Abstract
We sought to characterize sex-based differences in access to deceased donor liver transplantation. Scientific Registry of Transplant Recipients data was used to analyze n=78,998 adult candidates listed before (8/1997–2/2002) or after (2/2002–2/2007) implementation of Model for End-stage Liver Disease (MELD)-based liver allocation. The primary outcome was deceased donor liver transplant. Cox regression was used to estimate covariate-adjusted differences in transplant rates by sex. Females represented 38% of listed patients in the pre-MELD era and 35% in the MELD era. Females had significantly lower covariate-adjusted transplant rates in the pre-MELD era (by 9%; p<0.0001) and in the MELD era (by 14%; p<0.0001). In the MELD era, the disparity in transplant rate for females increased as waiting list mortality risk increased, particularly for MELD scores ≥15. Substantial geographic variation in sex-based differences in transplant rates was observed. Some areas of the U.S. had more than a 30% lower covariate-adjusted transplant rate for females compared to males in the MELD era.
In conclusion, the disparity in liver transplant rates between females and males has increased in the MELD era. It is especially troubling that the disparity is magnified among patients with high MELD scores and in certain regions of the U.S.
Keywords: sex-related differences, disparities, gender, liver transplantation, access to transplantation, end stage liver disease, health policy
Introduction
Each year, thousands of patients with end stage liver disease die awaiting liver transplant. Since the organ supply is overwhelmed by more than 17,000 candidates waiting for a transplant, complex organ allocation rules have developed, predicated on ensuring a fair distribution of these resources to a heterogenous patient population. Since 2002, deceased donor livers have been allocated to candidates with chronic liver disease at the highest risk of death, determined by the Model for End-stage Liver Disease (MELD) scoring system (1). MELD is based on three laboratory tests – international normalized ratio (INR) of the prothrombin time, total serum bilirubin, and serum creatinine – and is a reliable clinical tool for consistent, accurate, and objective estimation of short-term mortality in a wide range of chronic liver disease patients, including those awaiting liver transplantation (1–6). However, MELD scores may underestimate mortality risk in women, as their smaller stature and lesser muscle mass skews estimation of their level of renal dysfunction, which is based solely on serum creatinine (7–9). As a result, female priority for liver transplantation may be devalued under MELD-based allocation.
Previous authors have reported compromised access among females in more general examinations of liver transplant waiting list outcomes (10, 11), and studies also show that sex-based disparities exist in access to kidney transplantation (12). However, a comprehensive analysis focusing on the association between sex and liver transplant rates has not been published. Improved understanding of inequities in the liver transplant process requires a thorough understanding of liver allocation and distribution, which may create unintentional disparities when the focus is optimizing organ placement. In liver transplantation, access to transplant is not solely dependent on candidate medical urgency as assessed by MELD score. Organs are offered first to candidates listed at transplant programs within a geographic distribution unit that represents the donation service area (DSA) of the donor hospital. Differential organ acceptance practices and geographic differences in organ availability generate substantial variation in transplant rates within each of the 50 DSAs in the U.S. where liver transplants are performed (13, 14). Analyses of liver allocation and disparities in its process should therefore account for the DSA as well as MELD.
In this study, we characterized the effect of sex on rates of liver transplantation among listed candidates. We hypothesized that female liver transplant candidates are less likely to receive liver grafts, even after controlling for confounders such as patient differences, medical urgency and disease progression, and geography. We also sought to examine the complex relationships among geography, diagnosis, and sex to investigate potential mechanisms underlying sex-based disparities in successful matriculation from the liver transplant waiting list.
Methods
We used data from the Scientific Registry of Transplant Recipients (SRTR) based on patient-level data submitted by all U.S. transplant centers to the Organ Procurement and Transplantation Network (OPTN). The observational study cohort was comprised of n=78,998 adult candidates with end-stage liver disease. The cohort and follow-up was divided into pre-MELD and MELD eras based on the date of candidate registration. Pre-MELD patients were those initially registered on the waiting list between February 28, 1997 and February 27, 2002. The OPTN adopted a MELD-based liver allocation policy in February 2002, and patients were classified as MELD era if they were initially added to the waiting list between February 28, 2002 and February 27, 2007. Status 1, pediatric patients, and patients with hepatocellular carcinoma were excluded, as were patients with MELD exception scores at initial waiting list registration.
The primary exposure variable was sex. Candidate follow-up time at risk began on the date of initial waiting list registration and continued until the earliest of deceased donor liver transplant (the event of interest), death, granting of a MELD exception score not based on laboratory values, non-death removal from the waiting list, receipt of a living donor liver transplant, or the end of the observation period. Aside from deceased donor liver transplant, all other waiting list outcomes (e.g., death, non-death removal, granting of an exception score) were treated as non-events. Follow-up time at risk was suspended during any periods when candidates were designated as temporarily inactive, and observation was subsequently re-initiated upon reactivation, if applicable. Borrowing terminology from competing risks statistics (15), our analysis compared, by sex, cause-specific deceased-donor liver transplant rates; i.e., rates of transplant among patients actually eligible for transplant: alive, not removed and active on the wait list.
