Abstract
Background
Alcohol abuse is associated with impaired circadian rhythms and sleep. Ethanol administration disrupts circadian clock phase-resetting, suggesting a mode for the disruptive effect of alcohol abuse on the circadian timing system. In this study, we extend previous work in C57BL/6J mice to: 1) characterize the SCN pharmacokinetics of acute systemic ethanol administration; 2) explore the effects of acute ethanol on photic and non-photic phase-resetting; and 2) determine if the SCN is a direct target for photic effects.
Methods
First, microdialysis was used to characterize the pharmacokinetics of acute i.p. injections of 3 doses of ethanol (0.5, 1.0 and 2.0 g/kg) in the mouse suprachiasmatic (SCN) circadian clock. Second, the effects of acute i.p. ethanol administration on photic phase-delays and serotonergic ([+]8-OH-DPAT-induced) phase-advances of the circadian activity rhythm were assessed. Third, the effects of reverse-microdialysis ethanol perfusion of the SCN on photic phase-resetting were characterized.
Results
Peak ethanol levels from the 3 doses of ethanol in the SCN occurred within 20–40 min post-injection with half-lives for clearance ranging from 0.6–1.8 hr. Systemic ethanol treatment dose-dependently attenuated photic and serotonergic phase-resetting. This treatment also did not affect basal SCN neuronal activity as assessed by Fos expression. Intra-SCN perfusion with ethanol markedly reduced photic phase-delays.
Conclusions
These results confirm that acute ethanol attenuates photic phase-delay shifts and serotonergic phase-advance shifts in the mouse. This dual effect could disrupt photic and non-photic entrainment mechanisms governing circadian clock timing. It is also significant that the SCN clock is a direct target for disruptive effects of ethanol on photic shifting. Such actions by ethanol could underlie the disruptive effects of alcohol abuse on behavioral, physiological, and endocrine rhythms associated with alcoholism.
Keywords: Circadian, Ethanol, Serotonin, Photic, Microdialysis, Suprachiasmatic Nucleus
INTRODUCTON
Reliance on alcohol to alleviate the malaise of sleep/wake cycle disturbance is common among shift workers and others exposed to challenging circadian environments (Roehrs and Roth, 2001; Smart, 1979; Trinkoff and Storr, 1998). Alcohol abuse can create a self-perpetuating cycle of alcohol dependence and chronobiological disturbance by disrupting circadian rhythms in hormone release, metabolism, core body temperature, and sleep/wake consolidation (Chen et al., 2004; Wasielewski and Holloway, 2001; Brower, 2001; Sack et al., 2007; Röjdmark et al., 1993; Iranmanesh 1989; Kühlwein et al. 2003, Fonzi et al. 1994; Mukai et al., 1998). There is also a disruption of central glutamatergic transmission caused by ethanol's inhibition of glutamate signaling at the NR2B subunit of the N-methyl-D-aspartate (NMDA) receptor (Krystal et al., 2003; Dodd et al., 2000). In abstaining alcoholics, this situation can promote a hyperglutamatergic condition, precipitating relapse, seizures, cognitive impairments and neural necrosis (Dodd et al., 2000; Floyd et al., 2003). Ethanol has also been shown in animal models to alter serotonergic activity, with increases following acute administration and decreases during chronic exposure (reviewed in Rosenwasser, 2001; LeMarquand et al., 1994).
The disruptive effects of ethanol on glutamate and serotonin (5-HT) have a strong impact on circadian timing, since both of these transmitters play key roles in regulating the circadian clock of the suprachiasmatic nucleus (SCN). Specifically, glutamate mediates photic entraining input from the retina to the SCN clock (Johnson et al., 1988; Meijer et al., 1988; reviewed in Challet and Pévet, 2003), and 5-HT from the midbrain raphe modulates photic input and mediates behavior-induced non-photic signaling to the clock (Dudley et al., 1998, 1999; Glass et al., 2000, 2003; Meyer-Bernstein et al., 1997; Rea et al., 1994; Grossman et al., 2000; Mistlberger and Antle, 1998; reviewed in Challet and Pévet, 2003). We and others have demonstrated that ethanol can block both photic and serotonergic circadian phase-resetting in Syrian hamsters (Ruby et al., 2009a, b; Seggio et al., 2007). In the C57BL/6 mouse, chronic ethanol drinking impairs photic phase-resetting and photic entrainment in vivo (Ruby et al., 2009a, b; Brager et al., 2010) and acute ethanol treatment blocks glutamatergic phase-resetting and enhances serotonergic phase-resetting in the in vitro SCN slice preparation (Prosser et al., 2008). We have also shown that the mouse SCN slice quickly develops tolerance to ethanol, with rapid (≤15 min) loss of the differential ethanol effects on glutamatergic and serotonergic phase-resetting responses (Prosser and Glass, 2009).
Despite these studies, little is known of the in vivo circadian effects of acute ethanol administration in the mouse. To fill this knowledge gap, a multifold approach was used in mice to: 1) characterize the pharmacokinetic profile of i.p. injected ethanol in the SCN; 2) explore the effects of i.p. ethanol administration on photic and serotonergic phase-resetting; and 3) determine if the SCN is a direct target of ethanol on photic phase-resetting using reverse microdialysis application of ethanol to the SCN. Results from these experiments would expand our knowledge of the nature of ethanol actions on the mammalian circadian timing system.
MATERIALS AND METHODS
Animals
Adult (~8 week-old) male C57BL/6J mice were obtained from The Jackson Laboratory (Bar Harbor,ME). Animals were singly housed in polycarbonate cages under a 12:12 LD photoperiod at a light intensity of 270 lux in a temperature-controlled vivarium (23°C) with food (Prolab 3000, PMI Feeds, St. Louis, MO) and water provided ad libitum. The experiments followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Kent State Institutional Animal Care and Use Committee.
