Abstract
Background
Certain patterns can induce perceptual illusions/distortions and visual discomfort in most people, headaches in patients with migraine, and seizures in patients with photosensitive epilepsy. Visual stimuli are common triggers for migraine attacks, possibly because of a hyperexcitability of the visual cortex shown in patients with migraine. Precision ophthalmic tints (POTs) are claimed to reduce perceptual distortions and visual discomfort and to prevent migraine headaches in some patients. We report an fMRI visual cortical activation study designed to investigate neurological mechanisms for the beneficial effects of POTs in migraine.
Methods
Eleven migraineurs and 11 age- and sex-matched non-headache controls participated in the study using non-stressful and stressful striped patterns viewed through gray, POT, and control coloured lenses.
Results
For all lenses, controls and migraineurs did not differ in their response to the non-stressful patterns. When the migraineurs wore gray lenses or control coloured lenses, the stressful pattern resulted in activation that was greater than in the controls. There was also an absence of the characteristic low-pass spatial frequency (SF) tuning in extrastriate visual areas. When POTs were worn, however, both cortical activation and SF tuning were normalized. Both when observing the stressful pattern and under more typical viewing conditions, the POTs reduced visual discomfort more than either of the other two lenses.
Conclusion
The normalization of cortical activation and SF tuning in the migraineurs by POTs suggests a neurological basis for the therapeutic effect of these lenses in reducing visual cortical hyperactivation in migraine.
Keywords: fMRI, migraine, precision ophthalmic tints, visual cortical hyperactivation
Introduction
Certain patterns, particularly gratings, are uncomfortable to look at and can induce headaches and seizures. The contrast threshold necessary for perception of a grating pattern varies with the size of the stripes on the retina (i.e. spatial frequency, SF of the grating), and it is easiest to see the stripes at low contrast when they have a SF of about 3 cycles per degree (cpd) (1). At high contrast, gratings with this SF are aversive to look at (2) and they induce illusions of colour, shape, and motion, to which some individuals, notably those with migraine, are more susceptible than others. Migraineurs show an abnormally large visual cortical activation in response to striped patterns measured with blood oxygenation level dependent (BOLD) functional magnetic resonance imaging (fMRI), and this pattern-induced visual cortical hyperactivation has been found to be associated with the pattern-provoked visual distortions and discomfort (3). A hyperexcitability of the visual cortex, evident in photosensitive epilepsy (4) and also postulated in migraine (5), may be responsible not only for the seizures but also the perceptual illusions/distortions and visual discomfort and perhaps visually triggered migraine headaches.
Coloured filters have been reported to improve reading in dyslexia and to reduce perceptual distortions, discomfort, and headaches from striped patterns (6–8). The use of individually prescribed precision ophthalmic tints (POTs) to treat perceptual distortion of text has recently become common in many schools in Britain, and this colour treatment has been reported to increase reading speed by more than 25% in at least 5% of children in mainstream education (9), provided the colour is selected by the individual to reduce the perceptual distortions. The chromaticity optimum for such reduction reportedly varies from individual to individual, and departures from this optimal chromaticity of 0.06 units in the uniform chromaticity scale (UCS) diagram of the Commission Internationale de I'Eclairage (CIE) (10) are reportedly sufficient to remove any advantage that the optimum colour conveys (11). The mechanisms for these beneficial effects from coloured filters and POTs remain obscure, contributing to controversy surrounding their use.
We report a BOLD-fMRI study that, for the first time, sheds light on the neurological basis for the beneficial effect of POTs. We investigate the effect of POTs on visual cortical hyperactivation induced by a stressful striped pattern in migraineurs.
Materials and methods
Inclusion and exclusion criteria
The inclusion criteria for migraine patients were: (1) diagnosed as having migraine with visual aura (MwA) or without aura (MwoA) according to the criteria of the International Headache Society; (2) age between 16 and 65 years; (3) recurrence of episodic migraine attacks no more than 10 times per month on average and no less than 12 times in the past year; and (4) no migraine headache at least 3 days prior to the fMRI scan. The inclusion criteria for non-headache control subjects were either no history of headache or tension-type recurring headaches no more than 3 times per year and controlled by using over-the-counter medication. The exclusion criteria for both groups included: (1) frequent tension headaches (one per week or more); (2) ill-defined head pain; (3) history of seizures; (4) prior head injury or brain surgery; (5) other diagnosed neurological and/or psychiatric disorders; (6) other diagnosed cardiovascular disorders; (7) other illness (e.g. cancer, diabetes, and anaemia); (8) implanted cardiac pacemakers or other electronic or metallic devices; (9) women who are pregnant or lactating; (10) neurological symptoms associated with migraine suggestive of prolonged or severe neurological deficit (e.g., aura lasts longer than 1 hour) or risk of stroke; and (11) subjects who use drugs that have a side effect of visual disturbance and/or light sensitivity. The University Institutional Review Board at Michigan State University approved the study, and written informed consent forms were obtained from all participants prior to the study.
Visual test for prescribing precision ophthalmic tints for migraineurs and fMRI participants
Prior to the fMRI study each migraine patient was assessed using the Intuitive Colorimeter (Cerium Visual Technologies, UK), an apparatus that illuminates text with coloured light and permits the separate manipulation of hue and saturation at constant luminance. The apparatus was used to obtain an optimal hue and saturation (chromaticity) of light that maximized visual comfort and reduced any perceptual distortion (7,12). The procedure involved: (1) a progressive increase then decrease in saturation at each of 12 hues with hue angles (huv) approximately 30 degrees apart; (2) a comparison at optimal saturation of any hues reported to be comfortable; and (3) successive adjustment of hue and saturation to optimize the best of these settings. Finally, the text was replaced with a stressful stripe pattern with a SF of ~3 cpd to confirm the optimal setting.
After obtaining the optimal setting for a patient, a computer program was used to prescribe a POT for the patient that would match the patient-selected shade of colour (chromaticity) under illumination with a correlated colour temperature of 4000 K (12). Two additional lenses were chosen as control lenses: both had a saturation similar to that of the prescribed POT. One was gray (G) and another was coloured (C) but differed in hue so that where possible the chromaticities of the POT and C filters were separated by 0.07 in the 1976 CIE UCS diagram (10). Based on the behavioural data, such chosen C filters were unlikely to have beneficial effect (11). In addition, patients were never permitted to see the combination of trial lenses that matched their chosen setting and were often unaware of the chosen shade of colour because of adaptation. Then MRI-compatible tints were ordered and used in the fMRI study.