Cox regression models were fitted to estimate the effect of sex on rates of deceased donor liver transplantation with adjustment for confounding covariates. We accounted for geographic variation and medical urgency using models stratified for these factors (16). We fitted separate models for the pre-MELD and MELD eras, and fitted a combined model with female × era interaction terms. Each model was stratified by geography (DSA) and medical urgency (vide infra). Stratified Cox proportional-hazards models assume varying hazard functions for receipt of a liver transplant within each stratum, which in the geographic context was represented by each of the 50 DSAs that participate in liver transplantation in the U.S. We assumed that patients in different DSAs had intrinsically different likelihoods of receiving a liver transplant based on the variation in organ donation, differences in waitlisting practices, and provider differences within DSAs.
Since sicker patients receive greater allocation priority, we also stratified the models on medical urgency. This prioritization was delineated by medical urgency status (Status 2A, 2B, or 3) in the pre-MELD era or MELD score in the MELD era. In the pre-MELD era, medical urgency status was defined by Child-Turcotte-Pugh (CTP) score and hospitalization category (1). Three status definitions were used for candidates in this era: Status 2A, for patients registered while being treated in the intensive care unit with a CTP score greater than 10; Status 2B, for patients hospitalized at the time of registration with CTP scores greater than 10; and Status 3, for outpatients with CTP scores from 7–9. In the MELD era, patients were grouped by integer MELD score: 6, 7, …, 39, 40.
The analysis was adjusted for covariates recorded in the SRTR liver transplant candidate file, including age, height, weight, race/ethnicity, diagnosis, blood type, diabetes, body mass index (BMI), hospitalization status at listing, receipt of dialysis (MELD era), albumin (MELD era) and prior malignancy. Status (in the pre-MELD era), MELD (in the MELD era), serum albumin, and requirement for dialysis were treated as time-dependent covariates, since they could be updated multiple times during patient follow-up. Less than 10% of all data was missing. Candidates with missing data elements were excluded from the analysis, and this was similar for both sexes. Results are expressed as differences in relative transplant rates, based on hazard ratios estimated relative to a reference group assigned a hazard ratio of 1.00.
All statistical analyses were performed using SAS v9.2 (SAS Institute; Cary, NC). Statistical significance was defined by p<0.05. This study was approved by the U.S. Health Resources and Services Administration (HRSA) SRTR project officer. HRSA has determined that this study satisfies the criteria for the IRB exemption described in the “Public Benefit and Service Program” provisions of 45 CFR 46.101(b)(5) and HRSA Circular 03.
Results
Baseline Demographics and Disease Severity
Of the n=78,998 candidates included in the analysis, 36.4% were female (n=28,759) and 63.6% were male (n=50,239). There were similar proportions of female candidates in the pre-MELD and MELD eras (37.8% and 35.0%, respectively). Table 1 displays the clinical characteristics of male and female candidates in the study cohort. Slightly more than half of all the females in the cohort were listed in the pre-MELD era. The reverse was true for males. Overall, female and male candidates were of similar age. African Americans and Hispanics had proportionally greater representation among female candidates. The proportion of females with cholestatic liver disease was two-fold higher than males, and fewer females had hepatitis C-related cirrhosis. Male and females had similar BMI and dialysis requirements at listing.
Table 1.
Clinical Characteristics of Male and Female Liver Transplant Waitlist Candidates in the Study Cohort
| Variable | Males (n=50,239) | Females (n=28,759) | p-value |
|---|---|---|---|
| Age at waitlist registration (yrs) (SD) | 51.23 (9.16) | 51.94 (10.56) | <0.0001 |
| Race | <0.0001 | ||
| White (%) | 38465 (76.56) | 20706 (72.00) | |
| African American (%) | 3155 (6.28) | 2490 (8.66) | |
| Hispanic (%) | 6226 (12.39) | 4221 (14.68) | |
| Asian (%) | 1998 (3.98) | 1047 (3.64) | |
| Other (%) | 395 (0.79) | 295 (1.03) | |
| Era | <0.0001 | ||
| Pre-MELD Era (n) | 24749 (49.26) | 15056 (52.35) | |
| MELD Era (n) | 25490 (50.74) | 13703 (47.65) | |
| Diagnosis | <0.0001 | ||
| Cholestatic (n) (%) | 3360 (6.69) | 4395 (15.28) | |
| Non-Cholestatic (n) (%) | 17524 (34.88) | 10260 (35.68) | |
| Acute Hepatic Necrosis (n) (%) | 897 (1.79) | 773 (2.69) | |
| Malignant Neoplasm (Non-HCC) (n) (%) | 1205 (2.40) | 428 (1.49) | |
