter (DAT) expression is relatively low [31,32], degradation by the enzyme catechol-O-methyltransferase (COMT) accounts for approximately 60% of DA removal [33]. Because 2OHE2 is also degraded by COMT to form the metabolite 2-methoxyestradiol (2ME2), and because its affinity for COMT is higher than that of DA [34], 2OHE2 may competitively inhibit the enzyme and therefore increase DA availability (Figure 3). Supporting this idea, inhibition of COMT by pharmacological means with the antagonist tolcapone increases DA in the PFC in response to food anticipation [26]. It is possible that administration of 2OHE2 increased DA availability in these regions by inhibiting COMT activity. Effects may have been specific to the INT rats due to repeated release of DA in binge rats relative to controls, as reported by others for sucrose in the accumbens [28].
Figure 3.
The possible mechanism by which 2OHE2 increases DA availability in the prefrontal cortex. (A) Normal degradation of DA to form the DA metabolite 3-methoxytyramine (3MT). (B) Competitive inhibition of COMT by 2OHE2 to form the metabolite 2-methoxyestradiol (2ME) may cause increased DA levels in the prefrontal cortex.
2OHE2 may also affect DA signaling via its effect on adenylyl cyclase. D2 receptor activation is known to inhibit adenylyl cyclase, lowering cAMP and acting in opposition to D1 receptors. 2OHE2, like D2 receptor activation, has been shown to inhibit adenylyl cyclase activity and lower intracellular cAMP. Importantly, this effect was markedly less potent or absent altogether with the parent compound, E2, or the metabolite 2ME2 [8], and occurs independently of D1 or D2 receptors [9]. Additional evidence suggests that 2OHE2 is able to bind directly to D2 receptors in pituitary with an affinity that is similar to DA [7]. 2OHE2 may, therefore, mimic or enhance the effects of D2 receptor activation. The inverted U-shaped function obtained in the male INT rats may reflect differential presynaptic vs. post-synaptic D2 effects.
While the mechanisms by which 2OHE2 stimulated binge intake remain to be elucidated, the findings of the current study contribute to the understanding of sex differences in the risk of binge eating. Though the involvement of an estrogen metabolite, specifically 2OHE2, seems contradictory, the results of this study along with previous literature provide evidence for a possible novel mechanism for this increased risk in females.
Supplementary Material
Research Highlights.
Female rats had faster and higher escalations in binge intake than did males.
2-hydroxyestradiol-enhanced male intakes were similar to female intakes after vehicle.
2-hydroxyestradiol-enhanced intake in binge rats only.
Acknowledgments
We would like to thank Dr. John Hayes for his guidance and help with the statistical analyses. Support for this study by 1 RO1 MH67943-04 (RLC)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
R.K. Babbs, Email: rkb145@psu.edu.
F.H.E. Wojnicki, Email: fhw3@psu.edu.
References
- 1.APA (American Psychiatric Association) Diagnostic and Statistical Manual-IV-Text Revision. Washington, DC: American Psychiatric Association; 2000. pp. 589–595. online BED. [Google Scholar]
- 2.Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The Prevalence and Correlates of Eating Disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckel LA. Estradiol: a rhythmic, inhibitory, indirect control of meal size. Physiol Behav. 2004;82(1):35–41. doi: 10.1016/j.physbeh.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42(4):461–71. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- 5.Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychol Med. 2006:1–11. doi: 10.1017/S0033291706008956. [DOI] [PubMed] [Google Scholar]
- 6.Breuer H, Koster G. Interaction between oestrogens and neurotransmitters at the hypophysial-hypothalamic level. J Steroid Biochemistry. 1974;(5):961–7. [Google Scholar]
- 7.Schaeffer JM, Hsueh AJW. 2-Hydroxyestradiol interaction with dopamine receptor binding in the rat anterior pituitary. J Bio Chem. 1978;254(13):5606–8. [PubMed] [Google Scholar]
- 8.Tofovic PS, Rosado BM, Dubey RK, Jackson EK. Effects of estradiol metabolites on cAMP production and degradation. Biol Med Sci. 2009;30:5–23. [PubMed] [Google Scholar]
- 9.Braun T. Inhibition of the soluble form of testis adenylate cyclase by catechol estrogens and other catechols. Proc Soc Exp Biol Med. 1990;194(1):58–63. doi: 10.3181/00379727-194-43055. [DOI] [PubMed] [Google Scholar]
- 10.Clopton J, Gordon JH. In vivo Effects of estrogen and 2-hydroxyestradiol on D2 dopamine receptor agonist affinity states in rat striaturn. J Neur Trans. 1986;66:13–20. doi: 10.1007/BF01262954. [DOI] [PubMed] [Google Scholar]
