Abstract
Background
The ideal solution for recovery of donor lungs remains unknown. Low potassium dextran (LPD) solution is most common, but University of Wisconsin (UW) solution is also used. The United Network for Organ Sharing (UNOS) database allows assessment of preservation solutions in a large cohort of lung transplant (LTx) patients.
Methods
We retrospectively reviewed the UNOS dataset for adult primary LTx patients (2005–2008) whose donor lungs were recovered with UW or LPD solution. Patients were stratified by UW vs. LPD, and secondarily grouped by quartiles of the lung allocation score (LAS) to examine high risk recipients. Kaplan-Meier (KM) short term mortality (30d, 90d, 1 yr), and rejection in the 1st yr, were examined for intervals with adequate follow-up. Cox proportional hazard regression using 11 variables examined all cause 1-yr mortality.
Results
Of 4455 patients, 4161 (93.4%) received LPD lungs and 294 (6.6%) received UW lungs. 1105 (24.8%) patients died during the study. There was no mortality difference based on flush solution with all patients examined together. However, patients in the upper two LAS quartiles (Q3:37.8-45.4, Q4:>45.4) receiving LPD lungs had greater 1 yr survival (81.5% vs.73.5%, p=0.02). On multivariable analysis, flush with UW solution resulted in an increased risk of 1 yr mortality (Hz ratio 1.77[1.21–2.58], p=0.003) compared to LPD. Preservation solution did not affect rejection rates in the year after LTx. KM modeling demonstrated the impact of flush solution on survival (p=0.02).
Conclusions
This study is the largest modern cohort to evaluate the effect of donor lung flush solutions on survival in adult LTx. UW solution increases the risk of 1 yr mortality in high risk LTx recipients.
Keywords: Lung Transplantation, UNOS, organ preservation
Background
Lung transplantation (LTx) has emerged as the standard therapy for patients with end stage lung disease, with improved survival and quality of life.1–3 However, early graft dysfunction (EGD) remains a common and devastating complication. EGD occurs in 10–25% of LTx and accounts for nearly 30% of deaths within 30 days.4–6 Experimental studies and clinical reports implicate ischemia-reperfusion injury in the development of EGD.4 Preservation technique is a putative mediator of early graft function, and much clinical and laboratory research has focused on optimal preservation solutions.
Preservation solutions simulate either intracellular or extracellular ion concentrations. Perfadex (Vitrolife, Englewood, CO) is a low potassium dextran (LPD) solution and is the most commonly used extracellular solution in the United States. University of Wisconsin (UW) solution (ViaSpan, DuPont Pharmaceuticals, Wilmington, DE) is an intracellular solution that has been used widely for solid organ preservation. Experimental animal studies have noted a beneficial effect of LPD compared to UW in ischemia-reperfusion injury and reactive oxygen species formation.7–9 Previous human studies have compared the intracellular solution LPD with various extracellular solutions, though no comparison between LPD and UW exists. These studies demonstrate LPD lungs have improved early graft function without a survival benefit, but are limited by low sample size.10–13
Implementation of the lung allocation score (LAS) has shifted the demographics of LTx recipients in the United States.14, 15 The United Network for Organ Sharing (UNOS) registry provides the opportunity to address this question in the post-LAS era. Therefore, we examined the UNOS dataset to test the hypothesis that high risk patients receiving LPD preserved lungs have a survival advantage over recipients of UW stored lungs.
Methods
Data Source
The UNOS Standard Transplant Analysis and Research (STAR) database represents an open cohort of prospectively collected data involving all United States patients receiving LTx from 1987 until December 2008, with follow-up extending until September 2009. Our institution deemed IRB approval unnecessary as no patient or center identifiers were included in this analysis.
Study Design
This study was a retrospective cohort design, including adult (>17 years) patients undergoing LTx in the post-LAS era (March 2005-present). Exclusion criteria included incomplete preservation solution information, heart-lung transplantation, and patients with prior LTx. The cohort was stratified according to whether donor lungs were preserved in LPD (LDS) or University of Wisconsin (UW) solution.
Variables Examined and Outcome Measures
Pertinent variables examined within the dataset included: demographic factors (age, gender, race, and education level); markers of pulmonary status (oxygen requirement, six minute walking distance, forced expiratory volume at 1 second (FEV1), forced vital capacity (FVC), FEV1 to FVC ratio, mechanical ventilation prior to LTx, and ICU care prior to transplant); co-morbidities (LAS score, diabetes mellitus, body mass index (BMI), preoperative creatinine levels and hypertension); and transplant variables (ischemic time, HLA mismatch, panel reactive antibody (PRA) level, year of transplant, and wait list times). We further examined donor variables including donor age, race, gender, cigarette use, and BMI.
