SUMMARY
Giardia lamblia, a protozoan parasite, infects a wide variety of vertebrates, including humans. Studies indicate that this anaerobic protist possesses a limited ability to synthesize lipid molecules de novo and depends on supplies from its environment for growth and differentiation. It has been suggested that most lipids and fatty acids are taken up by endocytic and non-endocytic pathways and are used by Giardia for energy production and membrane/organelle biosynthesis. The purpose of this article is to provide an update on recent progress in the field of lipid research of this parasite and the validation of lipid metabolic pathways through recent genomic information. Based on current cellular, biochemical and genomic data, a comprehensive pathway has been proposed to facilitate our understanding of lipid and fatty acid metabolism/syntheses in this waterborne pathogen. We envision that the current review will be helpful in identifying targets from the pathways that could be used to design novel therapies to control giardiasis and related diseases.
INTRODUCTION
Although identified by Antoni van Leeuwenhoek more than three centuries ago, Giardia has recently occupied a central stage of parasite research. The epidemiological studies conducted over the past few years indicate that a wide range of mammals, including humans and cattle, are infected by this parasite, causing a substantial burden on the global economy (Giangaspero et al. 2005). Current knowledge supports the proposal that giardiasis is a zoonotic disease and that contaminated water serves as one of the main sources of infection (Monis et al. 2003; Smith et al. 2006; Bajer 2008). Various species of Giardia are recognised (Thompson, 2009), and efforts have been made over the past few years to enhance the taxonomy using molecular tools (Hunter et al. 2005; Xiao et al. 2008; Thompson 2009). Based on such tools, 6 species of Giardia have been identified to date, representing 6 different assemblages, of which assemblages A and B infect humans and other mammals (Thompson, 2009).
In humans, Giardia infection can be symptomatic or asymptomatic. Symptomatic giardiasis can present with fatty diarrhoea, abdominal discomfort, vomiting, malabsorption and/or weight loss (Kamda et al. 2009). In some cases, giardiasis resolves rapidly, but in other cases, it can result in chronic infection (Faubert, 2000). Both cell-mediated and humoral immune responses in the host against Giardia have been reported, and adaptive responses have been shown to be critical for controlling giardiasis (Faubert, 2000). Non-immune systems such as secretory immunoglobulins also play a role in the severity of the disease (Nayak et al. 1987).
This parasite has a simple life cycle, with two morphological forms - i.e., trophozoites and cysts. Following ingestion, cysts pass through the stomach (being exposed to stomach acid), after which trophozoites are released and colonise the small intestine by longitudinal binary fission (Ghosh et al. 2001). The Giardia trophozoite (12–15 μm long) (Fig. 1, panel A), is non-invasive, and contains a ventral disc made of cytoskeletal proteins that provide support to Giardia for attachment to the enterocyte wall (Holberton, 1973, Ghosh et al. 2001). The resistant cysts (7–10 μm long) with thick cyst walls (Fig. 1, panel B) are responsible for the transmission of giardiasis via contaminated food or water. The cyst wall of Giardia contains insoluble filamentous materials that consist of glycoprotein, glycolipids, and amino-sugar containing oligo- and polysaccharides (Das and Gillin, 1996; Sener et al., 2004; Ratner et al., 2008). Three encystation-specific cyst-wall proteins (CWP-1, -2, and -3) are expressed at the time of encystation and concentrated within encystation-specific vesicles (ESVs) before they are targeted to the cyst wall. Besides these three CWPs, a high-cysteine non-variant cyst protein (HCNCp) is present in trophozoites and may participate in cyst production (Davids et al. 2006).
Fig. 1.
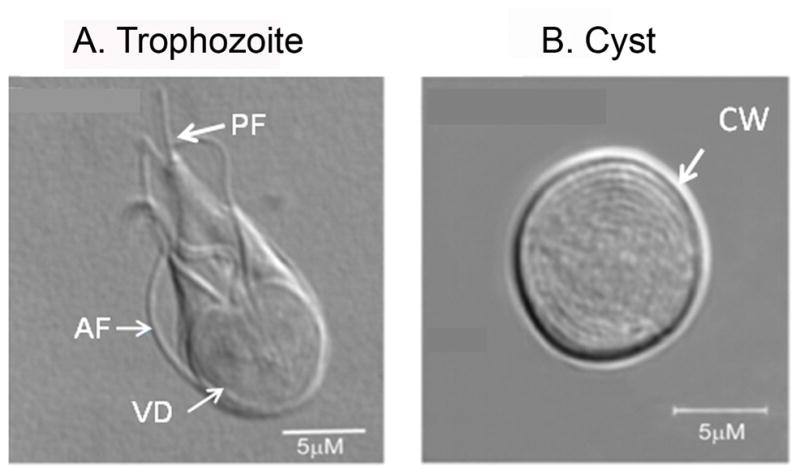
Direct interference contrast (DIC) microscopy pictures of Giardia trophozoite (panel A) and water-resistant cyst (panel B) cultured in the laboratory. The trophozoites (12–15 μm long) contain two nuclei (not visible in the picture) and a ventral disc (VD) made of cytoskeletal proteins that provide support to Giardia for attachment to the intestinal cell wall. The water-resistant cysts (7–10 μm long) with thick cyst walls (CW) are responsible for the transmission of giardiasis via contaminated water. VD, ventral disc; AF, anterior flagellum; PF, posterior flagellum; CW, cyst wall. Bar: 5 μM.
Studies conducted in recent years indicate that intestinal lipids and fatty acids influence the growth and encystation of Giardia (Farthing et al. 1985; Gillin et al. 1987, 1988; Lujan et al. 1996). Most lipids are taken up by this parasite from its environment and used as required (Kaneda and Goutsu, 1988; Mohareb et al. 1991). However, contrary to the earlier notion that Giardia is unable to synthesize its own lipids de novo (Jarroll et al. 1981), results from our laboratory suggest that selective phospholipids can be produced by this parasite via de novo and/or remodelling reactions (Gibson et al. 1999; Das et al. 2001, 2002). The recently established Genome Database (www.giardiadb.org, Morrison et al. 2007), which revealed the presence of lipid synthesis and metabolic genes, further validates our observations. The focus of this article is to review progress in the field of lipid research of Giardia and to validate lipid metabolic pathways by comparison to genomic sequence information. A possible lipid biosynthesis pathway for Giardia has also been proposed.
INTERACTIONS WITH INTESTINAL LIPIDS AND FATTY ACIDS
Because Giardia is continuously exposed to bile acids and dietary fats in the small intestine, it was proposed that lipids and fatty acids play important roles in regulating growth, encystation and excystation. Fatty acids from the intestine kill Giardia in vitro, whereas mucous and bile salts protect the parasite from being killed by fatty acids and other small intestinal factors (Reiner et al. 1986; Das et al. 1988). Bile acids were also proposed to facilitate the transport of intestinal lipids into Giardia by forming mixed micelles (Das et al. 1997). The intestinal factors include aggregated and non-aggregated fats, lipases and secretory immunoglobulins (Farthing et al. 1985; Reiner et al. 1986). Free fatty acids generated from phospholipids and triglycerides are detrimental to the growth of Giardia (Reiner et al. 1986; Das et al. 1988). Studies suggest that dodecanoic (C12:0) acid (also known as lauric acid) possesses an anti-giardial property at a reasonably low concentration (Rayan et al. 2005). This medium-chain fatty acid accumulates inside trophozoites and alters membrane permeability and integrity. Giardia has the machinery to neutralize the toxic effects of free fatty acids by forming complex with membrane proteins, lipids, and carbohydrates (Das et al. 1991; Gibson et al. 1999; Touz et al. 2005).
The role of bile and fatty acids in inducing the encystation of Giardia was first proposed by Frances D. Gillin. In classic experiments, Gillin and her colleagues showed that a mixture of primary bile acid (glycocholate) and fatty acid (oleic acid or myristic acid) promotes in vitro encystation (Gillin et al. 1987, 1988). Subsequently, cholesterol and an excess amount of bovine bile, which Giardia obtains from the growth medium, were shown to induce encystation (Kane et al. 1991; Lujan et al. 1996). Interestingly, the homologues of sterol regulatory-element-binding proteins (SREBPs) were identified in Giardia and found to regulate the expression of cwp genes during encystation (Worgall et al. 2004). Giardia expresses four genes linked to cholesterol biosynthesis, which are up-regulated during its differentiation into cyst (Hernandez et al. 2006). Several proteins of the parasite can undergo post-translational modification by the intermediate of cholesterol (isoprenyl-group) biosynthetic pathway (Lujan et al. 1995), and it is possible that these modifications of giardial proteins are important for maintaining membrane integrity and functions.