Recruitment was directed mainly towards MwA patients. A total of 25 migraineurs (16 MwA and 9 MwoA) underwent assessment with the colorimeter, and all patients except one identified an individually optimal chromaticity of light. Of the 24 patients, three chose to not participate in the MRI scan and a further four could not do so (three had metal implants and one was pregnant). This left a total of 17 patients enrolled in the fMRI study.
Stimulus
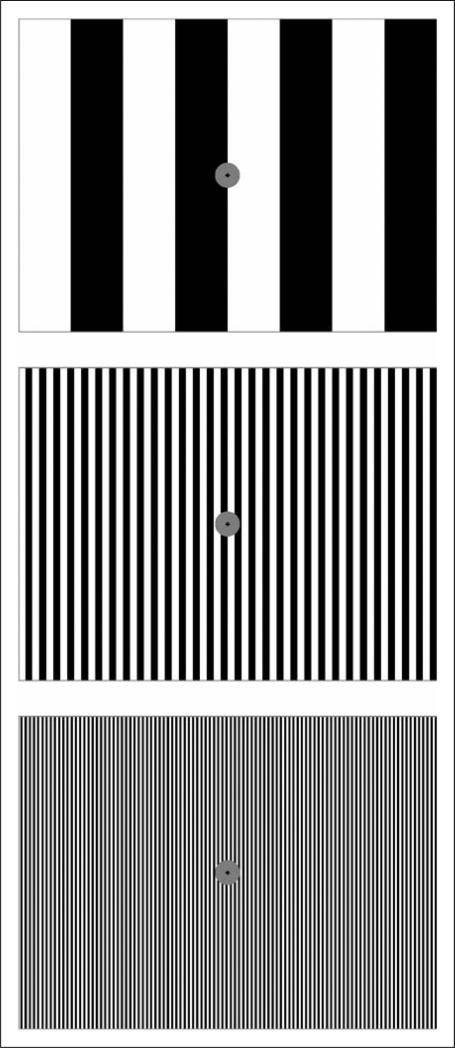
Three black-and-white vertically striped patterns (square-wave luminance profile) with a mean luminance of 121 cd/m2 and a contrast of 98% were used; one with a low SF of 0.31 cpd (a non-stressful control pattern that does not induce distortions and discomfort), one with a mid-range SF of 2.5 cpd (a stressful pattern that maximally induces distortions and discomfort), and one with a high SF of 7.9 cpd (Figure 1). The stimuli subtended a 10° × 13° (height × width) visual angle and had a fixation mark inside a gray circle (diameter 0.5° ) at the centre of the visual field. The low and high SF patterns were used as controls for examining the effect of POTs on the cortical hyperactivation produced in the patients by the stressful pattern.
Figure 1.
Illustration of three black-and-white stripes with a low spatial frequency (SF) (top), a medium SF (middle), and a high SF (bottom). When the width of the whole pattern is about 8 cm and the viewing distance is about 36 cm, the corresponding SF values for the three stripes are approximately 0.31, 2.5, and 7.9 cpd, respectively. The experimental patterns were larger than those shown, subtending 10° × 13° (height × width) at the eye.
Functional MRI protocols
Functional brain images covering the whole occipital cortex were acquired on a GE 3.0 T clinical scanner using a gradient echo Echo-Planar-Imaging pulse sequence (TE/TR = 45.3/2000 ms, flip angle 80° , FOV 22 cm, matrix 96 × 96, slice thickness 3.0 mm, number of slices 20). The three selected stimuli with SFs 0.31, 2.5, and 7.9 cpd were presented via a 32-inch LCD monitor (Salvagione Design, Sausalito, CA) placed at the back of the scanner. The stimulation presentation was controlled by a PC equipped with E-Prime (Psychology Software Tools, Pittsburgh, PA) and synchronized with MRI acquisition. A 5-button MRI-compatible keypad was used to record participant responses. For the participants who needed vision correction, MRI-compatible lenses were used. The participants viewed the monitor through a mirror mounted on top of the head coil. To hold the tinted lenses, a transparent plastic frame was placed between the eyes and the mirror and mounted on the head coil, making it convenient to switch the lenses between the scans with minimum interference with the participants. The stimulation sequence consisted of 12 10-s long stimulation blocks interleaved with 12 24-s fixation blocks, each SF presented four times in a pseudo-random order. Each participant had three visual activation scans; the participant had G lenses for the first scan and then had either POT or C lenses for the next two scans (counter-balanced across the participants). For the two scans with coloured lenses, the participant was not informed which lenses (POT or C lenses) were used for which scan. (The study was therefore single-masked.) Each paired patient and control participant wore the lenses in the same order, and the yoked control subject was tested with the same three lenses as the migraine patient, having the chromaticities shown in Figure 2. Note that using the same three lenses as the migraine patients enabled us to test whether the POTs produced similar effects on cortical activation in the control subjects. Each stimulation paradigm started with a blank screen lasting for 10 s and the corresponding images were discarded, resulting in a total of 204 volume images per anatomic location for each scan. During the scan, the fixation mark at the centre of the visual field randomly changed from square to cross or vice versa at a mean rate of 3.6 s (a total of 114 fixation mark changes occurred in each functional scan). The participant was instructed to respond by pressing a button on the keypad when a change occurred, and the response was recorded and instantly displayed to the investigator for monitoring the participant's attention during the whole scan. Both the response time for each response and the total number of responses during the whole scan were recorded for later analysis.
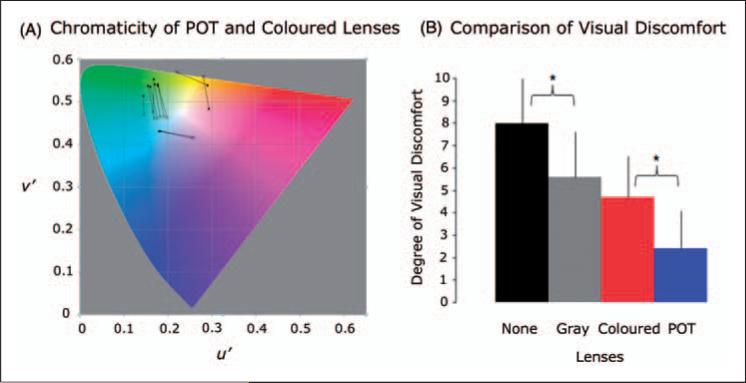
Figure 2.