SCN pharmacokinetics of acute systemic ethanol administration
In vivo microdialysis was used to characterize the pharmacokinetics of EtOH within the SCN in freely-behaving mice following i.p. EtOH administration. The SCN microdialysis procedures have been described previously (Dudley et al., 1998; Ruby et al., 2009a, b). Briefly, concentrically designed probes were constructed from a 26-gauge stainless steel outer cannula into which was inserted 32-gauge fused silica tubing. Hemicellulose dialysis tubing (230 µm outer diameter; 12kDa MW cutoff; Spectra-por; Fisher) was affixed to the outer cannula with epoxy glue. The active dialysis length was 1.0 mm. Two days prior to experimentation, the animals were pretreated with Marcaine (0.25% bupivicaine; 0.05 ml) in the scalp area and atropine subcutaneously (0.09%; 0.10 ml) to reduce localized pain and respiratory occlusion, respectively, and subsequently anesthetized with pentobarbital sodium (Nembutal; 35 mg/kg). The probe was stereotaxically aimed at the lateral margin of the SCN (anteroposterior −0.46 mm from bregma; lateral +0.2 mm from midline; horizontal 5.5 mm from dura) to avoid damaging the nucleus. Probes were secured to the skull with dental cement and stainless steel screws. The 2 day recovery period was used, as this is considered optimal for tissue recovery and probe function. Microdialysis sampling was undertaken 1 h before, and extended 4.3 h after an i.p. injection of ethanol (0.5 g/kg, 1.0 g/kg and 2.0 g/kg each diluted to 20% v/v in physiological saline; n=3/group). The sampling interval was 20 min at a flow rate of 1.0 µl/min. Ethanol in 5 µl aliquots of microdialysate was measured with an Analox AM1 ethanol analyser (Analox Instruments, Lunenburg, MA). Probe efficiency was estimated in vitro by measuring the yield of ethanol from probes immersed in a 50 mM ethanol solution at 37°C and averaged ~10%. Pharmacokinetic measurements included peak EtOH concentration (mM), time of peak EtOH concentration (Tmax), and the half-life (t1/2 elimination) of EtOH elimination. These values were used to determine the optimum timing for ethanol administration prior to a phase-resetting treatment. Following experimentation, probe placement was verified from 60 µm-thick frozen sections stained with cresyl violet.
Effects of acute systemic administration of ethanol on photic phase-resetting
This experiment was undertaken to determine if ethanol inhibits photic phase-resetting in vivo as previously reported in hamsters (Ruby et al., 2009a). Mice under LD were individually caged and their general locomotor activity rhythm was measured over a 2 wk pre-experimentation period using overhead passive infrared motion detectors interfaced with a computerized data acquisition system (Clocklab: Coulbourn Instruments, Whitehall, PA). Data was collected in 1 min bins, and activity onset associated with lights-off (designated as zeitgeber time [ZT] 12) was defined by the initial 6 min period that 1) coincided with an intensity of activity that exceeded 10% of the maximum rate for the day; 2) preceded by at least 4 hr of activity quiescence; and 3) followed by at least 60 min of sustained activity. Phase shifts were calculated as the difference between the projected times of activity onset of baseline entrainment and days following EtOH-photic treatment as determined by 1) back extrapolation of the least-squares line through activity onsets on days 3–7 after EtOH-photic treatment and 2) extrapolation of the least-squares line calculated from activity onset data collected the last 5 days of baseline entrainment.
On the day of experimentation, animals received an i.p. injection one of three doses of ethanol (0.5 g/kg, 1.0 g/kg or 2.0 g/kg [all 20% v/v in physiological saline]; n=7/group) or saline alone preceding a 30 min phase-delaying light pulse (25 lux) delivered from ZT 16–16.5. Immediately following the light pulse, the animals were released into constant darkness (DD) for 2 wks to assess the extent of phase-delaying using an Aschoff Type II procedure (Daan and Pittendrigh, 1976). Under DD, activity onset is designated as circadian time (CT) 12 and is the phase reference point for the onset of the subjective night.
Effects of SCN ethanol reverse microdialysis perfusion on photic phase-resetting
In this experiment, ethanol was administered directly to the SCN region using reverse microdialysis perfusion to determine if the SCN is a direct target of ethanol's inhibitory effect on photic phase-resetting. Animals under LD were singly caged, and their circadian activity rhythms were measured over a 2 wk period prior to experimentation. Two days before experimentation, the animals were outfitted with a microdialysis probe stereotaxically aimed at the lateral margin of the SCN as described above. On the day of experimentation, microdialysis probes were perfused with ACSF alone or ACSF+ethanol (500 mM) from a syringe pump. Based on in vitro probe efficiency of 10% this provided a theoretical ethanol concentration of ~ 50mM outside the probe. Continuous perfusion of ACSF or ACSF+ethanol commenced 30 min before, and extended throughout a 30 min phase-delaying light pulse (25 lux) (or no light pulse for controls) administered at ZT 14 (n=4 for both groups). Immediately following this treatment, the animals were released into DD to assess phase-shifting responses using the Aschoff Type II procedure. Following experimentation, probe placement was verified histologically from fixed frozen sections stained with cresyl violet.
Effects of acute systemic ethanol administration on serotonergic phase-resetting
The effect of ethanol on serotonergic phase-resetting was explored to determine if ethanol suppresses non-photic phase regulation in vivo as previously reported in hamsters (Ruby et al., 2009a). Mice under LD were individually caged, and their circadian locomotor activity rhythms measured over a two week period prior to experimentation. On the day of the experiment, animals received an i.p. injection of one of three doses of ethanol (0.5 g/kg, 1.0 g/kg or 2.0 g/kg) 30 min before i.p. injection of the serotonin1A/7 agonist (+)8-OH-DPAT (10.0 mg/kg; Sigma Chemical Co., St. Louis MO) dissolved in dimethyl sulfoxide (DMSO) or DMSO alone at ZT 6 (n=7/group) which coincides with the phase-advancing portion of the non-photic PRC. Immediately following drug injection, the animals were released into constant darkness to assess phase-advancing responses using the Aschoff Type II procedure.
Effects of acute systemic ethanol administration on Fos expression in the SCN
This experiment was undertaken to determine if ethanol affects basal SCN neural activity at midday. Mice received an i.p. injection of ethanol (2.0 g/kg) or saline vehicle at ZT 6 (n=5/group). One hundred minutes after injection, the animals were deeply anesthetized with Nembutal and intracardially perfused with 100 ml of 4% buffered paraformaldehyde (pH = 7.3). The brains were extracted, and immersion-fixed in 4% paraformaldehyde for 24 h, followed by immersion in 30% sucrose for 24 h at 4°C. Cryostat sections (40µm-thick) were incubated with a rabbit polyclonal IgG antibody (c-fos (4); Santa Cruz Biotechnology; Santa Cruz, CA), and Fos expression was visualized using Vectastain Elite ABC kit with 3,3-diaminobenzidine tetrahydrochloride as chromagen (Vector Labs, Burlingame, CA). Sections were mounted with permount (Fisher), and Fos expression was quantified using ImageJ (National Institutes of Health, Bethesda, MD). Counts of immunostained nuclei were undertaken blindly from within the entire mid-posterior region of the SCN.
Statistics
Multivariate analysis of variance was used to compare the pharmacokinetic profiles of the different ethanol doses. The effects of ethanol on photic and non-photic phase-resetting were assessed by one-way ANOVA. Student Neuman-Keuls post-hoc comparisons were utilized when the analysis of variance revealed significant treatment effects. The effect of ethanol on SCN Fos expression was assessed using an unpaired t-test. The level of significance was set at p<0.05.
