Abstract
Rationale
Drug-associated cues and stress increase craving and lead to greater risk of relapse in abstinent drug users. Animal models of reinstatement of drug seeking have been utilized to study the neural circuitry by which either drug-associated cues or stress exposure elicit drug seeking. Recent evidence has shown a strong enhancing effect of yohimbine stress on subsequent cue-elicited reinstatement; however, there has been no examination of the neural substrates of this interactive effect.
Objectives
The current study examined whether inactivation of the bed nucleus of the stria terminalis (BNST), an area previously implicated in stress activation of drug seeking, would affect reinstatement of cocaine seeking caused by conditioned cues, yohimbine stress, or the combination of these factors.
Methods
Male rats experienced daily IV cocaine self-administration, followed by extinction of lever responding in the absence of cocaine-paired cues. Reinstatement of responding was measured during presentation of cocaine-paired cues, following pretreatment with the pharmacological stressor, yohimbine (2.5 mg/kg, IP), or the combination of cues and yohimbine.
Results
All three conditions led to reinstatement of cocaine seeking, with the highest responding seen after the combination of cues and yohimbine. Reversible inactivation of the BNST using the gamma-aminobutyric acid receptor agonists, baclofen+muscimol, significantly reduced all three forms of reinstatement.
Conclusion
These results demonstrate a role for the BNST in cocaine seeking elicited by cocaine-paired cues, and suggest the BNST as a key mediator for the interaction of stress and cues for the reinstatement of cocaine seeking.
Keywords: Cocaine, Yohimbine, Reinstatement, Relapse, Bed nucleus stria terminalis, Norepinephrine, Neurocircuitry, Stress, Conditioned, Self-administration
Introduction
Treatment of cocaine addiction is impeded by high rates of relapse to drug seeking and drug taking in chronic users. Relapse can be triggered by many factors, including exposure to drug-associated cues and contexts (such as drug paraphernalia or drug-associated environments) or stressful life events that occur during periods of abstinence. Clinical laboratory studies have shown that cocaine-associated stimuli increase craving in abstinent users (Childress et al. 1993), as does exposure to stress-provoking stimuli (Sinha et al. 1999). The triggering of relapse in abstinent users has been modeled in animals using the self-administration and reinstatement paradigm. Animals trained to self-administer cocaine will reinstate responding on the previously drug-paired lever after exposure to cocaine-associated cues (See 2002), stress (Shalev et al. 2000), or a priming dose of cocaine (de Wit and Stewart 1981). The reinstatement model of relapse (Katz and Higgins 2003) has allowed for the investigation of the neural circuitry underlying cocaine-seeking behavior triggered by each of these factors. Evidence to date suggests that cues and stress share some of the same neural pathways in mediating reinstatement of cocaine seeking, most notably the nucleus accumbens core and the dorsomedial prefrontal cortex (Capriles et al. 2003; Fuchs et al. 2004a; McFarland et al. 2004; McLaughlin and See 2003). However, some structures (e.g., basolateral amygdala) have been found to be important for cue- but not footshock stress-induced reinstatement of cocaine seeking (McFarland et al. 2004; Meil and See 1997).
While almost all previous studies of the triggering events that initiate reinstatement of drug seeking have limited their focus to separate factors, we have recently demonstrated that footshock or yohimbine stress activation potentiates conditioned cue-induced reinstatement of cocaine seeking (Buffalari and See 2009a; Feltenstein and See 2006). The mechanisms by which this interaction occurs likely involve a convergence of activity in the neural pathways that reinitiate drug seeking. Among the structures that mediate drug seeking, the bed nucleus of the stria terminalis (BNST) is a key component of the extended amygdala that may be a critical point of convergence for cue and stress interactions. Studies of the BNST have indicated that it plays a key role in addictive drug actions, including cocaine (Dumont et al. 2005; Kash et al. 2008). Inactivation of the BNST with sodium channel blockers (Erb and Stewart 1999) or gamma-aminobutyric acid (GABA) receptor agonists (McFarland et al. 2004), as well as beta norepinephrine (NE) receptor antagonism (Leri et al. 2002), have been shown to block reinstatement of cocaine seeking caused by acute footshock stress. However, it is not yet known if the BNST plays a role in conditioned cue-induced reinstatement of cocaine seeking, or reinstatement caused by other stressors.
Several recent studies have successfully used systemic injections of yohimbine to trigger stress-induced reinstatement of drug seeking in rats (Banna et al. 2010; Bongiovanni and See 2008; Feltenstein and See 2006; Shepard et al. 2004) and nonhuman primates (Lee et al. 2004), and elicit drug craving in human addicts (Stine et al. 2002). Yohimbine increases NE in terminal regions via antagonism of α-2 NE receptors (Galvez et al. 1996; Tjurmina et al. 1999). However, nothing is yet known about the neural circuitry underlying reinstatement caused by systemic yohimbine, or any other drugs that may act as stressors. Both intermittent footshock (Galvez et al. 1996) and yohimbine (Forray et al. 1997) increase NE in the amygdala, including the extended amygdala and BNST, and NE receptor blockade in the BNST disrupts cocaine seeking caused by footshock stress (Leri et al. 2002), supporting the possibility that stress activation via yohimbine may rely on intact BNST function.