(A) CIE 1976 UCS diagram (10) showing the colour appearance (chromaticities) of the precision ophthalmic tint (POT) and coloured lenses used by the 11 migraine patients and their control subjects in the fMRI study. The chromaticity of each POT is marked by a solid circular point, and a line connects the point to the chromaticity of its paired coloured lens (cross). (B) The effects of the gray, the POT, and the coloured lenses in reducing visual discomfort relative to that without lenses when viewing the stressful pattern out of doors in direct sunlight. The degree of visual discomfort was self-scored using a 0–10 scale with 0 representing no visual discomfort and 10 representing severe visual discomfort. Overall, the POT lenses had the most significant reduction in visual discomfort followed by the coloured lenses and then the gray lenses. The reduction with the POT lenses was significantly larger than that with the coloured lenses (t-test, p = 0.005). The reduction with the coloured lenses showed no difference compared to that of the gray lenses (p = 0.253). Although the effect was the smallest among the three lens types, the reduction with the gray lenses was significant (p = 0.027) compared to that without lenses. Error bars indicate the standard deviations.
Visual discomfort rating test
After the fMRI session, the stressful pattern was viewed out of doors in direct sunlight with each of the lenses and without. The viewing conditions were therefore realistic and extreme, and although luminance was uncontrolled, it was similar for all test conditions. The degree of visual discomfort was self-scored using a 0–10 scale with 0 representing no visual discomfort and 10 representing severe visual discomfort.
Visual area delineation
Each participant had a retinotopic mapping protocol for delineating visual areas (V1, V2, V3, V3A, and V4) using phase-encoded polar and eccentricity coordinate stimuli (13). The stimuli consisted of a 30° ray-shaped black-white checker wedge and a black-white checker ring with an approximate ring width of 0.54° (14). The black and white checkers alternated every 200 ms. The wedge started from the lower vertical meridian in the visual field, rotated around the centre, and completed one full cycle every 24 s. It first rotated clockwise for four cycles, followed by a black screen for 24 s, and then rotated counter-clockwise for four cycles. The ring first dilated for four cycles, followed by a black screen for 24 s, and then contracted for four cycles. It completed one cycle in 24 s, same as the wedge rotation. To maintain fixation and attention, the colour of fixation mark at the centre randomly changed among red, green, or blue at a rate of 2.5 s, and the participant was instructed to respond to the three colours by pressing three different buttons.
In addition, T1-weighted whole-brain MRI images were also collected using a 3D IR-fspgr pulse sequence with a voxel resolution of 0.90 ×0.90 × 2.0 mm3.
Image processing and data analysis
Image preprocessing of the functional images was performed using AFNI (http://afni.nimh.nih.gov/afni), including (1) slice-timing correction of the image acquisition time difference from slice to slice; (2) motion correction of the images for alignment volume by volume; (3) normalization of a signal intensity time course by dividing it with its mean signal intensity value voxel-by-voxel for each scan; and (4) temporal drift correction to remove slow linear and parabolic baseline shift in the signal intensity time course voxel by voxel. After the preprocessing steps, further image analysis was carried out using in-house developed Matlab-based software algorithms.
Retinotopic mapping
The polar and eccentricity phases of the periodic activation corresponding to the spatial location of the rotating wedge and the dilating/contracting ring were obtained by discrete Fourier analysis of the signal intensity time courses for the two stimuli, yielding the polar and eccentricity retinotopic maps. These maps were visualized using SUMA (http://afni.nimh.nih.gov/afni/suma) on the cortical surface that was reconstructed from the T1-weighted high-resolution whole brain images using FreeSurfer software (http://surfer.nmr.mgh.harvard.edu/). The borders of visual areas were manually drawn, based on phase reversals in the polar map, and the visual areas (V1, V2, V3, V3A, and V4) were identified for each hemisphere. Then, these visual areas on the cortical surface were used for constructing the 3D volume masks corresponding to the visual areas. For each surface node in a visual area, a 3-mm line segment perpendicular outward to the surface of the white/gray matter boundary was generated in the 3D image space and used to examine voxels for constructing a 3D volume mask. For a given voxel, the intersections of the voxel with all segments from the visual areas were first examined and then the voxel was assigned to the visual area with the most segments intersected by the voxel. Voxels not intersected with any segment were excluded from the 3D volume masks.
Activation in visual areas
This study aimed to investigate the effect of the POTs on the cortical activation induced by the stressful pattern in each of the visual areas. For each stimulus pattern, functional images from the three scans with the three different lenses were first sorted and then concatenated to form a time course from all trials across the three lenses. The cross-correlation coefficient (ccc) of the concatenated time course with a reference response function was computed voxel-by-voxel. The reference response function was obtained with the convolution of the stimulus presentation pattern with a gamma density function f(t) = tδ exp(−t/τ) with δ = 8.6, τ = 0.547, t in unit of second (15). Activated voxels were chosen with the threshold level of ccc > 0.24 (estimated p < 0.0006, uncorrected for multiple comparisons), yielding activated voxels unbiased to the three lenses. For each visual area, a region of interest (ROI) was defined as those activated voxels within the 3D volume mask of the visual area. For each lens condition, signal intensity time courses for the trials with the same stimulus pattern were first averaged, then averaged over the voxels within the ROI of a visual area combining both the dorsal and ventral pathways in one hemisphere, and further averaged over the same visual area for the left and right hemispheres to yield a mean cortical area response curve for the stimulus pattern. To improve accuracy, the baseline value for each response curve was computed as the mean of the last four time point values of the curve. The height of the response peak relative to the baseline was further calculated as the mean value over the two time points with the maximum values at the peak in the response curve. This height was used as the metric for quantifying cortical area response to each stimulus pattern under different lens conditions.