IMPORT OF LIPIDS AND FATTY ACIDS BY GIARDIA
Phospholipids and fatty acids are important constituents of all eukaryotic membranes, including those of Giardia. Because of its limited lipid synthesis ability (Das et al. 2002), lipids in Giardia are acquired from the small intestine of the host, in which the trophozoites are exposed to free and conjugated fatty acids, various sterols, phospholipids and bile acids (Stevens et al. 1997). Lujan et al. (1994) showed that lipoprotein-like receptor molecules are present in trophozoites, which allow them to internalize serum-lipoproteins through a cytochalasin-D-sensitive pathway. Using fluorescent lipid analogues, we have shown that trophozoites are able to internalize lipids directly from the culture medium and transport them to various locations, including the plasma and nuclear membranes, the cytoplasm and the endoplasmic reticulum (ER). The cellular localisation of each particular lipid analogue studied is distinct. We confirmed that the incorporations of fluorescently-labelled lipid analogues are not dependent on respective fluorophores (i.e., BODIPY or NBD), rather solely on the intrinsic properties (hydrophobicity and hydrophilicity) of lipid probes (Stevens et al. 1997; Gibson et al. 1999; Das et al. 2001). Results indicate that ceramide and phosphatidylglycerol (PG) show preferential localisation at perinuclear membranes, whereas phosphatidylcholine (PC) is incorporated into plasma and flagellar membranes. Palmitic acid (PamA) and sphingomyelin (SM) label the nuclear envelopes and the plasma membrane. Phosphatidylethanolamine (PE) is localised to the plasma membrane and in certain cytoplasmic structures adjacent to the plasma membrane.
As cytoskeletal components (i.e., actin filaments and microtubules) are involved in transporting lipid molecules in a range of eukaryotes, we investigated whether the giardial cytoskeleton also participates in the uptake and recycling of fluorescent lipid molecules from plasma- to endo-membranes. In Giardia, microtubules constitute numerous structures in trophozoites, including the ventral disc, basal bodies, flagella, paraflagellar rods and median body (Crossley et al. 1986; Elmendorf et al. 2003). The basal bodies are the major microtubule organizing centre and functional equivalent of the centrosome of higher eukaryotes (Nohynkova et al. 2000; Correa et al. 2004; Davids et al. 2008). A large set of kinesin homologues are present (Iwabe et al. 2002; Richardson et al. 2006), but, thus far, no putative homologue of myosin has been identified (Elmendorf et al. 2003). Giardia contains a single copy of the actin gene (Morrison et al. 2007), and its protein sequence reveals an ~58% nucleotide identity to other eukaryotic actin sequences (Elmendorf et al. 2003).
We have observed that the uptake and inter-organelle transport of fluorescently labelled SM, PC, and PG are interrupted by anti-actin and anti-microtubule agents. Cytochalasin-D, an actin-depolymerising drug, induces the formation of several tubular/vesicular structures and blocks the intracellular trafficking of ceramide and SM (Hernandez et al. 2007a; Castillo et al. 2009). Furthermore, vinorelbine (a microtubule depolymerising agent) is effective in significantly lowering the intracellular incorporation of fluorescently labelled ceramide and SM. These observations indicate that both ceramide and SM are taken up by cells through cytoskeletal-dependent processes which require intact actin and microtubule structures. On the contrary, the uptake of PC is not dependent on cytoskeleton, because cytochalasin-D and other microtubule-depolymerising drugs neither alter nor reduce the localisation pattern of PC. Like PC, PamA intake is also not affected by anti-cytoskeleton agents (Castillo et al. 2009). We have also observed that anti-microtubule depolymerising agents (e.g., cholchicine, albendazole and nocodazole) blocked the release of PG from the ER/perinuclear regions, suggesting that an intact microtubule structure could be essential not only for uptake and transport, but also for the recycling of PG from the ER to the cytoplasm and plasma membranes (Castillo et al. 2009). The results for cytoskeletal-based lipid transport and trafficking experiments (Castillo et al. 2009) have been summarized in a model (Fig. 2), which suggests that fluorescently labelled ceramide, SM and PG are mainly taken up by actin-dependent endocytic mechanisms (Hernandez et al. 2007a; Castillo et al. 2009). It can be postulated that soon after endocytic vesicles are released from the plasma membranes encapsulating lipid molecules, they reach the ER/perinuclear membranes on microtubule rails. Lipids, such as ceramide, SM, PC and PamA, are possibly taken up by the cells through non-endocytic pathways, but, at this stage, it is not clear whether PC localised on the outer cell membrane originates from the ER/perinuclear membranes or from the inner plasma membrane (Fig. 2). Giardial lipid and fatty-acid transport proteins may also participate in translocating lipid molecules that may travel along the microtubules to reach perinuclear membranes. The presence of a fatty-acid binding protein (~8 KD) has been reported in Giardia (Hassan et al. 2005). The transport of ER/perinuclear PG via exocytic vesicles may be regulated by microtubule filaments and not by actin cytoskeleton (Fig. 2), but more, in-depth experiments must be carried out to fully elucidate the lipid transport and trafficking in this organism.
Fig. 2.
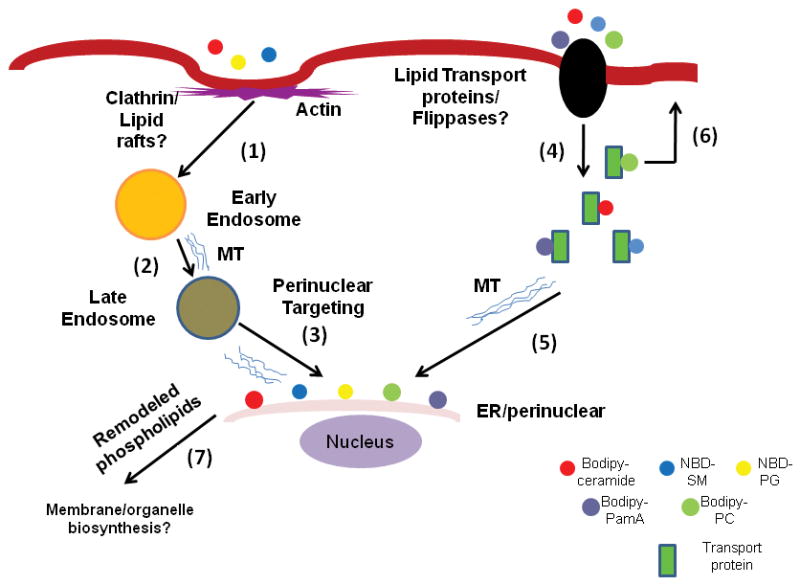
Lipid import and trafficking by Giardia. The figure shows that BODIPY-ceramide, NBD-SM, and NBD-PG could be imported by actin-dependent endocytic pathways and targeted to ER/perinuclear membranes (steps 1–3) (Hernandez et al. 2008; Castillo et al. 2009). Membrane lipids and fatty acids like ceramide, SM, PC, and PalmA can also be taken up by a flippase-dependent, non-vesicular mechanism and migrated intracellularly. Lipid-binding proteins and microtubule filaments may participate in this process (steps 4–5). Membrane phospholipids like PC, which is mostly localised in the plasma membrane (Das et al. 2001), can be flipped back to the plasma membrane (step 6), although the mechanism of this outward movement is not known. It is possible that the internalised lipids are remodelled at the ER/perinuclear regions (step 7) and utilised by the parasite for the synthesis of membranes and organelles. SM, sphingomyelin; PG, phosphatidylglycerol; PalmA, palmitic acid; PC, phosphatidylcholine; MT, microtubule.
