Abstract
Background
Endovascular technologies represent major advancements in treating descending thoracic aortic aneurysms(DTAA). We compared hospital charges of open thoracic aortic replacement(OTAR) with endovascular repair of thoracic aortic aneurysms(TEVAR).
Methods
Retrospective analysis of hospital charges related to repair of DTAA(2000–2009). Charges were inflation-adjusted for dollars in 2009.
Results
There were 50 OTAR and 50 TEVAR patients. OTAR charges were $64,531(IQR:49,000–108,515) versus $61,909(IQR:41,307–92,109) for TEVAR(p = 0.4). 10 patients(10%) died before discharge, with zero TEVAR deaths(p<0.05). For OTAR, supply charges($9,167) accounted for 13% of total charges versus 56% for TEVAR($40,468), p <.01. OTAR LOS was 12d(6d ICU stay); bed charges comprised 40% of total charges. TEVAR had lower LOS(5d with 2d ICU stay, p <0.001).
Conclusions
DTAA repair remains a formidable operation with significant resource utilization. TEVAR does not significantly reduce overall hospital charges due to device costs, but demonstrates improved mortality, ICU and total LOS.
Keywords: Aortic Aneurysm, Endovascular, Health Economics
Introduction
Refinements in endovascular technology have expanded the application of this therapy to include descending thoracic aortic aneurysms (DTAA).1 Since the United States Food and Drug Administration (FDA) approval of the first thoracic endograft device in 2005, there have been several studies comparing outcomes of thoracic endovascular aneurysm repair (TEVAR) with open thoracic aneurysm repair (OTAR).2–5 These studies reveal encouraging results for TEVAR, citing less pneumonia, shorter hospital stay, and improved aneurysm-related mortality, though none has been a randomized study.6, 7 Despite these reported clinical advantages, there are concerns regarding the costs of this new therapy.
As enthusiasm for endovascular techniques to repair aneurysms continues to change treatment paradigms in the thoracic aorta, it is important to elucidate the costs of delivering comprehensive care. Indeed, with increasing scrutiny of the cost of delivery of competing therapies, both immediate and long-term costs are important in determination of comparative effectiveness. Our study seeks to compare the hospital charges and resource utilization of descending thoracic aortic care in our institution between endovascular and open surgical techniques, and to identify the specific hospital-based areas which contribute to total charges.
Methods
Patient Data
We screened the Maryland state database by International Classification of Diseases (ICD-9) and procedure codes to identify all procedures involving the aorta at the Johns Hopkins Hospital between the years 2000–2009, and all charts of these patients were available for review. A query of the institutional medical record office was performed to assess consistency of reporting, and any coding errors were resolved immediately using clinic and operative records. Further direct medical chart review identified the procedures as related to the descending thoracic aorta, and these were further categorized as open or endovascular as appropriate. Patients who underwent operative repair of a DTAA only were included in the present analysis. Any patient with ascending, arch, or thoraco-abdominal aortic pathology was excluded. Hospital charges as reported to the Maryland State Health Services Cost Review Commission (HSCRC) were recorded.
Patient records were retrospectively reviewed following Institutional Review Board approval (NA_00035810). Demographic variables including age, gender, race, and cardiovascular co-morbidities were extracted from the medical record. Operative records were reviewed to gather details of the operations performed, including OTAR versus TEVAR approach. For endovascular procedures, the number of device pieces implanted and the need for adjunct procedures such as carotid-left subclavian bypass were recorded as well. Length of stay (LOS), intensive care unit (ICU) LOS, disposition to a rehabilitation center, and in-hospital mortality were recorded. The computerized medical record was queried to attain information regarding long-term follow-up. The Social Security Death Index was queried by social security number to ascertain survival status of all patients in this study.