The primary endpoint was the incidence of 1-year mortality. Secondary outcomes examined were short term mortality (30-day and 90-day), as well as rejection requiring treatment within the first year following LTx.
Statistical Analysis
We compared baseline characteristics among the LPD and UW groups by the student’s t-test (continuous variables) and the chi-square test (categorical variables). 30-day, 90-day, and 1-year survival were estimated using the Kaplan-Meier method, as these time intervals have adequate follow-up in the post-LAS era. To compare survival estimates according to preservation solution, the Mantel-Cox log-rank test was used. The entire cohort was analyzed according to the Kaplan-Meier method. Separate Kaplan-Meier analysis was performed in the upper two quartiles of LAS to assess the impact of preservation solution in high risk patients.
A multivariable Cox proportional hazards regression model estimated risk of death with censoring for death, loss to follow-up, and administrative reasons. To construct the multivariable model, independent covariates with potential for confounding were first tested in a univariate fashion. In addition to variables associated with mortality on exploratory analysis (p<0.1), those with biological plausibility and previously recognized risk factors were incorporated in a forwards and backwards stepwise fashion into the multivariable model. The likelihood ratio test and Akaike’s information criterion in a nested model approach were used to identify which covariates increased the explanatory power of the model. As the multivariable model was developed with case-wise deletion, all covariates with greater than 15% missing data were not included. The final model incorporated the following covariates: storage solution, recipient age ≥ 65, creatinine level, ICU prior to transplant, hospitalization prior to transplant, final LAS calculation, organ ischemic time, donor cigarette use, donor age, and donor CMV status.
For all analyses, a p-value of less than 0.05 (two-tailed) was considered significant. Means are displayed with standard deviations. Hazard ratios are presented with 95% confidence intervals (CI). Statistical testing was performed using STATA software (version 9.2 SE, StataCorp LP, College Station, TX).
Results
Cohort Statistics
From 2005 to 2008, 5,712 patients receiving LTx were included in the UNOS database. Patients with previous transplants (n= 272), absent storage solution information (n=334), and children (n=175) were excluded. Thus the final study population was 4,459. The mean age of the cohort was 53±13 years with 41.7% females (n=1,860).
Recipient race distribution was: 84.6% Caucasian (n=3,774), 8.6% African American (n=385), 4.8% Hispanic (n=213), and 2.0% Other (n=87). The donor race distribution was: 62.8% Caucasian (n=2,802), 18.0% African American (n=802), 15.4% Hispanic (n=688), and 3.8% Other (n=167). During the study period 1,106 patients died, and 697 patients did not survive one year. One year incidence of death was 18.5 deaths/100 person-years. The mean follow-up was 19±12 months. Throughout the 4 year study period, the number of adult LTx’s remained constant, with 1,400 in 2005 and 1,467 in 2008.
Baseline Characteristics
Baseline characteristics were evenly distributed in the LPD versus UW groups, with few exceptions. Patients receiving LPD preserved lungs tended to be elderly, have diabetes, and have donor cigarette use; whereas patients receiving UW preserved lungs had higher BMI, longer waitlist times, poorer pulmonary function testing, and greater HLA mismatch. Though statistically significant, the absolute differences in these categories were small and unlikely to be of clinical relevance. Ischemic time was similar in both groups. There were no differences based on LAS in the two groups, nor was there any difference in the rate of pre-LTx mechanical ventilation, ICU care, or hospitalization between the two groups. Idiopathic pulmonary fibrosis was the most common indication for LTx in both groups (Table 1).
Table 1.