SYNTHESES OF NEW LIPIDS AND FATTY ACIDS
The synthesis and metabolism of phospholipids and fatty acids in Giardia was first investigated by Edward Jarroll and his colleagues almost three decades ago (Jarroll et al. 1981). Using radioactive acetate, glucose, glycerol, threonine, cholesterol and glycerol-3-phosphate, his group monitored the incorporations, utilisation and subsequent conversions of these lipids into downstream metabolic products. Interestingly, it was reported that none of these radioactive precursors were converted into other lipids, and it was postulated that Giardia has little or no ability to synthesize lipids de novo. It was thus suggested that Giardia obtains most of its phospholipids and fatty acids from bovine serum and bile supplemented to the growth medium or present in dietary lipids which are abundant in the human small intestine (Farthing et al. 1985; Gillin et al. 1986). This proposal was further supported by Kaneda and Goutsu (1988) and Mohareb et al. (1991), who showed that the lipid composition in Giardia is similar to that of the growth medium. Thin-layer chromatographic analyses revealed that four phospholipids — i.e., PC, PE, SM, and PG are present in both encysting and non-encysting cells and remain unaltered throughout the process of encystation (Ellis et al. 1996).
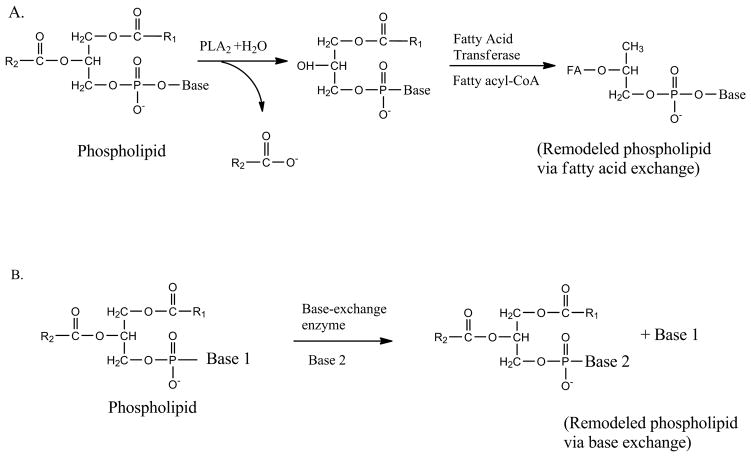
Recently, we carried out detailed analyses of phospholipids in Giardia with the help of electrospray ionization quadrupole time-of-flight mass spectrometry (ESI-qTOF-MS) (Yichoy et al. 2009). The results indicated that PCs and PGs are the major phospholipids in this parasite. Analyses in negative-ion mode revealed that at least 17 different species of PGs are present with various combinations of odd- and even-numbered, carbon-containing fatty acids. Quantitative analyses further elucidated that two PG species containing C18:1/C16:0 and C18:1/C16:0 were most abundant, followed by C16:0/C16:0 and/or C18:0/C14:0. Although we detected more species of PCs (19 in positive-ion mode and 6 in negative-ion mode), only one of them (C18:1/C18:1) was abundant. In addition to the PGs and PCs, 6 species of PEs, 3 species of SMs and 2 species of PIs were also detected (Yichoy et al. 2009). Interestingly, except for lyso-PCs and PCs, no other phospholipids are present in bile and serum, suggesting that many of these phospholipids (specifically PG and PE) in Giardia could be synthesized de novo via CDP-DAG and/or fatty acid and head-group remodelling pathways (Das et al. 2001). This proposal can be further supported by the finding that radiolabelled fatty acids are directly incorporated into membrane phospholipids (Blair et al. 1987; Stevens et al. 1997; Gibson et al. 1999; Vargas-Villarreal et al. 2007), indicating that Giardia has the cellular machineries to synthesize new phospholipids. Radiolabelled bases (i.e., choline, inositol, ethanolamine, serine and glycerol) are also incorporated into respective phospholipids of trophozoites when added to the culture medium (Subramanian et al. 2000; Das et al. unpublished). A schematic diagram of the synthesis of new phospholipids by fatty acid and headgroup exchange reactions (Das et al. 2001; Das et al. 2002) is shown in Fig. 3. In the future, it will also be interesting to investigate whether some of the phospholipids in Giardia, particularly PGs, are synthesized via the CDP-DAG de novo pathway.
Fig. 3.
Generation of new lipids by fatty acid and headgroup remodelling reactions. Panel (A) shows fatty acid remodelling by deacylation/reacylation reaction (the Lands cycle), in which phospholipase A2 and fatty acyl CoA transferase enzymes are involved. Panel B indicates the generation of new lipids by headgroup or base-exchange reactions (Das et al. 2001; Das et al. 2002).
Several studies suggest that sphingolipid (SL) metabolic pathways are critical for encystation process, and that inhibition of their syntheses blocks the production of cysts in culture (Hernandez et al. 2008; Sonda et al. 2008; Stefanic et al. 2010). Only five SL metabolic genes have been annotated in the Giardia Genomic Database (www.giardiadb.org), and they are all transcribed differentially between trophozoites and encysting cells. These genes are: (i) giardial serine-palmitoyltransferase-1 and -2 subunit genes (gspt-1 and gspt-2), (ii) glucosylceramide synthase or glucosylceramide transferase 1 (gglct-1), and (iii) two acid sphingomyelinase genes (gasmase-1 and -2). The enzymatic activities of serine-palmitoyltransferases (gSPTs) and glucosylceramide transferase1 (gGlcT1) were measured and found to be up-regulated during encystation. It was observed that gSPTs (synthesize 3-ketosphinganine—the first rate-limiting step of SL biosynthesis) regulate ceramide endocytosis, which is important because Giardia is unable to synthesize ceramide de novo (Hernandez et al. 2008). On the other hand, gGlcT1 (catalyzes the synthesis of glucosylceramide or GlcCer) is involved in encystation and cyst production by modulating the synthesis of CWPs and ESVs. Inhibition of the synthesis of GlcCer interferes with trophozoite replication and cyst formation (Hernandez et al. 2008; Sonda et al. 2008). Recently, it has been demonstrated that the inhibition of GlcCer production causes cellular abnormalities, including the formation of enlarged lysosomes, clathrin localization and cell-cycle progression before blocking the overall cyst production (Stefanic et al. 2010). Although the function of giardial SMase has yet to be elucidated, it is possible that this enzyme is involved in scavenging ceramide from SM present in the growth medium or in the milieu of the small intestine. These results indicate that ceramide and other SLs play important roles in giardial biology and differentiation.
A comprehensive analysis of fatty acids by Ellis et al. (1996) revealed that major fatty acids in Giardia were C16:0 followed by C18:0, C18:1, and C18:2. Small amounts of C14:0, C15:0, C17:0, C18:3, C19:0, C20:0, C22:0, C24:0, C26:0, and C28:0 were also detected. Interestingly, no dramatic differences were observed between the fatty acid content of non-encysting and encysting Giardia. The authors also determined the fatty acid compositions of low-bile (1% bile-containing growth medium) and high-bile (10% bile-containing encystation medium) and noticed that major fatty acids (i.e., C16:0, C18:0 and C18:1)were present in both media, although some quantitative differences were recorded (Ellis et al. 1996). A detailed analysis of fatty acids by gas chromatography-mass spectrometry (GC-MS) showed that C16:0, C18:0, and C18:1 are indeed the major fatty acids in Giardia and that they remained essentially unaltered during the transition from vegetative forms to encysting (0–48 h) and mature cysts (Yichoy et al. 2009). Traces of shorter-chain fatty acids—i.e., C10:0, C12:0, C14:0, and C15:0 — were also detected. It is interesting that C12:0 and C14:0 were found to be present in the adult bovine serum and bovine bile, the major sources of lipids in giardial growth and encystation medium (Yichoy et al. 2009).
Our results (Yichoy et al., 2009) and those of Ellis et al. (1996) further supported the proposal that very long-chain fatty acids (i.e., C20:0, C20:1, C21:0, C22:0, C23:0, C24:0, and C24:1) that are present in Giardia are not taken up from bile and serum and thus could be generated by the action of fatty acid elongase activity, because a similarity (BLAST) search of the giardial genome predicted the presence of fatty-acid elongase 1 gene (gelo) (Yichoy et al. 2009). Free and esterified cholesterols were found to be the major neutral lipids in non-encysting and encysting stages. In addition, the presence of cholesterylesters and small quantities of ergosterol and glycerides were reported (Ellis et al. 1996). However, the GC-MS analyses (Yichoy et al. 2009) suggest that cholesterol is the only sterol present in trophozoites, encysting cells and cysts, and that it is obtained directly from the growth medium.