Normalization of filter-induced activation variations
A colour change to a stimulus redistributes the three cone excitations at the level of the retina and consequently affects the visual cortical response to the stimulus. In addition, there were small and unavoidable differences in transmission of the three lenses that may have provoked variations in the cortical response over and above those variations due to the differences in colour. These colour- and transmission-induced cortical response variations vary from filter to filter. For a given filter, however, they are expected to remain the same from stimulus to stimulus. For the non-stressful control stimulus condition (SF 0.31 cpd) the three coloured lenses were not expected to have any significant differential effect on the cortical activation. Accordingly, for each lens, to remove the colour- and transmission-induced cortical response variations, the height of the cortical area response curve to the control stimulus was used to normalize the height of the corresponding cortical area response curve of each of the other two stimuli by computing the ratio of the latter to the former. Then the relative heights for the three lenses were compared to evaluate the effect of the POTs on visual cortical hyperactivation produced by the stressful stimulus in the patients.
Results
The chromaticities of POT and paired C lenses, and visual discomfort test
Of the 17 migraine patients enrolled in the fMRI study, two could not complete their MRI scans due to claustrophobia. Four patients’ fMRI data were excluded from further analysis; one patient suffered bipolar disorder, one patient's frequency of migraine attacks was ~15 times per month and had a migraine headache within 2 days prior to her fMRI session, one patient had only one functional eye, and one failed to respond to fixation mark changes as instructed during the fMRI scan. This resulted in a total of 11 patients satisfying all the criteria for the fMRI study (seven MwA and four MwoA, aged from 29 to 49 years old with mean ± SD = 40.3 ± 6.3). Eleven age- and sex-matched non-headache control subjects (aged from 30 to 49 years old with mean ± SD = 39.3 ± 5.9) were also enrolled in the fMRI study. The control subjects were not photophobic and satisfied the criteria above.
Figure 2A shows the colour appearance of POT and C lenses expressed in terms of the lens chromaticities, joined by separate lines for each of the 11 migraine patients included in the fMRI study. The mean and standard deviation of photopic transmission are 25.6 ± 5.7% for the G lenses, 25.6 ± 5.2% for the POT lenses, and 25.4 ± 7.2% for the C lenses. Ten out of the 17 migraine patients who participated in the fMRI session were able to take the visual discomfort rating after the fMRI session. The group mean and standard deviation of visual discomfort were 8.0 ± 2.1 without lenses, 5.6 ± 2.0 with the G lenses, 4.7 ± 1.8 with the C lenses, and 2.4 ± 1.7 with the POT lenses (Figure 2B). The G lenses significantly reduced the degree of visual discomfort by 30% compared to that without lenses (t-test, p = 0.027). The C lenses also significantly reduced the degree of visual discomfort by 41% but showed no difference compared with the G lenses (p = 0.253). The POT lenses, however, had the most significant reduction of 70% and this reduction was significant in comparison to that of the C lenses (p = 0.005).
All of the 11 migraine patients who participated in the fMRI study reported photophobia during their migraine attacks (Table 1). Ten of them indicated that they were sensitive to light or certain visual patterns between headaches. Nine of them reported that some of their migraine attacks were triggered by visual stimuli. Stress was a triggering factor for eight patients. The seven MwA patients reported that their migraine headaches were often preceded by still or moving visual aura of scotoma or scintillating spots/lines/coloured lights. When viewing the stressful striped pattern, all patients reported illusions and distortions and claimed that viewing the pattern for some time would trigger a migraine attack.
Table 1.
Characteristics of the migraineurs participating in the fMRI study
| Patient no. | Sex | Age (y) | Visual aura, affected visual field | Headache location | Photophobia | Sensitive to LoVP | Triggering factors | MPD |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 41 | Scotoma, RVHF, moves from periphery to visual centre | R, front | Yes | Yes | Stress, light, visual pattern, etc. | No |
| 2 | F | 49 | No | R, front, lateral side | Yes | Yes | Stress, visual pattern, etc. | No |
| 3 | F | 38 | Scotomas, LVHF and RVHF, move from peripheries to visual centre | R, front | Yes | Yes | Stress, light, visual pattern, etc. | No |
| 4 | M | 42 | Scotoma, visual centre | B, front | Yes | No | No | Yes |
| 5 | F | 40 | Scotoma, visual periphery | B, front | Yes | Yes | Stress, light, visual pattern | No |
| 6 | F | 29 | No | B, front | Yes | Yes | Stress, light, visual pattern, etc. | Yes |
| 7 | F | 48 | Scintillating lines/spots, RVHF, move from periphery to visual centre | B, front | Yes | Yes | Stress, light | No |
| 8 | F | 30 | Scintillating blue/yellow lights, LVHF, move from visual centre to periphery | R, front | Yes | Yes | Light, visual pattern, etc. | Yes |
| 9 | F | 40 | No | B, back | Yes | Yes | Menstrual cycles, seasonal effect | No |
| 10 | F | 42 | No | L, back | Yes | Yes | Stress, light, visual pattern, etc. | No |
| 11 | F | 44 | Scintillating coloured lights, LVHF, periphery | B, back | Yes | Yes | Stress, light, visual pattern, etc. | Yes |
B, bilateral; F, female; L, left; M, male; LoVP, light or visual pattern; LVHF, left visual hemifield; MPD, migraine prophylactic drug; RVHF, right visual hemifield; R, right.
Participants’ responses during the fMRI scans
The group mean and standard deviation of response time and response rate for the control subjects were 645 ± 49 ms and 94.3 ± 2.8% for the G lenses, 695 ± 56 ms and 93.2 ± 2.7% for the POTs, and 699 ± 54 ms and 92.9 ± 2.3% for the C lenses, respectively. The corresponding values for the migraine patients were 676 ± 46 ms and 94.3 ± 3.1% for the G lenses, 658 ± 54 ms and 90.5 ± 6.4% for the POTs, and 680 ± 55 ms and 93.0 ± 3.8% for the C lenses, respectively. These responses showed no statistical difference between the control subjects and the migraine patients. They also showed no statistical difference among the three lenses within each participant group.
