Abstract
Background. Visceral leishmaniasis (VL) is caused by Leishmania donovani and Leishmania infantum chagasi. Genome-wide linkage studies from Sudan and Brazil identified a putative susceptibility locus on chromosome 6q27.
Methods. Twenty-two single-nucleotide polymorphisms (SNPs) at genes PHF10, C6orf70, DLL1, FAM120B, PSMB1, and TBP were genotyped in 193 VL cases from 85 Sudanese families, and 8 SNPs at genes PHF10, C6orf70, DLL1, PSMB1, and TBP were genotyped in 194 VL cases from 80 Brazilian families. Family-based association, haplotype, and linkage disequilibrium analyses were performed. Multispecies comparative sequence analysis was used to identify conserved noncoding sequences carrying putative regulatory elements. Quantitative reverse-transcription polymerase chain reaction measured expression of candidate genes in splenic aspirates from Indian patients with VL compared with that in the control spleen sample.
Results. Positive associations were observed at PHF10, C6orf70, DLL1, PSMB1, and TBP in Sudan, but only at DLL1 in Brazil (combined P = 3 × 10−4 at DLL1 across Sudan and Brazil). No functional coding region variants were observed in resequencing of 22 Sudanese VL cases. DLL1 expression was significantly (P = 2 × 10−7) reduced (mean fold change, 3.5 [SEM, 0.7]) in splenic aspirates from patients with VL, whereas other 6q27 genes showed higher levels (1.27 × 10−6 < P < .01) than did the control spleen sample. A cluster of conserved noncoding sequences with putative regulatory variants was identified in the distal promoter of DLL1.
Conclusions. DLL1, which encodes Delta-like 1, the ligand for Notch3, is strongly implicated as the chromosome 6q27 VL susceptibility gene.
Ninety percent of visceral leishmaniasis (VL) cases caused by the Leishmania donovani complex occur in India/Bangladesh/Nepal, Brazil, and Sudan. The disease is characterized by parasitization of the spleen, liver, lymph nodes, and bone marrow and is fatal if left untreated in the 10%–20% of infected individuals who are susceptible. VL is listed as a neglected disease by the World Health Organization. Large-scale epidemics with high mortality rates have occurred in rural areas of Sudan and India. Outbreaks in Brazil are often peri-urban.
Host genetic factors are indicated by familial clustering [1, 2], ethnic differences [3], and high sibling risk ratios [4]. Family-based genome-wide linkage studies (GWLSs) (reviewed by Blackwell et al [5]) identified chromosome 6q26-q27 as a region of interest in eastern Sudan [6], with a major village-specific peak of linkage (Logarithm of odds [LOD], 3.58; P = 2.5 × 10−5) observed for El-Rugab village at D6S281 on 6q27. This result was replicated (LOD, 0.97; P = .017) precisely in a GWLS of an ethnically admixed population from northeast Brazil [7].
In this study, we investigated the candidacy of genes PHF10, C6orf70, DLL1, FAM120B, PSMB1, and TBP under the 6q27 peak of linkage. Specific replication in Brazil for one of several loci associated with VL in Sudan provides initial evidence to pinpoint DLL1 as the causal gene. This finding is supported by linkage disequilibrium (LD), haplotype, and resequencing data, as well as quantitative expression profiles in splenic aspirates and in silico analysis of putative etiological variants located in regions of conserved noncoding sequence (CNS).
METHODS
Study Sites
In Sudan, 193 VL cases from 85 families were studied: 42 families from El-Rugab village (108 cases) and 43 families from Um-Salala village (85 cases) (Table 1). These villages lie 40 km apart and 400 km southeast of Khartoum on the eastern bank of the River Rahad (Galabat Province, Gadaref State), as described elsewhere [6]. The people of these villages are Nilosaharan-speaking Masalit who are recent immigrants to the area [8]. These villages have been under annual surveillance for VL by the Institute of Endemic Diseases since the mid-1980s; a Médecins Sans Frontières treatment center was established in 1996. Epidemiological and demographic details are described elsewhere [8–10]. Sampling was performed by means of noninvasive buccal swabs, with genomic DNA extracted from buccal lysate. Some DNA samples were replenished using whole genome amplification (Repli-g; Qiagen). Ethical approval was obtained from the institutional review board of the University of Khartoum (Khartoum, Sudan). Informed consent was obtained from adults and from parents of children <18 years old.
Table 1.
Structure of Sudanese and Brazilian Families
| Sudanese families |
||||
| Family structure | El-Rugab | Um-Salala | Total | Brazilian families |
| Families | 42 | 43 | 85 | 80 |
| Nuclear families | 45 | 48 | 93 | 99 |
| Nuclear families with 1 affected sibling | 11 | 27 | 38 | 27 |
| Nuclear families with 2 affected siblings | 18 | 10 | 28 | 57 |
| Nuclear families with 3 affected siblings | 12 | 9 | 21 | 9 |
| Nuclear families with 4 affected siblings | 1 | 2 | 3 | 2 |
| Nuclear families with 5 affected siblings | 3 | 0 | 3 | 1 |
| Total affected individuals | 108 | 85 | 193 | 194 |
| Affected offspring | 102 | 82 | 184 | 178 |
| Affected parents | 6 | 1 | 7 | 9 |
| Total individuals | 182 | 216 | 398 | 456 |
NOTE. Data are no. of families or individuals. Details of the structure of the Sudanese and Brazilian visceral leishmaniasis multicase families and trios are given. Numbers are given for successfully genotyped individuals.
Replication was performed for samples from 194 VL patients from 80 Brazilian families (Table 1) whose samples were collected during the Belém Family Study, which was conducted from 1991 through 1994 [4]. Families were ascertained from medical records of the Fundacão National de Saude in the States of Para, Maranhão, and Piaui with the use of data from the epidemics of 1983–1985 and 1993–1994. These populations are long-term (>200 years) admixtures of Caucasian, African, and Native American ethnic backgrounds [11]. All families were of equivalent socioeconomic status. Epidemiological and demographic information is described elsewhere [4]. Blood samples were collected by venepuncture, and genomic DNA was prepared from Epstein–Barr virus–transformed B cells [7]. Whole genome amplification (Repli-g kit; Qiagen) was used to replenish some DNA stocks. Ethical approval was obtained originally from the local ethics committee at the Instituto Evandro Chagas, Belém, Pará, Brazil [4]. Approval for continued use of samples has been granted from the local institutional review board at the Universidade Federal do Rio Grande do Norte (Natal, Brazil; CEP-UFRN 94-2004), nationally from the Comissão Nacional de Ética em Pesquisa (CONEP; 11019), and from the Ministério da Ciência e Tecnologia for approval to ship samples out of Brazil (portaria 617; 28 September 2005). Informed consent was obtained from adults and from parents of children <18 years old.
