Abstract
Introduction
Frailty is a state of increased vulnerability to health-related stressors and can be measured by summing the number of frailty characteristics present in an individual. Discharge institutionalization (rather than discharge to home) represents disease burden and functional dependence following hospitalization. Our aim was to determine the relationship between frailty and need for post-operative discharge institutionalization.
Study Design
Subjects ≥65 years undergoing major elective operations requiring post-operative ICU admission were enrolled. Discharge institutionalization was defined as need for institutionalized care at hospital discharge. Fourteen pre-operative frailty characteristics were measured in six domains: co-morbidity burden, function, nutrition, cognition geriatric syndromes and extrinsic frailty.
Results
223 subjects (mean age 73±6 years) were studied. Discharge institutionalization occurred in 30% (66). Frailty characteristics related to need for post-operative discharge institutionalization included: older age, Charlson ≥3, Hematocrit <35%, any functional dependence, Up-and-Go ≥15 seconds, albumin <3.4 mg/dL, Mini-Cog ≤3, and having fallen within six-months (p<.0001 for all comparisons). Multivariate logistic regression retained prolonged timed up-and-go (p<.0001) and any functional dependence (p<.0001) as the variables most closely related to need for discharge institutionalization. An increased number of frailty characteristics present in any one subject resulted in increased rate of discharge institutionalization.
Conclusions
Nearly one in three geriatric patients required discharge to an institutional care facility following major surgery. The frailty characteristics of prolonged up-and-go and any functional dependence were most closely related to the need for discharge institutionalization. Accumulation of a higher number of frailty characteristics in any one geriatric patient increased their risk of discharge institutionalization.
INTRODUCTION
More than half of all operations performed in the United States are on patients older than 65 years.1 Frailty describes physiologic vulnerability which is unique to the geriatric population. Frailty is defined as a state of reduced physiologic reserve associated with increased susceptibility to disability.2 Frailty is most often measured by summing the number of frailty characteristics present in an individual.3 By definition, frailty implies poor health care outcomes. With an aging population, understanding the relationship of frailty to surgical outcomes is becoming increasingly important.4
Major operation in elderly individuals results in functional decline.5 The need for discharge to an institutional care facility (rather than home) suggests impairment of function necessary to live independently. Types of post-discharge institutions include rehabilitation centers, skilled nursing facilities and nursing homes. Following a major operation, 20–44% of older adults require discharge to an institutional care facility prior to returning home.6–8 Evidence of the national importance of discharge care facilities is the fact that Medicare allocates 10% of its annual budget (or more than 40 billion dollars annually) to pay for institutional care following hospital discharge.9
The possibility of correlating baseline frailty, measured in pre-operative clinic, to the need for institutional discharge following the post-operative hospital stay has clinical utility. Anticipating which older adults will require discharge to an additional care facility (rather than home) following a major operation is important for pre-operative counseling of expected outcomes, and pre-operative care planning for both the elderly patient and their family. The specific aims of this study were to: (1) determine the incidence of post-discharge institutionalization for geriatric patients following major elective operations; (2) describe the relationship between the presence of individual pre-operative frailty characteristics and the outcome of discharge institutionalization; (3) determine which baseline pre-operative frailty characteristics are most closely related with need for discharge to an institution; and (4) describe the relationship between the number of accumulated frailty characteristics in any one individual and the need for discharge institutionalization.
METHODS
This is a prospective cohort study performed at the Denver Veteran Affairs Medical Center. Inclusion criteria were subjects 65 years and older undergoing a major elective operation requiring post-operative surgical intensive care unit admission. Subjects were recruited from the services of general surgery, cardiac surgery, thoracic surgery, urology and vascular surgery services. Exclusion criteria were pre-operative residence at an institutional facility, emergent operations and subjects with recent hemorrhage resulting in acute blood loss anemia (to distinguish subjects with anemia of chronic disease from acute blood loss anemia). Subjects were recruited between January 2007 and March 2010. Regulatory approval was obtained through the Colorado Multi-Institutional Review Board (COMIRB 08-1071). We previously reported on frailty and both six-month mortality (primary outcome variable) and discharge institutionalization (secondary outcome variable) in 110 of the 223 subjects included in this study.