Baseline demographics stratified by flush solution
| 4,459 Patients | Perfadex N=4,165 |
UW N=294 |
P-Value* |
|---|---|---|---|
| Recipient Demographics and Comorbidities | |||
| Age | 53.5 (±12.8) | 52.5 (±12.3)† | 0.2 |
| Age≥65 | 1696/4165 (40.7%) | 98/294 (33.3%) | 0.01 |
| Male | 2434/4165 (58.4%) | 165/294 (56.1%) | 0.4 |
| Gender-matched | 2898/4165 (69.6%) | 214/294 (72.3%) | 0.2 |
| Caucasian | 3522/4165 (85.6%) | 252/294 (85.7%) | 0.6 |
| African-American | 359/4165 (8.6%) | 26/294 (8.8%) | 0.9 |
| Hispanic | 202/4165 (4.8%) | 11/294 (3.7%) | 0.4 |
| Other | 82/4165 (2.0%) | 5/294 (1.7%) | 0.7 |
| Diabetes | 707/4127 (17.1%) | 33/294 (11.2%) | 0.009 |
| Hypertension | 538/2255 (23.9%) | 41/192 (21.4%) | 0.4 |
| Creatinine | 0.88 (±0.54) | 0.86 (±0.31) | 0.4 |
| Body Mass Index (BMI) | 24.9 (±66.0) | 26.7 (±13.8) | <0.001 |
| Days on wait list | 249 (±432) | 330 (±465)† | <0.001 |
| End LAS calculation | 42.82 (±14.09) | 42.38 (±13.77) | 0.6 |
| Quartile 1 | 1029/4165 (24.7%) | 84/294 (28.6%) | 0.1 |
| Quartile 2 | 1048/4165 (25.2%) | 70/294 (23.8%) | 0.6 |
| Quartile 3 | 1043/4165 (25.0%) | 71/294 (24.1%) | 0.7 |
| Quartile 4 | 1045/4165 (25.1%) | 69/294 (23.5%) | 0.5 |
| Recipient Hemodynamics and Pulmonary Function | |||
| Pre-LTx Mean PAP (mmHg) | 27.3 (±10.7) | 26.4 (±9.8) | 0.2 |
| Pre-LTx PVR (dyn·s/cm5) | 3.4 (±2.7) | 2.9 (±2.0) | <0.001 |
| Pre-LTx ventilation | 185/4165 (4.4%) | 9/294 (3.1%) | 0.2 |
| Pre-LTx ICU care | 296/4165 (7.1%) | 19/294 (6.5%) | 0.7 |
| Pre-LTX hospitalization | 596/4165 (14.3%) | 34/294 (11.6%) | 0.2 |
| O2 requirement - mean (±sd) | 3.6 (±3.0) | 3.5 (±2.9) | 0.5 |
| Six minute walking distance < 150 ft | 222/4165 (5.3%) | 25/294 (8.5%) | 0.02 |
| FEV1 %predicted – mean (±sd) | 37.8 (±21.2) | 34.8 (±20.1) | 0.02 |
| FVC %predicted – mean (±sd) | 49.0 (±17.6) | 46.6 (±16.2) | 0.02 |
| FEV/FVC – mean (±sd) | 0.58 (±0.26) | 0.57 (±0.26) | 0.6 |
| Recipient Insurance and Education | |||
| Private Insurance/self pay | 2648/4165 (63.6%) | 182/294 (61.9%) | 0.6 |
| Medicare | 1071/4165 (25.7%) | 72/294 (24.5%) | 0.6 |
| Medicaid | 310/4165 (7.4%) | 23/294 (7.8%) | 0.8 |
| Other Insurance | 135/4165 (3.2%) | 17/294 (5.85%) | 0.02 |
| College or Graduate | 1920/3538 (54.2%) | 137/264 (51.9%) | 0.5 |
| Pre-college | 1618/3538 (45.7%) | 127/264 (48.1%) | 0.5 |
| Primary Diagnosis | |||
| COPD | 1266/4165 (30.4%) | 90/294 (30.6%) | 0.9 |
| CF | 532/4165 (12.8%) | 37/294 (12.6%) | 0.9 |
| IPF | 1365/4165 (32.8%) | 91/294 (31.0%) | 0.5 |
| Other | 1002/4165 (24.1%) | 76/294 (25.9%) | 0.5 |
| Donor Variables | |||
| Donor diabetes | 220/4156 (5.3%) | 15/293 (5.1%) | 0.9 |
| Donor age | 33.6 (±14.4) | 31.4 (±14.2) | 0.8 |
| Cigarette use | 650/4137 (15.7%) | 29/293 (9.9%) | 0.008 |
| HLA-mismatch | 2057/3498 (58.0%) | 109/194 (56%) | 0.5 |
| CMV mismatch | 1093/4165 (26.2%) | 79/294 (26.9%) | 0.8 |
| Ischemic time (hours) | 5.0 (±1.6) | 5.0 (±1.9) | 0.8 |
Survival
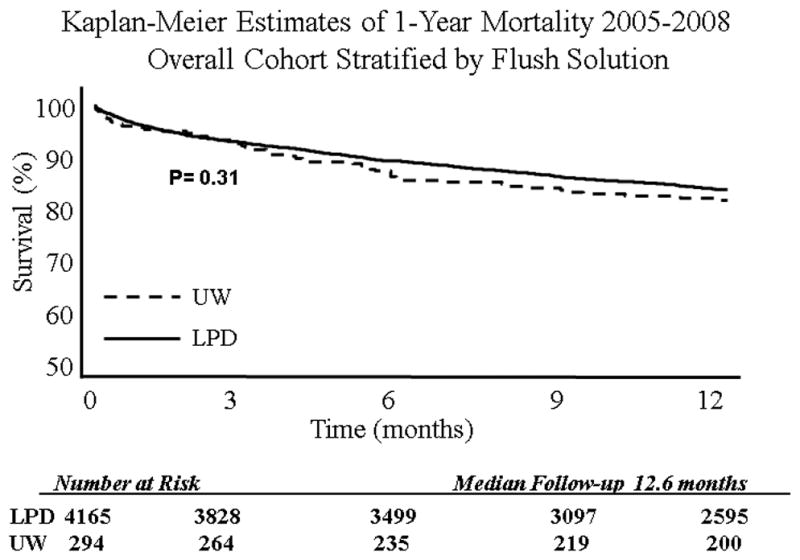
When the entire cohort was analyzed without stratification, overall survival at 1 year was 82.5%. After stratification by preservation solution, in the entire cohort there was no difference in 30-day, 90-day, or 1-year KM survival. However, when examining 1-year KM survival in high risk patients (LAS Q 3–4), there was a benefit conferred by LPD stored lungs (81.2% vs 73.3%, p=0.02) (Table 2). There was no difference in 30-day or 90-day survival in high risk LAS patients when stratified by storage solution. The 1-year incidence rate for death in LPD (18.3 deaths/ 100-person years) stored lungs versus UW (21.2 deaths/100-person years) stored lungs did not differ significantly. Survival curves are presented in Figure 1–2.