| Metabolic Disease (n) (%) | 1022 (2.03) | 447 (1.55) | |
| Hepatitis C (n) (%) | 22912 (45.61) | 9771 (33.98) | |
| Other (n) (%) | 3319 (6.61) | 2685 (9.34) | |
| MELD Score at Listing, (mean) (SD) | 14.55 (7.86) | 13.57 (7.76) | <0.0001 |
| MELD Score at Listing, (median) (25th,75th %ile) | 13 (8, 18) | 12 (7, 17) | |
| MELD Score at Transplant (median) (25th, 75th %tile) | 18 (13, 25) | 18 (13,25) | |
| Pre-MELD era Status | <0.0001 | ||
| Status 2A | 964 (3.90) | 521 (3.46) | |
| Status 2B | 6678 (26.98) | 3245 (21.55) | |
| Status 3 | 17107 (69.12) | 11290 (74.99) | |
| Hospitalization in Intensive Care Unit at Registration (n) (%) | 1872 (3.7) | 1174 (4.1) | 0.01 |
| Mechanical Ventilation Support at Registration (n) (%) | 644 (1.3) | 443 (1.5) | 0.003 |
| Diabetes mellitus (n) (%) | 6717 (13.4) | 4076 (14.2) | 0.002 |
| Mean Body Mass Index | 28.38 (6.21) | 28.29 (7.07) | 0.07 |
| Renal Failure (% dialysis) | 1290 (2.57) | 609 (2.12) | <0.0001 |
| Angina (n) (%) | 1224 (2.4) | 456 (1.6) | <0.0001 |
| Chronic Obstructive Pulmonary Disease (n) (%) | 756 (1.5) | 413 (1.4) | 0.44 |
| Cerebral Vascular Disease (n) (%) | 310 (0.6) | 181 (0.6) | 0.83 |
| Peripheral Vascular Disease (n) (%) | 507 (1.0) | 140 (0.5) | <0.0001 |
There were significant differences in disease severity at the time of listing between males and females. In the pre-MELD era, similar proportions of female and male candidates were initially listed as Status 2A (3.5% vs. 3.9%, respectively), but 21.6% of females were listed as Status 2B compared to 27.0% of males. Females had proportionally more Status 3 designations at the time of waitlisting, which represented the minimum listing criteria in the pre-MELD era (75.0% vs. 69.1%, respectively). In the MELD era, females were listed with a lower median MELD score compared to males (12 vs. 13, p<0.0001). Above MELD 15, proportionally fewer females were listed at each individual MELD score. In both the pre-MELD and MELD eras, females tended to be placed on the waiting list with less severe liver disease. Clinically, rates of ICU hospitalization and mechanical ventilation at registration were similar between the sexes, but due to large sample size, were significantly different statistically. Males and females had statistically significant differences in medical co-morbidities including body mass index, dialysis dependence, angina, chronic obstructive pulmonary disease, and diabetes mellitus, but these respective differences were not clinically significant. Males had higher rates of peripheral vascular disease at registration versus females.
Access to Liver Transplantation in the Pre-MELD and MELD Eras
As illustrated in Figure 1, after adjusting for clinical factors, medical urgency status designation, and DSA in the pre-MELD era, females had a significant 9% (p<0.0001) lower transplant rate compared to males, with a covariate-adjusted hazard ratio (HR) of 0.91 and 95% confidence interval (CI) (0.87, 0.94). This pre-MELD era disparity increased to 14% (p<0.0001) for females in the MELD era, adjusted for the same clinical factors, MELD score, and DSA; HR=0.86 (0.83, 0.89). The increase in the disparity from the pre-MELD era to the MELD era was significant (p=0.004). Sex-race interactions were not significant. Interactions between primary diagnosis and sex were also tested, and were non-significant.
Figure 1.
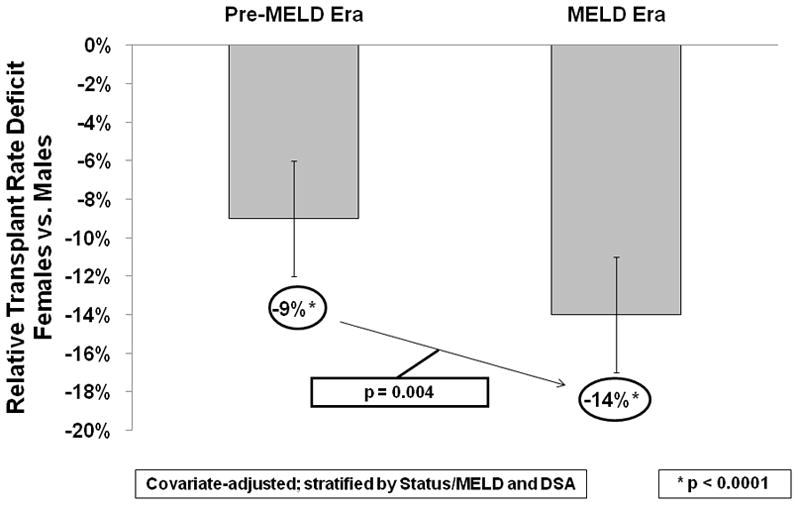
Sex-Based Disparities in Liver Transplant Rates in the Pre-MELD and MELD Eras. After adjusting for several patient-level factors and stratifying by the DSA and Status or MELD score of the patient, females had lower relative transplant rates in both eras compared to men, with a 9% deficit in the pre-MELD era and a 14% deficit in the MELD era. This widening of the gap in liver transplant rates between males and females was significantly worse in the MELD era, based on female × era interaction models (p=0.004).
The pre-MELD era disparity was largest among Status 2B candidates, where females had a 12% lower transplant rate (p<0.05) compared to their male counterparts; HR=0.88 (0.84, 0.92) (Figure 2). Transplant rates were also lower among females listed as Status 2A and Status 3, but these differences were not statistically significant. In the MELD era, females with MELD scores less than 15 did not have significantly different transplant rates than males with similar medical urgency, but the disparity was marked and significant among females with MELD scores ≥15 (Figure 3). Transplant rates were 20% lower for females with MELD scores 20–29 (p<0.05), with HR=0.80 (0.70, 0.90). Even among candidates with the highest MELD scores (MELD 30–40), females had a 12% lower (p<0.05) transplant rate, with HR=0.88 (0.84, 0.92).
Figure 2.