Operative Technique for OTAR
In the day prior to the procedure, patients routinely undergo a spinal arteriogram to identify intercostal or lumbar arteries for re-implantation, and additionally placement of a cerebrospinal fluid drainage (CSFD) catheter. Our approach to repair of the thoracic and thoracoabdominal aorta has been described.8 Introduction of routine spinal drainage began in 1995, and open operative management has remained constant throughout the study period. In brief, to facilitate visualization of the thoracic aorta and mediastinal structures for partial left heart bypass, the anesthesia team places a double lumen endotracheal tube. A standard thoracoabdominal incision is made. Pleural entry is made through the 5th and/or 8th interspaces, using a single skin incision. The extent of involved aorta is freed from the parietal pleura. Adequate proximal and distal clamping zones are identified, and partial bypass is accomplished using the left inferior pulmonary vein for outflow and the left femoral artery for distal perfusion. Partial heart bypass maintains distal perfusion with moderate hypothermia at 32°C. When circulatory arrest is employed, patients are further systemically cooled to 20°C. Motor-evoked potential monitoring is routinely employed. Re-warming begins after the proximal anastomosis and intercostal inclusion patch are completed. After the distal anastomosis is complete, the diaphragm is re-approximated when necessary, and the remaining closure performed in standard fashion.
Endovascular Technique for TEVAR
At Johns Hopkins, patients undergoing TEVAR routinely receive preoperative CSFD. The CSFD catheter is placed on the first preoperative day. Currently, if the left subclavian artery will need to be covered due to anatomic constraints for proximal device deployment, a carotid-subclavian bypass is routinely performed prior to the endovascular procedure in a staged fashion. In our early experience, carotid-subclavian bypass was performed in select situations. All TEVAR procedures are performed in the endovascular operating suite using the Gore TAG (W.L. Gore & Associates, Flagstaff, Arizona) endovascular prosthesis. A standard cut-down is performed over the right femoral artery, and if the vessel is suitable for clamping, typically a 22-French sheath is inserted. Percutaneous left transfemoral access is obtained using Seldinger technique and a 5-French sheath is placed. A wire is negotiated into the abdominal aorta under fluoroscopy and pigtail catheter is positioned above the aortic bifurcation. Patients are then administered systemic heparin. Diagnostic angiograms confirm aortic dimensions prior to device deployment. Post-deployment angiograms are performed to document proper placement and absence of endoleak. Standard closures are performed, and the spinal catheter remains in place for 24–48 hours.
Charges Data
Hospital charges are obtained through the hospital billing department as reported to the Maryland State authorities, and represent all hospital charges for the index admission only including charges incurred during the operation. Charges data are divided into the following categories: Routine charges, operating room facility use, operating room supply use, pharmacy charges, laboratory charges, radiology charges, physical therapy charges, and other charges. Charges for carotid-subclavian bypass patients who required left subclavian artery coverage are included in the total hospital charges for TEVAR therapy. The majority of patients who underwent carotid-subclavian bypass underwent their TEVAR procedure in a staged fashion but as part of the same hospitalization. Entire admission charges for the carotid-subclavian bypass in the single patient who was discharged and re-admitted for the TEVAR operation were added to total charges for that individual patient. All charges information was inflation-adjusted according to the United States Department of Labor Consumer Price Index in United States dollars for the year 2009. Charges for TEVAR patients requiring re-intervention are reported separately from index admission charges, and not part of the total charges comparison between OTAR and TEVAR.
Statistical Analysis
Differences between OTAR and TEVAR patients were compared using two-tailed student’s t-test for continuous variables, and chi-square test for categorical variables. Continuous variables are presented with the mean ± standard deviation (SD). Categorical variables are shown in whole numbers and percentages. All charges data are presented in median and interquartile range (IQR), as hospital charges were not normally distributed. Wilcoxon rank-sum test compared charges data between the two groups. One-way analysis of variance (ANOVA) compared annual OTAR and TEVAR charges over time. Because charges data were not normally distributed, a logarithmic transformation of these data was performed in order to perform a linear regression to further assess any correlation with charges over time.
Clinical outcomes examined included discharge to a rehabilitation facility and in-hospital mortality. Univariate analysis was used in an exploratory fashion to identify risk factors associated with each outcome. Variables with a p-value<0.1 were then incorporated into separate multivariable logistic regression models identifying risk factors significantly associated with discharge to a rehabilitation center and in-hospital mortality. Odds ratios (OR) are presented with 95% confidence intervals (CI). 1-year survival was estimated using the Kaplan-Meier method. The log-rank test was used to compare survival estimates between the OTAR and TEVAR groups. Cox proportional hazards regression examined the hazard of death within one year following the index operation. Independent covariates identified as potential confounders were tested in a univariate model. Variables achieving significance on an exploratory level (p<0.1) were incorporated into the final Cox model. Independent variables comprised within the final Cox model included: Age, chronic renal insufficiency (CRI), chronic obstructive pulmonary disease (COPD), coronary artery disease (CAD), OTAR versus TEVAR, and aortic rupture. Results are presented as Hazard Ratios (HR) with 95% CI. P-values less than 0.05 were considered statistically significant for all tests. Analysis was performed using Stata statistical software, version 9.1 (StataCorp, College Station, Texas).