LIPID SYNTHESIS AND METABOLIC GENES
Giardia is polyploid, and its genome is very much like the eukaryotic genome that includes linear chromosomes flanked by telomere sequences (TAGGG). The five chromosomes, ranging in size from 1.6 to 3.8 Mb, are constituted of 1.34 × 108 bp, which predicts up to 8–12 copies of each chromosome compared with the haploid genome (Yu et al. 2002). The trophozoite stage of the parasite has two morphologically indistinguishable nuclei that both replicate at approximately the same time and that are transcriptionally active. Each nucleus contains approximately the same copy numbers of ribosomal RNA genes on a single chromosome (chromosome 1), which indicates that this chromosome is present in both nuclei and contains the same complement of DNA (Adam, 2001). To understand the biology of the organism as well as to identify new drug targets, the Giardia Genome Project was initiated (www.GiardiaDB.org) in 1998 by Mitchell Sogin and his colleagues at the Marine Biological Laboratory, Woods Hole, MA, with the support from the National Institutes of Health, USA (McArthur et al. 2000; Morrison et al. 2007). This genome project (Morrison et al. 2007) has assisted in identifying several putative homologues of lipid synthesis and metabolic genes in assemblages A (isolate WB), B (isolate GS) and C (isolate P 15) of Giardia. Table 1 demonstrates the classes of phospholipid syntheses/metabolic genes that were annotated in GenBank (http://www.ncbi.nlm.nih.gov/genbank/). These classes represent the putative genes encoding phosphatidylinositol synthase (PIS), phosphatidylglycerolphosphate synthase (PGPS), phosphatidylserine synthase (PSS) and decarboxylase (PSD). The presence of these genes in the database, together with our earlier lipidomic study that PG, PE, and PI are not obtained from the growth medium (Yichoy et al. 2009), supports the hypothesis that Giardia has the ability to synthesize selective phospholipids de novo. We have observed that Giardia has a strong PSD activity and that it converts [14C]-PS to [14C]-PE instantly (Das et al. unpublished). It is likely that Giardia utilises the product of the gpss gene to synthesize PS from PC and PE, respectively. In mammalian cells, two pss genes are present—pss-1 and pss-2. While pss-1 facilitates the formation of PS from PC, pss-2 converts PE to PS (Kent 1995; Dowhan 1997). Because Giardia is considered an early diverging eukaryote (Sogin et al. 1989), its pss gene may encode an enzyme that functions both as gPSS1 and gPSS2. Similarly, PG may be synthesized from CDP-DAG in a reaction catalyzed by PGPS, encoded by the gpgps gene expressed throughout its life cycle (Yichoy et al. 2009).
Table-1.
Lipids and Fatty Acid Metabolic Genes Annotated in GS, WB and P15 Isolates in Giardia
| Classification | Homologues | NCBI Accession Number | GiardiaDB ORF | ||
|---|---|---|---|---|---|
| 50803 (WB) | 50581 (GS) | 50803 (WB | P15 | ||
| Phospholipid | PI Synthase/CDP-DAG-inositol 3-phosphatidyltransferase (gPIS) | XP_001707169 | 831 | 9829 | 1650 |
| PI transfer protein alpha isoform (PITPα) | XP_001705528 | 3968 | 4197 | 2564 | |
| PGP synthase/CDP-DAG-glycerol-3-phosphate-3- phosphatidyltransferase (gPGPS) | XP_001707005 | 4006 | 7529 | 1650 | |
| PS decarboxylase (gPSD) | XP_001707910 | 1294 | 16495 | 5211 | |
| PS synthase (gPSS) | XP_001707737 | 928 | 17427 | 660 | |
| Phospholipid-transporting ATPase IA, putative (gPLTATPase 1A) | XP_001704967, XP_001710085 | 177, 3570 | 8182, 16958, 10019 | 2350, 2846, 1870 | |
| Phospholipid-transporting ATPase IIB, putative (gPLTATPase IIB) | XP_001704293, XP_001707954 | 900, 2201 | 137725, 101810, 38104 | 2493, 1733 | |
| Fatty Acid | 1-acyl-sn-glycerol-3-phosphate acyl transferase (gAGPAT4) | XP_001704656 | 7126 | ||
| gAGPAT3 | XP_001704595 | 2692 | |||
| gAGPAT2 | XP_001707326 | 12109 | |||
| Lysophosphatidic acid acyltransferase, putative (gLPAAT) | XP_001707002 | 4004 | 14403 | 1652 | |
| Fatty acid elongase 1 (gFAELO) | XP_001708101 | 2228 | 92729 | 536 | |
| Long chain fatty acid CoA ligase 5 (gLCFACL5) | XP_001705891, XP_001705009, XP_001706424, XP_001707853 | 2104, 2579, 2829, 3493 | 9062, 2118, 15063, 17170 | 2620, 2964, 109, 5157 | |
| Long chain fatty acid CoA ligase 4 (gLCFACL5) | XP_001708520 | 3754 | 30476 | 1330 | |
| Long chain fatty acid CoA ligase, putative (gLCFACL) | XP_001709411 | 113892 | 4443 | ||
| Acetyl-CoA carboxylase (gACC)/pyruvate carboxylase fusion protein, putative | XP_001705655 | 1829 | 113021 | 184 | |
| Sterol | Lecithin-cholesterol acyl transferase, putative (gLCCAT) | XP_001705338, XP_001706263 | 1681, 4525 | 5746, 16286 | 297, 1123 |
| Neutral Lipid | Phospholipase B (gPLB) | XP_001704922, XP_001709220 | 128, 380 | 93548, 17277 | 2398, 711, 1744 |
| Sphingolipids | Ceramide glucosyltransferase (gGlcT1) | XP_001704299 | 2206 | 11642 | 1728 |
| Acid sphingomyelinase-like phosphodiesterase 3b (gASMase) | XP_001709364, XP_001705202 | 370, 4397 | 16737, 8360 | 757, 809 | |
| Serine palmitoyltransferase-1(gSPT1) | XP_001707207 | 798 | 23015 | 2116 | |
| Serine palmitoyltransferase-2 (gSPT2) | XP_001704960 | 1960 | 14374 | 1863 | |
| Signaling Lipids | Phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase (gPI3Pase) | XP_001709198 | 1218 | 16728 | 1550 |
| Type II inositol-1,4,5-trisphosphate 5-phosphatase precursor (gITP5Pase) | XP_001705945 | 3898 | 14787 | 3004 | |
| Inositol 5-phosphatase 4 (gI5Pase) | XP_001709238 | 146 | 9077 | 363 | |
| Phosphoinositide-3-kinase, class 3 (gPI3K) | XP_001708644 | 2073 | 17406 | 3357 | |
| PI-3-kinase, catalytic, alpha polypeptide (gPI3Kα) | XP_001709235 | 144 | 14855 | 361 | |
| Phosphatidylinositol-4-phosphate 5-kinase, putative (gPI4P5K) | XP_001709292, XP_001705538 XP_001707008, XP_001705604, XP_001709854, XP_001710017 | 649, 950, 1909, 3977, 4008, 4088 | 14628, 24712, 7261, 2622, 13606, 11897 | 2573, 606, 2900, 1648, 4395, 2048 | |
| PI-4-kinase (gPI4K) | XP_001706660 | 1085 | 16558 | 1075 | |
| Phosphatidylinositol-glycan biosynthesis, class O protein (gPIG) | XP_001709629 | 1892 | 14975 | 2782 | |
The genome database (Morrison et al. 2007) also suggests the presence of several classes of phospholipid-transport (PLT) ATPases or flippases (FLIPs) that allow the parasite to internalize phospholipids (particularly amino-phospholipids, which include PC and PE) from the environment in the small intestine. As the database suggests, there are several flippase genes in the WB, GS and P15 isolates of Giardia (Table 1). In an unpublished observation, we found that all of these flippase genes in the WB isolate are active and expressed differentially in trophozoites and encysting stages of the parasite’s life cycle (K. Y. Aguilera and S. Das, unpublished). Although presently the reason for the existence of so many flippases is not known, it can be presumed that Giardia has evolved an efficient mechanism to internalize amino-phospholipids, particularly PC, from the intestinal environment.