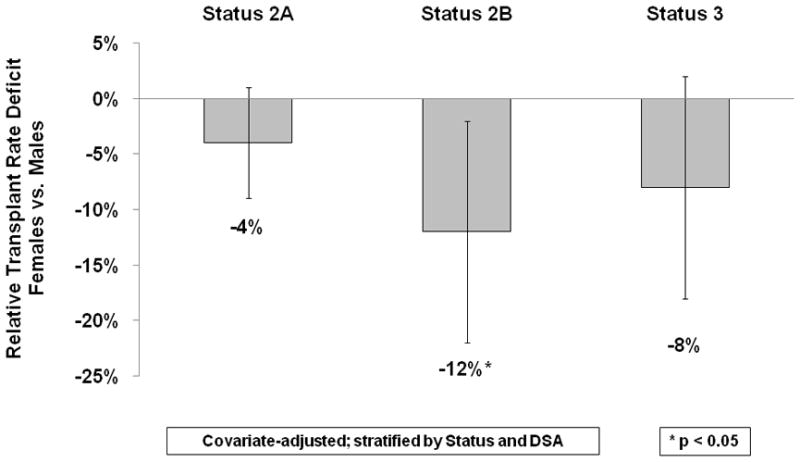
Lower Relative Transplant Rates for Status 2B Females in the Pre-MELD Era. Among the 3 status designations included in the study (Status 2A, 2B, and 3), females only had significantly lower relative transplant rates than men when registered as Status 2B. By definition, Status 2B candidates were hospitalized at the time of registration with Child-Turcotte-Pugh scores of 7–10.
Figure 3.
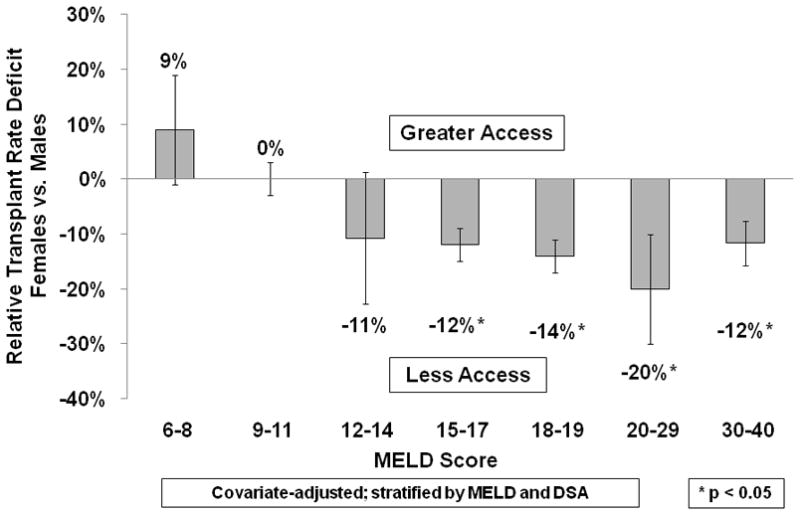
Females with Higher MELD Scores Have Less Access to Liver Transplant than Males. In the MELD era, the disparity in transplant rates between men and women was visible only in those candidates with MELD scores greater than 15. As the MELD scores increased, the deficit between men and women widened. These lower transplant rates were not accompanied by any changes in the adjusted relative rates of any other waiting list events, including death, removal, or inactivation.
We further evaluated the association between candidate sex and other waiting list events in both eras, including death on the waiting list, inactivation, and non-transplant removal from the waiting list. Prior to the MELD era, females had a 13% lower adjusted waiting list mortality rate compared to males (HR=0.87, p=0.0004), but this disparity was not seen in the MELD era (HR=1.08, p=0.196). Inactivation rates were similar between males and females in both eras. Female sex was associated with a 9% lower non-transplant removal rate versus males in the pre-MELD era (HR=0.91, p<0.0001). We observed this in the MELD era as well (HR=0.89, p<0.0001). Figure 4 summarizes these effects for MELD era candidates.
Figure 4.
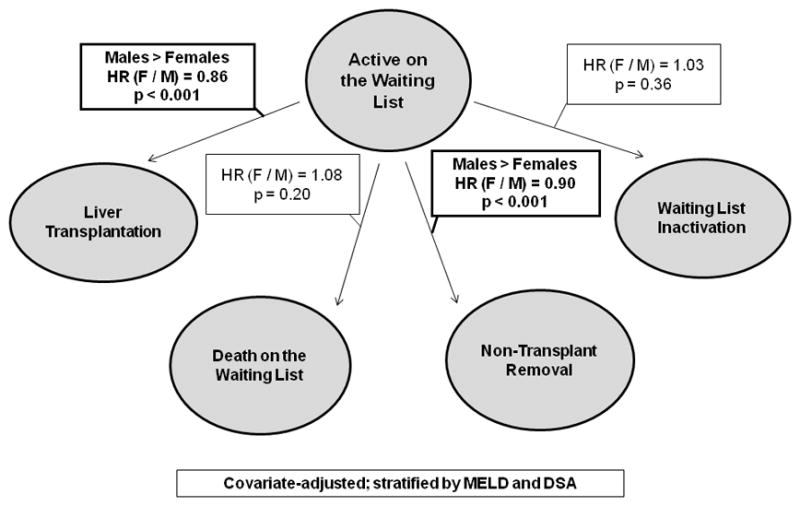
The Effect of Candidate Sex on Liver Transplant Waiting List Events in the MELD Era. Several events may occur after registration on a liver transplant waiting list, including receipt of a liver transplant, death while waiting on the list, being removed from the list due to an illness precluding transplantation, or temporary inactivation for a period of time. We have modeled the effect of sex on each of these transitions, which is captured by the hazard ratios above each arrow. As in Figure 1, males have significantly higher transplant rates than females, and also have a significantly higher relative rate of removal from the waiting list. There are no statistically significant sex-based differences in waiting list mortality or inactivation, after adjusting for MELD score and geography.