Results
From 2000–2009, 100 patients were treated for DTAA. 50 patients underwent OTAR; and 50 patients received the TEVAR approach. 20 (40%) of the OTAR patients had their operation after FDA approval of the Gore TAG device for TEVAR in 2005. Overall, the mean age was 65.6±13.2yrs. Patients tended to be older in the TEVAR group: 68.3±12.4 versus 63.0±13.5yrs, p=0.04. There were 59 (59%) men in the entire series. 4(8%) of the patients in the OTAR group had a diagnosis of connective tissue disorder, and more patients in the TEVAR group had a diagnosis of COPD. In the TEVAR group, patients received a mean of 2.1±1.1 endograft devices. The remaining clinical variables were evenly distributed between the two treatment groups (Table 1).
Table 1.
Baseline demographics stratified by OTAR vs TEVAR
| Variables | OTAR (#=50) | TEVAR (#=50) | P-value |
|---|---|---|---|
| Mean age, years (SD) | 63.0.(13.5) | 68.3 (12.4) | 0.04 |
| Male gender, # (%) | 34 (68) | 25 (50) | 0.06 |
| Diabetes mellitus, # (%) | 4 (8) | 9 (18) | 0.1 |
| Coronary artery disease, # (%) | 8 (16) | 16 (32) | 0.06 |
| Smoking history, # (%) | 23 (46) | 26 (52) | 0.5 |
| Chronic obstructive pulmonary disease, # (%) | 5 (10) | 14 (28) | 0.02 |
| Hypertension, # (%) | 33 (66) | 41 (82) | 0.07 |
| Peripheral vascular disease, # (%) | 11(22) | 13 (26) | 0.6 |
| Chronic renal insufficiency, # (%) | 7 (14) | 12 (24) | 0.1 |
| Connective tissue disorder, # (%) | 4 (8) | 0 (0) | 0.2 |
| Left subclavian coverage, # (%) | -- | 18 (36) | -- |
| Left subclavian bypass, # (%) | -- | 8 (16) | -- |
Comparing acuity of presentation revealed equivalent rates of patients who presented with acutely symptomatic thoracic aortic pathology: 24 (48%) patients in the OTAR group versus 21 (42%) patients in the TEVAR group, p =0.5. 6 (12%) patients in the OTAR group presented with a contained rupture, compared with 7 (14%) patients receiving endovascular intervention (p=0.8). DTAA was the most common presenting condition in both groups (Table 2). 14 (28%) patients in the OTAR group presented with Type B aortic dissection compared with 4(8%) in TEVAR group, and these patients underwent operations for either aneurysmal degeneration or acute evidence of malperfusion. Four patients in the TEVAR group underwent subsequent interventions in order to address endoleaks(Table 3).
Table 2.
Acuity of presentation and description of aortic pathology stratified by OTAR vs TEVAR
| Variables | OTAR (N=50) | TEVAR (N=50) | P-value |
|---|---|---|---|
| Symptomatic, # (%) | 24 (48) | 21 (42) | 0.5 |
| Rupture, # (%) | 6 (12) | 7 (14) | 0.8 |
| Traumatic, # (%) | 3 (6) | 3 (6) | 0.9 |
| Aortic Pathology | 0.06 | ||
| DTAA, # (%) | 31 (62) | 41 (82) | |
| Type B dissection, # (%) | 14 (28) | 4 (8) | |
| Pseudoaneurysm, # (%) | 1 (2) | 3 (6) | |
| Other, # (%) | 4 (8) | 2 (4) |
19 (38%) of the TEVAR patients required coverage of the left subclavian artery to achieve an adequate proximal landing zone. Of these 19 patients, 9 (47%) underwent carotid-subclavian bypass prior to the definitive TEVAR procedure. The first 4 TEVAR patients that required left subclavian artery coverage did not undergo carotid-subclavian bypass. None of these four patients developed postoperative arm ischemia. The trend in our later experience has been for patients requiring left subclavian artery coverage to have routine carotid-subclavian bypass in a staged fashion.