Several phosphatidylinositoI kinase (PIK) - and phosphatidylinositol phosphatase (PIP)-related lipid signalling genes were also annotated in the genome database for Giardia (Table 1), and many of them were shown to be involved in regulating the growth and encystation. An example is the giardial target of rapamycin (TOR), which is an analogue of the FAKB-rapamycin associated protein (FRAP)/TOR of eukaryotes expressed in dividing parasites and is not inhibited by rapamycin (Morrison et al. 2007). The bioinformatic analyses of three giardial PIKs genes (gpiks) [two gpi3ks (gpi3k-1 and gpi3k-2), and one gpi4k] were also carried out (Cox et al. 2006; Hernandez et al. 2007b). The analyses revealed that giardial PI3Ks, unlike higher eukaryotes, contain only catalytic (p110) but not regulatory subunits (p85) (Hernandez et al. 2007b). Transcriptional analyses demonstrated that gpiks are expressed in Giardia and are differentially regulated during encystation. In addition, two PI3K inhibitors, wortmannin and LY 294002, have been shown to inhibit the replication of trophozoites in culture, supporting the notion that the activities of PIKs could be linked to the growth and encystation of Giardia (Cox et al. 2006; Hernandez et al. 2007b). Thus, signal-transducing phospholipid molecules are synthesized in Giardia and participate in cell growth and differentiation.
As shown in Table 1, only five SL metabolic genes have been annotated in the Giardia genomic database, including the genes that encode serine-palmitoyltransferases 1 & 2 (gspt-1 and -2)—glucosylceramide transferase or GlcT-1 (gglct-1), and two separate acid sphingomyelinase enzymes (gasmases). All five genes are reported to be expressed differentially between the two different stages of the life cycle of Giardia, suggesting that SL pathways could be involved in modulating the growth and differentiation of this waterborne pathogen (Hernandez et al. 2008).
With regard to fatty acids (FAs), genomic information for Giardia infers the presence of 9 fatty-acid transport, synthesis and metabolic genes (Table 1). Three 1-acyl-sn-glycerol-3-phosphate acyltransferases (AGPATs) have been annotated, suggesting that Giardia might use the products of these genes to import fatty acids from its surrounding environment. Additional FA genes annotated are putative lysophosphatidic acid acyltransferase (glaat), elongase 1 (gelo), several long-chain fatty-acid (LCFA)-CoA ligases—LCFA-CoA ligase (glcfal), LCFA-CoA ligase 4 (glcfal4), and three different forms of LCFA-CoA ligase 5 (glcfal5)—and acetyl-CoA/pyruvate carboxylase (gacpc). The presence of these FA genes further indicates that a very basic but essential FA metabolism is present in Giardia, which is linked to transferring fatty acids across the membranes, forming reactive fatty-acid species (fatty acyl-CoA), acylating lysophosphatidic acid (LPA) to form phosphatidic acid (PA) and elongating and ligating fatty-acid chains (Table 1). Giardia contains two isoforms of secreted and cytoplasmic phospholipase B enzymes (gplb) which are responsible for the simultaneous removal of Sn1 and Sn2 fatty acids from a phospholipid (Morgan et al. 2004).
PROPOSED PATHWAY AND FUTURE PERSPECTIVES
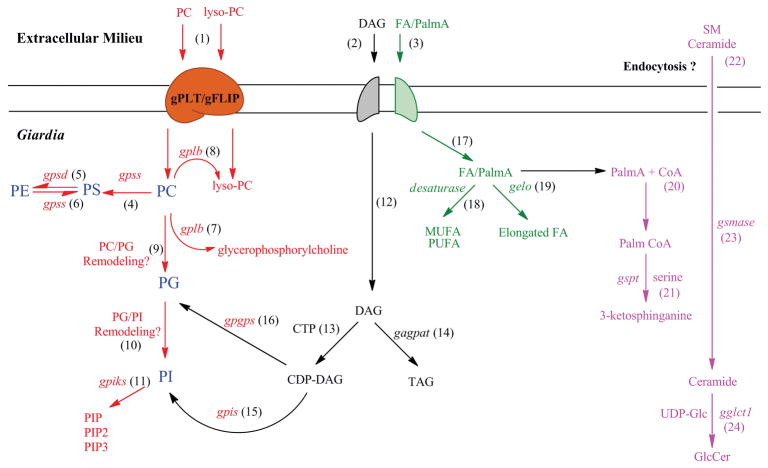
Based on biochemical, cell biology, and genomic information, we have inferred a comprehensive pathway describing the synthesis and metabolism of phospholipids, neutral lipids FAs and SLs in Giardia (Fig. 4). The model reveals that a vibrant and metabolically active trophozoite synthesizes putative gPLTs (or gFLIPs) that allow the parasite to import PC and lyso-PC from the external environment by facilitated diffusion and to convert these molecules into various downstream lipids. For example, PC can be converted to PS by the enzyme encoded by gpss. Table 1 also indicates that both gpsd and gpss are present in Giardia, and that the parasite has the ability to synthesize PS from PE and PE from PS by base-exchange reactions. PG is synthesized de novo, as proposed earlier (Yichoy et al. 2009), and PC may serve as a major precursor. It is likely that a novel PC-to-PG remodelling enzyme may exist and that the parasite uses this enzyme to synthesize PG directly from PC. Nevertheless, such an enzyme has yet to be identified and characterized. The gene gpgps (encoding PGPS) was identified and shown to express in non-encysting and encysting cells (Yichoy et al. 2009). However, it is not known whether this gene participates in the synthesis of new PG via the CDP-DAG (de novo) pathway.
Fig. 4.
Proposed lipid metabolic pathways in Giardia. The model proposes that PC and lyso-PC, which are abundant in the growth medium, can be taken up by Giardia with the help of gPLT or gFLIP (step 1). Diacylglycerol (DAG) and FA are internalised by specific transporter(s) from the growth medium (steps 2 & 3). Internalised PC can be converted to PS with the help of gPSS1-like enzyme encoded by putative gpss gene (step 4). Giardia expresses psd gene (Yichoy et al. 2009), and its possible encoded product (gPSD) may facilitate PE synthesis from PS (step 5). The putative gpss can also encode gPSS2-like enzyme for the synthesis of PS from PE (step 6). Because PG is the major phospholipid in Giardia and is not present in the growth media (Yichoy et al. 2009), it is likely that Giardia has the ability to synthesize PG not only by CDP-DAG pathway (step 16) but also by the headgroup remodelling reaction shown in step 9. Similarly, PI is synthesized from PC by base or headgroup exchange reactions from PG (step 10). However, the presence of these two pathways—i.e., PC -> PG and PG -> PI—is yet to be elucidated in Giardia or other eukaryotic cells. PI can be converted to various phosphinositides facilitated by gpiks (step 11) as mentioned before (Cox et al. 2006; Hernandez et al. 2007b). Giardial plb and lpl gene may be responsible for synthesizing glycerophosphorylcholine from PC and lyso-PC, respectively (steps 7 & 8). Diacylglycerol (DAG) obtained from the growth medium can be converted to CDP-DAG (step 13) and serves as a precursor for TAG (step 14), PG (step 15), and PI (step 16). Exogenous FAs can produce unsaturated FAs (MUFA and PUFA) and elongated FAs as depicted in steps 18 & 19. Exogenous PalmA can be used as a precursor to synthesize 3-ketosphinganine with the help of gspts (steps 20–21). Similarly, both ceramide and SM can be acquired from the growth medium (step 22) by endocytic and non-endocytic pathways. Exogenously obtained SM can be hydrolyzed by gsmases to produce ceramide (step 23), and ceramide can be used to synthesize GlcCer (step 24). PC, phosphatidylcholine; lyso-PC, lyso-phosphatidylcholine; PS, phosphatidylserine; PE, phosphatidylethanolamine; PIP, phosphatidylinositol phosphate; PIP2, phosphatidylinositol bisphosphate; PIP3, phosphatidylinositol triphosphate; DAG, diacylglycerol; TAG, triacylglycerol; CTP, cytidine triphosphate; FA, fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SM, sphingomyelin; GlcCer, glucosylceramide. For better understanding, phospholipid pathways are shown in red, neutral lipids (DAG and TAG) in black, FAs in green, and SLs in pink.