In India, a sample of splenic aspirate collected as part of routine diagnostic procedures was obtained from each of 10 patients with VL at the Kala-Azar Hospital, Muzaffarpur, Bihar State, Northern India. Approval was provided by the ethical committee of the Institute of Medical Sciences, Banaras Hindu University (Varanasi, India). Informed consent was obtained from adults and from parents of children <18 years old.
Individuals were classified as affected if they received diagnoses of clinical VL that responded to specific antileishmanial treatment. Data on subclinical disease or asymptomatic infections were not included. Diagnosis of clinical VL was made on the basis of clinical, parasitological, and serological criteria as described elsewhere [4, 8, 10]. In Sudan, isoenzyme typing of isolates from cases during the 1995–1996 season showed 3 zymodemes in this area, corresponding to L. donovani sensu stricto, Leishmania infantum, and Leishmania archibaldi [8]. In Brazil, the causative agent of VL in a subset of patients (∼10% over 4 states) was confirmed as L. infantum chagasi [4].
Genotyping
High-throughput single-nucleotide polymorphisms (SNPs) at PHF10, C6orf70, DLL1, FAM120B, PSMB1, and TBP were genotyped using TaqMan SNP genotyping assays (Applied Biosystems). SNPs were chosen on the basis of proximity to genes and public domain information on allele frequencies for the HapMap Yoruba (YRI) population in Ibadan, Nigeria [12], by use of the Haploview tagging SNP application (http://www.broadinstitute.org/haploview) [13]. Taqman assays were analyzed using an ABI Prism 7900HT sequence detection system (SDS) and SDS software (version 1.2; Applied Biosystems). Twenty-four SNPs (Table 1; available online) were genotyped in 85 Sudanese families. Eight SNPs with positive association in El-Rugab (P < .05) were genotyped in 80 Brazilian families. The only notable allele frequency difference between cases in Brazil and those in Sudan was at rs9459988 near DLL1 (common T allele 0.87 in Brazil and 0.52 in El-Rugab). This marker therefore had less power to detect association in the Brazilian study (see below). Marker rs4716396 at C6orf70 was not available for genotyping in Brazil and was replaced by rs4145078, which was in strong LD (D′ = 1; r2 = 0.76) with rs4716396 in El-Rugab.
Data Analyses
A blind double-scoring system was implemented for genotype clustering of SNPs. Mendelian inconsistencies were identified in PedCheck [14] and removed. Since familial relationships within Sudanese and Brazilian families had been assessed during GWLSs [6, 7], PedCheck errors were due to allele miscalling. Minor allele frequencies (MAFs) were determined from unrelated individuals in families by use of Splink (http://www-gene.cimr.cam.ac.uk/clayton/software/splink.txt) [15]. Noninformative markers with a MAF of <0.1 were excluded from further analysis (Table 1; available online). Deviation from Hardy–Weinberg equilibrium (HWE) was determined using 168 and 136 unrelated unaffected individuals from Sudanese and Brazilian families, respectively (Table 1; available online). Tests for HWE were carried out within Stata (version 8.0; http://www.stata.com/) using the GenAssoc package (http://www-gene.cimr.cam.ac.uk/clayton/software/stata/). Markers out of HWE (determined using a conservative cutoff of P < .05) were excluded. Patterns of LD were determined for unrelated individuals using Hedrick’s definition of Lewontin’s D′ statistic and r2 as implemented in Haploview [13].
Power calculations for trios were performed according to the method of Knapp [16]. This method provides an estimate of power for independent trios, which would be the case for trios in families for a true functional variant, or a marker in complete LD with it [17]. We determined statistical power for the number of full parent-offspring trios for each population. For El-Rugab, 86 trios had ≥80% power to detect an odds ratio (OR) of 2 at P = .05 for SNPs with a MAF of ≥0.2, and ≤58% power for SNPs with a MAF of ≤0.1. For Um-Salala, 71 trios had ≥72% power to detect an OR of 2 (P = .05) for SNPs with a MAF of ≥0.2, and ≤50% power for SNPs with a MAF ≤0.1. For Brazil, 176 trios had ≥93% power to detect an OR of 2 (P = .01) for SNPs with a MAF of ≥0.2, and ≤71% power for SNPs with a MAF of ≤0.1. All SNPs used had a MAF of ≥0.2. Since markers used for association analysis were unlikely to represent causal variants or to be in complete LD with a functional variant, robust association tests were performed to take account of multiple trios within a pedigree (see below).
Association analyses were performed under an additive model using family-based case-pseudocontrol (CPC) analysis [18], in which each affected offspring is matched to 1–3 pseudocontrols that derive from the remaining possible genotypes of the parental mating. ORs, 95% confidence intervals (CIs) and P values are calculated using conditional logistic regression (CLOGIT) models employing a robust sandwich estimator of variance and a Wald χ2 test statistic to control for clustering of trios within pedigrees. CPC was implemented in Stata (version 8.0; http://www.stata.com/). Combined P values using the Fisher trend test were calculated using MetaP (http://people.genome.duke.edu/∼dg48/metap.php) [19].
Haplotype analysis was performed in TRANSMIT (http://www-gene.cimr.cam.ac.uk/clayton/software/transmit.txt) [20], a generalization of the transmission disequilibrium test that investigates transmission of multilocus genotypes when phase is unknown and parental genotypes are missing. Association analyses were performed for all significantly associated 6q27 SNPs with χ2 statistics, corresponding P values (global and 1 degree of freedom), and haplotype frequencies computed for all possible 2-, 3-, and 4-SNP haplotypes. The robust estimate of the variance of the score factor implemented in the −ro flag option was utilized to allow for multiple affected siblings within a family in the presence of linkage.