Hospital charges and disposition
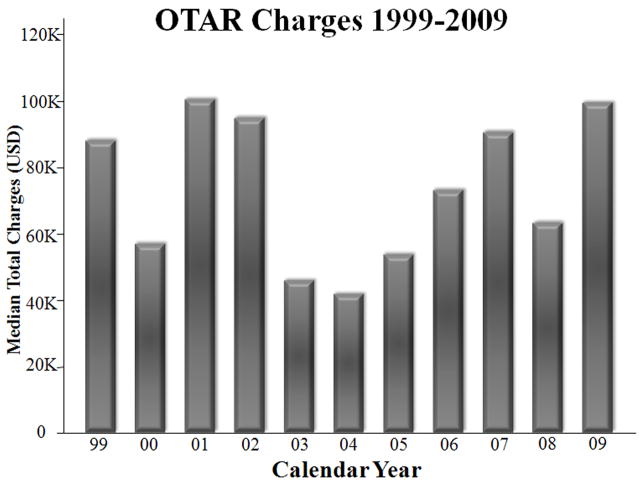
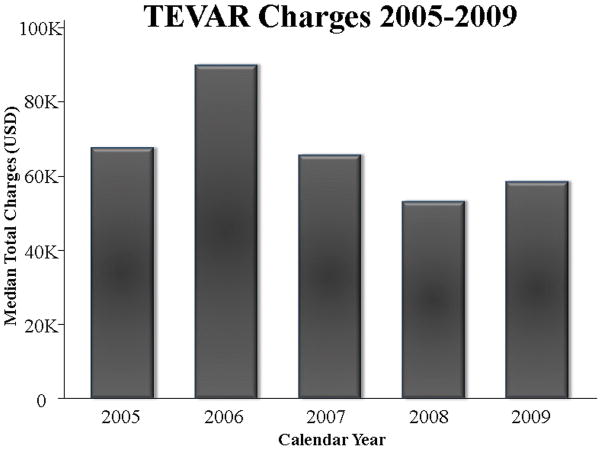
There was no difference in median total hospital charges between the two surgical approaches: $61,909 (IQR:41,307–92,109) for TEVAR compared with $64,531 (IQR49,000–108,515) for OTAR, p=0.4. Sub-analysis was performed including only OTAR patients since FDA approval of TEVAR in 2005, and there was still no difference in total hospital charges (p=0.4). Total hospital charges over time for OTAR are depicted in Figure 1. When performing a linear regression of charges data after logarithmic transformation, there was no significant correlation between OTAR charges and year of operation (correlation coefficient: 0.002, 95% CI −0.05–0.06, p=0.9). Median hospital charges for TEVAR appeared to be lowering with time, but this was not statistically significant (Figure 2).
Figure 1.
Figure 2.
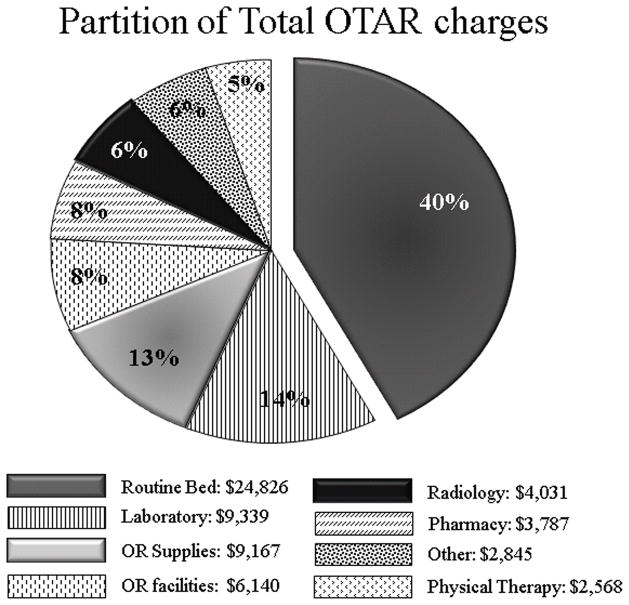
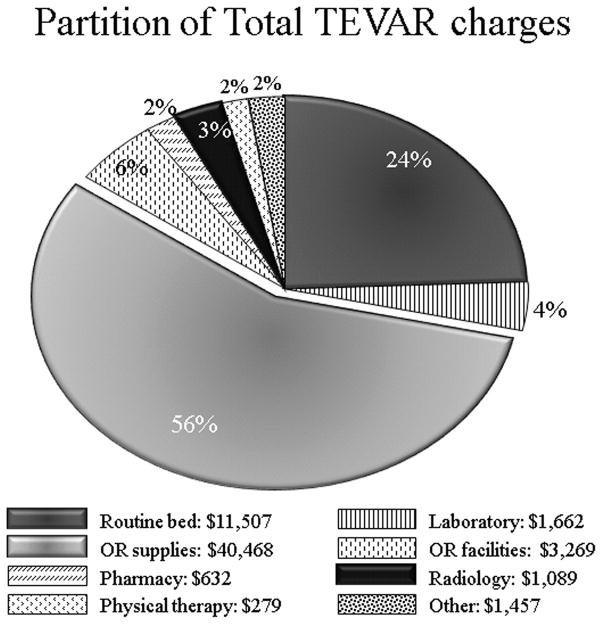
Hospital charges according to category were compared between TEVAR and OTAR. Reflecting the high cost of endografts and related supplies, operating room supplies charges were significantly greater in the TEVAR group: $40,468(IQR23,368–51,089) for TEVAR versus $9,167(IQR5,766–12,592) for OTAR, p<0.001. Conversely, patients who underwent OTAR were more likely to utilize hospital resources in the form of ICU and ward care, manifest by greater routine bed charges: $24,826(IQR17,845 –41,638) for OTAR versus $11,507(IQR6,801–24,345) in the TEVAR group, p<0.001 (Figures 3 and 4).
Figure 3.
Figure 4.