RESULTS
Ethanol pharmacokinetics in the SCN
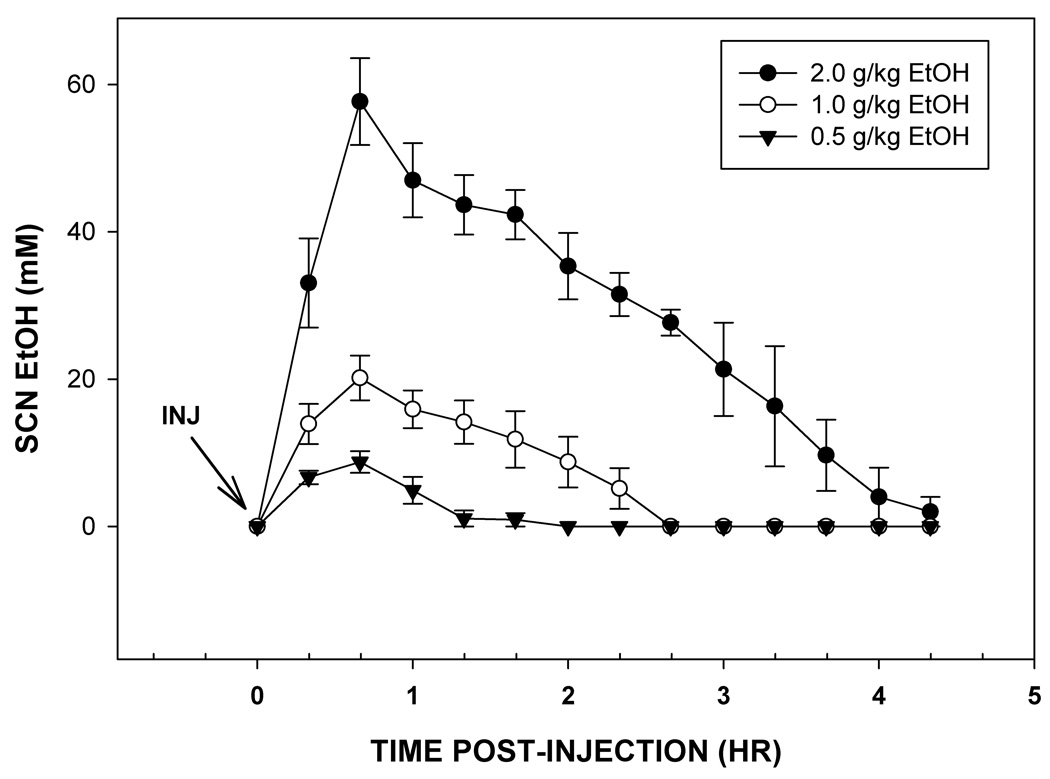
The pharmacokinetic profiles of ethanol in the SCN following acute i.p. injections of ethanol (0.5 g/kg to 2.0 g/kg) as assessed in freely-behaving mice by microdialysis are presented in Fig. 1. Peak levels of ethanol in the SCN were independent of dose, and all occurred within 20–40 min post-administration. These levels, estimated using a 10% probe efficiency, were 57.7±5.9 mM, 20.2±3.0 mM, and 8.8±1.5 mM, for the 2.0, 1.0 and 0.5 g/kg doses, respectively. The half-lives of the absorbed ethanol (t1/2 elimination) were 1.8±0.2 hr, 1.1±0.5 hr, and 0.6±0.1 hr, for the same doses, respectively.
Figure 1.
Pharmacokinetic profiles of ethanol in the SCN following i.p. injections of three doses of ethanol. For each dose, peak levels occurred within 20–40 min of injection. Clearance rate was dose-dependent. Time points are the means ± S.E.
Acute systemic EtOH administration dose-dependently attenuates photic phase-resetting
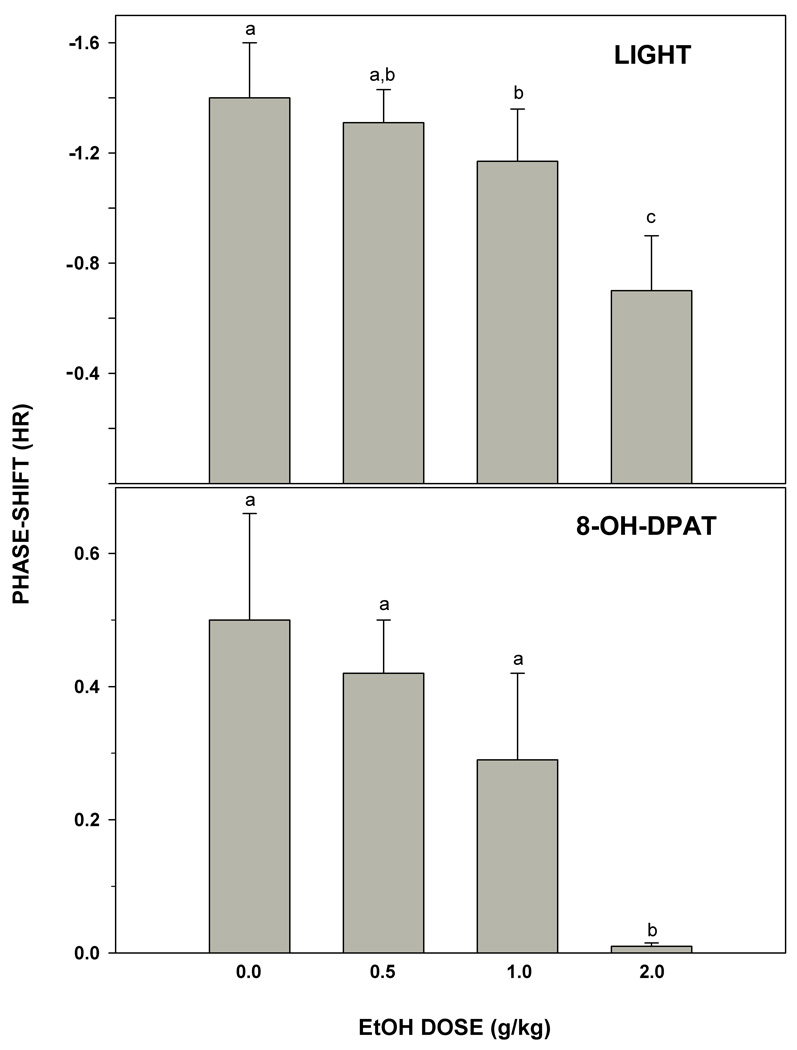
Photic phase-delay shifts at ZT 16 were significantly attenuated by i.p. injection of ethanol in a dose-dependent manner (F3,24=3.89; p<0.03; Fig. 2). Vehicle controls receiving i.p. saline injection had phase-delay shifts averaging 1.4±0.2 hr, while animals receiving i.p. injection of ethanol at doses of 0.5, 1.0, and 2.0 g/kg showed reduced phase delays averaging 1.3±0.1 hr, 1.2± 0.2 hr, and 0.7±0.2 hr, respectively. The two higher doses significantly inhibited photic phase resetting (both p<0.05 vs. vehicle). Representative actograms for vehicle- and ethanol-treated animals are shown in Fig. 3.