Table 2.
Effect of flush solution on unadjusted Kaplan-Meier estimates of survival (with 95% confidence intervals) in LTx recipients
| Survival Time | Perfadex | UW | P-Value |
|---|---|---|---|
| Overall cohort | (N=4,459) | (N=294) | |
| 30 day | 95.8% (95.2–96.4) | 95.2% (92.0–97.1) | 0.6 |
| 90 day | 92.5% (91.7–93.2) | 91.7% (87.9–94.4) | 0.7 |
| 6 month | 89.0% (88.0–89.9) | 85.3% (80.7–88.9) | 0.07 |
| 1-Year | 83.3% (82.1–84.5) | 81.2% (76.1–85.4) | 0.3 |
| LAS Q1 – Q2 | (N=2,077) | (N=154) | |
| 30 day | 96.3% (95.4–97.1) | 97.4% (93.1–99.0) | 0.6 |
| 90 day | 93.9% (92.8–94.9) | 96.0% (91.4–98.2) | 0.3 |
| 6 month | 90.6% (89.2–91.8) | 90.0% (84.0–93.8) | 0.8 |
| 1-Year | 85.4% (83.8–86.9) | 88.4% (82.0–92.6) | 0.4 |
| LAS Q3 – Q4 | (N=2,088) | (N=140) | |
| 30 day | 92.8% (87.0–96.1) | 95.3% (94.3–96.2) | 0.2 |
| 90 day | 91.1% (89.8–92.3) | 87.0% (80.2–91.6) | 0.1 |
| 6 month | 87.4% (85.9–88.8) | 80.2% (72.5–86.0) | 0.02 |
| 1-Year | 81.2% (79.4–83.0) | 73.3% (64.8–80.1) | 0.02 |
Figure. 1.
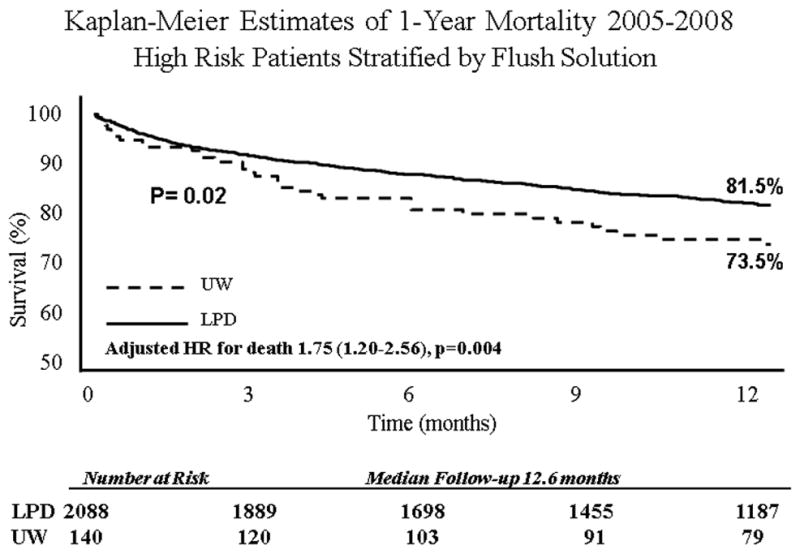
Figure. 2.