Median LOS was significantly greater in the OTAR group compared with TEVAR:12d(IQR10–21) versus 5d(IQR3–10), p<0.001, respectively. In addition, patients experienced shorter ICU LOS in the TEVAR group: 2d(IQR1–3) versus 6d (IQR3–11), p<0.001. Delayed paraplegia occurred in 2(4%) patients after TEVAR repair; no TEVAR patients required renal replacement therapy(RRT). 7(14%) patients developed paraplegia in the OTAR group, and 7(14%) patients experienced severe kidney dysfunction requiring RRT. In-hospital charges relating to these complications are included in the charges analysis. Patients in the TEVAR group were more likely to be discharged directly to home compared with patients in the OTAR group: 40(80%) patients versus 26(52%), p<0.01. Multivariable logistic regression revealed age(OR: 1.07, 95% CI 1.02–1.12) increased the odds of discharge to a rehabilitation facility, whereas TEVAR approach lowered the likelihood of post-discharge inpatient rehabilitation(OR: 0.19, 95% CI 0.05–0.70).
Mortality analysis
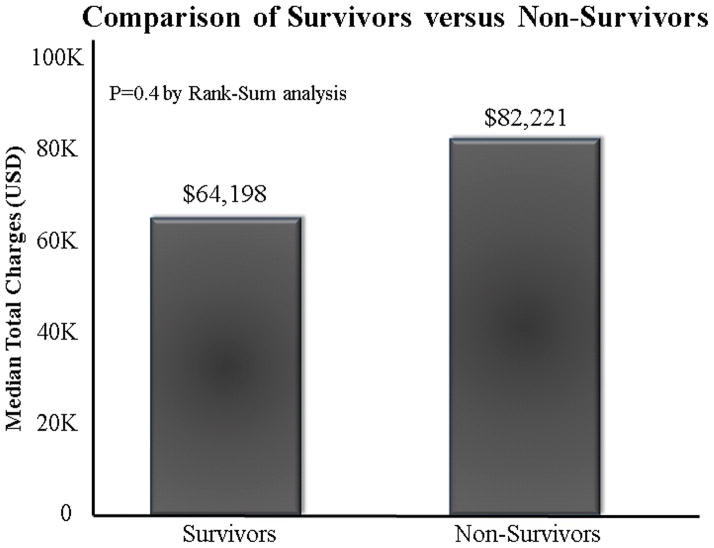
There were 10(10%) in-hospital deaths, all in patients undergoing open repair. Six of these patients had their operations performed in an emergency setting for aortic rupture (aneurysm-related or post-traumatic) or dissection with evidence of malperfusion; the remaining 4 patients underwent elective repairs. There was no difference in total hospital charges when comparing non-survivors with those who survived to hospital discharge: $82,221(IQR48,305 –218,430) versus $64,198(IQR49,776–102,989), p=0.4 (Figure 5). Multivariable logistic regression analysis demonstrated age(OR:1.08,95% CI 1.01–1.16), hypertension(OR:0.05, 95%CI 0.01–0.40), and aortic rupture(OR:9.0, 95% CI 1.58–51.2) as predictors associated with in-hospital mortality.