Additionally, we evaluated potential interactions between body size and female sex. In particular, we fitted models which allowed the female/male contrast in transplant rates to depend on height quartile, and results were highly non-significant. It thus appears that the reduction in liver transplant rates among females, relative to males, does not depend on candidate height (data not shown).
Geographic Variation in Sex-Based Disparities in Liver Transplant Rates
We evaluated which geographic areas of the U.S. had the largest sex-based disparities in transplant rates in both the pre-MELD and MELD eras by evaluating disease severity and DSA-adjusted transplant rates within the 11 OPTN regions used in organ allocation (Figure 5). In the pre-MELD era, three regions displayed more than 10% sex-based disparity in liver transplant rates – Regions 1, 2, and 10, representing New England, parts of the middle Atlantic, and the states of Indiana, Michigan, and Ohio, respectively. These areas displayed 16% to 22% lower transplant rates for females compared to males with similar disease burden in the same region. There were no significant differences between females and males in the other eight OPTN regions in the pre-MELD era.
Figure 5.
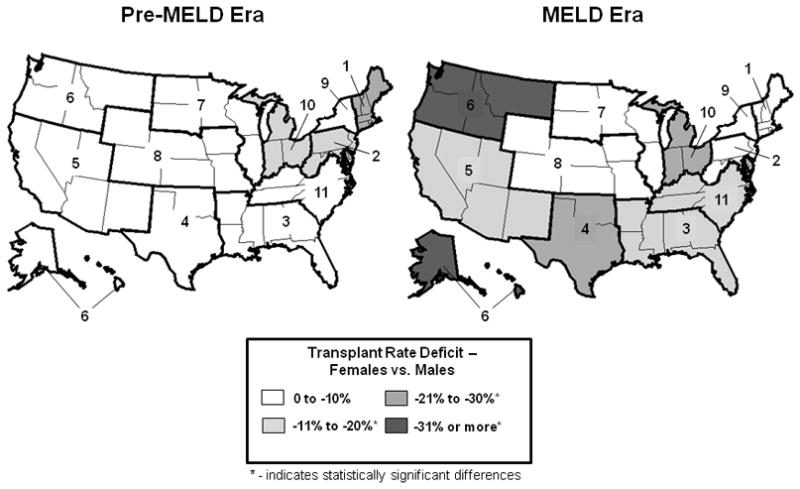
Geographic Variation in Female Access to Liver Transplantation.
In the pre-MELD era, the majority of the U.S. did not display any deficits in the relative liver transplant rates between men and women. In three Organ Procurement and Transplant Network (OPTN) regions, there was at least a 10% lower transplant rate for women. Region 1 displayed a greater than 20% lower liver transplant rate for women compared to men during that period. In the MELD era, sex-based differences in adjusted relative liver transplant rates were common across the United States. The majority of OPTN Regions displayed lower transplant rates for women. Only one region, Region 10, displayed failures in sex equity in both eras.
In the MELD era, the geographic distribution of sex-based disparities in relative transplant rates was more pronounced. Females had significantly lower transplant rates in six of the 11 OPTN regions, with a maximum deficit of 35% in the Pacific Northwest (Region 6). The geographic areas demonstrating these disparities included the southeastern and southwestern U.S., Texas, Oklahoma, California, the Pacific Northwest, Alaska, Hawaii, parts of the Midwest, the Carolinas, Virginia, Tennessee, and Kentucky. Only Region 10 had a significant sex-based transplant rate disparity in both eras. Sex-based disparities in access to liver transplantation in the MELD era were therefore both geographically extensive and of variable magnitude.
Discussion
The results of this study demonstrate that female candidates have markedly lower liver transplant rates than their male counterparts. This disparity is geographically widespread and has worsened under recent changes in deceased donor liver allocation policy in the U.S. Furthermore, the deficit in the transplant rate among females was concentrated among those candidates at the highest risk of death on the waiting list, including those whose transplants are associated with the most survival benefit (17). Under the current allocation system, both sexes had similar respective risk-adjusted waiting list mortality rates and inactivation, but females were less likely to be removed from the waiting list. This suggests that females are remaining on the waiting list, while males are more likely to receive a transplant or are removed for being too sick.
Studies published to date on sex-based disparities in access to liver transplantation have provided limited understanding of this important problem. Three studies have demonstrated reduced access for female candidates (10, 11, 18). We found a somewhat smaller magnitude difference in sex-specific transplant rates, but have shown that the difference has significantly worsened, rather than improved, under current allocation rules. This novel observation was likely revealed by our carefully considered adjustment strategy for geography and MELD, and is opposite to the temporal trend described by Moylan et al. (10). The previous studies underestimated how transplant rates are affected by disease progression over time and the local allocation environment.
Importantly, our findings of significantly lower transplant rates among females with MELD scores above 15 are especially concerning. At MELD scores above 12–15, the calculated life years from a liver transplant exceed the projected lifetime without one (17, 19). This survival benefit increases as MELD increases. Our findings therefore suggest that female candidates lose a major survival benefit as a result of the observed disparity in transplant rates. Provider and practice alterations in response to current allocation rules may be responsible for these observations, by unintentionally keeping females on the waiting list, while maintaining lower transplant and removal rates compared to males. Differences in MELD progression by sex may also be present, but this has not been validated and should be studied further. Secular changes in donor characteristics may also play a role, as size constraints are a clear part of transplant surgical decision-making. In summary, although there is not a precise explanation for worsened sex inequity in the MELD era, this observation remains ripe for further investigation.