Therefore, in the current study, we examined the role of the BNST in reinstatement of cocaine seeking caused by yohimbine-induced stress alone, conditioned cues alone, or a combination of yohimbine and cues in rats with a history of chronic cocaine self-administration and extinction. Based on the reported role of the BNST in both stress activation and drug seeking, we predicted that the BNST would be critical for mediating stress-induced reinstatement via yohimbine, as well as the enhancing effects of yohimbine on conditioned cue-induced reinstatement of cocaine seeking.
Materials and methods
Subjects
Male Sprague–Dawley rats (initial weight 275–300 g; Charles River, Wilmington, MA, USA) were individually housed in a temperature- and humidity-controlled vivarium on a reverse 12-h light–dark cycle (lights on 6 PM–6 AM). Animals received water and standard rat chow (Harlan, Indianapolis, IN, USA) ad libitum, with the exception of 2–3 days of food restriction during initial cocaine self-administration (animals never received <10 g food/day). Housing and care of the rats were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) revised 1996.
Surgery
Rats received chronically indwelling catheters into the right jugular vein as previously described (Fuchs et al. 2004b). Briefly, male rats were anesthetized using a mixture of ketamine hydrochloride and xylazine (66 and 1.33 mg/kg, respectively, IP) followed by equithesin (0.5 ml/kg, IP). Catheters (constructed using previously described methods; Fuchs et al. 2004b) were inserted into the right jugular vein. To maintain patency, catheters were flushed with heparin and cefazolin solutions daily. Immediately following catheter surgery, animals were placed into a stereotaxic frame (Stoelting, Wood Dale, IL, USA). Bilateral stainless steel guide cannulae (26 gauge; Plastics One, Inc.) were inserted dorsal to the BNST (±3.5 M/L, −0.4 A/P, −4.6 D/V, 15° angle). Three small screws and cranioplastic cement secured the guide cannulae to the skull. Stylets (Plastics One, Inc.) were placed into the guide cannulae and catheter to prevent occlusions. To verify catheter patency, rats occasionally received a 0.12-ml infusion of methohexital sodium (10.0 mg/ml IV; Eli Lilly and Co., Indianapolis, IN, USA), a short-acting barbiturate that produces a rapid loss of muscle tone when administered intravenously.
Cocaine self-administration
Rats self-administered cocaine (cocaine hydrochloride dissolved in 0.9% sterile saline; cocaine provided by the National Institute on Drug Abuse, Research Triangle Park, NC, USA) during daily 2-h sessions according to a fixed ratio-1 (FR 1) schedule of reinforcement as previously described (Feltenstein et al. 2007b; Fuchs et al. 2004b). Briefly, lever presses on the active lever resulted in cocaine infusions along with presentation of a light+tone complex and were followed by a 20-s timeout. Inactive lever presses had no consequences, but were recorded. Daily cocaine self-administration continued until each rat had obtained the self-administration criterion of 10 sessions with at least 10 infusions per session.
Extinction and reinstatement of cocaine seeking
Following chronic self-administration and before the first reinstatement test, rats underwent daily 2-h extinction sessions as previously described (Feltenstein et al. 2007a; Fuchs et al. 2004b). Once extinction criterion was reached (defined here as a minimum of seven extinction sessions with ≤15 active lever responses per session for the last two consecutive days before testing), each rat underwent six separate reinstatement tests. Prior studies have successfully utilized similar repeated reinstatement testing designs (Feltenstein and See 2006; Kippin et al. 2006). Rats experienced six total reinstatement tests examining three reinstatement factors before and after intracranial vehicle and B/M infusions. The following three reinstatement triggers were used: conditioned cues, yohimbine administration (IP), and conditioned cues+yohimbine. Each test was given twice with either vehicle or baclofen–muscimol (B/M) infusions into the BNST immediately prior to the test. Both drug treatment (vehicle or B/M) and reinstatement test type were counterbalanced. Animals that did not finish all six reinstatement tests (e.g., due to cannulae blockade) were not utilized for data analysis. Animals were extinguished to criterion between reinstatement tests (≤15 active lever responses per session for two consecutive days). Yohimbine injections (2.5 mg/kg, IP) were given 30 min prior to testing, and saline vehicle was given prior to conditioned cue reinstatement tests. The yohimbine dose was based on previous reinstatement studies in rats (Feltenstein and See 2006; Shepard et al. 2004). Conditioned cue-induced reinstatement tests involved contingent presentation of the light+tone stimulus previously associated with the active lever press during self-administration. Cue presentation was followed by a 20-s time-out, during which lever presses were recorded, but had no programmed consequences.