Baseline frailty characteristics were measured pre-operatively. Frailty is recognized as a multi-dimensional, multi-system impairment across numerous physiologic domains.10 The frailty characteristics that were measured covered six domains: burden of co-morbidity, function, nutrition, cognition/mental, geriatric syndromes and extrinsic frailty.
Burden of co-morbidity was quantified using: (1) Charlson Index contains 19 categories of co-morbidity and assigns a weighted value to each co-morbidity based on the risk of one-year mortality.11 (2) American Society of Anesthesiologists (ASA) score represents an individual’s physical health and predicts post-operative morbidity and ranges from 0 (lowest risk) to five (highest risk).12 (3) Number of outpatient medications (polypharmacy) each subject was taking immediately prior to surgery was counted; a measure recognized as a surrogate for burden of co-morbidity.13 (4) Hematocrit was measured to quantify anemia, a recognized characteristic of frailty.14–15 A hematocrit of less than 35% was chosen to define anemia based on our previous work on frailty and post-operative mortality.4
Function was quantified using: (1) The Katz Activity of Daily Living (ADL) Score which assesses for any dependence versus independence in the six activities of daily living.16 Scores range from 6 (independence in all ADLs) to 0 (dependence in all six ADLs). (2) A Timed Up-and-Go was performed to evaluate lower extremity strength and gait speed. This timed test starts with the subject standing from a chair, walking 10 feet, returning to the chair and then ends after the subject sits back down in the chair.
Nutrition was quantified using. (1) Albumin level measured within 30 days of the operation. Hypoalbuminemia signifies malnutrition and is a recognized frailty biomarker.17 (2) Body mass index calculated by height and weight (kilogram / meters squared). A low body mass index, suggestive of malnutrition, signifies frailty.18 (3) Recent under-nutrition was defined as ten or more pounds of weight loss in the six months prior to the operation.19
Cognition/mental function was quantified using: (1) The Mini-Cog test combines a three item recall with a clock draw to define cognitive function.20 Scores range from 5 (normal cognition) to 0 (severe cognitive impairment). (2) Depression was assessed with the Two-Question Depression Screen which inquires about depressed mood and anhedonia.4 A positive response to either question signifies possible depression.
Geriatric syndromes and extrinsic frailty were quantified using: (1) Geriatric syndromes are clinical phenomena unique to geriatric patients and signify physiologic compromise across multiple systems.21 Falls represent a geriatric syndrome.22 The presence of falls was determined by asking the subject if they had fallen one or more time in the six-months prior to surgery. One or more falls was considered positive for falling. (2) Extrinsic frailty, also called social vulnerability, recognizes social factors (including social isolation, lack of a close family network) that increase mortality in nursing home patients.23 Living alone, without a spouse or companion, was defined as positive for extrinsic frailty.
The primary outcome variable was discharge to an institutional care facility. An institutional care facility was defined as a nursing home, a skilled nursing facility or rehabilitation center. Discharge to an institutional care facility, rather than home, almost always signifies dependence in activities of daily living.
Univariate and logistic regression analysis were employed to determine the relationship of individual frailty characteristics to the outcome of discharge to an institution. Correlations between the explanatory and predictor variables were performed using Spearman rank correlation coefficients. To identify variables that predict discharge to an institution, a logistic regression model was developed using backwards selection with significance level equal to 0.05. All statistical analyses were performed using SAS version 9.2 (Cary, NC).
The seven frailty characteristics most closely related to the outcome of post-discharge institutionalization by initial statistical analysis were subsequently used to determine the relationship of accumulated frailty characteristics in an individual subject to need for discharge institutionalization. Cutoff values used to convert continuous variables to dichotomous variables were described in our previous work.4 Using these cutoff values, the number of total frailty characteristics were counted in individual subjects. The sum, or accumulation, of total frailty characteristics present in an each subject was then correlated to post-discharge institutionalization.
RESULTS
This study included 223 subjects with a mean age of 73±6 years and 215 (96%) were male. Six patients (3%) who met inclusion criteria did not have frailty characteristics measured pre-operatively and were excluded. Operations performed included: abdominal 47% (104/223), cardiac 34% (75/223), vascular 10% (23/223) and non-cardiac thoracic 9% (21/223). Average length of intensive care unit stay was 7.2±8.3 days. Average length of hospital stay was 11.7±11.4 days.