Multivariable Analysis
Following risk adjustment with Cox multivariable analysis, storage with LPD increased the hazard of 1-year mortality in high risk patients (Table 3). LTx recipients of UW stored lungs had a 75% increase in the risk of adjusted 1-year mortality compared to patients receiving LPD stored lungs (HR1.75, CI[1.20–2.56], p=0.004). Additional predictors of mortality on multivariable analysis included: age>65 years, recipient creatinine level, pre-LTx hospitalization, and pre-LTx ICU care. A diagnosis of cystic fibrosis was found to be protective.
Table 3.
Univariate and multivariable Cox regression analysis for 1-year mortality after LTx in High Risk LAS patients
| Variables of interest | Univariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P-value* | HR (95% CI) | P-value† | |
|
Additional Variables | ||||
| University of Wisconsin | 1.52 (1.07–2.16) | 0.03 | 1.75 (1.20–2.56) | 0.004 |
| Age | 1.02 (1.01–1.03) | <0.001 | ||
| Age > 65 | 1.46 (1.20–1.78) | <0.001 | 1.43 (1.10–1.89) | 0.007 |
| BMI | 1.01 (0.99–1.01) | 0.4 | ||
| Recipient creatinine | 1.13 (1.07–1.20) | 0.004 | 1.10 (1.04–1.18) | 0.002 |
| Recipient diabetes | 0.88 (0.69–1.15) | 0.36 | ||
| Recipient HTN | 1.36 (0.99–0.84) | 0.06 | ||
| Recipient diagnosis | ||||
| COPD | 1.06 (0.71–1.58) | 0.8 | ||
| CF | 0.63 (0.47–0.87) | 0.003 | 0.58 (0.41–0.82) | 0.002 |
| IPF | 1.05 (0.86–1.28) | 0.62 | ||
| Other | 0.97 (0.91–1.04) | 0.4 | ||
| Acuity | ||||
| LAS‡ | 1.01 (1.00–1.02) | <0.001 | 0.99 (0.99–1.01) | 0.3 |
| O2 requirement | 1.07 (1.03–1.11) | <0.001 | ||
| Six minute walking distance < 150 ft | 1.11 (0.92–1.34) | 0.3 | ||
| FEV1 % predicted | 1.01 (1.00–1.01) | 0.001 | ||
| FVC % predicted | 1.01 (1.00–1.01) | 0.05 | ||
| FEV/FVC – mean (±sd) | 1.69 (1.39–2.05) | <0.001 | ||
| Ventilator prior to LTx | 2.12 (1.56–2.86) | <0.001 | ||
| ICU prior to LTx | 3.14 (2.49–3.96) | <0.001 | 2.26 (1.52–3.38) | <0.001 |
| Hospitalized prior to LTx | 2.39 (1.94–2.94) | <0.001 | 2.42 (1.83–3.04) | 0.01 |
| Donor and Immunology | ||||
| HLA mismatch (0 or 1 antigens matched) | 1.02 (0.82–1.28) | 0.8 | ||
| Age of donor (yrs) | 1.01 (1.00–1.01) | 0.04 | 1.00 (0.99–1.01) | 0.3 |
| Donor BMI | 0.98 (0.96–1.01) | 0.3 | ||
| Donor cigarette use | 1.25 (0.96–1.64) | 0.1 | 1.21 (0.90–1.62) | 0.2 |
| Donor diabetes | 1.29 (0.84–1.97) | 0.25 | ||
| BLT | 0.84 (0.68–1.04) | 0.1 | ||
| Ischemic time > 6 hrs | 0.95 (0.75–1.21) | 0.7 | 1.05 (0.98–1.12) | 0.1 |
Rejection
For all LAS quartiles, the UW group had a higher risk of having a rejection episode in the year after transplant on univariate analysis (41.3% versus 30.6%, p<0.01). In the risk-adjusted multivariable logistic regression model this difference persisted (OR1.84, CI[1.17–2.88], p=0.007). Although a greater proportion of patients in the UW stored lung group had HLA-mismatch, this variable was also accounted for in the regression model, and found to be independently associated with risk of rejection (OR1.29, CI[1.03–1.62], p=0.03. Other significant risk factors for rejection were donor age and ischemic time.
Discussion
This analysis used prospectively collected UNOS data in the post-LAS era to evaluate the impact of preservation solution on survival in United States LTx patients. During the study period, the majority of patients in the US received lungs stored with LPD solution, with a smaller proportion of patients receiving UW preserved lungs. Patients were stratified based on LPD versus UW preservation solution, and 1-year survival was examined. Because high risk patients according to LAS calculations are known to have worse 1-year survival, we performed a sub-analysis in this cohort of patients to assess any impact on survival.16 After risk-adjustment, UW preservation solution imparts a 75% increase in the risk of 1-year mortality in the upper two quartiles of LAS patients. In high risk patients receiving UW solution, the absolute difference in 1-year survival was 7.9%.