Resequencing
Resequencing of all 11 exons of DLL1, along with ∼100 bp of intron-exon boundaries, intron 1, 1 kb upstream, and 1 kb downstream was performed for 20 affected and 2 unaffected individuals from El-Rugab (1 case per family) with use of genomic DNA. CNSs (see above) upstream of DLL1 were resequenced and analyzed using PreGap and Gap4 in Staden (http://staden.sourceforge.net/overview.html) [21].
Real-time Polymerase Chain Reaction
Gene expression in splenic aspirates from 10 Indian patients with VL was compared with that in normal human spleen (BD Biosciences). RNA from aspirates, preserved in 0.5–1 mL of RNAlater reagent (Qiagen), was isolated using the QIAshredder homogenizer and RNeasy mini kit (Qiagen). Complementary DNA (cDNA) was synthesised using Moloney murine leukemia virus reverse transcriptase (Invitrogen). Primers and fluorescently labeled probes were designed using Primer3 (http://frodo.wi.mit.edu/) [22]. Real-time polymerase chain reactions were performed on 12.5 ng of cDNA and analyzed using an ABI Prism 7700HT Fast Real-Time PCR system and SDS software (version 2.3; Applied Biosystems). Expression of target genes was normalized to 18S ribosomal RNA or β-actin endogenous controls and further calibrated to control human spleen samples by use of the standard curve method for relative quantification according to ABI’s instructions. A 1-sample T test was performed to compare expression values for the splenic aspirates against the control spleen samples as a population mean.
Bioinformatics
CNSs were identified for all 6q27 genes and intergenic regions flanked by PHF10 and PDCD2. Genomic sequences and associated gene annotations for human, mouse, rat, dog, and opossum were exported from Ensembl (National Center for Biotechnology Information build 36; Ensembl release 43) in FASTA and General Feature File (GFF) format, respectively. Global alignment of genomic sequences was performed in Multi-LAGAN (http://lagan.stanford.edu/lagan_web/index.shtml) [23, 24]. The annotated alignment was visualized in SynPlot (http://hscl.cimr.cam.ac.uk/syn_plot.html), and CNSs of a specified percentage identity value based on the degree of exonic conservation in the region were identified in SynPlot-Peaks (http://hscl.cimr.cam.ac.uk/syn_plot_peaks.html) [25]. To search for putative transcription factor binding sites (TFBSs) at SNP locations, we used the online tools PROMO (http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3) [26], AliBaba (version 2.1; http://www.gene-regulation.com/pub/programs.html) [27], and MatInspector (http://www.genomatix.de/online_help/help_matinspector/matinspector_help.html) [28].
Accession Numbers
The Swissprot accession numbers for the genes studied here are PHF10:Q8WUB8; C6orf70:Q5T6L9; DLL1:O00548; FAM120B:Q96EK7; PSMB1:P20618; and TBP:P20226.
RESULTS
Association in Sudan
Individuals from 42 El-Rugab and 43 Um-Salala families were genotyped for 24 SNPs (Figure 1; online only). Eighteen SNPs passed quality control tests (MAF, >0.1; HWE P > .05) for association analysis (Table 2). On the basis of our GWLS [6], we hypothesized that associations at 6q27 would be specific to El-Rugab village. Accordingly, using a robust CPC [18], we found the following: (1) 8 markers associated with VL in El-Rugab (Table 2), and (2) no markers associated with VL in Um-Salala (Table 2; available online). Application of a strict Bonferroni correction for 18 SNPs provides a significance cutoff of P ≤ .003 (ie, P = .05/18), which is achieved at rs17860704. Given that the 18 SNPs are not all independent (see below) (Figure 1), this result is overconservative and could lead to loss of power. A less stringent correction that takes account of nonindependence of markers provides a cutoff of P ≤ .006 (ie, P = .05/8; 4 LD blocks and 4 independent markers). The adjacent SNP rs9459988 is robust to this correction. Both robust associations are in SNPs immediately 5′ of DLL1. Effect sizes (OR, >2) (Table 2) are also consistent with the predicted power for El-Rugab. These are large effect sizes for a complex disease, which is consistent with the high LOD score (LOD, 3.58; P = 2.5 × 10−5) for linkage in El-Rugab [6].
Table 2.
Robust CLOGIT Analysis of Associations in El-Rugab Village, Sudan, and in Brazil
| Marker | Disease-associated allele | Allele frequency | No. of case-pseudocontrol sets | Pa | OR (95% CI) |
| El-Rugan village, Sudan | |||||
| PHF10/rs4073926 | T | 0.44 | 37 | .010b | 2.45 (1.24–4.86) |
| PHF10/rs9371126 | T | 0.75 | 43 | .809 | 1.14 (0.39–3.37) |
| C6orf70/rs4145078 | G | 0.3 | 54 | .154 | 1.67 (0.83–3.36) |
| C6orf70/rs4716396 | C | 0.37 | 58 | .043b | 2.07 (1.02–4.21) |
| DLL1/rs1028488 | G | 0.51 | 48 | .355 | 1.21 (0.81–1.80) |
| DLL1/rs1884190 | T | 0.56 | 22 | .085 | 1.5 (0.95–2.38) |
| DLL1/rs2738822 | T | 0.39 | 35 | .021b | 1.75 (1.09–2.82) |
| DLL1/rs2738820 | A | 0.32 | 46 | .019b | 1.69 (1.09–2.62) |
| DLL1/rs9459988 | T | 0.52 | 49 | .004b | 2.20 (1.29–3.76) |
| DLL1/rs17860704 | C | 0.81 | 31 | .001b | 3.25 (1.61–6.57) |
| FAM120B/rs9366198 | C | 0.55 | 65 | .203 | 1.39 (0.84–2.31) |
| FAM120B/rs9460106 | C | 0.48 | 46 | >.999 | 1 (0.53–1.90) |
| FAM120B/2103816 | A | 0.43 | 53 | .192 | 1.38 (0.85–2.22) |
| FAM120B/rs9295407 | A | 0.91 | 39 | .071 | 3.5 (0.9–13.6) |
| PSMB1/rs6914744 | C | 0.29 | 56 | .008b | 2.40 (1.25–4.60) |
| PSMB1/rs12717 | C | 0.26 | 58 | .254 | 1.57 (0.72–3.42) |
| TBP/rs13207114 | G | 0.4 | 51 | .026b | 1.79 (1.07–2.99) |
| TBP/rs6937840 | G | 0.21 | 67 | .91 | 1.05 (0.47–2.32) |
| Brazil | |||||
| PHF10/rs4073926 | C | 0.49 | 59 | .903 | 1.04 (0.55–1.95) |
| C6orf70/rs4145078 | A | 0.68 | 39 | .804 | 1.07 (0.62–1.85) |
| DLL1/rs2738822 | T | 0.46 | 79 | .383 | 1.24 (0.77–1.98) |
| DLL1/rs2738820 | A | 0.46 | 94 | .56 | 1.14 (0.73–1.79) |
| DLL1/rs9459988 | G | 0.13 | 76 | .402 | 1.56 (0.55–4.38) |
| DLL1/rs17860704 | C | 0.91 | 80 | .047b | 3 (1.01–8.86) |
| PSMB1/rs6914744 | A | 0.62 | 45 | .131 | 1.53 (0.88–2.65) |
| TBP/rs13207114 | A | 0.67 | 56 | .428 | 1.27 (0.7–2.31) |
NOTE. Robust CLOGIT odds ratios (ORs) and 95% confidence intervals (CIs) for allele-wise associations are shown. Single-nucleotide polymorphisms genotyped in the Brazilian sample were those with evidence for positive associations in El-Rugab village in Sudan.