Intracranial infusions
For intracranial infusions, stainless steel injection cannulae (33 gauge, Plastics One) were inserted to a depth of 2 mm below the tip of the guide cannulae immediately prior to placement into the chamber. The injection cannulae were connected to 10-ml Hamilton syringes (Hamilton Co., Reno, NV, USA) mounted on an infusion pump (Harvard Apparatus, South Natick, MA, USA). A combination of B/M (1.0/0.1 mM, 0.2 ul volume) or phosphate-buffered saline vehicle (pH=7.0 for both solutions) was infused bilaterally over a 2-min time period. Dose–response analyses have shown that this concentration of B/M site-selectively attenuates cocaine-primed (McFarland and Kalivas 2001) or conditioned cue-induced reinstatement (Fuchs et al. 2004b) of cocaine seeking, and this dose of B/M has previously been used to selectively inactivate the BNST (Rogers et al. 2008). The injection cannulae were left in place for 1 min prior to and after the infusion.
To assess the effects of B/M inactivation on general motor activity, subsequent to completion of reinstatement testing, a subset of animals were tested for locomotor activity after vehicle or B/M infusions (n=10/group) into the BNST. Infusions occurred immediately before placement into clear Plexiglas chambers (22×43×33 cm). Each chamber was equipped with a Digiscan monitor (Omnitech Electronics, Columbus, OH, USA) containing a series of 16 photobeams (eight on each horizontal axis) that tabulated total distance (cm) traveled by each animal. Beam breaks were detected by a Digiscan analyzer and recorded by DigiPro software (Verson 1.4). Immediately following a 1-h testing period, animals were returned to their home cages.
Histology and data analysis
After completion of reinstatement testing, the rats were deeply anesthetized with equithesin and transcardially perfused with PBS and 10% formaldehyde solution and processed as previously described (Fuchs et al. 2004b). The most ventral point of the microinjector tips were mapped onto schematics from a rat brain atlas (Paxinos and Watson 1997). Reinstatement of responding from extinction levels and the effects of B/M inhibition of the BNST on reinstatement were analyzed using separate one-way analysis of variance (ANOVA) for each reinstatement condition, followed by pairwise comparisons with the Student–Newman–Keuls test. Locomotor activity was measured in 12 5 min time bins and analyzed with a two-way ANOVA (group×time). Data points were not included if they were three standard deviations beyond the group mean. Analyses were considered statistically significant at p<0.05. All data are presented as mean±SEM.
Results
Histology
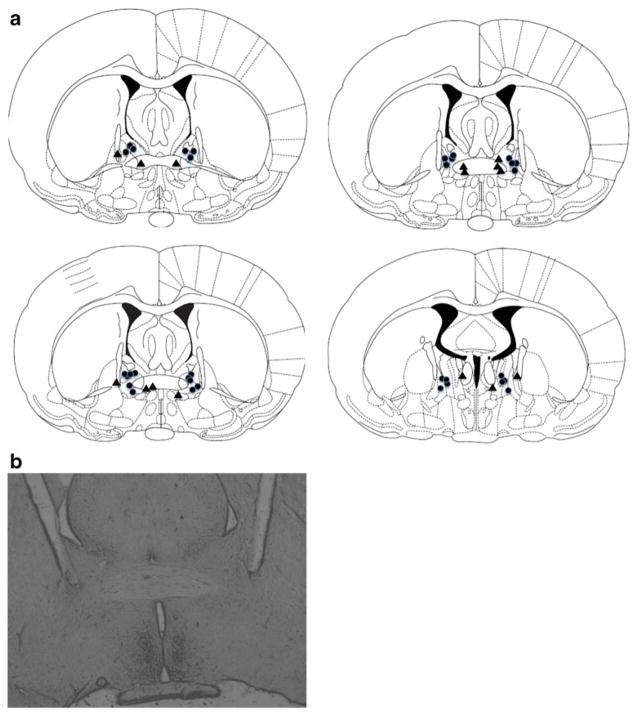
Inspection of injection cannulae tip locations showed that all correct BNST placements were within the dorsomedial portions of the more posterior BNST, including the anterolateral and anteromedial nuclei (Fig. 1a). A representative photomicrograph is shown in Fig. 1b. Note that due to overlap, some points indicate injector locations for multiple animals. A total of n=18 animals had correct injector placements in the BNST. Animals (n=8) with inaccurate cannulae placements served as anatomical controls to validate the specificity of BNST inactivation on reinstatement. These placements targeted the anterior commissure at various rostral/caudal levels, as well as the internal capsule and the nucleus of the commissural stria terminalis. It is important to note that the animals with placement outside the BNST did not show any effect of B/M treatment on reinstatement responding (shown below).