The incidence of discharge institutionalization was 30% (66/223). The length of institutional care stay was 25±21 days (range 3 to 112). Of the subjects discharged home, 17% (27/157) were prescribed home care visits immediately after discharge. Baseline pre-operative frailty characteristics were compared in the group discharged to home to the group discharged to an institutional care facility. (See Table 1) Multivariate logistic regression found timed up and go >15 seconds, any functional dependence, Charlson score of 3 or higher and hematocrit less than 35% were the predictor variables most closely related to need for discharge institutionalization. (See Table 2) Intra-operative variables (which represent potential confounding variables) were similar in the group discharged home in comparison to the group discharged to institutional care facilities. (See Table 3)
Table 1.
Baseline Preoperative Frailty and Postoperative Discharge Institutionalization
| Baseline preoperative frailty traits | After discharge care | Spearman rank correlations with discharge location | ||
|---|---|---|---|---|
| Home (n=157) | Institution (n=66) | Correlation coefficient | p Value | |
| Age, y | 72±6 | 77±6 | −0.3570 | <0.0001 |
| Burden chronic disease | ||||
| Charlson Index ≥ 3* | 42% (66/157) | 86% (57/66) | −0.4068 | <0.0001 |
| ASA Scorea | 2.8±0.5 | 3.0±0.3 | −0.2182 | 0.0010 |
| Outpatient medicines, n | 5.8±3.2 | 6.0±2.9 | −0.0586 | 0.3839 |
| Hematocrit < 35%* | 6% (9/157) | 44% (29/66) | −0.4639 | <0.0001 |
| Function | ||||
| Any functional dependence* | 16% (25/157) | 76% (50/66) | 0.5781 | <0.0001 |
| Timed up-and-go ≥ 15 sec* | 8% (12/157) | 67% (44/66) | −0.6213 | <0.0001 |
| Nutrition | ||||
| Albumin < 3.4, g/dL* | 10% (15/147) | 66% (43/65) | −0.4938 | <0.0001 |
| Body Mass Index, kg/m2 | 27±5 | 26±5 | 0.1110 | 0.0986 |
| ≥10 lbs weight loss/before 6 mo | 10% (16/155) | 22% (14/64) | 0.1324 | 0.0504 |
| Mental | ||||
| Mini-cognitive ≤ 3* | 29% (46/157) | 65% (43/66) | −0.3342 | <0.0001 |
| Depressed mood | 35% (54/156) | 41% (27/66) | 0.0598 | 0.3756 |
| Geriatric Syndromes | ||||
| ≥ 1 fall in prior 6-mo* | 17% (26/156) | 61% (40/66) | −0.4394 | <0.0001 |
| Extrinsic frailty, lives alone | 33% (52/157) | 61% (40/66) | 0.2549 | 0.0001 |
Variable used to assess accumulation of frailty characteristics and the need for discharge to an institution.
ASA, American Society of Anesthesiology.
Table 2.
Baseline Frailty and Postoperative Discharge Institutionalization (Multivariate Logistic Regression)
| Effect | Odds Ratio Estimates | p Value | ||
|---|---|---|---|---|
| Point estimate | 95% Confidence | Limit | ||
| Timed up-and-go ≥ 15 sec | 13.026 | 5.145 | 32.980 | <0.0001 |
| Any functional dependence | 5.662 | 2.371 | 13.519 | <0.0001 |
| Charlson Index ≥ 3 | 3.954 | 1.575 | 9.927 | 0.0034 |
| Hematocrit ≤ 35% | 3.520 | 1.383 | 8.955 | 0.0083 |
Table 3.