Figure 5.
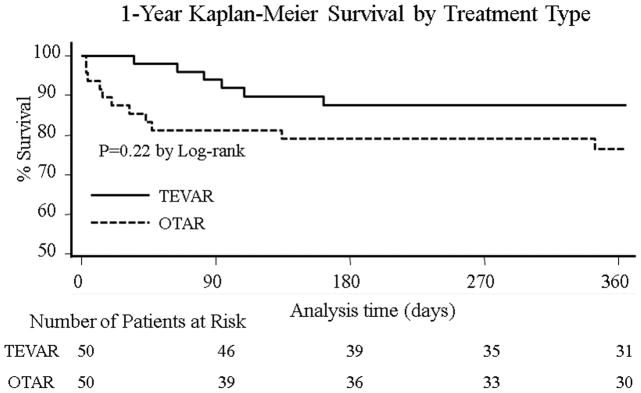
Average follow-up was 18(±26) months. 19 patients (19%) died within one year of the initial operation. Log-rank analysis revealed no significant difference in the 1-year survival curves between patients who received OTAR versus TEVAR (Figure 6). On Cox multivariable regression analysis, age (HR:1.06,95% CI 1.01–1.12) and aortic rupture (HR:6.46, 95 % CI 1.91–21.8) increased the hazard of death within the first year following aortic repair. In the risk-adjusted multivariable model the TEVAR approach lowered the hazard of death in the first year following repair(HR:0.14, 95% CI 0.04–0.51).
Figure 6.
Discussion
The efficacy of TEVAR versus OTAR to reduce early morbidity and mortality, with similar long-term survival has been established.6 In light of this and other perceived benefits, we have employed endovascular techniques to address thoracic aortic disease with enthusiasm. In this study, since FDA approval of the GORE TAG device and more recently other devices, our practice has shifted to perform TEVAR over OTAR on a 2:1 basis. Indeed, a recent analysis of the Nationwide Inpatient Sample(NIS) by Orandi and co-authors revealed in late 2005, TEVAR was being performed three times more often than OTAR.9 It may be anticipated as the collective national experience grows, the fraction of OTAR patients will continue to decrease as devices proliferate and longevity can be demonstrated. Notable exceptions to the application of TEVAR are first, those patients currently affected by connective tissue disorders(who constitute a significant percentage of our practice), and second, those afflicted with acute and chronic dissections for which consensus documents rightly describe the inherent limitation of FDA approved devices to achieve suitable outcomes.10
Traditional supply-and-demand models of economies of significant scale suggest device cost should decrease with increasing market competition of available devices. In the arena of endovascular repair of abdominal aortic aneurysms(EVAR), now over 10 years into widespread practice, device costs have continued to escalate despite market presence of more companies. Indeed, prior EVAR reports suggested insufficient coverage of Medicare reimbursement due to high device costs.11 In a later report, Noll continued to reveal high device costs as a contributor to total cost of delivery of care in the long-term.12 In our assessment of TEVAR, 56% of total costs was derived from OR supply charges which include all stent-graft devices and adjunctive endovascular supplies. Clearly, early cost effectiveness would be likely demonstrated with a reduced device charge for TEVAR, but to date, all available devices are entering the market with similar costs. Our study builds on the experience of two prior reports comparing TEVAR with OTAR. Orandi et al. found no difference in OTAR versus TEVAR costs in a large cohort of patients.9 In contrast, Beaver et al. found higher operating room costs for the TEVAR group, but overall hospitalization costs were greater in the OTAR group due to increased utilization of anesthesia, pharmacy, and radiology services.13 This study is more specific in relating the charge buckets underlying the total expenditures; and due to the inability to account for cost-shifting in other studies, this report may be more accurate given the unique payment system in the state of Maryland.