Short-term mortality and having a treated rejection episode within one year were examined as secondary outcome measures. 30-day and 90-day survival analysis revealed no significant differences in high risk patients or in the cohort as a whole. Rejection was more frequent in patients receiving UW versus LPD stored lungs, occurring in 41.3% and 30.6% of patients, respectively. On univariate analysis, HLA-mismatch was more common in patients receiving UW stored lungs. This finding is prominent as HLA-mismatch is known to affect the odds of rejection.17 However, after risk-adjustment with multivariable logistic regression, recipients of UW stored lung had an 84% increase in the odds of a rejection episode requiring treatment. HLA-mismatch also independently increased the odds of rejection by 29%.
Multivariable Analysis
A multivariable Cox hazard regression model was used to assess the impact of potential confounding variables on 1-year survival. When high risk patients were included in the model, age>65, recipient creatinine, hospitalization before LTx, and intensive care unit before LTx were all significant predictors of 1-year mortality. Older age and pre-LTx hospitalization are known risk factors for worse 1-year survival.18 With the exception of age>65, which was more common in the LPD group, the remainder of these variables was evenly distributed between the two study groups. A LTx diagnosis of cystic fibrosis (CF) improved 1-year survival with a 42% reduction in the hazard of death. There was an equal proportion of CF patients in the UW and LPD groups.
Previous Work
Previously published studies comparing LPD against UW solution involve experimental animal models only. Oka et al. published the first comparison of LPD versus UW storage solution using a rabbit lung transplantation model.19 All donor organs were reperfused after hypothermic storage for 30 hrs. LPD stored lungs demonstrated improved oxygenation and less pulmonary edema compared with standard UW solution. This study revealed short-term information, however, as reperfusion continued for a total duration of 10 minutes. A third group of rabbits receiving modified low potassium UW solution was examined also. Results in this group were comparable to the LPD group, supporting other reports that implicate high potassium content in endothelial dysfunction and generation of reactive oxygen species.9, 20
Hausen et al. compared graft performance in a rat lung transplantation model in which donor organs were cold stored with either LPD, UW, or Euro-Collins solution. In the extended (16 hrs) ischemia model, rat lungs stored with LPD solution exhibited improved pulmonary compliance compared with UW solution.8 This finding is consistent with the worse pulmonary edema that was seen in the earlier report by Oka et al. Another experimental study by Chien et al. also found improved oxygenation for donor lungs stored in LPD compared with UW in a rat lung transplantation model.7 However, in contrast to earlier rat models, this study demonstrated improved pulmonary edema in UW stored lungs. The conclusion is that more impermeant contents improve cellular swelling, although the authors emphasize that less pulmonary edema did not translate into improved pulmonary function, as UW lungs demonstrated worse gas exchange and pulmonary hemodynamics.
In human studies, several single institution reports compared LPD solutions with other intracellular type solutions (Euro-Collins). LPD stored lungs demonstrated improvement in post-LTx oxygenation,13, 21, 22 pulmonary compliance,23 or EGD.11, 24 In contrast, a study by Aziz et al. did not detect any statistically significant clinical benefit, though there was a trend toward fewer deaths due to primary organ failure in the LPD group.25 This study was hampered by low sample size as only 69 patients were included.
Ganesh et al. conducted the only multi-institutional cohort study in 681 LTx recipients to examine survival differences between LPD and Euro-Collins storage solutions.10 There was no significant survival benefit with either preservation method, though there was a trend toward improved 3-year survival in LPD stored lungs. This study lacked the breakdown of ischemic time by preservation solution; however this issue was subsequently addressed in a response to the editor. Furthermore, in the setting of bilateral lung transplantation, graft ischemic time was reported as time to reperfusion of the first lung which is not consistent with other studies examining survival after LTx.
This study adds to the existent literature by examining data in the post-LAS era using a large multi-institutional modern cohort of LTx recipients. This series provides an overview of the modern practice of preservation technique for donor lungs in the United States. Furthermore, no previous study has directly compared the two most commonly used preservation solutions in the United States—LPD and UW. These results reinforce the importance of preservation technique, and are consistent with many of the findings in experimental animal models.
Limitations
Because of the retrospective cohort approach, it is not possible to certify that all possible confounders have been considered. A strength of the UNOS dataset is the large number of variables available for analysis, however there is a possibility of potential important variables being absent from this analysis. Furthermore, large multi-institutional databases rely on accurate coding. It is difficult to verify that coding errors are not present. However, the assumption is that any coding errors present in the database will occur randomly and thus do not render any bias. If this assumption is false, there is the possibility of residual bias in our conclusions. There were relatively few patients who received UW stored lungs compared with LPD solution, but this finding reflects the current practice of storage technique in the United States. Our methodology attempted to control for this imbalance. As the information included in the UNOS database only involves clinical information, this study does not purport to establish the mechanisms by which storage solution affects survival. That topic has been the focus of many in vivo animal model studies.