As shown in Table 1, and also reported earlier (Morgan et al. 2004), Giardia has the genes that encode phospholipase B (PLB). The hydrolysis of Sn1 and Sn2 FAs from PC by PLB produces lyso-PC and soluble glycerophosphorylcholine. The presence of lyso-phosphatidic acid acyltransferase (LPAAT) gene (glpaat) in the genomic database suggests that this parasite also has the ability to convert lyso-PA to PA.
The pathway also proposes that most FAs can be taken up by simple and facilitated diffusion (Gibson et al. 1999). Once internalized, FAs undergo elongation and/or desaturation reactions. The presence of a giardial FA desaturase was reported earlier by Ellis et al. (1996); the gene (gelo) that is likely to encode elongase was annotated in the database (Table 1). Diacylglycerol (DAG) or other neutral lipids present in the medium (Yichoy et al. 2009) can also be obtained by membrane diffusion and/or via transport proteins. Intracellular DAG can form triacylglycerol (TAG) by agpat gene products, and diacylglycerol (DAG) can be activated by cytidine diphosphate (CDP) to produce CDP-DAG, which then can be used as a precursor to synthesize PI. Newly synthesized PI can be utilized to generate PIP, PIP2, and PIP3 by giardial PIKs for cellular signalling (Cox et al. 2006; Hernandez et al. 2007b).
As mentioned, Giardia expresses gspt, gglct1, and gsmase genes, indicating a limited SL synthesis/metabolic pathway. It is possible that PalmA obtained from the growth medium is converted to Palm-CoA by acyl-CoA ligase and then is used by the parasite to synthesize 3-ketosphinganine by the action of serine-palmitoyltransferase enzymes encoded by gspts. We have proposed earlier (Hernandez et al., 2008) that 3-ketosphinganine regulates ceramide uptake in Giardia by controlling its endocytic machinery, and this is important because ceramide is not synthesized by this parasite de novo. The newly acquired ceramide is then used by Giardia as precursors to synthesize GlcCer by glucosylceramide synthase (encoded by gglct1) which may serve as a key regulator of encystation and cyst production (Hernandez et al. 2008). Taken together, it has been proposed that ceramide uptake and GlcCer synthesis is important for the encystation of Giardia (Hernandez et al. 2008; Sonda et al. 2008; Stefanic et al. 2010).
We foresee that the current review not only contributes to our understanding of the lipid pathways in Giardia but also should assist researchers in identifying unique targets for developing effective therapies in the future. One of these targets might be the enzymes of PG biosynthesis, because PG appears to be the major phospholipid in Giardia (Gibson et al. 1999; Yichoy et al. 2009). Lipid transport and lipid-based cell signalling could be another important area for future investigation. As mentioned above, Giardia has evolved mechanisms to import exogenous lipids and cholesterol by receptor-mediated endocytosis (Lujan et al. 1994) and traffic via clathrin-mediated and actin/microtubule-dependent pathways (Hernandez et al. 2007a). Therefore, the identification of lipoprotein-like receptors and the study of lipid transport vesicles in Giardia should open a new research area that might lead to the discovery of unique pathways and mechanisms of lipid sorting and targeting. At present, it is not fully understood how extracellular signals regulate the growth and differentiation of Giardia. Future investigation may suggest that PI3K-based signalling is associated with this phenomenon and drives the process of encystation and excystation. Finally, it would also be fascinating to investigate whether giardial lipids and lipid metabolic enzymes are involved in host-parasite interactions.
Acknowledgments
The authors were supported by the National Institutes of Health (S.D., grant no. S06GM008012). Mass spectrometric analyses of lipids were carried out (in collaboration with Professor Igor C. Almeida) in the Biomolecule Analysis Core Facility at BBRC/UTEP supported by the NIH/NCRR/RCMI (grant no. 5G12RR008124). Dr Mayte Yichoy was supported in part by the Dodson Dissertation Fellowship from UT-El Paso. Mr. Trevor T. Duarte, Mr. Tavis L. Mendez and Ms. Kristina Y. Aguilera were supported by the RISE and MARC (NIH) programs, respectively. Mr. Debarshi Roy was supported by a grant from the Howard Hughes Medical Institute.
References
- Adam RD. Biology of Giardia lamblia. Clinical Microbiology Reviews. 2001;14:447–475. doi: 10.1128/CMR.14.3.447-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajer A. Cryptosporidium and Giardia spp. infections in humans, animals and the environment in Poland. Parasitology Research. 2008;104:1–17. doi: 10.1007/s00436-008-1179-x. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Weller PF. Uptake and esterification of arachidonic acid by trophozoites of Giardia lamblia. Molecular and Biochemical Parasitology. 1987;25:11–1. doi: 10.1016/0166-6851(87)90013-2. [DOI] [PubMed] [Google Scholar]
- Castillo C, Hernandez Y, Roychowdhury S, Das S. Cytoskeleton-based lipid transport in a parasitic protozoan, Giardia lamblia. In: Ortega-Pierres G, Caccio S, Fayer R, Mank T, Smith H, Thompson RCA, editors. Giardia and Cryptosporidium: From Molecules to Disease. CABI International; UK: 2009. pp. 292–307. [Google Scholar]
- Chen XM, Splinter PL, Tietz PS, Huang BQ, Billadeau DD, Larusso NF. Phosphatidylinositol 3-kinase and frabin mediate Cryptosporidium parvum cellular invasion via activation of Cdc42. Journal of Biological Chemistry. 2004;279:31671–31678. doi: 10.1074/jbc.M401592200. [DOI] [PubMed] [Google Scholar]
- Correa G, Morgado-Diaz JA, Benchimol M. Centrin in Giardia lamblia - ultrastructural localization. FEMS Microbiology Letters. 2004;233:91–96. doi: 10.1016/j.femsle.2004.01.043. [DOI] [PubMed] [Google Scholar]
- Cox SS, Van Der Giezen M, Tarr SJ, Crompton MR, Tovar J. Evidence from bioinformatics, expression and inhibition studies of phosphoinositide-3 kinase signaling in Giardia intestinalis. BMC Microbiology. 2006;6:45. doi: 10.1186/1471-2180-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley R, Marshall J, Clark JT, Holberton DV. Immunocytochemical differentiation of microtubules in the cytoskeleton of Giardia lamblia using monoclonal antibodies to alpha-tubulin and polyclonal antibodies to associated low molecular weight proteins. Journal of Cell Science. 1986;80:233–252. doi: 10.1242/jcs.80.1.233. [DOI] [PubMed] [Google Scholar]
- Das S, Reiner DS, Zenian J, Hogan DL, Koss MA, Wang CS, Gillin FD. Killing of Giardia lamblia trophozoites by human intestinal fluid in vitro. Journal of Infectious Diseases. 1988;157:1257–1260. doi: 10.1093/infdis/157.6.1257. [DOI] [PubMed] [Google Scholar]
- Das S, Traynor-Kaplan A, Reiner DS, Meng TC, Gillin FD. A surface antigen of Giardia lamblia with a glycosylphosphatidylinositol anchor. Journal of Biological Chemistry. 1991;266:21318–21325. [PubMed] [Google Scholar]
- Das S, Gillin FD. Giardia lamblia: increased UDP-N-acetyl-D-glucosamine and N-acetyl-D-galactosamine transferase activities during encystation. Experimental Parasitology. 1996;83:19–29. doi: 10.1006/expr.1996.0045. [DOI] [PubMed] [Google Scholar]
- Das S, Schteingart CD, Hofmann AF, Reiner DS, Aley SB, Gillin FD. Giardia lamblia: evidence for carrier-mediated uptake and release of conjugated bile acids. Experimental Parasitology. 1997;87:133–141. doi: 10.1006/expr.1997.4197. [DOI] [PubMed] [Google Scholar]