In contradistinction, Glade and colleagues, in 2005, demonstrated cost efficacy in their assessment of the index TEVAR procedure over OTAR(€ 20,663 vs €33,770).14 Interestingly, their comparison shadows our charge categories, with the notable exception of a much reduced prosthesis charge of only €10,000 for two devices – yielding a conversion to US dollars of $11,720 by a contemporaneous 2005 Interbank Rate Conversion to USD. Hence, considering the multiplicity of devices available in the European Union in 2005 which lowered their costs, it is unclear how to reconcile the current state of device pricing in the United States market. In the current era of cost-effectiveness, it may become incumbent on the cardiovascular surgery community in the US to assume a more proactive role in lowering device costs.
Costs of TEVAR therapy include both the initial cost of placement and also the long-term cost of follow-up and surveillance. Endoleak formation and aneurysm expansion are well-known occurrences after endovascular interventions on the aorta, and contribute to the long-term cost of follow-up.14 Patients undergoing TEVAR require routine, lifelong surveillance, typically with computerized tomography(CT), and many require secondary interventions. Addressing the issue of long-term costs requires ongoing monitoring and longer follow-up than is currently available in the published literature- only recent studies document the postplacement costs of endovascular therapy in the abdominal aorta.12 In their report of EVAR, postplacement cost of EVAR was increased eight fold with requirement for secondary procedure($31, 696) versus those without procedures($3,668). Our charge assessment of the secondary procedures in Table 3 parallels the experience of EVAR. Our secondary reintervention rate is 7.8%, higher than the 3.6% reintervention rate of the 5yr followup of the Gore TAG Pivotal trial.6 The higher rate of reintervention in our study likely reflects our experience in managing patients with difficult proximal fixation zones, and accordingly, a higher event rate. Our costs on the secondary interventions are largely dependent on the nature of the intervention, but again, device costs are the majority of the expenditure(data not shown).
The limitations of the current study are the use of hospital charges as an index of cost. It is important to cite the unique insurance structure present in the State of Maryland which serves to neutralize this issue. The Maryland HSCRC was established by the state legislature in 1971 and supported by the hospital industry. The HSCRC was authorized to establish hospital payment rates to promote cost containment, thus improving access to care. The HSCRC sets all payment rates for insurers, both private and public, including Medicare and Medicaid within all Maryland hospitals. Thus, the common practice of “cost shifting” by overcharging privately insured patients is absent, making our charge data consistent and predictable. The authors’ institution HSCRC rate for charge payment has been cost + 1–3% over the study interval. This formula applies evenly to each charge category included in this analysis. Accordingly, we believe this study represents the most accurate assessment of TEVAR and OTAR costs to date. Similar reports have cited costs as equivalent, but alluded to increased charges consistent with “cost shifting.” In a non-rate controlled state, charges were $119,932 for open TAAA repair, but cost was cited as equal.9
Exact charges for post-discharge inpatient rehabilitation care were not available for rigorous analysis from the billing office. However, estimates for one month of inpatient physical therapy, which was more common in the OTAR group, are $30,000(Personal communication rehabilitation facility executive). The purpose of this paper was to compare the costs of delivering care for each form of therapy during the index hospitalization. As well, slightly different time frames were studied between the two groups, but significant resource utilization for OTAR is still the rule.
Conclusion
By either an open or endovascular approach, thoracic aortic repair remains a formidable operation associated with significant resource utilization. Endovascular thoracic aortic repair does not significantly reduce hospital costs due to devices costs, but demonstrates improved mortality and ICU usage in comparison to open repairs. Despite the costs of the thoracic aortic devices and their potential re-interventions, it is likely market forces, both patient and industry driven, that will continue to drive the expansion of TEVAR. Long-term follow-up is necessary to determine if secondary intervention costs in TEVAR will significantly offset the historically stable cost of OTAR, and lead to changes in delivery of thoracic aortic care in this current climate of increasing scrutiny of high cost therapies without demonstrable survival benefit.