One degree of freedom.
Positive associations at nominal P < .05.
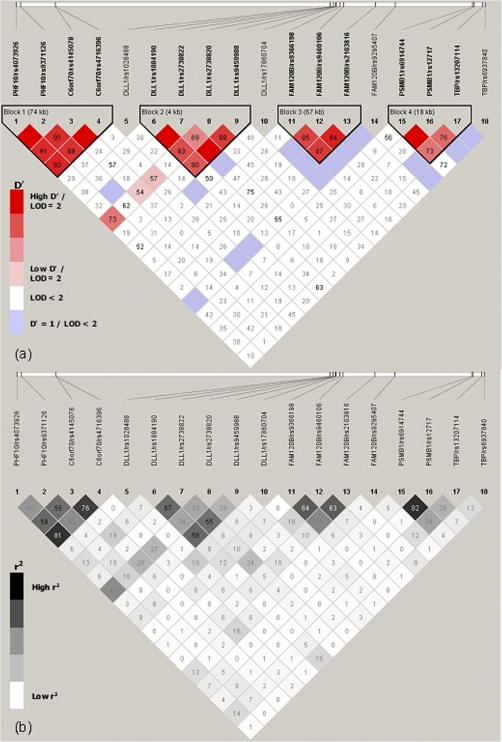
Figure 1.
Pairwise D′ (a) and r2 (b) linkage disequilibrium measures across the18 biallelic markers in the 6q27 region. Linkage disequilibrium was determined in 65 unrelated unaffected individuals from the families in El-Rugab, Sudan, by means of the default parameters in Haploview (http://www.broadinstitute.org/haploview).
LD (Figure 1) across the 18 SNPs showed 4 distinct LD blocks for El-Rugab defined by markers near (1) PHF10 and C6orf70, (2) DLL1, (3) FAM120B, and (4) PSMB1 and TBP. Some associations in El-Rugab can thus be attributed to LD between neighboring genes. Long-range LD across the 4 blocks is observed only between rs2738820 at DLL1 and all SNPs of the PHF10-C6orf70 block. The most associated SNP upstream of DLL1, rs17860704, is not in strong LD with any other markers.
Replication at DLL1 in Brazil
SNPs positively associated with VL in El-Rugab were genotyped in 80 Brazilian families. A robust CPC study confirmed a positive association upstream of DLL1 at rs17860704 (nominal P = .047) (Table 2). The combined P value across El-Rugab and Brazil is = 3 × 10−4.
Haplotype Analyses
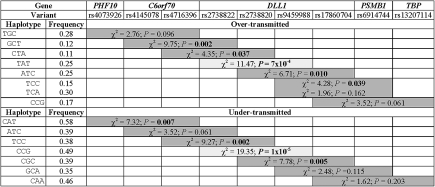
To define the 6q27 region of association more precisely, 2-, 3-, and 4-marker haplotype analyses were performed in TRANSMIT (Figure 2) for positive SNPs (nominal P < .05) in El-Rugab. Only haplotypes with a frequency of >0.1 and associations based on >10 informative transmissions were considered. The most significant associations were observed for 3-marker haplotypes across the DLL1 SNPs rs2738822-rs2738820-rs9459988, including both overtransmitted (TAT; frequency, 0.25; P = 7 × 10−4) and undertransmitted (CCG; frequency, 0.49; P = 1 × 10−5) haplotypes. This finding is consistent with observed LD (Figure 1) and strongly supports candidacy of DLL1 as the putative susceptibility gene at 6q27.
Figure 2.
TRANSMIT associations for 3-marker haplotypes across 6q27 in the families from El-Rugab, Sudan. One degree of freedom χ2 tests and corresponding P values are shown for haplotypes that are over- or undertransmitted from heterozygous parents to affected offspring. Nominal P values of <.05 are in boldface. All χ2 tests shown are based on >10 informative transmissions, and data are only shown for haplotypes with frequencies of >0.1.
Resequencing DLL1
In view of the association mapping to DLL1, we resequenced the samples from 22 individuals from El-Rugab for DLL1 and identified 3 novel noncoding SNPs, only 1 of which has a MAF of ≥0.1 (Table 3; available online). No novel or known SNPs with potential functional relevance to VL were identified within coding sequences, in the 5′ untranslated region, or in the 500 bp immediately upstream of DLL1, which would usually be associated with strong promoter activity. This finding indicates that the functional variant or variants controlling DLL1 expression might lie within a more distal upstream regulatory region. SNPs rs2738820, rs9459988, and rs1786074 all lie in this region.
Differential DLL1 Expression in Patients With VL
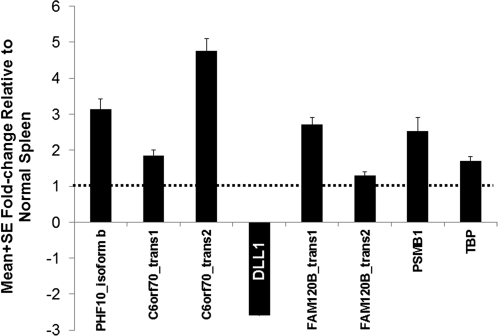
To determine whether a search for regulatory elements at DLL1 would be profitable, we sought further biological evidence by quantitative assessment of DLL1 expression in splenic aspirates relative to the other 6q27 genes (Figure 3). The latter all showed higher expression (1.27 × 10−6 < P < .01) in VL aspirates compared with that in control spleen samples. On the contrary, DLL1 expression was uniformly (P = 2 × 10−7) reduced (mean fold change, 3.5 [SEM, 0.7]) in splenic aspirates from VL patients compared with that in control spleen samples, encouraging the search for a putative upstream regulatory polymorphism.