The breadth and diversity of geographic elements of the sex-based disparity in the MELD era is another important finding. Geographic variation in overall access to transplantation is well recognized in both kidney and liver transplantation (20–22), but these studies were not designed to examine the interactions between a candidate’s sex, race, location, and transplant access. We have demonstrated that in 28 U.S. states, females had more than a 10% lower transplant rate than males with the same MELD scores. Previous analyses by the SRTR also suggest that wait-list events rates vary by geography (13), so it is not surprising that a disparity in these event rates also differs based on location. However, geographic differences in female liver transplant rates could represent otherwise concealed unrecognized sex-biased practice patterns and provider behaviors that may arise in the acceptance of donor livers, and be integrated into local patterns of clinical care. Testing of this hypothesis would require data beyond what is currently available to the SRTR, but could be addressed using survey methodology, mixed methods, or other approaches.
Female sex plays a role in the navigation of the transplant process for other organs (23). In kidney transplantation, females with end stage renal disease had lower waitlisting and transplant rates compared to males (12, 24, 25). Disparate access to health care for women also extends beyond transplantation. In cardiovascular disease and oncology, female patients were less likely to undergo recommended invasive cardiac procedures, and less likely to receive optimal cancer treatments compared to males (26–31).
Health disparities may arise from unequal treatment driven by conscious or less explicit provider behaviors (29, 32, 33). In the case of liver transplantation, receipt of a liver allograft is dependent not only on the candidate being sick enough to warrant a transplant. She must be at the top of the waiting list, receive an offer of a suitable donor liver, and have that offer accepted on her behalf by the transplant surgeon. Numerous factors contribute to the decision to accept or decline a liver offer. For example, the donor liver may be declined if it is deemed too large for a small-sized candidate, or a higher risk graft may be declined for a particular candidate. Transplant surgeons may deem female candidates to be not as medically urgent as their MELD scores suggest in the absence of major complications of cirrhosis or portal hypertension. Even in the absence of overtly identifiable provider bias against female candidates, isolation of the presumptively independent association of sex with organ offer decisions will soon be possible. With the recent adoption of DonorNet, a nationwide electronic organ offer system used by all U.S. transplant centers (34), data on all organ offers are being captured and may be used to directly test this hypothesis.
Our analysis showed striking inequalities in transplant access by sex, but the results are subject to some limitations. We considered only adults with chronic liver disease in our model and patients with hepatocellular carcinoma were excluded because of the existence of a separate preference pathway for access to donor organs. Hepatocellular carcinoma is a common indication for adult deceased donor liver transplantation (35), and sex-based disparities in transplant access may be present to a different degree in this patient group. Our approach specifically accounted for allocation-related factors, but was not designed to assess the role of donor factors. Despite these limitations, our study is the most comprehensive to date, and contributes to our knowledge on the effect of sex on access to liver transplantation from the waiting list.
From a methodological standpoint, our analysis was designed to determine whether sex-based disparities exist in the transition from liver transplant candidate to transplant recipient. Several factors affect the rate of this transition, and several outcomes are possible for liver transplant candidates: deactivation, removal, transplant and death. These outcomes can be considered competing risks (15). Our primary interest was in determining differences in transplant rates between males and females. To this end, several statistical approaches can be applied. One approach would be to compare the sex-specific cumulative incidence of liver transplantation (36), i.e., the probability that a patient undergoes transplantation. Comparisons of cumulative incidence can be difficult to interpret since, in the context of the liver transplant waiting list, differences in the sex-specific cumulative incidence of liver transplantation could result from differences in mortality, removal for non-death causes, deactivation, and/or actual disparities present in the process (37). To remove this potential source of bias, we analyzed cause-specific rates of the liver transplant event, i.e., the rate of transplant, given that no other competing risk has occurred (15). This approach provides an accurate estimate of the sex-based differences in transplant rates among patients who are alive, on the wait list, not removed, and not deactivated, or more plainly, the transplant rate differences among patients actually eligible for transplant. Unlike the cumulative incidence approach, the cause-specific rates do not “interact” with each other. For example, compared to females, knowing that males have higher rates of removal while listed and alive need not imply that they have lower rates of transplant while alive and not removed. From this perspective, comparisons of cause-specific transplant rates were more relevant to our objectives and are easier to interpret. Additionally, since our interest was in the mechanics of the allocation system, and since organs are allocated only to alive, non-removed, active candidates, it makes sense to compare sex-specific transplant rates using this approach.
Surgical advances and new policy development may help remediate the transplant rate deficit for female candidates. For example, female liver transplant candidates may consider the option of living donor liver transplantation (38–41). The use of split liver grafts from deceased donors for smaller adult female recipients is also gaining favor, but requires further study (42). Finally, the transplant community has the opportunity and responsibility to amend liver allocation rules as circumstances warrant. Thus, several tools are available to increase equity between the sexes in liver transplantation.