Intraoperative Variables (Potential Confounders)
| Home (n=157) | Institution (n=66) | p Value | |
|---|---|---|---|
| Operative characteristics | |||
| Length of operation, min | 282±120 | 301±113 | 0.273 |
| Blood loss, mL | 534±713 | 654±576±6 | 0.227 |
| Intraoperative blood transfusion, U | 1.0±2.1 | 1.5±2.2 | 0.111 |
| Type of operation | |||
| Abdominal | 46% (73/157) | 47% (31/66) | 1.000 |
| Cardiac | 35% (55/157) | 30% (20/66) | 0.537 |
| Non-cardiac thoracic | 10% (15/157) | 12% (8/66) | 0.631 |
| Vascular | 9% (14/157) | 11% (7/66) | 0.802 |
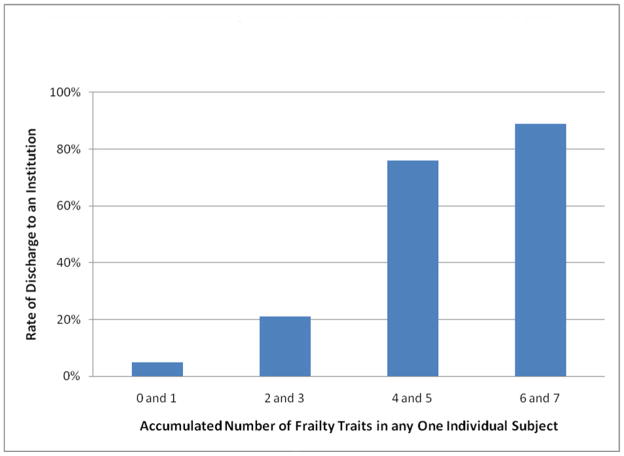
Subjects were grouped based on the number of frailty characteristics present in an individual subject, the relationship of accumulation of frailty characteristics and need for discharge institutionalization is depicted in Figure 1. Three or more frailty characteristics in any one subject predicted discharge institutionalization with a sensitivity of 82% (95% Confidence Interval 70% to 90%) and specificity of 84% (95% Confidence Interval 77% to 89%).
Figure 1.
Accumulation of frailty characteristics and the rate of postoperative discharge to an institution. The presence or absence of the seven frailty characteristics found to be most closely related to discharge institutionalization (asterisks on Table 1 denotes the 7 frailty characteristics used) were assessed in each subject. The presence of a frailty characteristic was scored as 1 characteristic. The number of positive frailty characteristics was summed to create a number that represented the accumulated amount of frailty characteristics present in each subject. (p<0.01 for all comparisons except 4 and 5 versus 6 and 7 [p=0.31]).
DISCUSSION
Following major elective surgery in subjects 65 years and older, summing the accumulation of frailty characteristics present in an individual subject has utility in predicting the need for discharge institutionalization. Baseline, pre-operative frailty characteristics most closely related to discharge institutionalization by univariate analysis were: high burden of chronic disease (Charlson Index ≥ 3), anemia (hematocrit ≤ 35%), any functional dependence (Katz score < 6), prolonged Timed Up-and-Go (≥ 15 seconds), low albumin level (< 3.4 mg/dL), cognitive dysfunction (Mini-Cog ≤ 3) and the presence of the geriatric syndrome of falling (≥ 1 fall within six-months of an operation). Logistic regression found prolonged Timed Up-and-Go ≥ 15 seconds, any functional dependence and Charlson Index ≥ 3 to be the three pre-operative baseline variables most closely related to the need for post-operative institutionalization. Comparing the number of frailty traits present in any individual subject and the need for discharge institutionalization revealed that an accumulation of a higher number of frailty characteristics correlated with increased need for discharge to an institution.
Institutional placement following hospitalization became an important part of U.S. healthcare following the federal government’s passing of the Medicare Prospective Payment System (PPS).24 This landmark legislation shifted healthcare reimbursement from a retrospective fee-for-service system to a prospective system in which hospitals receive a fixed amount determined by the patient’s diagnosis related group (DRG). In reaction, hospitals shortened length of stay to optimize financial gains. Elderly patients who previously relied on a few extra days in the hospital to regain the functional skills necessary to return home, now found themselves without that buffer. The result, three decades later, is a $40 billion industry of institutional care facilities aimed to bridge patients with functional dependence following their hospital stay (a group disproportionately represented by the elderly) until they regain the ability to return home.9, 25
Frailty (defined by assessing the accumulation of abnormalities in the domains of burden of co-morbidity, function, nutrition, cognition and geriatric syndromes) measured prior to elective surgery has been associated with adverse post-operative outcomes in elderly patients. Our group found the presence of four or more frailty characteristics pre-operatively was related to six-month post-operative mortality with a sensitivity of 81% and a specificity of 86% in 110 subjects 65 years and older undergoing major elective operations.4 The present study as well as our previous work includes subject 65 years and older, an age cutoff that might be considered young from a geriatric standpoint. Dasgupta and colleagues studied subjects 70 years and older undergoing elective inpatient operations (mostly orthopedic) and measured accumulation of frailty characteristics using the Edmonton Frail Scale. Increased burden of frailty characteristics was associated with increased post-operative complications, longer hospital stay and decreased chance of discharge to home.26 Kristjansson and colleagues used the Comprehensive Geriatric Assessment to assess 178 subjects 70 years and older undergoing a colorectal operation and found that frail individuals were at greater risk for severe complications.27 There is mounting evidence relating frailty to adverse surgical outcomes.