Figure 2.
Dose-dependent inhibition of phase-delay responses to a light pulse delivered at ZT 16 (top) and phase-advance responses to i.p. injection of (±)8-OH-DPAT delivered during the middle of the light-phase (ZT 6; bottom). Bars with different letters are significantly different (p<0.05). Bars represent means ± S.E.
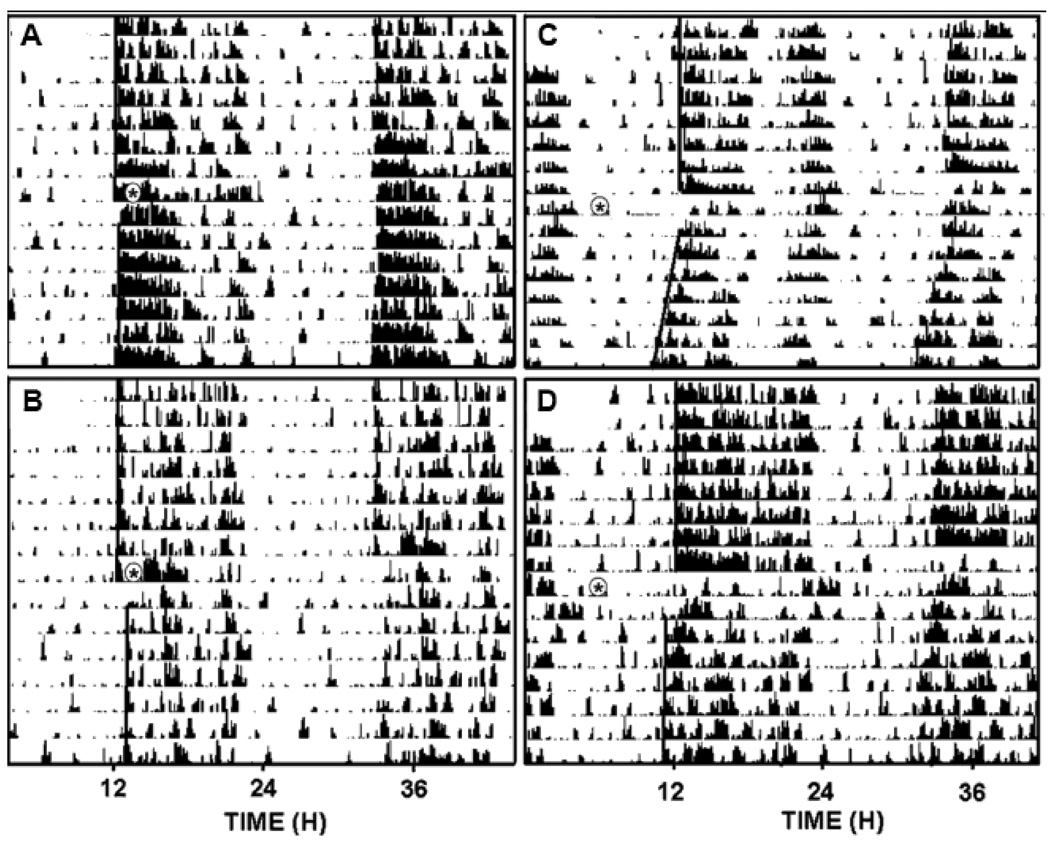
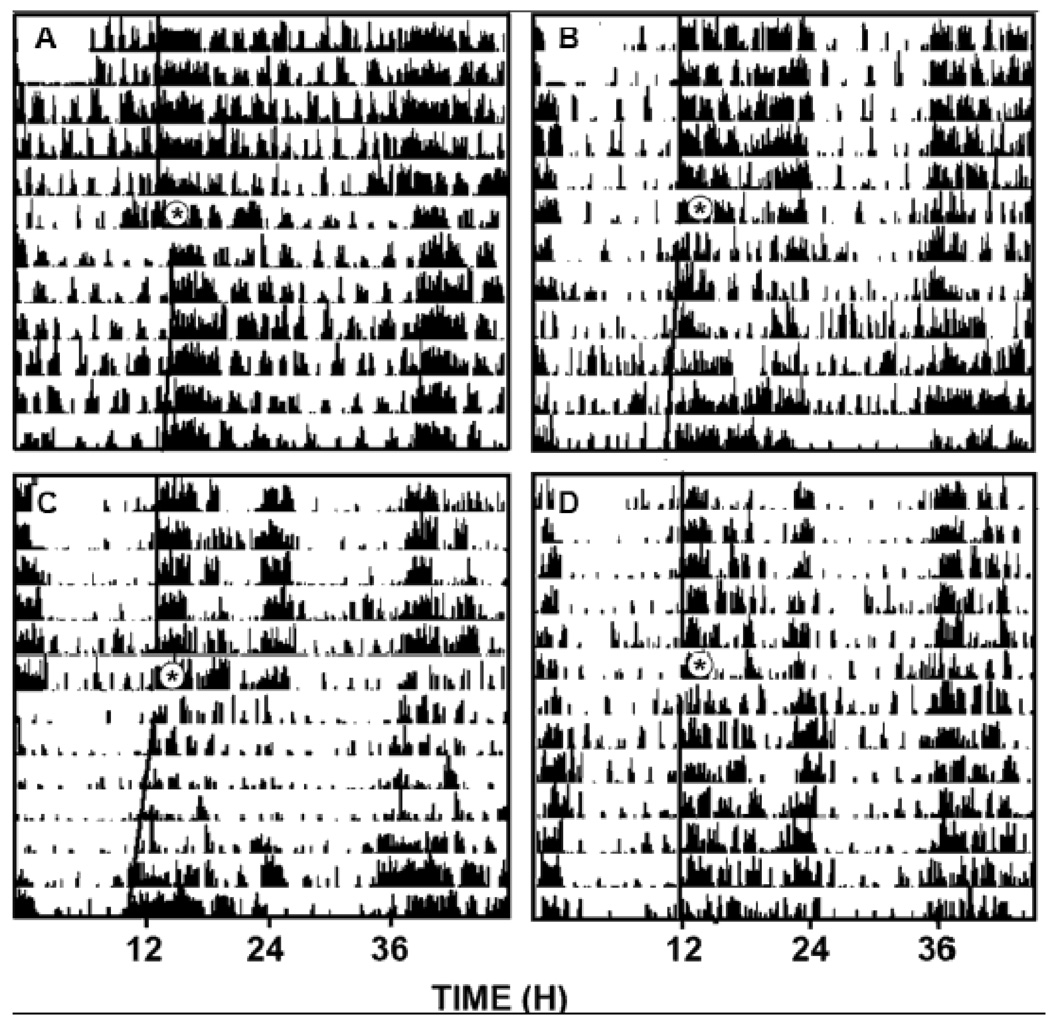
Figure 3.