- Das S, Castillo C, Stevens TL. Phospholipid remodeling/generation in Giardia: the role of the Lands cycle. Trends in Parasitology. 2001;17:316–319. doi: 10.1016/s1471-4922(01)01901-8. [DOI] [PubMed] [Google Scholar]
- Das S, Stevens TL, Castillo C, Villasenor A, Arredondo H, Reddy K. Lipid metabolism in mucous-dwelling amitochondriate protozoa. International Journal for Parasitology. 2002;32:655–675. doi: 10.1016/s0020-7519(02)00006-1. [DOI] [PubMed] [Google Scholar]
- Davids BJ, Reiner DS, Birkeland SR, Preheim SP, Cipriano MJ, McArthur AG, Gillin FD. A new family of giardial cysteine-rich non-VSP protein genes and a novel cyst protein. PLoS One. 2006;1:e44. doi: 10.1371/journal.pone.0000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids BJ, Williams S, Lauwaet T, Palanca T, Gillin FD. Giardia lamblia aurora kinase: a regulator of mitosis in a binucleate parasite. International Journal of Parasitology. 2008;38:353–369. doi: 10.1016/j.ijpara.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Dowhan W. Molecular basis for membrane phospholipid diversity: why are there so many lipids? Annual Review of Biochemistry. 1997;66:199–232. doi: 10.1146/annurev.biochem.66.1.199. [DOI] [PubMed] [Google Scholar]
- Ellis JE, Wyder MA, Jarroll EL, Kaneshiro ES. Changes in lipid composition during in vitro encystation and fatty acid desaturase activity of Giardia lamblia. Molecular and Biochemical Parasitology. 1996;81:13–25. doi: 10.1016/0166-6851(96)02677-1. [DOI] [PubMed] [Google Scholar]
- Elmendorf HG, Dawson SC, Mccaffery JM. The cytoskeleton of Giardia lamblia. International Journal for Parasitology. 2003;33:3–28. doi: 10.1016/s0020-7519(02)00228-x. [DOI] [PubMed] [Google Scholar]
- Farthing MJ, Keusch GT, Carey MC. Effects of bile and bile salts on growth and membrane lipid uptake by Giardia lamblia. Possible implications for pathogenesis of intestinal disease. Journal of Clinical Investigation. 1985;76:1727–1732. doi: 10.1172/JCI112162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubert G. Immune response to Giardia duodenalis. Clinical Microbiology Reviews. 2000;13:35–54. doi: 10.1128/cmr.13.1.35-54.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Frisardi M, Rogers R, Samuelson J. How Giardia swim and divide. Infection and Immunity. 2001;69:7866–7872. doi: 10.1128/IAI.69.12.7866-7872.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GR, Ramirez D, Maier J, Castillo C, Das S. Giardia lamblia: incorporation of free and conjugated fatty acids into glycerol-based phospholipids. Experimental Parasitology. 1999;92:1–11. doi: 10.1006/expr.1999.4389. [DOI] [PubMed] [Google Scholar]
- Gillin FD, Gault MJ, Hofmann AF, Gurantz D, Sauch JF. Biliary lipids support serum-free growth of Giardia lamblia. Infection and Immunity. 1986;53:641–645. doi: 10.1128/iai.53.3.641-645.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin FD, Reiner DS, Gault MJ, Douglas H, Das S, Wunderlich A, Sauch JF. Encystation and expression of cyst antigens by Giardia lamblia in vitro. Science. 1987;235:1040–1043. doi: 10.1126/science.3547646. [DOI] [PubMed] [Google Scholar]
- Gillin FD, Reiner DS, Boucher SE. Small-intestinal factors promote encystation of Giardia lamblia in vitro. Infection and Immunity. 1988;56:705–707. doi: 10.1128/iai.56.3.705-707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangaspero A, Paoletti B, Iorio R, Traversa D. Prevalence and molecular characterization of Giardia duodenalis from sheep in central Italy. Parasitology Research. 2005;96:32–37. doi: 10.1007/s00436-005-1317-7. [DOI] [PubMed] [Google Scholar]
- Hassan SM, Maache M, De La Guardia RD, Cordova OM, Garcia JR, Galiana M, Castroviejo DA, Martins M, Osuna A. Binding properties and immunolocalization of a fatty acid-binding protein in Giardia lamblia. Journal of Parasitology. 2005;91:284–292. doi: 10.1645/GE-3352. [DOI] [PubMed] [Google Scholar]
- Hernandez PC, Wasserman M. Do genes from the cholesterol synthesis pathway exist and express in Giardia intestinalis? Parasitology Research. 2006;98:194–199. doi: 10.1007/s00436-005-0039-1. [DOI] [PubMed] [Google Scholar]
- Hernandez Y, Castillo C, Roychowdhury S, Hehl A, Aley SB, Das S. Clathrin-dependent pathways and the cytoskeleton network are involved in ceramide endocytosis by a parasitic protozoanGiardia lamblia. International. 2007a doi: 10.1016/j.ijpara.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez Y, Zamora G, Ray S, Chapoy J, Chavez E, Valvarde R, Williams E, Aley SB, Das S. Transcriptional analysis of three major putative phosphatidylinositol kinase genes in a parasitic protozoan, Giardia lamblia. Journal of Eukaryotic Microbiology 54, 29–32. Journal for Parasitology. 2007b;37:21–32. doi: 10.1111/j.1550-7408.2006.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez Y, Shpak M, Duarte TT, Mendez TL, Maldonado RA, Roychowdhury S, Rodrigues ML, Das S. Novel role of sphingolipid synthesis genes in regulating giardial encystation. Infection and Immunity. 2008;76:2939–2949. doi: 10.1128/IAI.00116-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holberton DV. Fine structure of the ventral disk apparatus and the mechanism of attachment in the flagellate Giardia muris. Journal of Cell Science. 1973;13:11–41. doi: 10.1242/jcs.13.1.11. [DOI] [PubMed] [Google Scholar]
- Hunter PR, Thompson RC. The zoonotic transmission of Giardia and Cryptosporidium. International Journal for Parasitology. 2005;35:1181–1190. doi: 10.1016/j.ijpara.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Iwabe N, Miyata T. Kinesin-related genes from diplomonad, sponge, amphioxus, and cyclostomes: divergence pattern of kinesin family and evolution of giardial membrane-bounded organella. Molecular Biology and Evolution. 2002;19:1524–1533. doi: 10.1093/oxfordjournals.molbev.a004215. [DOI] [PubMed] [Google Scholar]
- Jarroll EL, Muller PJ, Meyer EA, Morse SA. Lipid and carbohydrate metabolism in Giardia lamblia. Molecular and Biochemical Parasitology. 1981;2:187–196. doi: 10.1016/0166-6851(81)90099-2. [DOI] [PubMed] [Google Scholar]
- Kamda JD, Singer SM. Phosphoinositide 3-kinase-dependent inhibition of dendritic cell interleukin-12 production by Giardia lamblia. Infection and Immunity. 2009;77:685–693. doi: 10.1128/IAI.00718-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane A, Ward HD, Keusch GT, Pereira MEA. In vitro encystation of Giardia lamblia: large-scale production of in vitro cysts and strain and clone differences in encystation efficiency. Journal of Parasitology. 1991;77:974–981. [PubMed] [Google Scholar]
- Kaneda Y, Goutsu T. Lipid analysis of Giardia lamblia and its culture medium. Annals of Tropical Medicine and Parasitology. 1988;82:83–90. doi: 10.1080/00034983.1988.11812213. [DOI] [PubMed] [Google Scholar]
- Kent C. Eukaryotic phospholipid biosynthesis. Annual Review of Biochemistry. 1995;64:315–343. doi: 10.1146/annurev.bi.64.070195.001531. [DOI] [PubMed] [Google Scholar]
- Lujan HD, Byrd LG, Mowatt MR, Nash TE. Serum Cohn fraction IV-1 supports the growth of Giardia lamblia in vitro. Infection and Immunity. 1994;62:4664–4666. doi: 10.1128/iai.62.10.4664-4666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan HD, Mowatt MR, Byrd LG, Nash TE. Cholesterol starvation induces differentiation of the intestinal parasite Giardia lamblia. Proceedings of the National Academy of Science. 1996;93:7628–7633. doi: 10.1073/pnas.93.15.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan HD, Mowatt MR, Chen GZ, Nash TE. Isoprenylation of proteins in the protozoan Giardia lamblia. Molecular and Biochemical Parasitology. 1995;72:121–127. doi: 10.1016/0166-6851(94)00070-4. [DOI] [PubMed] [Google Scholar]