The importance of the present study is twofold. First, the majority of previous research on post-operative discharge institutionalization utilized large administrative datasets to recognize advancing age as a risk for increased need for discharge institutionalization.6–8 While our data confirms the finding that older age is associated with discharge institutionalization; this study found multiple patient characteristics more closely related to discharge institutionalization than age. Moreover, these characteristics (e.g., prolonged Timed Up-and-Go, functional dependence and high burden of co-morbidity) can be measured with ease and brevity in pre-operative clinic; thereby contributing to surgical decision-making. Secondly, the present study adds to a growing body of evidence that measurement of baseline, pre-existing frailty characteristics has value in predicting surgical outcomes in geriatric patients.4, 26–27 Using frailty as a pre-operative tool for post-operative risk stratification is a paradigm shift from traditional pre-operative evaluation techniques which focus on single end organ function (e.g., the American Heart Association cardiac clearance guideline)28 rather than physiologic vulnerabilities unique to the elderly aged. Both of the characteristics which make this study valuable center on the fact that gathering information on frailty characteristics can improve the clinician’s ability to accurately counsel the older adults and their families on anticipated post-operative outcomes and can modify surgical recommendations.
Major limitations of this study are threefold. First, two contesting views of what defines frailty exist and our dataset represents only one of the two existing strategies. In brief, the approach used to collect our data recognizes frailty as a composite of clinical risk factors (termed frailty characteristics) that predict future adverse events.29 This approach is criticized by gerontologists who believe phenotypic frailty (the centerpiece of which is slowing mobility and exhaustion) is distinct from disability and co-morbidity.19, 30 We chose the former because of its clinical utility and potential for easy administration in a surgeon’s pre-operative clinic to provide that surgeon with timely information to influence pre-operative decision-making. Second, the majority of our subjects were male. As a result, this study cannot detect potential gender differences of frailty and the need for discharge institutionalization in women. Our study population’s gender distribution reflects the patient population at the Denver Veterans Affairs Medical Center and not a selection bias. Third, this study included major operations from the standpoints of prolonged operative time, high blood loss and long intensive care unit stays. The extensive nature of these operative stresses likely leads to higher a higher rate of discharge institutionalization in comparison to lesser operative stresses.
Our study found that the pre-operative presence of multiple frailty characteristics is related to an increased need for discharge institutionalization in subjects 65 years and older undergoing major elective operations. Given that the population is aging and that geriatric patients require a disproportionate amount of operations, the knowledge to accurately risk stratify post-operative outcomes in elderly individuals will become increasingly important. The ability for the clinician to provide accurate pre-operative counseling to older adults about their risk of needing additional institutional care following their hospitalization is practical to the patient and their family in terms of appropriate expectations prior to an elective operation. Future directions include incorporation of all clinical characteristics used to define the frail older individual into a single study to determine which characteristic most accurately predicts post-discharge institutionalization and to perform validation studies including those specific to surgical specialties to determine if frailty predicts discharge institutionalization across surgical specialties.
Acknowledgments
The study was supported by the Paul B Beeson Award – NIA K23AG034632 (TNR); Dennis W Jahnigen Award - American Geriatrics Society (TNR); and Dr Moss received an NIH grant, K24-HL-089223.
Footnotes
Presented at the Western Surgical Association 118th Scientific Session, Chicago, IL, November 2010.