Cortical activation in response to the non-stressful control stimulus
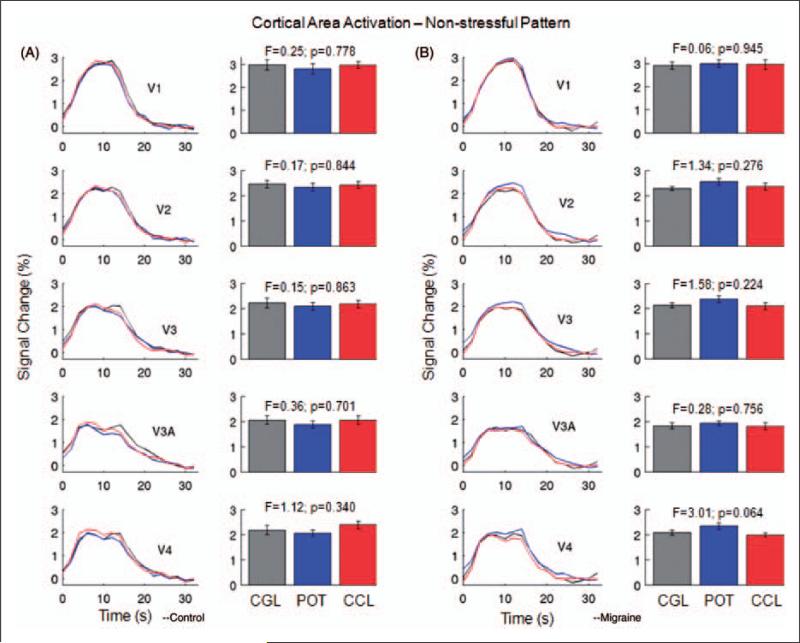
Left columns in Figure 3A and 3B show the visual cortical area activation curves resulting from the non-stressful control striped pattern with SF 0.31 cpd for the control subjects and the migraine patients. Right columns compare the peak heights of these cortical area activation curves. For both the control and patient groups, cortical activation in response to the control pattern showed no difference between the neutral gray lens, the control coloured lens, and the POT in V1, V2, V3, V3A, and V4, demonstrating that these three types of lenses had no differential effect on the cortical activation. These cortical activations also showed no difference between the migraine patients and the control subjects.
Figure 3.
Activation in visual areas V1, V2, V3, V3A, and V4 from the non-stressful striped pattern (SF 0.31 cpd) for the control subjects (A) and the migraine patients (B). Left columns in (A) and (B), averaged cortical activation curves for the three lenses; right columns in (A) and (B), comparison of the peak heights of the cortical activation curves in the left columns. No significant difference in activation was observed in any area. Error bars indicate the standard errors of the means. CCL, control coloured lens; CGL, control gray lens; POT, precision ophthalmic tint.
Cortical activation in response to the stressful stimulus
The normalized cortical area response curve to the stressful pattern with SF 2.5 cpd relative to that for the non-stressful control stimulus (0.31 cpd) was plotted in each visual area for each lens (see Methods). The left column in Figure 4A shows the normalized visual cortical area activation curves resulting from the stressful pattern for the control subjects, and the right column compares the peak heights of these cortical area activation curves. The cortical activation in any visual area showed no differences between the neutral gray lens, the control coloured lens, and the POT. Furthermore, the POT showed no difference compared to the coloured lens.
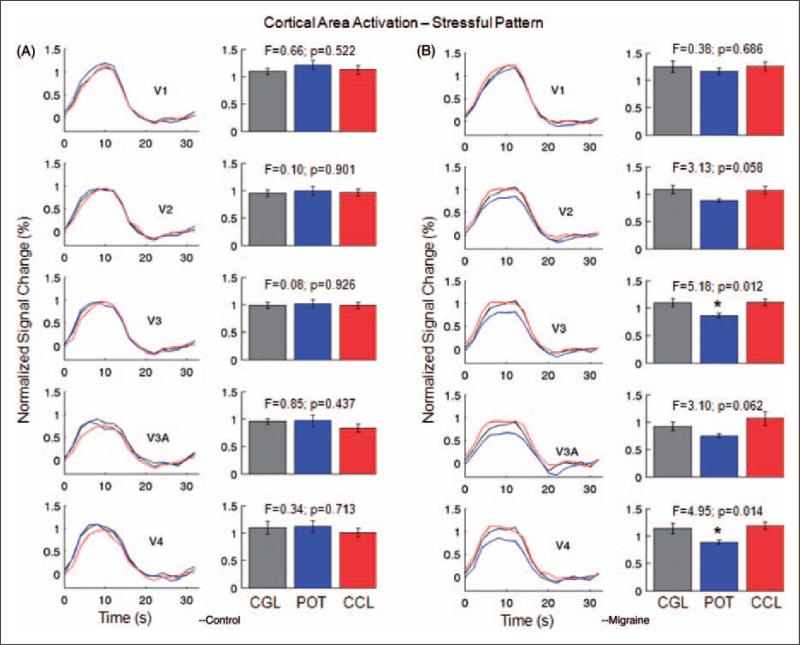
Figure 4.
Normalized activation in visual areas V1, V2, V3, V3A, and V4 from the stressful striped pattern (SF 2.5 cpd) for the control subjects (A) and the migraine patients (B). Left columns in (A) and (B), normalized cortical area activation curves; right columns in (A) and (B), comparison of the peak heights of the normalized cortical area activation curves in the left columns. (Note that, to reduce the filter-induced activation variations, the cortical area response curve to the stressful pattern was normalized by dividing the height of the corresponding cortical area response curve to the non-stressful pattern for each lens.) For the control subjects, cortical area activation showed no difference in any visual area among the three lenses. For the migraine patients, however, the POTs produced significant reductions to cortical activation in V3 and V4. The POTs also reduced the cortical activation in V2 and V3A, though the differences were not statistically significant. Error bars indicate the standard errors of the means. CCL, control coloured lens; CGL, control gray lens; POT, precision ophthalmic tint.