Figure 3.
Mean fold change (± SEM) in 6q27 gene expression levels in splenic aspirates from Indian patients with visceral leishmaniasis (n = 12) relative to those in the normal spleen sample (n = 1). A 1-sample T test was used to compare expression levels for each gene in visceral leishmaniasis case aspirates against those in the control spleen sample as a population mean. DLL1 expression was significantly (P = 2 × 10−7) reduced (mean fold change, 3.5 [SEM, 0.7]) in splenic aspirates from patients with visceral leishmaniasis, whereas other 6q27 genes showed higher levels (1.27 × 10−6 < P < .01) than those in the control spleen sample.
Bioinformatics Analyses for CNSs
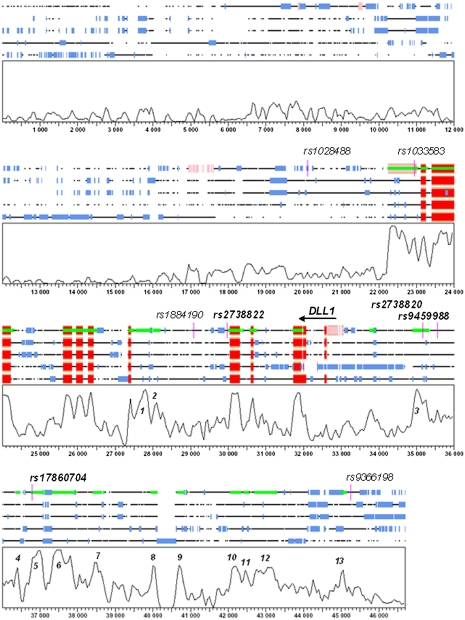
Genomic variation within CNSs is associated with phenotypic variability [29, 30] due to regulatory elements [31]. The location of associated 6q27 variants with respect to CNSs is therefore of interest. To identify CNSs, comparative sequence analysis was performed for the region flanked by PHF10 and PDCD2. A number of CNSs were identified across 6q27, few of which qualify as functionally conserved according to the criteria of 70% similarity over at least 100 bp of ungapped alignment [29, 32]. The most highly conserved cluster of CNSs was observed upstream of DLL1 and spanned ∼8.8 kb commencing 1.4 kb upstream of the 5′ untranslated region (labeled as peaks 3–13) (Figure 4). Three of the VL-associated SNPs are located within this cluster, specifically in CNSs labeled as peaks 3, 5, and 6 in Figure 4. DLL1 rs2738820 (nominal P = .019; El-Rugab) is positioned within peak 3, 1.5 kb upstream of DLL1, which shows 75% identity across species over 378 nucleotides of alignment. SNP rs9459988 (nominal P = .004; El-Rugab) does not fall within a CNS but is positioned 155 bp proximal to peak region 3. SNP rs17860704, for which the association with VL in El-Rugab (nominal P = .001) was replicated in Brazil (combined P = 3 × 10−4), is located within peak 5. This peak shows 79% identity over 317 nucleotides of virtually ungapped alignment of human, mouse, rat, dog, and opossum sequences. However, rs17860704 does not itself qualify as the putative etiological variant because the most significant over- and undertransmitted haplotypes in El-Rugab (Figure 2) both carry the C allele at this SNP. Hence, it is likely that rs17860704 tags the true etiological variant or variants in Sudan and Brazil.
Figure 4.
Graphical representation of the DLL1 multiple alignment generated in SynPlot. The sequences of each species are rendered as lines interrupted by spaces corresponding to the gaps inserted for optimum global alignment. Sequences are displayed, from top to bottom, for human, mouse, rat, dog, and opossum. Underneath the alignment lies a plot of the degree of sequence conservation across all species analyzed. The horizontal axis represents the distance from the start of the alignment, and the vertical axis represents the percentage identity score generated by SynPlot (scale, 0%–100% sequence identity across all species). The sequences in the graphs are annotated with blocks of untranslated regions in pink, coding sequences in red, and repeat elements in blue. All conserved sequences above a minimum identity specified for each alignment are represented in lime green, whereas the position of single-nucleotide polymorphisms (SNPs) genotyped in the Sudanese population is depicted in magenta. SNPs associated with visceral leishmaniasis in El-Rugab, Sudan, are highlighted in boldface. Peak regions that correspond to conserved noncoding sequences, as opposed to coding sequences or repeat features, are numbered.
To find other putative causal variants in CNSs upstream of DLL1, peaks 1, 3, 5, and 6 (Figure 4) were resequenced in samples from 22 El-Rugab individuals. No novel SNPs were identified across these regions. Eighteen known SNPs fall within CNSs across the 8.8-kb region upstream of DLL1 (Table 3). Only 6 of these are located within the highly conserved peaks 3, 5, and 6, including the 2 associated SNPs rs2738820 and rs17860704 and 4 SNPs (rs17860703, rs57618361, rs34733288, and rs73790663) that are not polymorphic in the 22 Sudanese samples that were sequenced. Twelve other variants lie in more distal CNSs (peaks 7, 8, 10, 11, 12, and 13) (Table 3). Further genotyping is required to determine whether these SNPs are associated with VL in Sudan or Brazil. However, the haplotype analysis suggests that association falls off at and beyond SNP rs17860704, which focuses the search for an etiological variant in the region containing SNP rs2738820, the proximal upstream region, or within the DLL1 intronic regions. We therefore determined what TFBS might be localized at rs2738820 and how the polymorphism might modify the ability of transcription factors to bind to this location. Of the TFBSs lost or gained at rs2738820 (data not shown), FAST1 (FOXH1) is of singular interest as a binder of SMAD2.
Table 3.