Conclusions
This study represents the largest cohort of LTx recipients in the post-LAS era in which the effect of preservation solution on survival has been examined. High risk patients receiving UW stored lungs have decreased 1-year survival when compared to patients receiving LPD stored lungs. In all patients, the risk of rejection is higher in patients receiving UW lungs. LTx centers and organ procurement organizations should give strong consideration to preserving lungs with LPD solution, especially in high risk recipients. As a limited donor pool remains a significant barrier to increasing the number of LTx performed annually, improved preservation technique may further expand the application of this therapy.
Acknowledgments
Dr. Arnaoutakis is the Irene Piccinini Investigator in Cardiac Surgery and Dr. Allen is the Hugh R. Sharp Cardiac Surgery Research Fellow. This work was supported in part by a Health Resources and Services Administration contract 231-00-0115 and by a Ruth L. Kirschstein National Research Service Award(NIH 2T32DK007713-12 ESW). The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Footnotes
To be presented at: The International Society for Heart and Lung Transplantation 30th Annual Meeting and Scientific Sessions, April 21-24, 2010, Chicago, IL
Conflicts: The authors have no conflicts of interest to disclose.
References
- 1.Geertsma A, Ten Vergert EM, Bonsel GJ, de Boer WJ, van der Bij W. Does lung transplantation prolong life? A comparison of survival with and without transplantation. J Heart Lung Transplant. 1998;17:511–516. [PubMed] [Google Scholar]
- 2.TenVergert EM, Essink-Bot ML, Geertsma A, van Enckevort PJ, de Boer WJ, van der Bij W. The effect of lung transplantation on health-related quality of life: a longitudinal study. Chest. 1998;113:358–364. doi: 10.1378/chest.113.2.358. [DOI] [PubMed] [Google Scholar]
- 3.Arcasoy SM, Kotloff RM. Lung transplantation. The New England journal of medicine. 1999;340:1081–1091. doi: 10.1056/NEJM199904083401406. [DOI] [PubMed] [Google Scholar]
- 4.King RC, Binns OA, Rodriguez F, Kanithanon RC, Daniel TM, Spotnitz WD, et al. Reperfusion injury significantly impacts clinical outcome after pulmonary transplantation. The Annals of thoracic surgery. 2000;69:1681–1685. doi: 10.1016/s0003-4975(00)01425-9. [DOI] [PubMed] [Google Scholar]
- 5.Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Lung and Heart-Lung Transplantation Report-2009. J Heart Lung Transplant. 2009;28:1031–1049. doi: 10.1016/j.healun.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Christie JD, Kotloff RM, Pochettino A, Arcasoy SM, Rosengard BR, Landis JR, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003;124:1232–1241. doi: 10.1378/chest.124.4.1232. [DOI] [PubMed] [Google Scholar]
- 7.Chien S, Zhang F, Niu W, Tseng MT, Gray L., Jr Comparison of university of wisconsin, euro-collins, low-potassium dextran, and krebs-henseleit solutions for hypothermic lung preservation. The Journal of thoracic and cardiovascular surgery. 2000;119:921–930. doi: 10.1016/S0022-5223(00)70087-0. [DOI] [PubMed] [Google Scholar]
- 8.Hausen B, Beuke M, Schroeder F, Poets CF, Hewitt C, DelRossi AJ, et al. In vivo measurement of lung preservation solution efficacy: comparison of LPD, UW, EC and low K+-EC following short and extended ischemia. Eur J Cardiothorac Surg. 1997;12:771–779. doi: 10.1016/s1010-7940(97)00205-4. discussion 779–780. [DOI] [PubMed] [Google Scholar]
- 9.Kelly RF, Murar J, Hong Z, Nelson DP, Hong F, Varghese A, et al. Low potassium dextran lung preservation solution reduces reactive oxygen species production. The Annals of thoracic surgery. 2003;75:1705–1710. doi: 10.1016/s0003-4975(03)00173-5. [DOI] [PubMed] [Google Scholar]
- 10.Ganesh JS, Rogers CA, Banner NR, Bonser RS. Does the method of lung preservation influence outcome after transplantation? An analysis of 681 consecutive procedures. The Journal of thoracic and cardiovascular surgery. 2007;134:1313–1321. doi: 10.1016/j.jtcvs.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 11.Oto T, Griffiths AP, Rosenfeldt F, Levvey BJ, Williams TJ, Snell GI. Early outcomes comparing Perfadex, Euro-Collins, and Papworth solutions in lung transplantation. The Annals of thoracic surgery. 2006;82:1842–1848. doi: 10.1016/j.athoracsur.2006.05.088. [DOI] [PubMed] [Google Scholar]