- Lauwaet T, Davids BJ, Reiner DS, Gillin FD. Encystation of Giardia lamblia: a model for other parasites. Current Opinion in Microbiology. 2007;10:554–559. doi: 10.1016/j.mib.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur AG, Morrison HG, Nixon JEJ, Passamaneck NQE, Kim U, Hinkle G, Crocker MK, Holder ME, Farr R, Reich CI, Olsen GE, Aley SB, Adam RD, Gillin FD, Sogin ML. The Giardia genome project database. FEMS Microbiology Letters. 2000;189:271–273. doi: 10.1111/j.1574-6968.2000.tb09242.x. [DOI] [PubMed] [Google Scholar]
- Mohareb EW, Rogers EJ, Weiner EJ, Bruce JI. Giardia lamblia: phospholipid analysis of human isolates. Annals of Tropical Medicine and Parasitology. 1991;85:591–597. doi: 10.1080/00034983.1991.11812614. [DOI] [PubMed] [Google Scholar]
- Monis PT, Thompson RC. Cryptosporidium and Giardia-zoonoses: fact or fiction? Infection, genetics and evolution. Journal of Molecular Epidemiology and Evolutionary Genetics in Infectious Diseases. 2003;3:233–244. doi: 10.1016/j.meegid.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Morgan CP, Insall R, Haynes L, Cockcroft S. Identification of phospholipase B from Dictyostelium discoideum reveals a new lipase family present in mammals, flies and nematodes, but not yeast. Biochemical Journal. 2004;382:441–449. doi: 10.1042/BJ20040110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison HG, Mcarthur AG, Gillin F, Aley SB, Adam RD, Olsen G, Best A, Cande WZ, Chen F, Mj C, Davids BJ, Dawson SC, Elmendorf HG, Hehl A, Holder ME, Huse S, Kim U, Lasek-Nesselquist E, Manning G, Niqam A, Nixon J, Palm D, Passamaneck NQE, Prabhu A, Reich CI, Reiner DS, Samuelson J, Svard SG, Sogin ML. Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science. 2007;317:1921–1926. doi: 10.1126/science.1143837. [DOI] [PubMed] [Google Scholar]
- Nayak N, Ganguly NK, Walia BN, Wahi V, Kanwar SS, Mahajan RC. Specific secretory IgA in the milk of Giardia lamblia-infected and uninfected women. Journal of Infectious Diseases. 1987;155:724–727. doi: 10.1093/infdis/155.4.724. [DOI] [PubMed] [Google Scholar]
- Nohynkova E, Draber P, Reischig J, Kulda J. Localization of gamma-tubulin in interphase and mitotic cells of a unicellular eukaryote, Giardia intestinalis. European Journal of Cell Biology. 2000;79:438–445. doi: 10.1078/0171-9335-00066. [DOI] [PubMed] [Google Scholar]
- Ratner DM, Cui J, Steffen M, Moore LL, Robbins PW, Samuelson J. Changes in the N-glycome, glycoproteins with Asn-linked glycans, of Giardia lamblia with differentiation from trophozoites to cysts. Eukaryotic Cell. 2008;7:1930–1940. doi: 10.1128/EC.00268-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayan P, Stenzel D, Mcdonnell PA. The effects of saturated fatty acids on Giardia duodenalis trophozoites in vitro. Parasitology Research. 2005;97:191–200. doi: 10.1007/s00436-005-1432-5. [DOI] [PubMed] [Google Scholar]
- Reiner DS, Wang CS, Gillin FD. Human milk kills Giardia lamblia by generating toxic lipolytic products. Journal of Infectious Diseases. 1986;154:825–832. doi: 10.1093/infdis/154.5.825. [DOI] [PubMed] [Google Scholar]
- Richardson DN, Simmons MP, Reddy AS. Comprehensive comparative analysis of kinesins in photosynthetic eukaryotes. BMC Genomics. 2006;7:18. doi: 10.1186/1471-2164-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener K, Shen Z, Newburg DS, Jarroll EL. Amino sugar phosphate levels in Giardia change during cyst wall formation. Microbiology. 2004;150:1225–1230. doi: 10.1099/mic.0.26898-0. [DOI] [PubMed] [Google Scholar]
- Smith A, Reacher M, Smerdon W, Adak GK, Nichols G, Chalmers RM. Outbreaks of waterborne infectious intestinal disease in England and Wales, 1992–2003. Epidemiology and Infection. 2006;134:1141–1149. doi: 10.1017/S0950268806006406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin ML, Gunderson JH, Elwood HJ, Alonso RA, Peattie DA. Phylogenetic meaning of the kingdom concept: an unusual ribosomal RNA from Giardia lamblia. Science. 1989;243:75–77. doi: 10.1126/science.2911720. [DOI] [PubMed] [Google Scholar]
- Sonda S, Stefanic S, Hehl AB. A sphingolipid inhibitor induces a cytokinesis arrest and blocks stage differentiation in Giardia lamblia. Antimicrobiological Agents and Chemotherapeutics. 2008 doi: 10.1128/AAC.01105-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanic S, Spycher C, Morf L, Fabrias G, Casas J, Schraner E, Wild P, Hehl AB, Sonda S. Inhibition of glucosylceramide synthesis affects cell cycle progression, membrane trafficking and stage differentiation in the minimized protozoan Giardia lamblia. Journal of Lipid Research. 2010 doi: 10.1194/jlr.M003392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens TL, Gibson GR, Adam RD, Maier J, Allison-Ennis M, Das S. Uptake and cellular localization of exogenous lipids by Giardia lamblia, a primitive eukaryote. Experimental parasitology. 1997;86:133–143. doi: 10.1006/expr.1997.4162. [DOI] [PubMed] [Google Scholar]
- Subramanian AB, Navarro S, Carrasco RA, Marti M, Das S. Role of exogenous inositol and phosphatidylinositol in glycosylphosphatidylinositol anchor synthesis of GP49 by Giardia lamblia. Biochimica et Biophysica Acta. 2000;1483:69–80. doi: 10.1016/s1388-1981(99)00171-7. [DOI] [PubMed] [Google Scholar]
- Thompson RCA. The Impact of Giardia on Science and Society. In: Ortega-Pierres G, Caccio S, Fayer R, Mank T, Smith H, Thompson RCA, editors. Giardia and Cryptosporidium: From Molecules to Disease. CABI Press; UK: 2009. pp. 1–11. [Google Scholar]
- Touz MC, Conrad JT, Nash TE. A novel palmitoyl acyl transferase controls surface protein palmitoylation and cytotoxicity in Giardia lamblia. Molecular Microbiology. 2005;58:999–1011. doi: 10.1111/j.1365-2958.2005.04891.x. [DOI] [PubMed] [Google Scholar]
- Vargas-Villarreal J, Escobedo-Guajardo BL, Mata-Cardenas BD, Palacios-Corona R, Cortes-Gutierrez E, Morales-Vallarta M, Sampayo-Reyes A, Said-Fernandez S. Activity of intracellular phospholipase A1 and A2 in Giardia lamblia. Journal of Parasitology. 2007;93:979–984. doi: 10.1645/GE-1038R3.1. [DOI] [PubMed] [Google Scholar]
- Worgall TS, Davis-Hayman SR, Magana MM, Oelkers PM, Zapata F, Juliano RA, Osborne TF, Nash TE, Deckelbaum RJ. Sterol and fatty acid regulatory pathways in a Giardia lamblia-derived promoter: evidence for SREBP as an ancient transcription factor. Journal of Lipid Research. 2004;45:981–988. doi: 10.1194/jlr.M400024-JLR200. [DOI] [PubMed] [Google Scholar]
- Xiao L, Fayer R. Molecular characterisation of species and genotypes of Cryptosporidium and Giardia and assessment of zoonotic transmission. International Journal for Parasitology. 2008;38:1239–1255. doi: 10.1016/j.ijpara.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Yichoy M, Nakayasu ES, Shpak M, Aguilar C, Aley SB, Almeida IC, Das S. Lipidomic Analysis Reveals That Phosphatidylglycerol and Phosphatidylethanolamine are Newly Generated Phospholipids in an Early-Divergent Protozoan, Giardia lamblia. Molecular and Biochemical Parasitology. 2009;165:67–78. doi: 10.1016/j.molbiopara.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LZ, Birky CW, Jr, Adam RD. The two nuclei of Giardia each have complete copies of the genome and are partitioned equationally at cytokinesis. Eukaryotic Cell. 2002;1:191–199. doi: 10.1128/EC.1.2.191-199.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]