Known Single-nucleotide Polymorphisms (SNPs) Within Conserved Noncoding Sequence (CNS) Peaks in the Region Spanning From the 5′ Untranslated Region to 8.8 kb Upstream of DLL1
| SNP | Physical position, bp | Position between markers, bp | CNS peaka | Alleles | MAFb |
| rs2738820c | 170601259 | 0 | 3 | C/A | CEU, 0.37; YRI, 0.32; DIL, 0.45; El-Rugab, 0.36 |
| rs17860704c | 170602429 | 1170 | 5 | T/C | YRI, 0.1; DIL, 0.01; El-Rugab, 0.19 |
| rs17860703 | 170602438 | 9 | 5 | G/A | DIL, 0.01; El-Rugab, 0 |
| rs57618361 | 170602477 | 39 | 5 | A/G | El-Rugab, 0 |
| rs34733288 | 170602979 | 502 | 6 | C/A | El-Rugab, 0 |
| rs73790663 | 170603272 | 293 | 6 | G/A | El-Rugab, 0 |
| rs9348307 | 170603985 | 713 | 7 | G/C | CEU, 0.2; YRI, 0; DIL, 0.16 |
| rs12200136 | 170605058 | 1073 | 8 | G/A | |
| rs77087564 | 170605086 | 28 | 8 | G/A | CEU, 0.17 |
| rs79819098 | 170606572 | 1486 | 10 | G/T | |
| rs35020594 | 170606633 | 61 | 10 | C/A | |
| rs76301666 | 170606709 | 76 | 11 | G/T | CEU, 0.14 |
| rs78981869 | 170607126 | 417 | 12 | G/T | YRI, 0.02 |
| rs76345779 | 170607215 | 89 | 12 | T/G | |
| rs77088136 | 170607218 | 3 | 12 | A/T | |
| rs34203352 | 170607258 | 40 | 12 | −/A | |
| rs79394597 | 170608750 | 1492 | 13 | G/A | YRI, 0.04 |
| rs60935272 | 170608860 | 110 | 13 | G/A |
NOTE. Physical positions are given for build 37.1 of the human genome. MAF, minor allele frequency.
Peaks are numbered in Figure 4.
Includes data from HapMap Utah residents with ancestry from northern and western Europe (CEU) and Yoruba individuals in Ibadan, Nigeria (YRI) [12], and data from 96 Caucasian European control individuals (DIL) [41].
SNPs genotyped in this study.
DISCUSSION
A gene at chromosome 6q27 that influences susceptibility to VL was previously indicated by a GWLS in Sudan [6] and Brazil [7]. Six genes are annotated under the direct linkage peak. We provide evidence from allelic and haplotype association analyses of SNPs across these genes in Sudan and Brazil, together with expression data from clinical samples from India, that DLL1 is the etiological gene influencing susceptibility to VL within this 6q27 gene cluster. In agreement with the GWLS [6], the association in Sudan is specific to El-Rugab village, which supports our previous interpretation that founder effect and consanguinity have influenced the pattern of genes controlling infection across villages recently inhabited by family groups migrating from Western Sudan. Haplotype analysis indicates that the etiological variant is positioned within or upstream of DLL1, and we highlighted a cluster of upstream CNSs likely to contain the regulatory elements influencing DLL1 expression following infection. Our data also point to alterations in TFBS activity by SNP rs2738820, which could be the variant influencing DLL1 expression. In particular, carriage of alternative alleles at rs2738820 results in loss or gain of the TFBS for FAST1 (FOXH1). Responses to transforming growth factor β (TGF-β), which is strongly implicated in regulating VL disease in the absence of interleukin 12 [33], are mediated by a DNA-binding complex formed by FAST1, SMAD2, and SMAD4 [34, 35]. Hence, a putative binding site for FAST1 within the distal highly conserved DLL1 promoter could point to DLL1 as a downstream target of TGF-β, which would explain a regulatory role played by rs2738820 in DLL1 expression during VL.
DLL1 encodes Delta-like 1, a human homolog of the Drosophila Notch ligand Delta that is the ligand for Notch3 [36, 37]. Notch signaling is one of the most conserved pathways in the regulation of cell differentiation and cell fate decisions [38, 39]. Given this evolutionarily conserved role, it is not surprising that there is a cluster of CNSs and regulatory elements upstream of DLL1, as reported by others [40, 41]. Experiments in transgenic mice have further demonstrated the presence of distinct positive and negative cis-regulatory elements within this conserved region directing tissue-specific expression of Delta-1 during early development [40].
Notch signaling plays an important role during development and is a major pathway involved in development and differentiation of immune cells. In bone marrow, Notch ligand Delta-1 completely inhibits differentiation of human hematopoietic progenitors into the B-cell lineage while promoting emergence of cells with a T-cell and natural killer precursor phenotype [42]. Bone marrow macrophage-like stromal cells are targeted by L. donovani and exhibit increased capacity for driving hematopoiesis [43]. The presence of the parasite could therefore directly influence the generation of T-cell precursors. In the spleen, Notch signaling is required to maintain CD8+ dendritic cells [44]. Blocking of Delta-like 1 alone has no effect on the maintenance of these dendritic cells, but blocking multiple Notch ligands, including Delta-like 1, Delta-like 4, Jagged1, and Jagged2, significantly decreases the number of CD8+ dendritic cells. Antigen presenting cells, including dendritic cells, also use Notch signaling to promote T helper cell differentiation in response to specific antigens [37]. Within the family of Notch ligands, Delta1 and Delta4 instruct antigen driven CD4 T-cell selection down the Th1 fate as opposed to the Th2 fate that is driven by Jagged1 and Jagged2 [37, 45]. The effects of polarized antigen-specific Th1 and Th2 responses on the outcome of leishmaniasis are well established in murine models [46, 47] and human disease [48, 49]. Clinical VL, in particular, has been associated with high Th2 cytokine responses [48], whereas Th1-generated interferon γ levels are higher in children infected with L. infantum chagasi that do not progress to clinical VL than in those who do [50]. The potential for Delta-1–driven Th1 differentiation to alter the course of infection has already been demonstrated for Leishmania major infection in BALB/c mice [36], making genetic regulation of DLL1 expression a highly plausible explanation for the genetic associations and downregulated splenic expression observed with this 6q27 gene.
Overall, our results strengthen the evidence for the role of 6q27 in VL susceptibility and point to DLL1 as the most plausible candidate based on genetic and functional data presented here and on its known immunological functions. Further investigations to definitively identify the causal variants and study DLL1 function in patients with VL and control individuals are needed. These studies will help unravel the molecular pathways that determine VL pathogenesis and provide important leads for development of improved strategies for vaccination and therapeutic intervention.