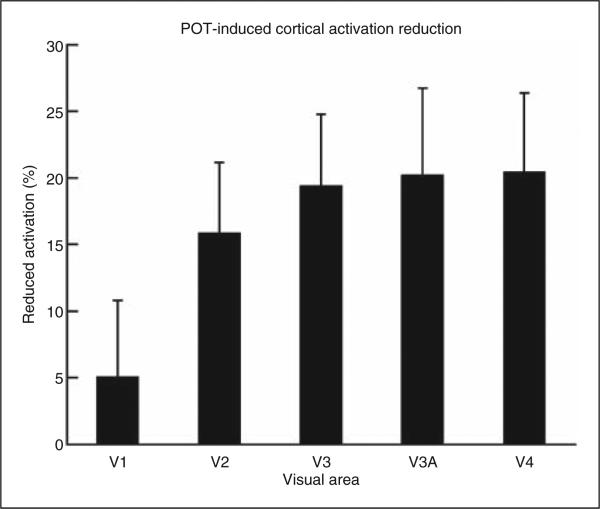
The left column in Figure 4B shows the normalized visual cortical area activation curves resulting from the stressful pattern for the migraine patients, and the right column compares the peak heights of these cortical area activation curves. Comparing the three lenses, the peak heights of the cortical activation were significantly different in visual areas V3 (F-test(2,30), p = 0.012) and V4 (p = 0.014), but not significantly different in V1 (p = 0.686), V2 (p = 0.058), and V3A (p = 0.062). Comparison between the gray lens and the control coloured lens showed no significant difference (t-test) in any of these visual areas; the p-values ranged from 0.28 to 0.93. Accordingly, the mean value of the peak heights for the gray and coloured lenses was computed for each visual area to represent the cortical activation in that visual area in response to the stressful pattern. This cortical activation in the migraine patients, however, was significantly suppressed by the POTs in the extra-striate visual areas: V2 (paired t-test, p < 0.01), V3 (p < 0.005), V3A (p < 0.01), and V4 (p < 0.01), but not significantly suppressed in the primary visual area V1 (p > 0.28). Figure 5 shows the POT-induced percentage reduction to the cortical activation in each visual area. For the extra-striate areas from V2 to V4, the POTs produced a 19% mean reduction to the cortical activation in comparison with a 5% reduction in the primary visual area, V1. This reduction effect was observed for every patient except one (data not presented).
Figure 5.
Precision ophthalmic tint-induced cortical activation reduction in the migraine patients relative to the mean cortical activation for the control gray and coloured lenses. Error bars indicate the standard errors of the means.
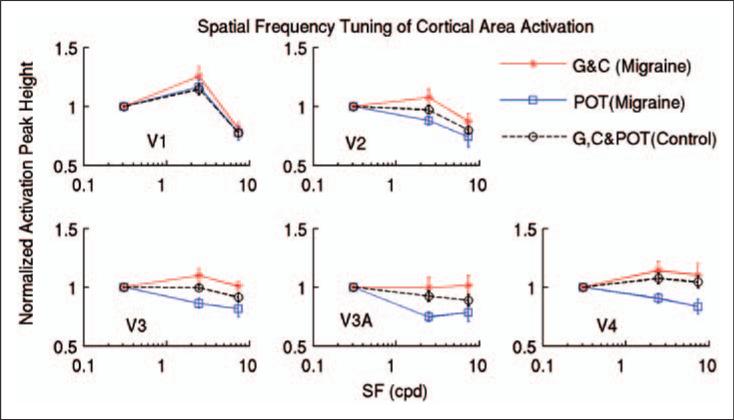
Characteristic SF tuning of cortical activation in V2
Figure 6 shows the comparison of cortical activation variation in each visual area as a function of the SF of the pattern of stripes. For the control subjects, the normalized cortical activation in any visual area showed no differences between the neutral gray lens, the control coloured lens, and the POT in response to the stressful pattern (Figure 4A) and the pattern with SF 7.9 cpd (data not presented). Accordingly, the mean value of the peak heights for these three lenses was computed for each visual area to represent the cortical activation in that visual area in response to the stressful pattern and the pattern with SF 7.9 cpd. The cortical activation in V1 peaked at the SF of 2.5 cpd (Figure 6, top left, dashed line), consistent with previous fMRI findings in normal participants (3,16). This characteristic SF tuning of cortical activation was noticeably different in V1 and V2: in V2 the cortical activation peak was shifted to the lower SF of 0.31 cpd (Figure 6, top right, dashed line).
Figure 6.
Comparison of cortical area activation between the control subjects and the migraine patients wearing control gray (G), coloured (C), and precision ophthalmic tint (POT) lenses, shown as a function of the spatial frequency (SF) of the pattern of stripes. The dashed-lines represent the mean peak heights of cortical area activation with the three lenses for the control subjects. The red solid lines represent the mean peak heights of cortical area activation of the G and C lenses for the migraine patients. The blue solid lines represent the peak heights of cortical area activation of the POTs for the migraine patients. Error bars indicate the standard errors of the means.
For the migraine patients, the normalized cortical activation in any visual area showed no differences between the neutral gray lens and the control coloured lens in response to the stressful pattern (Figure 4B) and the pattern with SF 7.9 cpd (data not presented). Accordingly, for the control gray and coloured lenses, the mean value of the peak heights for these two lenses was computed for each visual area to represent the cortical activation in that visual area in response to the stressful pattern and the pattern with SF 7.9 cpd. For the control lenses, unlike the control subjects, the migraine patients showed a similar SF tuning in both V1 (Figure 6, top left, red solid line) and V2 (Figure 6, top right, red solid line). In addition, in contrast to the control subjects, the cortical activation in response to the stressful pattern was augmented in both areas, reflecting the pattern-induced cortical hyperactivation in the migraine patients. When the POTs were worn, however, the cortical activation in both areas showed a SF tuning similar to that of the control subjects, and the augmented cortical activations were also reduced giving activation levels similar to those of the control subjects (Figure 6, top left and right, blue solid lines).
In all participants and experimental conditions, cortical responses in V3 behaved similarly to those in V2 (Figure 6, bottom left). For the control subjects and the migraine patients wearing the POTs, similar low-pass SF tuning was seen in both V2 and V3. The abnormal behaviour of the cortical responses in V2 in the migraine patients was also seen in V3 when the control gray and coloured lenses were worn (Figure 6, bottom left, red solid line). In comparison to V3, the cortical responses behaved similarly in V3A and V4 (Figure 6, bottom middle and right), though large response variations were present that could be due to relatively small BOLD signal changes in these areas.
Discussion
The stressful pattern is generally uncomfortable to look at (2). When viewing the stressful pattern, all the migraine patients reported illusions and distortions and claimed that viewing the pattern for some time would trigger a migraine attack. They found that their POTs maximally suppressed the illusions and distortions compared both with other colours and with white light, and two of them reported that their POTs almost completely eliminated the illusions and distortions. These claims were supported by the visual discomfort rating (Figure 2B). The reduced cortical activation in V2 by the POTs may have been responsible for the POT-induced suppression of the illusions and distortions, considering that V2 neurons but not V1 neurons in macaque monkey respond to illusory contour stimuli (17).