Fig. 1.
a Schematic diagram illustrating placements of injection cannulae as confirmed through histology (modified from Paxinos and Watson 1997). Coronal sections depicted are −0.2 to −0.6 mm from bregma along the A/P coordinates. Placements are shown within (circles) or outside of (triangles) the BNST. Please note some overlap in placements. The majority of BNST placements were in the dorsomedial portions of the BNST, with a few placed in more ventral locations. b A representative photomicrograph of BNST placements
Cocaine self-administration and extinction
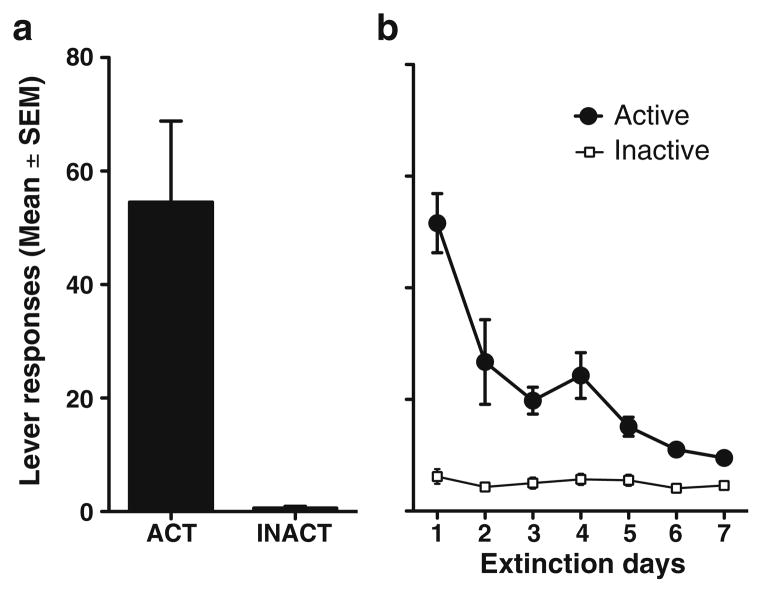
Rats readily acquired cocaine self-administration and demonstrated stable patterns of active lever responding and cocaine intake throughout the maintenance phase of the experiment. Figure 2a indicates the mean lever responding for the last 2 days of cocaine self-administration before extinction and reinstatement testing (mean active lever responses=54.5±14.3 and mean daily cocaine intake= 20.7±2.3 mg/kg). Furthermore, all animals decreased responding during extinction sessions in the absence of cocaine infusions and cue presentations (Fig. 2b). Active lever responding reached the established extinction criterion (<15 active lever responses over 2 days) in a mean of 8.7± 0.4 days before subsequent reinstatement testing. Mean lever presses before the first reinstatement test was measured across animals (mean active lever responses= 9.5±1.1). Inactive lever responding showed uniformly very low levels throughout the study, and no significant differences were found between treatment conditions during reinstatement trials (data not shown). Spontaneous locomotor activity measured after intracranial infusions of either vehicle or B/M (n=10/group) showed no significant effects of BNST inactivation on total locomotor activity (data not shown).
Fig. 2.
Responses (mean±SEM) on the active (ACT) and inactive (INACT) levers during cocaine self-administration (left panel) and the last 7 days of extinction responding before reinstatement (right panel)
Reinstatement testing
Reinstatement data for animals with correct placements in the BNST are shown in Fig. 3. Four out of 162 datapoints were excluded on the basis of outlier criterion (>3 SD). Presentation of conditioned cues led to significant reinstatement of responding on the previously cocaine-paired lever during testing (F2,51=18.28, p<0.0001). Post hoc analyses revealed significant reinstatement over extinction levels (p<0.05) and blockade of conditioned cue reinstatement by intracranial B/M administration (p<0.05). Yohimbine administration alone led to modest, but significantly increased reinstatement responding (F2,48=3.94, p<0.05) above that of extinction levels (p<0.05). Yohimbine-induced reinstatement was significantly attenuated by BNST inactivation (p<0.05). As previously reported (Feltenstein and See 2006), pretreatment with yohimbine increased cue-induced reinstatement of cocaine seeking beyond that seen with either cues or yohimbine alone. Analyses revealed a significant main effect (F2,50=17.32, p<0.0001), with pronounced reinstatement over extinction levels (p<0.05) as well as inhibition of the cue+yohimbine-induced reinstatement by B/M (p<0.05). In animals with cannulae placements outside the BNST, infusion of B/M had no effect on reinstatement of cocaine seeking under any of the three conditions (Fig. 4), with significant responding over extinction levels seen for cue-, yohimbine-, and cue+ yohimbine-induced reinstatement after either vehicle or B/M infusions (p<0.05).
Fig. 3.
Responses (mean±SEM) on the active (previously cocaine-paired) lever for the last day of extinction responding before testing (EXT), and following saline vehicle or baclofen–muscimol (B/M) infusions into the BNST for conditioned cue-induced reinstatement tests (CUE), yohimbine-induced reinstatement tests (YOH), and yohimbine+cue-induced reinstatement tests (CUE+YOH). Significant differences (Student–Newman–Keuls test) are indicated for reinstatement compared with extinction level responding (*p<0.05) or vehicle vs B/M (†p<0.05)
Fig. 4.