In summary, sex-based disparities in liver transplant rates have increased over the last decade. Under current allocation rules, there are geographically pervasive disparities in female access to deceased donor liver transplants, and these disparities are concentrated among those candidates most likely to benefit from liver transplantation. Our analysis of other waiting list event rates suggests that females may be disproportionately surviving on the waiting list. Future efforts should be directed toward understanding the genesis of these inequities, with the goal of achieving more equitable access to liver transplantation for all candidates.
Acknowledgments
We would like to acknowledge Craig Lake for his assistance in graphics production. Funding support was obtained from the University of Michigan Center for Integrated Approaches in Health Disparities (AKM), and in part by National Institutes of Health grants 2R01DK070869 (DES), T32 CA009672-18 (AKM), and L60 MD002968-01 (AKM). The SRTR is funded by a contract from the Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Freeman RB, Jr, Wiesner RH, Roberts JP, McDiarmid S, Dykstra DM, Merion RM. Improving liver allocation: MELD and PELD. Am J Transplant. 2004;4 (Suppl 9):114–131. doi: 10.1111/j.1600-6135.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 2.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 3.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 4.Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 5.Wiesner RH. Evidence-based evolution of the MELD/PELD liver allocation policy. Liver Transpl. 2005;11(3):261–263. doi: 10.1002/lt.20362. [DOI] [PubMed] [Google Scholar]
- 6.Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, Kremers WK, et al. MELD and PELD: application of survival models to liver allocation. Liver Transpl. 2001;7(7):567–580. doi: 10.1053/jlts.2001.25879. [DOI] [PubMed] [Google Scholar]
- 7.Cholongitas E, Marelli L, Kerry A, Nair D, Patch D, Burroughs AK. MELD and gender in the waiting list for liver transplantation. Transplantation. 2008;85(10):1509–1510. doi: 10.1097/TP.0b013e31816ff2c0. author reply 1510. [DOI] [PubMed] [Google Scholar]
- 8.Cholongitas E, Marelli L, Kerry A, Senzolo M, Goodier DW, Nair D, et al. Different methods of creatinine measurement significantly affect MELD scores. Liver Transpl. 2007;13(4):523–529. doi: 10.1002/lt.20994. [DOI] [PubMed] [Google Scholar]
- 9.Cholongitas E, Marelli L, Kerry A, Goodier DW, Nair D, Thomas M, et al. Female liver transplant recipients with the same GFR as male recipients have lower MELD scores--a systematic bias. Am J Transplant. 2007;7(3):685–692. doi: 10.1111/j.1600-6143.2007.01666.x. [DOI] [PubMed] [Google Scholar]
- 10.Moylan CA, Brady CW, Johnson JL, Smith AD, Tuttle-Newhall JE, Muir AJ. Disparities in liver transplantation before and after introduction of the MELD score. JAMA. 2008;300(20):2371–2378. doi: 10.1001/jama.2008.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volk ML, Choi H, Warren GJ, Sonnenday CJ, Marrero JA, Heisler M. Geographic variation in organ availability is responsible for disparities in liver transplantation between Hispanics and Caucasians. Am J Transplant. 2009;9(9):2113–2118. doi: 10.1111/j.1600-6143.2009.02744.x. [DOI] [PubMed] [Google Scholar]
- 12.Schaubel DE, Stewart DE, Morrison HI, Zimmerman DL, Cameron JI, Jeffery JJ, et al. Sex inequality in kidney transplantation rates. Arch Intern Med. 2000;160(15):2349–2354. doi: 10.1001/archinte.160.15.2349. [DOI] [PubMed] [Google Scholar]
- 13.Roberts JP, Dykstra DM, Goodrich NP, Rush SH, Merion RM, Port FK. Geographic differences in event rates by model for end-stage liver disease score. Am J Transplant. 2006;6(10):2470–2475. doi: 10.1111/j.1600-6143.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- 14.US Scientific Registry of Transplant Recipients. 2009 August 5; 2009]; Available from: http://www.ustransplant.org.
- 15.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. 2. Hoboken, N.J: Wiley; 2002. [Google Scholar]
- 16.Cox DR. Regression Models and Life Tables (with Discussion) Journal of the Royal Statistical Society. 1972;(34):187–220. Series B. [Google Scholar]
- 17.Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2005;5(2):307–313. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 18.Bryce CL, Angus DC, Arnold RM, Chang CC, Farrell MH, Manzarbeitia C, et al. Sociodemographic Differences in Early Access to Liver Transplantation Services. Am J Transplant. 2009 doi: 10.1111/j.1600-6143.2009.02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaubel DE, Sima CS, Goodrich NP, Feng S, Merion RM. The survival benefit of deceased donor liver transplantation as a function of candidate disease severity and donor quality. Am J Transplant. 2008;8(2):419–425. doi: 10.1111/j.1600-6143.2007.02086.x. [DOI] [PubMed] [Google Scholar]
- 20.Ashby VB, Kalbfleisch JD, Wolfe RA, Lin MJ, Port FK, Leichtman AB. Geographic variability in access to primary kidney transplantation in the United States, 1996–2005. Am J Transplant. 2007;7(5 Pt 2):1412–1423. doi: 10.1111/j.1600-6143.2007.01785.x. [DOI] [PubMed] [Google Scholar]