The cortical activation in response to the stripe with SF 0.31 cpd showed no difference between the migraine patients and the control subjects in each visual area, independent of the type of lenses, demonstrating a normal cortical activation of the patients in response to the non-stressful control pattern (Figure 3). For the control subjects, the cortical activation in response to the stressful stripe with SF 2.5 cpd showed no differences between the neutral gray lens, the control coloured lens, and the POT, demonstrating that both coloured and POT lenses did not produce a reduction in the cortical activation when compared with the neutral gray lens (Figure 4A). Furthermore, the POT showed no difference compared to the coloured lens. When the gray and control coloured lenses were worn, the migraine patients showed no difference in cortical activation in any visual area, demonstrating that a randomly selected colour does not have an effect of reducing the cortical hyperactivation in migraine (Figure 4B). This result is consistent with the self-scored visual discomfort test in which the gray lens and the control coloured lens also showed no difference (Figure 2B). However, the cortical activations in the extra-striate areas were suppressed by the POT lenses (Figure 4B). This suppressing effect of the POT to the cortical activation in the migraine patients is consistent with the effect of the POT in suppressing the visual discomfort (Figure 2B).
The SF tuning characteristics of cortical activation observed in V1 and V2 in the control subjects agree well with the SF selectivity of neurons in V1 and V2 of the macaque monkey, respectively (18). The optimum 2.5 cpd SF tuning in cortical activation in V1 in the humans matches with the optimum 2.2 cpd of SF selectivity of neurons in V1 in the macaque monkeys. The peak of SF selectivity of macaque V2 neurons was shifted to 0.65 cpd, consistent with the low-pass SF tuning of the cortical response in human V2. A previous fMRI study of normal participants using sine-wave gratings also found a similar low-pass SF tuned cortical activation in V2 (16). This marked low-pass SF tuning of the cortical response in V2 relative to V1 occurred despite our use of square-wave gratings and the SF harmonics they introduce. It signifies the importance of this low-pass SF tuning in normal visual cortical function. V2 receives major excitatory inputs from V1 (19). The absence of the low-pass SF tuning characteristic in V2 in the migraine patients is indicative of an abnormal neural activity, consistent with an insufficient inhibition of the output from V1 (Figure 6, top right, red solid line). The POTs were sufficient to normalize both the activation level and its SF tuning in V2 in the migraine patients (Figure 6, top right, blue solid line). Since the cortical responses in the other extra-striate areas (Figure 6, bottom panel) behaved similarly to those in V2 (Figure 6, top right), processing in these areas may have depended on that in V2. Overall, the POTs mainly affected cortical activation in V2 though they appeared also to have a relatively small, statistically non-significant suppressing effect on cortical activation in V1 (Figure 5).
V2 neurons in macaque monkey show a colour tuning characteristic and differ from V1 neurons in the linearity with which they summate cone signals (20). It was suggested that this difference between V2 and V1 may result from the interaction of different channel inputs via the laterally spreading connections within V2 (19,21). In macaque, Xiao et al. (22) have observed a spatially organized representation of colour in V2 similar to that in the CIE UCS diagram. A change in colour may therefore cause a change in the cortical topography of the response. Based on our experience, migraine patients who identified some beneficial colours also identified some offensive colours in general. Accordingly, we speculate that comfortable colours redistribute excitation in such a way as to reduce cortical hyperexcitation in V2. It remains to be explored whether this is responsible for the POT-induced normalized cortical response in the migraine patients.
The hypothesis that cortical spreading depression (CSD) underlies migraine visual aura has been supported by cerebral blood flow measurements of spreading hypoperfusion (23,24). Visually triggered headache and visual symptoms in patients with migraine have been found to be accompanied by an initial increase in occipital cortex oxygenation followed by spreading suppression of neuronal activation (25). This initial increase in occipital cortex oxygenation prior to the spreading suppression of neuronal activation was confirmed in an exercise-induced typical migraine visual aura (26), signifying its importance as regards the onset of migraine visual aura and migraine attacks. The study also demonstrated the source of aura-related BOLD signal changes to be located in extra-striate visual cortex (V3A) rather than in V1 in the patient, the location of the source consistent with the type of patient's typical aura (26). Another migraine patient studied using magnetoencephalography also had an extra-striate location for the onset of aura (27). The increased occipital cortex oxygenation prior to the onset of CSD could be a result of cumulative cortical hyperactivation directly induced by the visual stimulation or indirectly induced via the exercise. It is possible that a hyperexcitability of the visual cortex may contribute to the induction of spreading depression: in animals pre-treated with the pro-convulsant drug, metrazol, visual stimulation can precipitate spreading depression (28). The reduction in fMRI BOLD activation by POTs in extra-striate areas is therefore consistent with indications for the therapeutic potential of POTs in reducing migraine attacks. A re-analysis of a small-scale double-masked trial (8) of 17 patients (12 MwA and five MwoA) has shown that in 42% of the MwA patients, the frequency of migraine headaches was reduced 50% or more during the days in which the POTs were worn compared to the days in which the control coloured lenses were worn. On the basis of the current findings it is reasonable to suppose that a suppressed cortical activation reduces the chance for cumulative cortical activation to reach a level sufficient to initiate a CSD or migraine attack. We suppose that discomfort from strong cortical excitation is a reflection of homeostasis although the mechanisms are unclear. These mechanisms, however, may differ from those that underlie the photophobia that accompanies migraine headache for which image formation may not be required (29). The characteristic low-pass SF tuning of cortical activation in the extra-striate visual areas provides a potential biomarker for identifying those migraine patients suffering cortical hyperactivation, and this biomarker also has a potential to be used for evaluating therapeutic effects of POTs or drugs in reducing the cortical hyperactivation and preventing migraine in the patients.
Acknowledgements
We thank Cerium Visual Technologies for providing free precision ophthalmic tints used in this study. We appreciate Dr. E. J. Potchen and the Department of Radiology at Michigan State University for providing technical support. AW was supported by the Wellcome Trust (grant number 80274).
Funding This work was supported by the National Institutes of Health (grant number R21NS054202).
Footnotes
Conflicts of interest The UK Medical Research Council owns the rights to the Intuitive Colorimeter and POTs used in this study. AW receives from the Council a proportion of royalties on sales of the Intuitive Colorimeter as an ‘Award to Inventors’. No royalties are payable on POTs.