Funding
This work was supported by the Wellcome Trust (grants 074196/Z/04/Z to J. M. B.).
Acknowledgments
We thank the people of eastern Sudan, northeastern Brazil, and northern India for their participation in this study.
References
- 1.Cabello PH, Lima AM, Azevedo ES, Krieger H. Familial aggregation of Leishmania chagasi infection in northeastern Brazil. Am J Trop Med Hyg. 1995;52:364–5. doi: 10.4269/ajtmh.1995.52.364. [DOI] [PubMed] [Google Scholar]
- 2.Bucheton B, Kheir MM, El-Safi SH, et al. The interplay between environmental and host factors during an outbreak of visceral leishmaniasis in eastern Sudan. Microbes Infect. 2002;4:1449–57. doi: 10.1016/s1286-4579(02)00027-8. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim ME, Lambson B, Yousif AO, et al. Kala-azar in a high transmission focus: an ethnic and geographic dimension. Am J Trop Med Hyg. 1999;61:941–4. doi: 10.4269/ajtmh.1999.61.941. [DOI] [PubMed] [Google Scholar]
- 4.Peacock CS, Collins A, Shaw MA, et al. Genetic epidemiology of visceral leishmaniasis in northeastern Brazil. Genet Epidemiol. 2001;20:383–96. doi: 10.1002/gepi.8. [DOI] [PubMed] [Google Scholar]
- 5.Blackwell JM, Fakiola M, Ibrahim ME, et al. Genetics and visceral leishmaniasis: of mice and man. Parasite Immunol. 2009;31:254–66. doi: 10.1111/j.1365-3024.2009.01102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller EN, Fadl M, Mohamed HS, et al. Y chromosome lineage- and village-specific genes on chromosomes 1p22 and 6q27 control visceral leishmaniasis in Sudan. PLoS Genet. 2007;3:679–88. doi: 10.1371/journal.pgen.0030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamieson SE, Miller EN, Peacock CS, et al. Genome-wide scan for visceral leishmaniasis susceptibility genes in Brazil. Genes Immun. 2007;8:84–90. doi: 10.1038/sj.gene.6364357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalil EA, Zijlstra EE, Kager PA, El Hassan AM. Epidemiology and clinical manifestations of Leishmania donovani infection in two villages in an endemic area in eastern Sudan. Trop Med Int Health. 2002;7:35–44. doi: 10.1046/j.1365-3156.2002.00832.x. [DOI] [PubMed] [Google Scholar]
- 9.Zijlstra EE, el-Hassan AM. Leishmaniasis in Sudan: visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2001;95(suppl 1):S27–58. doi: 10.1016/s0035-9203(01)90218-4. [DOI] [PubMed] [Google Scholar]
- 10.Zijlstra EE, el-Hassan AM, Ismael A, Ghalib HW. Endemic kala-azar in eastern Sudan: a longitudinal study on the incidence of clinical and subclinical infection and post-kala-azar dermal leishmaniasis. Am J Trop Med Hyg. 1994;51:826–36. doi: 10.4269/ajtmh.1994.51.826. [DOI] [PubMed] [Google Scholar]
- 11.Ettinger NA, Duggal P, Braz RF, et al. Genetic admixture in Brazilians exposed to infection with Leishmania chagasi. Ann Hum Genet. 2009;73:304–13. doi: 10.1111/j.1469-1809.2009.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibbs RA, Belmont JW, Hardenbol P, et al. The International HapMap Project. Nature. 2003;426:789–96. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 13.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 14.O’Connell JR, Weeks DE. PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet. 1998;63:259–66. doi: 10.1086/301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmans P, Clayton D. Efficiency of typing unaffected relatives in an affected sib-pair linkage study with single locus and multiple tightly-linked markers. Am J Hum Genet. 1995;37:1221–32. [PMC free article] [PubMed] [Google Scholar]
- 16.Knapp M. A note on power approximations for the transmission disequilibrium test. Am J Hum Genet. 1999;64:1177–85. doi: 10.1086/302334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordell HJ. Properties of case/pseudocontrol analysis for genetic association studies: effects of recombination, ascertainment, and multiple affected offspring. Genet Epidemiol. 2004;26:186–205. doi: 10.1002/gepi.10306. [DOI] [PubMed] [Google Scholar]
- 18.Cordell HJ, Barratt BJ, Clayton DG. Case/pseudocontrol analysis in genetic association studies: a unified framework for detection of genotype and haplotype associations, gene-gene and gene-environment interactions, and parent-of-origin effects. Genet Epidemiol. 2004;26:167–85. doi: 10.1002/gepi.10307. [DOI] [PubMed] [Google Scholar]
- 19.Whitlock MC. Combining probability from independent tests: the weighted Z-method is superior to Fisher’s approach. J Evol Biol. 2005;18:1368–73. doi: 10.1111/j.1420-9101.2005.00917.x. [DOI] [PubMed] [Google Scholar]
- 20.Clayton D. A generalization of the transmission/disequilibrium test for uncertain-haplotype transmission. Am J Hum Genet. 1999;65:1170–7. doi: 10.1086/302577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staden R, Beal KF, Bonfield JK. The Staden package, 1998. Methods Mol Biol. 2000;132:115–30. doi: 10.1385/1-59259-192-2:115. [DOI] [PubMed] [Google Scholar]
- 22.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–86. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 23.Brudno M, Chapman M, Gottgens B, Batzoglou S, Morgenstern B. Fast and sensitive multiple alignment of large genomic sequences. BMC Bioinformatics. 2003;4:66. doi: 10.1186/1471-2105-4-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brudno M, Do CB, Cooper GM, et al. LAGAN and Multi-LAGAN: efficient tools for large-scale multiple alignment of genomic DNA. Genome Res. 2003;13:721–31. doi: 10.1101/gr.926603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottgens B, Gilbert JG, Barton LM, et al. Long-range comparison of human and mouse SCL loci: localized regions of sensitivity to restriction endonucleases correspond precisely with peaks of conserved noncoding sequences. Genome Res. 2001;11:87–97. doi: 10.1101/gr.153001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Messeguer X, Escudero R, Farre D, Nunez O, Martinez J, Alba MM. PROMO: detection of known transcription regulatory elements using species-tailored searches. Bioinformatics. 2002;18:333–4. doi: 10.1093/bioinformatics/18.2.333. [DOI] [PubMed] [Google Scholar]