- 21.Ellison MD, Edwards LB, Edwards EB, Barker CF. Geographic differences in access to transplantation in the United States. Transplantation. 2003;76(9):1389–1394. doi: 10.1097/01.TP.0000090332.30050.BA. [DOI] [PubMed] [Google Scholar]
- 22.Kemmer N, Safdar K, Kaiser T, Zacharias V, Neff GW. Impact of geographic location on access to liver transplantation among ethnic minorities. Transplantation. 2008;85(2):166–170. doi: 10.1097/TP.0b013e31816223f8. [DOI] [PubMed] [Google Scholar]
- 23.Mathur AK, Sonnenday CJ, Merion RM. Race and Ethnicity in Access to and Outcomes of Liver Transplantation: A Critical Literature Review. American Journal of Transplantation. 2009;9(12):2662–2668. doi: 10.1111/j.1600-6143.2009.02857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garg PP, Furth SL, Fivush BA, Powe NR. Impact of gender on access to the renal transplant waiting list for pediatric and adult patients. J Am Soc Nephrol. 2000;11(5):958–964. doi: 10.1681/ASN.V115958. [DOI] [PubMed] [Google Scholar]
- 25.Segev DL, Kucirka LM, Oberai PC, Parekh RS, Boulware LE, Powe NR, et al. Age and comorbidities are effect modifiers of gender disparities in renal transplantation. J Am Soc Nephrol. 2009;20(3):621–628. doi: 10.1681/ASN.2008060591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen JT, Berger AK, Duval S, Luepker RV. Gender disparity in cardiac procedures and medication use for acute myocardial infarction. Am Heart J. 2008;155(5):862–868. doi: 10.1016/j.ahj.2007.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parashar S, Katz R, Smith NL, Arnold AM, Vaccarino V, Wenger NK, et al. Race, gender, and mortality in adults > or =65 years of age with incident heart failure (from the Cardiovascular Health Study) Am J Cardiol. 2009;103(8):1120–1127. doi: 10.1016/j.amjcard.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raine R. Does gender bias exist in the use of specialist health care? J Health Serv Res Policy. 2000;5(4):237–249. doi: 10.1177/135581960000500409. [DOI] [PubMed] [Google Scholar]
- 29.Rathore SS, Chen J, Wang Y, Radford MJ, Vaccarino V, Krumholz HM. Sex differences in cardiac catheterization: the role of physician gender. JAMA. 2001;286(22):2849–2856. doi: 10.1001/jama.286.22.2849. [DOI] [PubMed] [Google Scholar]
- 30.Vaccarino V, Rathore SS, Wenger NK, Frederick PD, Abramson JL, Barron HV, et al. Sex and racial differences in the management of acute myocardial infarction, 1994 through 2002. N Engl J Med. 2005;353(7):671–682. doi: 10.1056/NEJMsa032214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paulson EC, Wirtalla C, Armstrong K, Mahmoud NN. Gender Influences Treatment and Survival in Colorectal Cancer Surgery. Dis Colon Rectum. 2009;52(12):1982–1993. doi: 10.1007/DCR.0b013e3181beb42a. [DOI] [PubMed] [Google Scholar]
- 32.Ayanian JZ, Cleary PD, Keogh JH, Noonan SJ, David-Kasdan JA, Epstein AM. Physicians’ beliefs about racial differences in referral for renal transplantation. Am J Kidney Dis. 2004;43(2):350–357. doi: 10.1053/j.ajkd.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 33.Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351(6):575–584. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 34.Tuttle-Newhall JE, Krishnan SM, Levy MF, McBride V, Orlowski JP, Sung RS. Organ donation and utilization in the United States: 1998–2007. Am J Transplant. 2009;9(4 Pt 2):879–893. doi: 10.1111/j.1600-6143.2009.02565.x. [DOI] [PubMed] [Google Scholar]
- 35.Scientific Registry of Transplant Recipients. 2009 Available from: http://www.ustransplant.org.
- 36.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 37.Ye Y. Doctoral Dissertation. Ann Arbor, MI: University of Michigan; 2006. Semiparametric Analysis of Correlated Recurrent and Terminal Events. [Google Scholar]
- 38.Berg CL, Gillespie BW, Merion RM, Brown RS, Jr, Abecassis MM, Trotter JF, et al. Improvement in survival associated with adult-to-adult living donor liver transplantation. Gastroenterology. 2007;133(6):1806–1813. doi: 10.1053/j.gastro.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freise CE, Gillespie BW, Koffron AJ, Lok AS, Pruett TL, Emond JC, et al. Recipient morbidity after living and deceased donor liver transplantation: findings from the A2ALL Retrospective Cohort Study. Am J Transplant. 2008;8(12):2569–2579. doi: 10.1111/j.1600-6143.2008.02440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghobrial RM, Freise CE, Trotter JF, Tong L, Ojo AO, Fair JH, et al. Donor morbidity after living donation for liver transplantation. Gastroenterology. 2008;135(2):468–476. doi: 10.1053/j.gastro.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olthoff KM, Merion RM, Ghobrial RM, Abecassis MM, Fair JH, Fisher RA, et al. Outcomes of 385 adult-to-adult living donor liver transplant recipients: a report from the A2ALL Consortium. Ann Surg. 2005;242(3):314–323. doi: 10.1097/01.sla.0000179646.37145.ef. discussion 323–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong JC, Yersiz H, Farmer DG, Duffy JP, Ghobrial RM, Nonthasoot B, et al. Longterm outcomes for whole and segmental liver grafts in adult and pediatric liver transplant recipients: a 10-year comparative analysis of 2,988 cases. J Am Coll Surg. 2009;208(5):682–689. doi: 10.1016/j.jamcollsurg.2009.01.023. discusion 689–691. [DOI] [PubMed] [Google Scholar]