Representative double-plotted actograms of general locomotor activity showing ethanol attenuation of photic phase-delay responses to a 30 min light pulse delivered at ZT 16 and phase-advance responses to an i.p. (±)8-OH-DPAT injection delivered at ZT 6. A, ethanol (2 g/kg) + light; B, saline + light; C, ethanol (2 g/kg) + 8-OH-DPAT; D, saline + 8-OH-DPAT. Asterisks denote the time of ethanol injection and subsequent light pulse or (+)8-OH-DPAT injection.
Intra-SCN EtOH administration attenuates photic phase-resetting
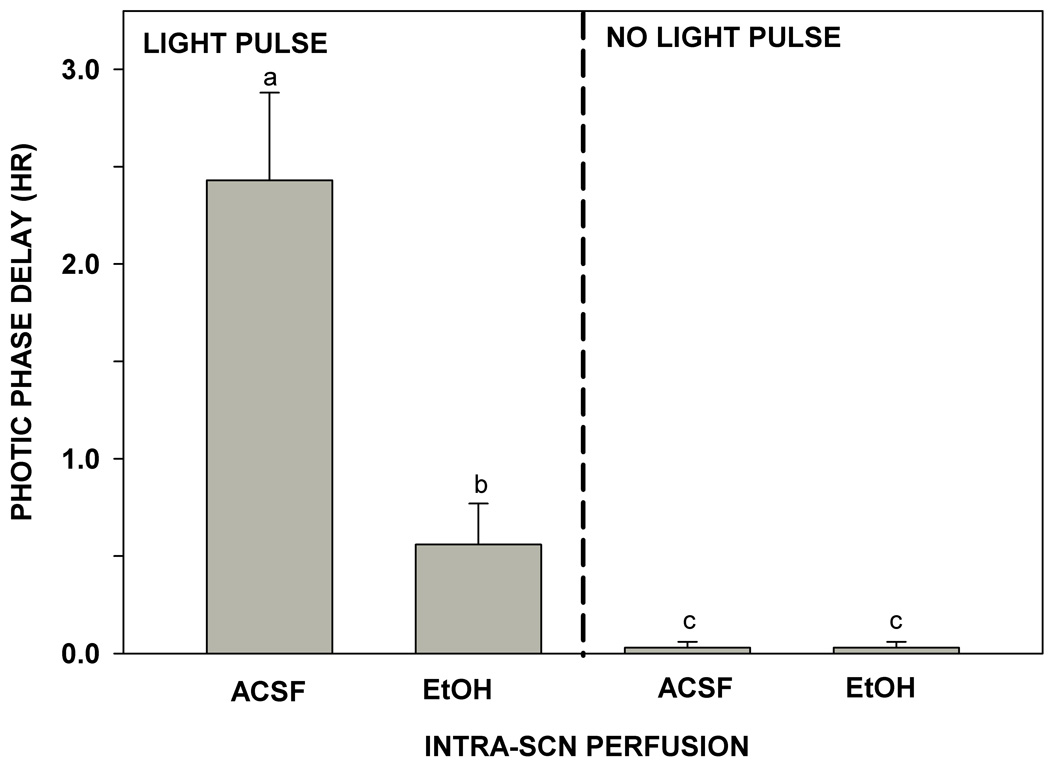
Reverse microdialysis perfusion of the SCN with ACSF containing ethanol (50 mM estimated tissue concentration) for 1 hr markedly inhibited photic phase-delay shifting at ZT 14. Controls receiving SCN ACSF perfusion alone had phase delays averaging 2.4±0.5 hr (Fig. 4), whereas mice receiving SCN ethanol perfusion exhibited much smaller phase delays averaging 0.6±0.2 hr (F1,12=16.56; p<0.01). Neither ACSF nor ethanol perfusions had phase-shifting effects in the absence of a light pulse. Representative actograms for ACSF and ethanol perfused mice are shown in Fig. 5.
Figure 4.
Inhibition of phase-delay responses to a light pulse delivered at ZT 14 by direct reverse-microdialysis perfusion of ethanol to the SCN. The ethanol and artificial cerebrospinal (ACSF) perfusions had no phase-resetting effect in the absence of a light pulse. For all treatments, bars with different letters are significantly different (pπ.05). Bars represent means ± S.E.
Figure 5.
Representative double-plotted actograms of general locomotor activity showing the inhibition by reverse-microdialysis perfusion of ethanol to the SCN of photic phase-delays induced by a light pulse delivered at ZT 14. A, vehicle (artificial cerebrospinal fluid); B, ethanol; C, vehicle/no light pulse; D, ethanol/no light pulse. Asterisks denote onset of the perfusions and subsequent light pulse (or no pulse).
Acute systemic EtOH administration attenuates serotonergic phase-resetting
Serotonergic phase-advance shifts induced by i.p. injection of 8-OH-DPAT at ZT 6 were significantly attenuated by i.p. injection of ethanol in a dose-dependent manner (F3,24=8.89; p<0.01; Fig. 2). Vehicle controls receiving i.p. DMSO injection had phase-advance shifts averaging 0.5±0.2 min, while animals receiving i.p. injection of ethanol at doses of 0.5, 1.0 and 2.0 g/kg showed reduced phase advances averaging 0.4±0.1 hr, 0.3±0.1 hr, and 0.0±0.0 hr, respectively. The highest dose significantly inhibited photic phase resetting (p<0.01 vs. vehicle). Representative actograms for vehicle- and ethanol-treated animals are shown in Fig. 3.