- 11.Mikolajczyk E, Grzywacz A, Samochowiec J. The association of catechol-O-methyltransferase genotype with the phenotype of women with eating disorders. Brain Research. 2010;1307:142–8. doi: 10.1016/j.brainres.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 12.Jimerson DC, Lesem MD, Kaye WH, Brewerton TD. Low serotonin and dopamine metabolite concentrations in cerebrospinal fluid from bulimic patients with frequent binge episodes. Arch Gen Psychiatry. 1992;49(2):132–8. doi: 10.1001/archpsyc.1992.01820020052007. [DOI] [PubMed] [Google Scholar]
- 13.Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav. 2004;82:123–130. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 14.Broft AI, Spanos A, Corwin RL, Mayer L, Steinglass J, Devlin MJ, Attia E, Walsh BT. Baclofen for binge eating: an open-label trial. Int J Eat Disord. 2007;40:687–691. doi: 10.1002/eat.20434. [DOI] [PubMed] [Google Scholar]
- 15.Corwin RL, Boan J, Peters K, Walsh BT, Ulbrecht J. Baclofen reduces binge frequency. Appetite. 2010;54:641. [Google Scholar]
- 16.Zacharia LC, et al. 2-Hydroxyestradiol is a prodrug of 2-methoxyestradiol. J Pharm Exp Ther. 2004;309(3):1093–7. doi: 10.1124/jpet.103.062505. [DOI] [PubMed] [Google Scholar]
- 17.Heusner AA. Body size and energy metabolism. Ann Rev Nutr. 1985;5:267–93. doi: 10.1146/annurev.nu.05.070185.001411. [DOI] [PubMed] [Google Scholar]
- 18.Corwin RL. Binge-type eating induced by limited access in rats does not require energy restriction on the previous day. Appetite. 2004;42:139–142. doi: 10.1016/j.appet.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Thomas MA, Rice HB, Weinstock D, Corwin RL. Effects of aging on food intake and body composition in rats. Physiol Behav. 2002;76:487–500. doi: 10.1016/s0031-9384(02)00800-4. [DOI] [PubMed] [Google Scholar]
- 20.Wojnicki FHE, Roberts DCS, Corwin RLW. Effects of baclofen on operant performance for food pellets and vegetable shortening after a history of binge-type behavior in non-food-deprived rats. Pharmacol Biochem Beh. 2006;84:197–206. doi: 10.1016/j.pbb.2006.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corwin RL, Wojnicki FH, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav. 1998;65(3):545–53. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- 22.Dimitriou SG, Rice HB, Corwin RL. Effects of limited access to a fat option on food intake and body composition in female rats. Int J Eat Disord. 2000;28(4):436–45. doi: 10.1002/1098-108x(200012)28:4<436::aid-eat12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 23.Wojnicki FH, Johnson DS, Corwin RL. Access conditions affect binge-type shortening consumption in rats. Physiol Behav. 2008;95(5):649–57. doi: 10.1016/j.physbeh.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 25.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 26.Lapish CC, Ahn S, Evangelista LM, So K, Seamans JK, Phillips AG. Tolcapone enhances food-evoked dopamine efflux and executive memory processes mediated by the rat prefrontal cortex. Psychopharmacology (Berl) 2009 Jan;202(1-3):521–30. doi: 10.1007/s00213-008-1342-1. [DOI] [PubMed] [Google Scholar]
- 27.Bassareo V, De Luca MA, Di Chiara G. Differential Expression of Motivational Stimulus Properties by Dopamine in Nucleus Accumbens Shell versus Core and Prefrontal Cortex. J Neurosci. 2002;(11):4709–19. doi: 10.1523/JNEUROSCI.22-11-04709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134(3):737–44. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 29.Liang N, Hajnal A, Norgren R. Sham feeding corn oil increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1236–9. doi: 10.1152/ajpregu.00226.2006. [DOI] [PubMed] [Google Scholar]
- 30.Avena NM, Rada P, Hoebel BG. Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr. 2009;139:623–628. doi: 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cass WA, Gerhardt GA. In vivo assessment of dopamine uptake in rat medial prefrontal cortex: comparison with dorsal striatum and nucleus accumbens. J Neurochem. 1995 Jul;65(1):201–7. doi: 10.1046/j.1471-4159.1995.65010201.x. [DOI] [PubMed] [Google Scholar]
- 32.Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998 Apr 1;18(7):2697–708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karoum F, Chrapusta SJ, Egan MF. 3-Methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in the frontal cortex, nucleus accumbens, and striatum by a simple two pool model. J Neurochemistry. 1994;63:972–9. doi: 10.1046/j.1471-4159.1994.63030972.x. [DOI] [PubMed] [Google Scholar]
- 34.Ball P, Knuppen R, Haupt M, Breuer H. Interactions between estrogens and catecholamines. 3 Studies on the methylation of catechol estrogens, catecholamines and other catechols by the catechol-O-methyltransferases of human liver. Journal Clin Met. 1972;34(4):736–46. doi: 10.1210/jcem-34-4-736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.