Responses (mean±SEM) on the active (previously cocaine-paired) lever for the last day of extinction responding before testing (EXT), and following saline vehicle or baclofen-muscimol (B/M) infusions directed at the BNST for conditioned cue-induced reinstatement tests (CUE), yohimbine-induced reinstatement tests (YOH), and yohimbine+cue-induced reinstatement tests (CUE+YOH) in animals with cannulae located outside of the BNST. Significant differences (Student–Newman–Keuls test) are indicated for reinstatement compared with extinction level responding (*p<0.05)
Discussion
The current study shows a role for the BNST in both conditioned cue and yohimbine-induced reinstatement of cocaine seeking in an animal model of relapse. Further, our results effectively demonstrate blockade of the interactive effects of yohimbine stress and cues on reinstatement, supporting the role of the BNST as a critical modulator when both types of reinstatement factors are applied. While the role of the BNST in footshock stress-induced reinstatement has previously been established (Erb and Stewart 1999; McFarland et al. 2004), examination of the neural substrates of conditioned cue-induced reinstatement has focused primarily on the basolateral and central regions of the amygdala, rather than the extended amygdala (See 2005). In addition to a role for the BNST in conditioned cue-induced reinstatement, our results indicate that the BNST is critical for the interaction of yohimbine stress and cues in the promotion of cocaine seeking, as BNST inactivation blocked this potent form of reinstatement. These results suggest that stress-cue interactions may be the result of the convergent activation of two separate, yet integrated stress and cue pathways that include the BNST.
Previous studies examining the neural circuitry underlying stress-induced reinstatement have generally utilized intermittent footshock stress (Ahmed and Koob 1997; Erb et al. 1996; Erb and Stewart 1999). Several other forms of stress (e.g., restraint stress) have been found to not produce reinstatement of drug seeking (Shaham et al. 2000). To date, studies have not directly examined the neural circuitry underlying yohimbine stress-induced reinstatement, or the degree to which patterns of neural activation may be similar during footshock or yohimbine stress-induced reinstatement. Interestingly, both of these stressors (but not several other types of stressors) induced similar increases in c-fos mRNA in the nucleus accumbens shell, and the basolateral and central amygdalar nuclei (Funk et al. 2006). However, other data indicate that yohimbine may promote reinstatement via mechanisms distinct from those underlying footshock stress-induced reinstatement. While footshock stress-induced reinstatement is thought to rely on interactions of CRF and NE within the BNST (Leri et al. 2002), recent studies suggest that yohimbine stress-induced reinstatement is unaffected by CRF receptor antagonists, or by NE α-2 agonists (Brown et al. 2009). However, we have recently found blockade of yohimbine-induced and conditioned cue-induced reinstatement by systemic administration of guanfacine, an α-2 receptor agonist (Buffalari and See 2009b). Furthermore, the current results suggest that yohimbine and footshock do both rely on intact function of the bed nucleus of the stria terminalis.
While yohimbine has been well characterized as an enhancer of NE activity via NE α-2 antagonist effects, other mechanisms in both the BNST and other brain regions may contribute to the effects of the drug, in particular the enhancing effect of yohimbine on cues. Yohimbine does have some affinity at serotonin (Millan et al. 2000) and dopamine (Scatton et al. 1980) receptor subtypes, any of which may contribute to reinstatement of drug seeking. Alternatively, NE increases in other terminal regions may contribute to reinstatement by both yohimbine stress alone and the enhanced reinstatement with simultaneous stress and cues. Yohimbine-induced increases in NE release in the basolateral amygdala (Buffalari and Grace 2009) may enhance the motivational salience of conditioned cues, while increased prefrontal NE tone (Garcia et al. 2004) could alter attentional processes and further modulate cue salience.
While yohimbine does not induce a stress state fully analogous to an external stress situation (e.g., social stress), the use of yohimbine as a stressor for an animal model of relapse offers several experimental and translational advantages. First, yohimbine has well-characterized stress and anxiety effects in humans (Southwick et al. 1999) including increased anxiety and activation of the hypothalamic–pituitary adrenal axis (Charney et al. 1987), elicitation of panic attacks, and increases in blood pressure (Vasa et al. 2009). Yohimbine causes stress and anxiety responses in animals as well, including increased cortisol in rats (Banihashemi and Rinaman 2006) and monkeys (Lee et al. 2004), and increased anxiety-like behaviors in several paradigms (File 1986; Johnston and File 1989; Bijlsma et al. 2010). Yohimbine therefore offers a homologous method of stress activation across species. Second, while not yet systematically tested for cocaine, yohimbine increases drug craving in opioid-dependent patients (Stine et al. 2002). Also, yohimbine reliably reinstates drug-seeking behavior in rats (Banna et al. 2010; Feltenstein and See 2006; Le et al. 2005; Shepard et al. 2004) and monkeys (Lee et al. 2004). Finally, yohimbine has a relatively long half-life of several hours (Hubbard et al. 1988), which allows for maintained stress activation across the duration of a reinstatement test session, with or without conditioned cue exposure. Further exploration of both the neural circuitry and pharmacological features of yohimbine stress-induced reinstatement offers a promising direction for future studies.