Acute systemic EtOH does not alter basal SCN Fos expression
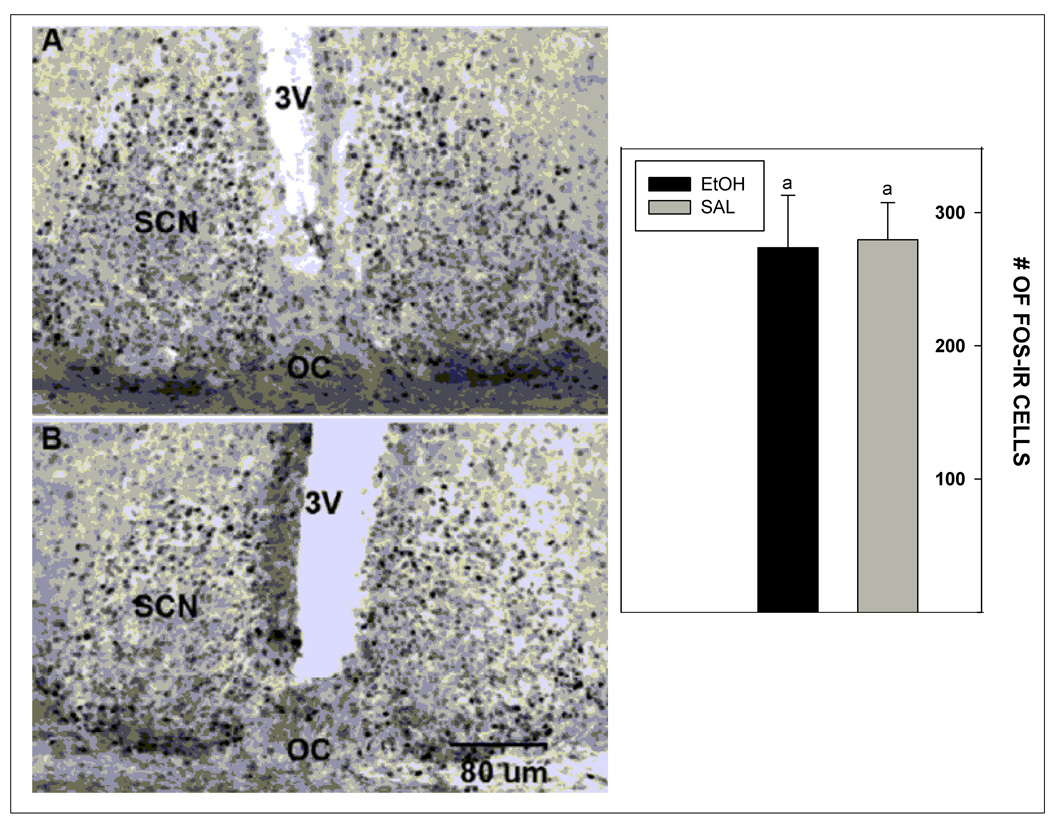
Acute i.p. injection of ethanol did not affect basal levels of Fos expression in the SCN when compared to saline injected controls (273.6±39.4 cells vs. 279.6±27.8 cells, respectively; p>0.05). Photomicrographs illustrating Fos expression in the SCN of mice receiving the EtOH and saline treatments are presented in Fig. 6.
Figure 6.
Photomicrographs and graph illustrating the lack of effect of i.p. ethanol injection (2 g/kg) on immunoreactive Fos expression in the SCN at midday (ZT 6; A, ethanol; B, saline) . 3V, third ventricle; OC, optic chiasm. Bars represent means ± S.E.
DISCUSSION
Alcohol abuse and subsequent withdrawal are highly disruptive to circadian clock-regulated homeostatic functions, including the sleep-wake cycle, metabolism, and feeding (Chen et al., 2004; Wasielewski and Holloway, 2001; Brower, 2001; Sack et al., 2007; Thiele et al., 2003, Sookoian et al., 2007; Röjdmark et al., 1993; Iranmanesh 1989; Kühlwein et al. 2003, Fonzi et al. 1994; Mukai et al., 1998). Chronic alcohol abuse can promote desynchronization of such rhythms with respect to the LD cycle and to internal SCN clock timing. This lack of internal rhythm coordination together with a loss of control from environmental timekeeping cues ultimately increases the risk of pathologies including diabetes, obesity, ulcers, sleep/circadian rhythm disorders, cardiovascular pathology and cancer (Thiele et al., 2003; Sookoian et al., 2007; Sack et al., 2007; Brower, 2001; Wasielewski and Holloway et al., 2001; Davis and Mirick, 2006). As the temporal regulation of circadian clock-generated rhythms is mediated by photic and non-photic entrainment inputs to the SCN, it is possible that these pathways could be vulnerable to the adverse chronobiological effects of ethanol. This assumption has been borne out by in vivo studies in hamsters and mice that have characterized ethanol's impairment of photic and non-photic signaling processes (Brager et al., 2010; Ruby et al., 2009a, b; al., Seggio et al., 2007, 2009). The present results extend these findings, showing that acute ethanol strongly inhibits phase-resetting responses of the mouse circadian clock to photic and non-photic (serotonergic) signals. Interestingly, the intra-SCN reverse microdialysis ethanol perfusion trials revealed that ethanol's inhibition of photic, but not serotonergic phase-resetting is manifested within the SCN itself. This latter result strongly implicates the SCN as a target for ethanol's disruption of photic phase-resetting, but that ethanol's inhibition of serotonergic phase resetting involves an extra-SCN site. Collectively, these findings are the first evidence that acute ethanol can affect phase-resetting mechanisms of the circadian clock in mice, and suggest that the SCN represents a critical target for ethanol's disruptive effects on photic but not non-photic phase-resetting mechanisms.
It is apparent that the extent of attenuation of photic shifting associated with the intra-SCN ethanol perfusion was greater than that produced by i.p. injection (~75% vs. ~50%, respectively). This result indicates an increased effectiveness of ethanol when delivered directly to the SCN. The concentrations of ethanol in the SCN (~40–60 mM) were equivalent for both applications, so the reason for this difference is unclear. Possibly it could have been due to the lack of behavioral/whole-body physiological responses to isolated SCN ethanol treatment that are manifest after systemic treatment. Another possibility is the difference in pharmacokinetics of ethanol in the SCN between the two treatment modes. Also, there may have been an effect of the different times of light+ethanol delivery (ZT 16 and ZT 14, respectively for the systemic and intra-SCN treatments), as control photic phase-delay responses were greater at ZT 14 than at ZT 16. Regardless of the magnitude of effects of the different treatments, our result suggests that intra-SCN ethanol treatment significantly attenuated photic phase-resetting.