References
- 1.Dake MD, Miller DC, Semba CP, Mitchell RS, Walker PJ, Liddell RP. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. The New England journal of medicine. 1994;331:1729–1734. doi: 10.1056/NEJM199412293312601. [DOI] [PubMed] [Google Scholar]
- 2.Makaroun MS, Dillavou ED, Kee ST, Sicard G, Chaikof E, Bavaria J, et al. Endovascular treatment of thoracic aortic aneurysms: results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J Vasc Surg. 2005;41:1–9. doi: 10.1016/j.jvs.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 3.Bavaria JE, Appoo JJ, Makaroun MS, Verter J, Yu ZF, Mitchell RS. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. The Journal of thoracic and cardiovascular surgery. 2007;133:369–377. doi: 10.1016/j.jtcvs.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 4.Matsumura JS, Cambria RP, Dake MD, Moore RD, Svensson LG, Snyder S. International controlled clinical trial of thoracic endovascular aneurysm repair with the Zenith TX2 endovascular graft: 1-year results. J Vasc Surg. 2008;47:247–257. doi: 10.1016/j.jvs.2007.10.032. discussion 257. [DOI] [PubMed] [Google Scholar]
- 5.Leurs LJ, Bell R, Degrieck Y, Thomas S, Hobo R, Lundbom J. Endovascular treatment of thoracic aortic diseases: combined experience from the EUROSTAR and United Kingdom Thoracic Endograft registries. J Vasc Surg. 2004;40:670–679. doi: 10.1016/j.jvs.2004.07.008. discussion679–680. [DOI] [PubMed] [Google Scholar]
- 6.Makaroun MS, Dillavou ED, Wheatley GH, Cambria RP. Five-year results of endovascular treatment with the Gore TAG device compared with open repair of thoracic aortic aneurysms. J Vasc Surg. 2008;47:912–918. doi: 10.1016/j.jvs.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Stone DH, Brewster DC, Kwolek CJ, Lamuraglia GM, Conrad MF, Chung TK, et al. Stent-graft versus open-surgical repair of the thoracic aorta: mid-term results. J Vasc Surg. 2006;44:1188–1197. doi: 10.1016/j.jvs.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Black JH., 3rd Technique for repair of suprarenal and thoracoabdominal aortic aneurysms. J Vasc Surg. 2009;50:936–941. doi: 10.1016/j.jvs.2009.02.215. [DOI] [PubMed] [Google Scholar]
- 9.Orandi BJ, Dimick JB, Deeb GM, Patel HJ, Upchurch GR., Jr A population-based analysis of endovascular versus open thoracic aortic aneurysm repair. J Vasc Surg. 2009;49:1112–1116. doi: 10.1016/j.jvs.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 10.Svensson LG, Kouchoukos NT, Miller DC, Bavaria JE, Coselli JS, Curi MA, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. The Annals of thoracic surgery. 2008;85:S1–41. doi: 10.1016/j.athoracsur.2007.10.099. [DOI] [PubMed] [Google Scholar]
- 11.Sternbergh WC, 3rd, Money SR. Hospital cost of endovascular versus open repair of abdominal aortic aneurysms: a multicenter study. J Vasc Surg. 2000;31:237–244. doi: 10.1016/s0741-5214(00)90154-x. [DOI] [PubMed] [Google Scholar]
- 12.Noll RE, Jr, Tonnessen BH, Mannava K, Money SR, Sternbergh WC., 3rd Long-term postplacement cost after endovascular aneurysm repair. J Vasc Surg. 2007;46:9–15. doi: 10.1016/j.jvs.2007.03.017. discussion 15. [DOI] [PubMed] [Google Scholar]
- 13.Walker KL, Lipori P, Lee WA, Beaver TM. Cost of thoracic endovascular aortic repair versus open repair and implications for the US health care system. The Journal of thoracic and cardiovascular surgery. 2009;139:231–232. doi: 10.1016/j.jtcvs.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Glade GJ, Vahl AC, Wisselink W, Linsen MA, Balm R. Mid-term survival and costs of treatment of patients with descending thoracic aortic aneurysms; endovascular vs. open repair: a case-control study. Eur J Vasc Endovasc Surg. 2005;29:28–34. doi: 10.1016/j.ejvs.2004.10.003. [DOI] [PubMed] [Google Scholar]