While previous studies have delineated various aspects of the neural circuitry underlying conditioned cue-induced reinstatement (Feltenstein and See 2008; See 2005), further studies will need to establish the links between amygdalar-based circuitry and the monoaminergic modulation of the corticostriatal circuitry necessary for reinstatement of drug seeking. The lack of a direct projection from the basolateral amygdala to the ventral tegmental area (VTA) suggests that the amygdala may modulate dopaminergic output in the prefrontal cortex via direct projections to the prefrontal cortex or indirect projections via the central amygdala regions and the BNST (Alheid 2003), which have glutamatergic, GABAergic, and peptidergic projections to dopamine neurons (Georges and Aston-Jones 2001; Morrell et al. 1984). Cue presentation may activate basolateral amygdala efferents to these regions, resulting in enhanced dopamine release in the prefrontal cortex. The importance of cortical dopamine function is evidenced from studies that have shown attenuation of both conditioned cue- (Ciccocioppo et al. 2001; See 2009) and footshock stress-induced (Capriles et al. 2003) reinstatement by dopamine receptor blockade. The BNST may modulate cortical dopamine levels via its projections to the VTA (Georges and Aston-Jones 2001, 2002).
Multiple changes in the BNST have been characterized after acute or repeated noncontingent cocaine, as well as cocaine self-administration (Koob 2003). Cocaine has been reported to increase dopamine in the BNST (Carboni et al. 2000) and enhance BNST excitatory transmission (Dumont et al. 2005). Dopamine has also been shown to enhance glutamatergic transmission in the BNST and modulate short-term NMDA-dependent plasticity (Kash et al. 2008). Plasticity within the neural circuitry associated with reinstatement has been implicated as a crucial component of cocaine-induced behavioral changes that may underlie relapse (Kauer and Malenka 2007). Further, chronic cocaine disrupts plasticity within the BNST, and leads to changes in NE transporter binding. NE in the BNST, which is critical for footshock-induced reinstatement (Leri et al. 2002), causes complex inhibitory and excitatory effects that are receptor and subregion dependent (Egli et al. 2005). Finally, the BNST has been heavily implicated in withdrawal from abused drugs (Koob 2003; Smith and Aston-Jones 2008), and withdrawal also disrupts long-term potentiation of intrinsic excitability within the BNST (Francesconi et al. 2009). Such studies complement the current results and suggest that the BNST is a critical region that may undergo changes relevant to chronic drug use, craving, withdrawal, and relapse.
The BNST is a complex brain region with as many as 12 anatomically identified subregions (Dumont 2009). However, the functional dissociation of different regions of the BNST, particularly with regard to reward and addiction-related functions, has yet to be delineated. Our placements primarily targeted the dorsomedial subdivisions, primarily in the posterior portions of BNST. However, this placement was not exclusive, and inactivation of other areas of the BNST was also effective. This is not surprising, in that other studies examining the role of the BNST in drug withdrawal, reinstatement, and reward have identified a role for multiple subregions of the BNST (Aston-Jones et al. 1999; Delfs et al. 2000; Harris and Aston-Jones 2003; Jalabert et al. 2009; Leri et al. 2002). Most recently, strong interactions between the prefrontal cortex, multiple BNST subregions, and dopamine neurons of the VTA have been identified (Jalabert et al. 2009). These interactions are relevant to the current results, as the VTA and prefrontal cortical regions have been shown to play a role in other forms of reinstatement of drug seeking (McFarland et al. 2004; See 2005).
We chose B/M infusions as a means of inactivation for four primary reasons: (1) sparing of effects on fibers of passage, (2) targeting of both GABA A and B receptors, (3) extensive previous research with this approach in determining the neural circuitry of reinstatement (McFarland et al. 2004; McFarland and Kalivas 2001), and (4) previous experience in our laboratory using B/M to specifically inactivate the BNST. However, as with any pharmacological approach, the use of B/M necessitates careful interpretation of the resultant behavioral effects. Often characterized as consisting of GABAergic interneurons and projection neurons, recent investigations (Jalabert et al. 2009) have suggested that this may be an oversimplification of the BNST. While combined GABA A and B agonists generally result in an overall inhibition, in a structure with many interconnected GABAergic interneurons (such as the BNST), more complex interactions could arise. Follow-up studies will need to target specific neurotransmitter receptors, with a primary focus on NE receptors due to yohimbine’s actions on the NE system and prior evidence of a role for BNST NE in footshock-induced reinstatement of drug seeking (Leri et al. 2002).
The current results have implications for the phenomena whereby heightened stress increases the likelihood of relapse in drug-dependent individuals in isolation, or when they experience stimuli or environments associated with prior drug use (Sinha et al. 2006). Whereas previous clinical and preclinical studies have almost exclusively focused on the isolated effects of stress or cues, users usually encounter multiple triggers for relapse during abstinence, and are more at risk during periods of greater stress or other maladaptive states, such as anxiety or depression (Brady et al. 2007). Thus, the use of animal models aimed at understanding the neurobiology of relapse need to incorporate the interactions that occur between factors that promote relapse. The discovery of novel neural substrates underlying the interaction of stress and cues in reinstatement will ultimately facilitate the development of more effective interventions that can successfully target both domains that contribute to relapse.