Ethanol disrupts photic phase-resetting
Treatment with ethanol in hamsters and mice has been shown to strongly suppress photic phase-resetting in a species-specific manner. In hamsters, acute or chronic ethanol administration inhibits phase-advances but not phase-delays, while in mice, chronic ethanol treatment inhibits phase-delays but not phase-advances (Ruby et al., 2009b; Brager et al., 2020; Seggio et al., 2009). Here we show further that photic phase-delays in mice are also inhibited by acute systemic and intra-SCN ethanol treatments. Importantly, this inhibition of photic phase-resetting by intra-SCN perfusion of ethanol in the mouse (present study) and in the hamster (Ruby et al., 2009a) as well as the inhibition of the phase-resetting action of glutamate on spontaneous electrical activity of the isolated mouse SCN slice (Prosser et al., 2008) strongly suggests that the inhibitory action of ethanol occurs within the SCN photic signaling cascade. One possible mechanism for this action is the inhibition of brain-derived neurotrophic factor (BDNF), since application of BDNF reverses the inhibitory action of ethanol on glutamate-induced shifts in the mouse SCN slice (Prosser et al., 2008).
Ethanol disrupts serotonergic phase-resetting
Serotonergic signaling from neurons of the midbrain raphe nuclear complex is implicated in behavioral phase-resetting (Glass et al., 2000, 2003; Grossman et al., 2000; Meyer-Bernstein et al., 1997) as well as modulating photic input to the SCN (Rea et al., 1994; Grossman et al., 2000; Mistlberger and Antle, 1998). The role for 5-HT in non-photic phase-resetting is supported in part by the potent shifting actions of 5-HT agonists, principally 8-OH-DPAT, in vivo (Ehlen et al., 2001; Horikawa and Shibata, 2004) and in vitro (Prosser et al., 1993, 2003; Shibata et al., 1992). This 5-HT1A,7 receptor agonist has been used widely to study non-photic mechanisms, and has a phase-response curve (PRC) similar to behavioral PRCs (Mrosovsky, 1988; Reebs and Mrosovsky, 1989; Grossman et al., 2000; reviewed in Mistlberger et al., 2000). It also produces shifts of similar magnitude as behavioral stimuli (Knoch et al., 2004; reviewed in Mistlberger et al., 2000). Consistent with our previous report in hamsters (Ruby et al., 2009a), acute i.p. ethanol injection in mice inhibits 8-OH-DPAT phase-advance shifting. In hamsters, a dose of 2 g/kg suppressed 8-OH-DPAT shifts by 72%, while in mice this dose completely blocked shifting. The basis of these strong inhibitory effects of ethanol is unclear, but this action seems contrary to observations that acute ethanol enhances serotonergic activity by increasing release and decreasing uptake of 5-HT (reviewed in Rosenwasser, 2001; LeMarquand et al., 1994). A possible explanation for this inhibition of 8-OH-DPAT shifting is that an ethanol-induced enhancement of serotonergic activity causes a rapid down-regulation of 5-HT receptors as reported in vitro (Prosser and Glass, 2009). In our in vivo systemic trials, ethanol was administered 30 min prior to 8-OH-DPAT injection, which (according to the present pharmacokinetic analyses) could have down-regulated 5-HT receptors prior to 8-OH-DPAT entry to the brain, thus attenuating its phase-advancing response. It also must be noted that the inhibition of 8-OH-DPAT-induced phase-advances by i.p. ethanol treatment contrasts with our earlier studies in the mouse SCN slice where ethanol potentiated, rather than inhibited, 8-OH-DPAT phase-advance shifting (Prosser et al., 2008). It was suggested that the potentiated in vitro (~30%) shifting was due to ethanol's inhibition of glutamate signaling, since glutamate agonists inhibit serotonergic phase-resetting in the SCN (Prosser, 2001). Why such an effect was not evident in vivo is not clear, but could relate to the heightened responsiveness of the deafferented slice to 5-HT agonists (Prosser et al., 2006).
In addition to assessing the actions of ethanol on non-photic phase-resetting, a trial involving the assessment of Fos expression was undertaken to determine if acute ethanol may affect cellular activity in the SCN during midday when spontaneous neuronal activity is high. Injection of ethanol (2 g/kg) at midday did not significantly affect the number of Fos-immunostained nuclei, indicating that ethanol neither increased nor decreased overall basal levels of neuronal activity in the SCN at this time. This result is in general agreement with those of other studies on ethanol effects on hypothalamic Fos expression, where there was little overall effect of ethanol in anterior and lateral hypothalamic areas, although such effects of ethanol were highly region- and dose-dependent (Bachtell et al., 2000; Ryabinin et al., 2000). With regard to photic phase-resetting effects of ethanol, it will be important in future experiments to determine if light-induced Fos expression in the SCN, used a marker for the activation of the photic signaling cascade (Kornhauser et al., 1990; Glass et al., 1994), is inhibited by ethanol. This would constitute additional evidence for an inhibitory action of ethanol registered in the signaling cascade.
SCN ethanol pharmacokinetics
Brain microdialysis assessment of the pharmacokinetic profile of ethanol in the SCN following i.p. injection was undertaken to verify that the timing of phase-resetting treatments coincided with sufficiently raised ethanol levels. It was also of interest to compare the pharmacokinetics of acute ethanol with that of other species. Similar to our previous measurements in the hamster (Ruby et al., 2009a), peak levels associated with a 2 g/kg dose of ethanol occurred within 20–40 min of injection (hamster, 50 mM; mouse 58 mM). In the mouse, however, the half-life for clearance was shorter than that of the hamster (1.8 hr vs. 3.0 hr, respectively). These measurements confirmed that the administration of the light and 8-OH-DPAT treatments 30 min following ethanol injection would have coincided with near-maximal ethanol levels. Consistent with our previous report (Ruby et al., 2009a), the pharmacokinetic profiles of acute ethanol in the mouse and hamster SCN is similar to that observed in the rat nucleus accumbens (Yan, 1999), striatum (Job et al., 2003) following i.p. injection of 2 g/kg ethanol, with peak levels (~60 mM) occurring 15–30 min post-injection. The half-life for clearance in these reports was 1.5 to 3.0 hrs, which is also similar to the present assessments. It is also notable from the present analyses of multiple doses of ethanol that the estimated SCN concentration was about 3 times higher at 2.0 g/kg compared to that at 1.0 g/kg. We believe that this is attributable to the physiological effects of the different does of i.p. ethanol. The lower doses (0.5 and 1.0 g/kg) caused behavioral excitation for ~1 hr, while the higher dose (2.0 g/kg) caused sedation and possible hypothermia for ~2 hr. It is thus plausible that the clearance/metabolism of the higher ethanol dose could be slowed by sedation, causing disproportionately higher peak levels and prolonged half-life compared to the lower doses.