Disclosure Information: Nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geriatric Review Syllabus - A Core Curriculum in Geriatric Medicine. 6. New York: American Geriatrics Society; 2006. [Google Scholar]
- 2.Buchner DM, Wagner EH. Preventing frail health. Clin Geriatr Med. 1992;8:1–17. [PubMed] [Google Scholar]
- 3.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson TN, Eiseman B, Wallace JI, Church SD. Redefining Geriatric Pre-Operative Assessment Using Frailty, Disability and Co-Morbidity. Ann Surg. 2009;250:449–455. doi: 10.1097/SLA.0b013e3181b45598. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence VA, Hazuda HP, Cornell JE, et al. Functional independence after major abdominal surgery in the elderly. J Am Coll Surg. 2004;199:762–772. doi: 10.1016/j.jamcollsurg.2004.05.280. [DOI] [PubMed] [Google Scholar]
- 6.Legner VJ, Massarweh NN, Symons RG, et al. The significance of discharge to skilled care after abdominopelvic surgery in older adults. Ann Surg. 2009;249:250–255. doi: 10.1097/SLA.0b013e318195e12f. [DOI] [PubMed] [Google Scholar]
- 7.Riall TS, Reddy DM, Nealon WH, Goodwin JS. The effect of age on short-term outcomes after pancreatic resection: a population-based study. Ann Surg. 2008;248:459–467. doi: 10.1097/SLA.0b013e318185e1b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finlayson E, Fan Z, Birkmeyer JD. Outcomes in octogenarians undergoing high-risk cancer operation: a national study. J Am Coll Surg. 2007;205:729–734. doi: 10.1016/j.jamcollsurg.2007.06.307. [DOI] [PubMed] [Google Scholar]
- 9.Services USDoHaH. CMS Financial Report - Fiscal Year 2007. 2007. [Google Scholar]
- 10.Rockwood K, Hogan DB, MacKnight C. Conceptualisation and measurement of frailty in elderly people. Drugs Aging. 2000;17:295–302. doi: 10.2165/00002512-200017040-00005. [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Cullen DJ, Apolone G, Greenfield S, et al. ASA Physical Status and age predict morbidity after three surgical procedures. Ann Surg. 1994;220:3–9. doi: 10.1097/00000658-199407000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajjar ER, Cafiero AC, Hanlon JT. Polypharmacy in elderly patients. Am J Geriatr Pharmacother. 2007;5:345–351. doi: 10.1016/j.amjopharm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Chaves PH, Semba RD, Leng SX, et al. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2005;60:729–735. doi: 10.1093/gerona/60.6.729. [DOI] [PubMed] [Google Scholar]
- 15.Ravaglia G, Forti P, Lucicesare A, et al. Development of an easy prognostic score for frailty outcomes in the aged. Age Ageing. 2008;37:161–166. doi: 10.1093/ageing/afm195. [DOI] [PubMed] [Google Scholar]
- 16.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10:20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 17.Hazzard WR. Depressed albumin and high-density lipoprotein cholesterol: signposts along the final common pathway of frailty. J Am Geriatr Soc. 2001;49:1253–1254. doi: 10.1046/j.1532-5415.2001.49245.x. [DOI] [PubMed] [Google Scholar]
- 18.Rolfson DB, Majumdar SR, Tsuyuki RT, et al. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35:526–529. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 20.Borson S, Scanlan JM, Chen P, Ganguli M. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51:1451–1454. doi: 10.1046/j.1532-5415.2003.51465.x. [DOI] [PubMed] [Google Scholar]
- 21.Cassell CK, Leipzig RM, Cohen HJ, et al. Geriatric Medicine: An Evidence Based Approach. New York: Springer-Verlag; 2003. [Google Scholar]
- 22.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–791. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrew MK, Mitnitski AB, Rockwood K. Social vulnerability, frailty and mortality in elderly people. PLoS ONE. 2008;3:e2232. doi: 10.1371/journal.pone.0002232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayes R. The origins, development, and passage of Medicare’s revolutionary prospective payment system. J Hist Med Allied Sci. 2007;62:21–55. doi: 10.1093/jhmas/jrj038. [DOI] [PubMed] [Google Scholar]
- 25.Stone J. Medicare’s Skilled Nursing Facility Payments. Congressional Research Service - Report for Congress; 2007. [Google Scholar]
- 26.Dasgupta M, Rolfson DB, Stolee P, et al. Frailty is associated with postoperative complications in older adults with medical problems. Arch Gerontol Geriatr. 2009;48:78–83. doi: 10.1016/j.archger.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Kristjansson SR, Nesbakken A, Jordhoy MS, et al. Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: A prospective observational cohort study. Crit Rev Oncol Hematol. 2009 doi: 10.1016/j.critrevonc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Kushner FG, Hand M, Smith SC, Jr, et al. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:2271–2306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 29.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27:17–26. doi: 10.1016/j.cger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]