Acknowledgments
This research was supported by the National Institute on Drug Abuse grants DA16511 and DA21690 (RES), 1F32 DA025411-01 (DMB), and NIH grant C06 RR015455. The authors thank Anthony Carnell, Alisha Henderson, and Bernard Smalls for technical assistance and data collection.
Contributor Information
Deanne M. Buffalari, Department of Neuroscience, University of Pittsburgh, Pittsburgh, PA 15260, USA
Ronald E. See, Email: seere@musc.edu, Department of Neurosciences, Medical University of South Carolina, BSB416B, 173 Ashley Avenue, Charleston, SC 29425, USA
References
- Ahmed SH, Koob GF. Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology. 1997;132:289–295. doi: 10.1007/s002130050347. [DOI] [PubMed] [Google Scholar]
- Alheid GF. Extended amygdala and basal forebrain. Ann NY Acad Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann N Y Acad Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Banihashemi L, Rinaman L. Noradrenergic inputs to the bed nucleus of the stria terminalis and paraventricular nucleus of the hypothalamus underlie the hypothalamic–pituitary–adrenal axis but not hypophagic or conditioned avoidance responses to system yohimbine. J Neurosci. 2006;26:11442–11453. doi: 10.1523/JNEUROSCI.3561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banna KM, Back SE, Do P, See RE. Yohimbine stress potentiates conditioned cue-induced reinstatement of heroin-seeking in rats. Behav Brain Res. 2010;208:144–148. doi: 10.1016/j.bbr.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlsma EY, de Jongh R, Olivier B, Groenink L. Fear-potentiated startle, but not light-enhanced startle, is enhanced by anxiogenic drugs. Pharmacol Biochem Behav. 2010;96:24–31. doi: 10.1016/j.pbb.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Bongiovanni M, See RE. A comparison of the effects of different operant training experiences and dietary restriction on the reinstatement of cocaine-seeking in rats. Pharmacol Biochem Behav. 2008;89:227–233. doi: 10.1016/j.pbb.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Verduin ML, Tolliver BK. Treatment of patients comorbid for addiction and other psychiatric disorders. Curr Psychiatry Rep. 2007;9:374–380. doi: 10.1007/s11920-007-0048-0. [DOI] [PubMed] [Google Scholar]
- Brown ZJ, Tribe E, D’Souza NA, Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology. 2009;203:121–130. doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Grace AA. Anxiogenic modulation of spontaneous and evoked neuronal activity in the basolateral amygdala. Neuroscience. 2009;163:1069–1077. doi: 10.1016/j.neuroscience.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Footshock stress potentiates cue-induced cocaine-seeking in an animal model of relapse. Physiol Behav. 2009a;98:614–617. doi: 10.1016/j.physbeh.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Guanfacine blockade of stress-induced and conditioend cue-induced cocaine-seeking in an animal model of relapse. Soc Neurosci Abstr. 2009b:387.5. doi: 10.1016/j.physbeh.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Carboni E, Silvagni A, Rolando MT, Di Chiara G. Stimulation of in vivo dopamine transmission in the bed nucleus of stria terminalis by reinforcing drugs. J Neurosci. 2000;20:RC102. doi: 10.1523/JNEUROSCI.20-20-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS, Woods SW, Goodman WK, Heninger GR. Neurobiological mechanisms of panic anxiety: biochemical and behavioral correlates of yohimbine-induced panic attacks. Am J Psychiatr. 1987;144:1030–1036. doi: 10.1176/ajp.144.8.1030. [DOI] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: Reversal by D1 antagonists. Proc Natl Acad Sci U S A. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Dumont EC. What is the bed nucleus of the stria terminalis? Prog Neuro-Psychopharmacol Biol Psychiatry. 2009;33:1289–1290. doi: 10.1016/j.pnpbp.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Mark GP, Mader S, Williams JT. Self-administration enhances excitatory synaptic transmission in the bed nucleus of the stria terminalis. Nat Neurosci. 2005;8:413–414. doi: 10.1038/nn1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli RE, Kash TL, Choo K, Savchenko V, Matthews RT, Blakely RD, Winder DG. Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuro-psychopharmacology. 2005;30:657–668. doi: 10.1038/sj.npp.1300639. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology. 1996;128:408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Altar CA, See RE. Aripiprazole blocks reinstatement of cocaine seeking in an animal model of relapse. Biol Psychiatry. 2007;61:582–590. doi: 10.1016/j.biopsych.2006.04.010. [DOI] [PubMed] [Google Scholar]
- File SE. Aversive and appetitive properties of anxiogenic and anxiolytic agents. Behav Brain Res. 1986;21:189–194. doi: 10.1016/0166-4328(86)90236-6. [DOI] [PubMed] [Google Scholar]