- 27.Grabe N. AliBaba2: context specific identification of transcription factor binding sites. In Silico Biol. 2002;2:S1–15. [PubMed] [Google Scholar]
- 28.Quandt K, Frech K, Karas H, Wingender E, Werner T. MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 1995;23:4878–84. doi: 10.1093/nar/23.23.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dermitzakis ET, Reymond A, Lyle R, et al. Numerous potentially functional but non-genic conserved sequences on human chromosome 21. Nature. 2002;420:578–82. doi: 10.1038/nature01251. [DOI] [PubMed] [Google Scholar]
- 30.Loots GG, Locksley RM, Blankespoor CM, et al. Identification of a coordinate regulator of interleukins 4, 13, and 5 by cross-species sequence comparisons. Science. 2000;288:136–40. doi: 10.1126/science.288.5463.136. [DOI] [PubMed] [Google Scholar]
- 31.Hardison RC, Oeltjen J, Miller W. Long human-mouse sequence alignments reveal novel regulatory elements: a reason to sequence the mouse genome. Genome Res. 1997;7:959–66. doi: 10.1101/gr.7.10.959. [DOI] [PubMed] [Google Scholar]
- 32.Duret L, Dorkeld F, Gautier C. Strong conservation of non-coding sequences during vertebrates evolution: potential involvement in post-transcriptional regulation of gene expression. Nucleic Acids Res. 1993;21:2315–22. doi: 10.1093/nar/21.10.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson ME, Recker TJ, Rodriguez NE, et al. The TGF-beta response to Leishmania chagasi in the absence of IL-12. Eur J Immunol. 2002;32:3556–65. doi: 10.1002/1521-4141(200212)32:12<3556::AID-IMMU3556>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 34.Zhou S, Zawel L, Lengauer C, Kinzler KW, Vogelstein B. Characterization of human FAST-1, a TGF beta and activin signal transducer. Mol Cell. 1998;2:121–7. doi: 10.1016/s1097-2765(00)80120-3. [DOI] [PubMed] [Google Scholar]
- 35.Cui Q, Lim SK, Zhao B, Hoffmann FM. Selective inhibition of TGF-beta responsive genes by Smad-interacting peptide aptamers from FoxH1, Lef1 and CBP. Oncogene. 2005;24:3864–74. doi: 10.1038/sj.onc.1208556. [DOI] [PubMed] [Google Scholar]
- 36.Maekawa Y, Tsukumo S, Chiba S, et al. Delta1-Notch3 interactions bias the functional differentiation of activated CD4+ T cells. Immunity. 2003;19:549–59. doi: 10.1016/s1074-7613(03)00270-x. [DOI] [PubMed] [Google Scholar]
- 37.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–26. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 38.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–6. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 39.Greenwald I. LIN-12/Notch signaling: lessons from worms and flies. Genes Dev. 1998;12:1751–62. doi: 10.1101/gad.12.12.1751. [DOI] [PubMed] [Google Scholar]
- 40.Beckers J, Caron A, Hrabe de Angelis M, Hans S, Campos-Ortega JA, Gossler A. Distinct regulatory elements direct delta1 expression in the nervous system and paraxial mesoderm of transgenic mice. Mech Dev. 2000;95:23–34. doi: 10.1016/s0925-4773(00)00322-1. [DOI] [PubMed] [Google Scholar]
- 41.Payne F, Smyth DJ, Pask R, et al. No evidence for association of the TATA-box binding protein glutamine repeat sequence or the flanking chromosome 6q27 region with type 1 diabetes. Biochem Biophys Res Commun. 2005;331:435–41. doi: 10.1016/j.bbrc.2005.03.203. [DOI] [PubMed] [Google Scholar]
- 42.Jaleco AC, Neves H, Hooijberg E, et al. Differential effects of Notch ligands Delta-1 and Jagged-1 in human lymphoid differentiation. J Exp Med. 2001;194:991–1002. doi: 10.1084/jem.194.7.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cotterell SE, Engwerda CR, Kaye PM. Leishmania donovani infection of bone marrow stromal macrophages selectively enhances myelopoiesis, by a mechanism involving GM-CSF and TNF-alpha. Blood. 2000;95:1642–51. [PubMed] [Google Scholar]
- 44.Sekine C, Moriyama Y, Koyanagi A, et al. Differential regulation of splenic CD8- dendritic cells and marginal zone B cells by Notch ligands. Int Immunol. 2009;21:295–301. doi: 10.1093/intimm/dxn148. [DOI] [PubMed] [Google Scholar]
- 45.Ong CT, Sedy JR, Murphy KM, Kopan R. Notch and presenilin regulate cellular expansion and cytokine secretion but cannot instruct Th1/Th2 fate acquisition. PLoS One. 2008;3:e2823. doi: 10.1371/journal.pone.0002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Locksley RM, Scott P. Helper T-cell subsets in mouse leishmaniasis induction, expansion and effector function. Immunol Today. 1991;12:A58–61. doi: 10.1016/S0167-5699(05)80017-9. [DOI] [PubMed] [Google Scholar]
- 47.Scott P, Pearce E, Cheever AW, Coffman RL, Sher A. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989;112:162–82. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 48.Sundar S, Reed SG, Sharma S, Mehrotra A, Murray HW. Circulating T helper 1 (Th1) cell- and Th2 cell-associated cytokines in Indian patients with visceral leishmaniasis. Am J Trop Med Hyg. 1997;56:522–5. doi: 10.4269/ajtmh.1997.56.522. [DOI] [PubMed] [Google Scholar]
- 49.Pirmez C, Yamamura M, Uyemura K, Paes-Oliveira M, Modlin RL. Cytokine patterns in the pathogenesis of human leishmaniasis. J Clin Invest. 1993;91:1390–5. doi: 10.1172/JCI116341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carvalho EM, Barral A, Pedral-Sampaio D, et al. Immunologic markers of clinical evolution in children recently infected with Leishmania donovani chagasi. J Infect Dis. 1992;165:535–40. doi: 10.1093/infdis/165.3.535. [DOI] [PubMed] [Google Scholar]