References
- 1.Campbell FW, Robson JG. Application of Fourier analysis to the visibility of gratings. J Physiol. 1968;197:551–566. doi: 10.1113/jphysiol.1968.sp008574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilkins AJ. Visual stress. Oxford University Press; Oxford: 1995. [Google Scholar]
- 3.Huang J, Cooper TG, Satana B, Kaufman DI, Cao Y. Visual distortion provoked by a stimulus in migraine associated with hyperneuronal activity. Headache. 2003;43:664–671. doi: 10.1046/j.1526-4610.2003.03110.x. [DOI] [PubMed] [Google Scholar]
- 4.Wilkins AJ, Bonanni P, Porciatti V, Guerrini R. Physiology of human photosensitivity. Epilepsia. 2004;45:7–13. doi: 10.1111/j.0013-9580.2004.451009.x. [DOI] [PubMed] [Google Scholar]
- 5.Aurora SK, Cao Y, Bowyer SM, Welch KMA. The occipital cortex is hyperexcitable in migraine: experimental evidence. Headache. 1998;39:469–476. doi: 10.1046/j.1526-4610.1999.3907469.x. [DOI] [PubMed] [Google Scholar]
- 6.Irlen H. Reading by the colors: overcoming dyslexia and other reading disabilities through the Irlen method. Avery Publishing Group; New York: 1991. [Google Scholar]
- 7.Wilkins A, Milroy R, Nimmo-Smith I, Wright A, Tyrrell R, Holland K, et al. Preliminary observations concerning treatment of visual discomfort and associated perceptual distortion. Ophthalmic Physiol Opt. 1992;12:257–263. doi: 10.1111/j.1475-1313.1992.tb00302.x. [DOI] [PubMed] [Google Scholar]
- 8.Wilkins AJ, Patel R, Adjamian R, Evans BJW. Tinted spectacles and visually sensitive migraine. Cephalalgia. 2002;22:711–719. doi: 10.1046/j.1468-2982.2002.00362.x. [DOI] [PubMed] [Google Scholar]
- 9.Wilkins AJ, Lewis E, Smith F, Rowland E, Tweedie W. Coloured overlays and their benefit for reading. J Res Read. 2001;24:41–64. [Google Scholar]
- 10.Hunt RWG. Measuring color. Fountain Press Ltd; London: 2001. [Google Scholar]
- 11.Wilkins AJ, Sihra N, Myers A. Increasing reading speed using colours: issues concerning reliability and specificity, their theoretical and practical implications. Perception. 2005;34:109–120. doi: 10.1068/p5045. [DOI] [PubMed] [Google Scholar]
- 12.Wilkins AJ, Sihra N. A colorizer for use in determining an optimal ophthalmic tint. Color Res Applic. 2000;26:246–253. [Google Scholar]
- 13.Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, et al. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- 14.Tootell RBH, Mendola JD, Hadjikhani NK, Ledden PJ, Liu AK, Reppas JB, et al. Functional analysis of V3A and related areas in human visual cortex. J Neurosci. 1997;17:7060–7078. doi: 10.1523/JNEUROSCI.17-18-07060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh KD, Smith AT, Greenlee MW. Spatiotemporal frequency and direction sensitivities of human visual areas measured suing fMRI. NeuroImage. 2000;12:550–564. doi: 10.1006/nimg.2000.0642. [DOI] [PubMed] [Google Scholar]
- 17.von der Heydt R, Peterhans E. Mechanisms of contour perception in monkey visual cortex. I. lines of pattern discontinuity. J Neuroscience. 1989;9:1731–1748. doi: 10.1523/JNEUROSCI.09-05-01731.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster KH, Gaska JP, Nagler M, Pollen DA. Spatial and temporal frequency selectivity of neurons in visual cortical areas V1 and V2 of the macaque monkey. J Physiol. 1985;365:331–363. doi: 10.1113/jphysiol.1985.sp015776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lund JS, Hendrickson AE, Ogren MP, Tobin EA. Anatomic organization of primate visual cortex area VII. J Comp Neurol. 1981;202:19–45. doi: 10.1002/cne.902020104. [DOI] [PubMed] [Google Scholar]
- 20.Levitt JB, Kiper DC, Movshon JA. Receptive fields and functional architecture of macaque V2. J Neurophysiol. 1994;71:2517–2542. doi: 10.1152/jn.1994.71.6.2517. [DOI] [PubMed] [Google Scholar]
- 21.Levitt JB, Yoshioka T, Lund JS. Intrinsic cortical connections in macaque visual area V2: evidence for interaction between different functional streams. J Comp Neurol. 1994;342:551–570. doi: 10.1002/cne.903420405. [DOI] [PubMed] [Google Scholar]
- 22.Xiao Y, Wang Y, Felleman DJ. A spatially organized representation of color in macaque cortical area V2. Nature. 2003;2003;421:535–539. doi: 10.1038/nature01372. [DOI] [PubMed] [Google Scholar]
- 23.Leao AAP. Spreading depression of activity in cerebral cortex. J Neurophysiol. 1944;7:359–390. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- 24.Olesen J, Larsen B, Lauritzen M. Focal hyperemia followed by spreading oligemia and impaired activation of rcbf in classic migraine. Ann Neurol. 1981;9:344–352. doi: 10.1002/ana.410090406. [DOI] [PubMed] [Google Scholar]
- 25.Cao Y, Welch KMA, Aurora S, Vikingstad SE. Functional MRI-BOLD of visually triggered headache in patients with migraine. Arch Neurol. 1999;56:548–554. doi: 10.1001/archneur.56.5.548. [DOI] [PubMed] [Google Scholar]
- 26.Hadjikhani N, Sanchez del Rio M, Wu O, Schwartz D, Bakker D, Fischl B, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci USA. 2001;98:4687–4692. doi: 10.1073/pnas.071582498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall SD, Barnes GR, Hillebrand A, Furlong PL, Singh KD, Holliday IE. Spatio-temporal imaging of cortical desynchronization in migraine visual aura: a magnetoencephalography case study. Headache. 2004;44:204–208. doi: 10.1111/j.1526-4610.2004.04048.x. [DOI] [PubMed] [Google Scholar]
- 28.Van Harreveld A, Stamm JS. Cortical responses to metrazol and sensory stimulation in the rabbit. Electroencephalogr Clin Neurophysiol. 1955;7:363. doi: 10.1016/0013-4694(55)90005-5. [DOI] [PubMed] [Google Scholar]
- 29.Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, Burstein R. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13:239–245. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]