- Forray MI, Bustos G, Gysling K. Regulation of norepinephrine release from the rat bed nucleus of the stria terminalis: in vivo microdialysis studies. J Neurosci Res. 1997;50:1040–1046. doi: 10.1002/(SICI)1097-4547(19971215)50:6<1040::AID-JNR15>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Francesconi W, Berton F, Repunte-Canonigo V, Hagihara K, Thurbon D, Lekic D, Specio SE, Greenwell TN, Chen SA, Rice KC, Richardson HN, O’Dell LE, Zorrilla EP, Morales M, Koob GF, Sanna PP. Protracted withdrawal from alcohol and drugs of abuse impairs long-term potentiation of intrinsic excitability in the juxtacapsular bed nucleus of the stria terminalis. J Neurosci. 2009;29:5389–5401. doi: 10.1523/JNEUROSCI.5129-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2004a;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004b;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–243. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Galvez R, Mesches MH, McGaugh JL. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiol Learn Mem. 1996;66:253–257. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- Garcia AS, Barrera G, Burke TF, Ma S, Hensler JG, Morilak DA. Autoreceptor-mediated inhibition of norepinephrine release in rat medial prefrontal cortex is maintained after chronic desipramine treatment. J Neurochem. 2004;91:683–693. doi: 10.1111/j.1471-4159.2004.02748.x. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci. 2001;21:RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Critical role for ventral tegmental glutamate in preference for a cocaine-conditioned environment. Neuropsychopharmacology. 2003;28:73–76. doi: 10.1038/sj.npp.1300011. [DOI] [PubMed] [Google Scholar]
- Hubbard JW, Pfister SL, Biediger AM, Herzig TC, Keeton TK. The pharmacokinetic properties of yohimbine in the conscious rat. Naunyn Schmiedebergs Arch Pharmacol. 1988;337:583–587. doi: 10.1007/BF00182736. [DOI] [PubMed] [Google Scholar]
- Jalabert M, Aston-Jones G, Herzog E, Manzoni O, Georges F. Role of the bed nucleus of the stria terminalis in the control of ventral tegmental area dopamine neurons. Prog Neuro-Psychopharmacol Biol Psychiatry. 2009;33:1336–1346. doi: 10.1016/j.pnpbp.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston AL, File SE. Yohimbine’s anxiogenic action: evidence for noradrenergic and dopaminergic sites. Pharmacol Biochem Behav. 1989;32:151–156. doi: 10.1016/0091-3057(89)90225-6. [DOI] [PubMed] [Google Scholar]
- Kash TL, Nobis WP, Matthews RT, Winder DG. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J Neurosci. 2008;28:13856–13865. doi: 10.1523/JNEUROSCI.4715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology. 2006;187:60–67. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology. 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res. 1997;87:139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Newman-Tancredi A, Audinot V, Cussac D, Lejeune F, Nicolas JP, Coge F, Galizzi JP, Boutin JA, Rivet JM, Dekeyne A, Gobert A. Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of fronto-cortical monoaminergic transmission and depressive states. Synapse. 2000;35:79–95. doi: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Morrell JI, Schwanzel-Fukuda M, Fahrbach SE, Pfaff DW. Axonal projections and peptide content of steroid hormone concentrating neurons. Peptides. 1984;5(Suppl 1):227–239. doi: 10.1016/0196-9781(84)90281-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3. Academic; New York: 1997. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scatton B, Zivkovic B, Dedek J. Antidopaminergic properties of yohimbine. J Pharmacol Exp Ther. 1980;215:494–499. [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- See RE. Dopamine D1 receptor antagonism in the prelimbic cortex blocks the reinstatement of heroin-seeking in an animal model of relapse. Int J Neuropsychopharmacol. 2009;12:431–436. doi: 10.1017/S1461145709000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shalev U, Highfield D, Yap J, Shaham Y. Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacology. 2000;150:337–346. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology. 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic–pituitary–adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick SM, Bremner JD, Rasmusson A, Morgan CA, 3rd, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- Stine SM, Southwick SM, Petrakis IL, Kosten TR, Charney DS, Krystal JH. Yohimbine-induced withdrawal and anxiety symptoms in opioid-dependent patients. Biol Psychiatry. 2002;51:642–651. doi: 10.1016/s0006-3223(01)01292-6. [DOI] [PubMed] [Google Scholar]
- Tjurmina OA, Goldstein DS, Palkovits M, Kopin IJ. Alpha2-adrenoceptor-mediated restraint of norepinephrine synthesis, release, and turnover during immobilization in rats. Brain Research. 1999;826:243–252. doi: 10.1016/s0006-8993(99)01281-0. [DOI] [PubMed] [Google Scholar]
- Vasa RA, Pine DS, Masten CL, Vythilingam M, Collin C, Charney DS, Neumeister A, Mogg K, Bradley BP, Bruck M, Monk CS. Effects of yohimbine and hydrocortisone on panic symptoms, autonomic responses, and attention to threat in healthy adults. Psychopharmacology. 2009;204:445–455. doi: 10.1007/s00213-009-1475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]