- 12.Van Raemdonck D. Current opinion in organ transplantation. Thoracic organs: current preservation technology and future prospects; part 1: lung. [DOI] [PubMed] [Google Scholar]
- 13.Fischer S, Matte-Martyn A, De Perrot M, Waddell TK, Sekine Y, Hutcheon M, et al. Low-potassium dextran preservation solution improves lung function after human lung transplantation. The Journal of thoracic and cardiovascular surgery. 2001;121:594–596. doi: 10.1067/mtc.2001.109703. [DOI] [PubMed] [Google Scholar]
- 14.Weiss ES, Allen JG, Merlo CA, Conte JV, Shah AS. Lung allocation score predicts survival in lung transplantation patients with pulmonary fibrosis. The Annals of thoracic surgery. 2009;88:1757–1764. doi: 10.1016/j.athoracsur.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Egan TM, Kotloff RM. Pro/Con debate: lung allocation should be based on medical urgency and transplant survival and not on waiting time. Chest. 2005;128:407–415. doi: 10.1378/chest.128.1.407. [DOI] [PubMed] [Google Scholar]
- 16.Merlo CA, Weiss ES, Orens JB, Borja MC, Diener-West M, Conte JV, et al. Impact of U.S. Lung Allocation Score on survival after lung transplantation. J Heart Lung Transplant. 2009;28:769–775. doi: 10.1016/j.healun.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 17.Schulman LL, Weinberg AD, McGregor C, Galantowicz ME, Suciu-Foca NM, Itescu S. Mismatches at the HLA-DR and HLA-B loci are risk factors for acute rejection after lung transplantation. American journal of respiratory and critical care medicine. 1998;157:1833–1837. doi: 10.1164/ajrccm.157.6.9707007. [DOI] [PubMed] [Google Scholar]
- 18.Kreider M, Kotloff RM. Selection of candidates for lung transplantation. Proceedings of the American Thoracic Society. 2009;6:20–27. doi: 10.1513/pats.200808-097GO. [DOI] [PubMed] [Google Scholar]
- 19.Oka T, Puskas JD, Mayer E, Cardoso PF, Shi SQ, Wisser W, et al. Low-potassium UW solution for lung preservation. Comparison with regular UW, LPD, and Euro-Collins solutions. Transplantation. 1991;52:984–988. doi: 10.1097/00007890-199112000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Chan BB, Kron IL, Flanagan TL, Kern JA, Hobson CE, Tribble CG. Impairment of vascular endothelial function by high-potassium storage solutions. The Annals of thoracic surgery. 1993;55:940–945. doi: 10.1016/0003-4975(93)90121-w. [DOI] [PubMed] [Google Scholar]
- 21.Muller C, Furst H, Reichenspurner H, Briegel J, Groh J, Reichart B. Lung procurement by low-potassium dextran and the effect on preservation injury. Munich Lung Transplant Group. Transplantation. 1999;68:1139–1143. doi: 10.1097/00007890-199910270-00014. [DOI] [PubMed] [Google Scholar]
- 22.Rabanal JM, Ibanez AM, Mons R, Gonzalez AM, Carbajo M, Ortega J, et al. Influence of preservation solution on early lung function (Euro-Collins vs Perfadex) Transplantation proceedings. 2003;35:1938–1939. doi: 10.1016/s0041-1345(03)00690-0. [DOI] [PubMed] [Google Scholar]
- 23.Struber M, Wilhelmi M, Harringer W, Niedermeyer J, Anssar M, Kunsebeck A, et al. Flush perfusion with low potassium dextran solution improves early graft function in clinical lung transplantation. Eur J Cardiothorac Surg. 2001;19:190–194. doi: 10.1016/s1010-7940(00)00631-x. [DOI] [PubMed] [Google Scholar]
- 24.Nath DS, Walter AR, Johnson AC, Radosevich DM, Prekker ME, Herrington CS, et al. Does Perfadex affect outcomes in clinical lung transplantation? J Heart Lung Transplant. 2005;24:2243–2248. doi: 10.1016/j.healun.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Aziz TM, Pillay TM, Corris PA, Forty J, Hilton CJ, Hasan A, et al. Perfadex for clinical lung procurement: is it an advance? The Annals of thoracic surgery. 2003;75:990–995. doi: 10.1016/s0003-4975(02)04491-0. [DOI] [PubMed] [Google Scholar]