In conclusion, the present study is the first to confirm that acute ethanol dose-dependently attenuates photic phase-delay shifts and serotonergic phase-advance shifts in the mouse. This dual effect theoretically could disrupt photic and non-photic entrainment mechanisms governing circadian clock timing. It is also significant that the SCN clock is a direct target for disruptive effects of ethanol on photic shifting. This suggests that ethanol could impair the activity of multiple transmitter systems and sites involved in circadian timing regulation. Such diversity of action by ethanol could underlie the disruptive effects of alcohol abuse on behavioral, physiological and endocrine rhythms associated with alcoholism.
Acknowledgments
This work was supported by National Institute on Alcohol Abuse and Alcoholism grants AA-015948 and AA-017898 to R.A. Prosser and J.D. Glass
REFERENCES
- Bachtell RK, Wang Y-M, Freeman P, Risinger FO, Ryabinin AE. Alcohol drinking produces brain region-selective changes in expression of inducible transcription factors. Brain Res. 2000;847:157–165. doi: 10.1016/s0006-8993(99)02019-3. [DOI] [PubMed] [Google Scholar]
- Brager AJ, Ruby CL, Prosser RA, Glass JD. Chronic ethanol disrupts circadian photic entrainment and daily locomotor activity in the mouse. Alcohol Clin Exp Res. 2010;34(7):1266–1273. doi: 10.1111/j.1530-0277.2010.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Res Health. 2001;25:110–125. [PMC free article] [PubMed] [Google Scholar]
- Challet E, Pévet P. Interactions between photic and nonphotic stimuli to synchronize the master circadian clock in mammals. Front Biosci. 2003;8:246–257. doi: 10.2741/1039. [DOI] [PubMed] [Google Scholar]
- Challet EK, Scarbrough K, Penev P, Turek FW. Roles of suprachiasmatic nuclei and intergeniculate leaflets in mediating the phase-shifting effects of a serotonergic agonist and their photic modulation during subjective day. J Biol Rhythms. 1998;13:410–421. doi: 10.1177/074873098129000237. [DOI] [PubMed] [Google Scholar]
- Chen CP, Kuhn P, Advis JP, Sarkar DK. Chronic ethanol consumption impairs the circadian rhythm of pro-opiomelanocortin and period genes mRNA expression in the hypothalamus of the male rat. J Neurochem. 2004;88(6):1547–1554. doi: 10.1046/j.1471-4159.2003.02300.x. [DOI] [PubMed] [Google Scholar]
- Cheng HM, Papp JW, Varlamova O, Dziema H, Russel B, Curfman JP, Nakazawa T, Shimizu K, Okamura H, Impey S, Obrietan K. microRNA modulation of circadian-clock period and entrainment. Neuron. 2007;54:813–829. doi: 10.1016/j.neuron.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daan S, Pittendrigh S. A functional analysis of circadian pacemakers in nocturnal rodents. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1976;106:291–331. [Google Scholar]
- Davis S, Mirick DK. Circadian disruption, shift work and the risk of cancer: a summary of the evidence and studies in Seattle. Cancer Causes Control. 2006;17(4):539–545. doi: 10.1007/s10552-005-9010-9. [DOI] [PubMed] [Google Scholar]
- Dippold RP, Vadigepalli R, Gonye GE, Hoek JB. Dyregulation of miRNAs by chronic ethanol contributes to repression of liver regeneration after partial hepatectomy. Alcohol Clin Exp Res. 2010;34(s2):88A. [Google Scholar]
- Dodd PR, Beckmann AM, Davidson MS, Wilce PA. Glutamate-mediated transmission, alcohol, and alcoholism. Neurochem Int. 2000;37:509–533. doi: 10.1016/s0197-0186(00)00061-9. [DOI] [PubMed] [Google Scholar]
- Dudley TE, DiNardo LA, Glass JD. Endogenous regulation of serotonin release in the hamster suprachiasmatic nucleus. J Neurosci. 1998;18(13):5045–5052. doi: 10.1523/JNEUROSCI.18-13-05045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley TE, DiNardo LA, Glass JD. In vivo assessment of the midbrain raphe nuclear regulation of serotonin release in the hamster suprachiasmatic nucleus. J Neurophysiol. 1999;81(4):1469–1477. doi: 10.1152/jn.1999.81.4.1469. [DOI] [PubMed] [Google Scholar]
- Ehlen JC, Grossman GH, Glass JD. In vivo resetting of the hamster circadian clock by 5-HT7 receptors in the suprachiasmatic nucleus. J Neurosci. 2001;21(14):5351–5357. doi: 10.1523/JNEUROSCI.21-14-05351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd DW, Jung KY, McCool BA. Chronic ethanol ingestion facilitates N-Methyl-D-aspartate receptor function and expression in rat lateral/basolateral amygdala neurons. J Pharmacol Exp Ther. 2003;307:1020–1029. doi: 10.1124/jpet.103.057505. [DOI] [PubMed] [Google Scholar]
- Fonzi S, Solinas GP, Costelli P, Parodi C, Murialdo G, Bo P, Albergati A, Montalbetti L, Savoldi F, Polleri A. Melatonin and cortisol circadian secretion during ethanol withdrawal in chronic alcoholics. Chronobiologia. 1994;21:109–112. [PubMed] [Google Scholar]
- Glass JD, DiNardo LA, Ehlen JC. Dorsal raphe nuclear stimulation of SCN serotonin release and circadian phase-resetting. Brain Res. 2000;859(2):224–232. doi: 10.1016/s0006-8993(00)01963-6. [DOI] [PubMed] [Google Scholar]
- Glass JD, Grossman JH, Farnbauch L, DiNardo L. Midbrain raphe modulation of nonphotic circadian clock resetting and 5-HT release in the mammalian suprachiasmatic nucleus. J Neurosci. 2003;23(20):7451–7460. doi: 10.1523/JNEUROSCI.23-20-07451.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Selim M, Rea MA. Modulation of light-induced C-Fos expression in the suprachiasmatic nuclei by 5-HT1A receptor agonists. Brain Res. 1994;638(1–2):235–242. doi: 10.1016/0006-8993(94)90655-6. [DOI] [PubMed] [Google Scholar]
- Grossman GH, Mistlberger RE, Antle MC, Ehlen CJ, Glass JD. Sleep deprivation stimulates serotonin release in the suprachiasmatic nucleus. Neuroreport. 2000;11(9):1929–1932. doi: 10.1097/00001756-200006260-00024. [DOI] [PubMed] [Google Scholar]
- Horikawa K, Shibata S. Phase-resetting response to (+)8-OH-DPAT, a serotonin 1A/7 receptor agonist, in the mouse in vivo. Neurosci Lett. 2004;368(2):130–134. doi: 10.1016/j.neulet.2004.06.072. [DOI] [PubMed] [Google Scholar]
- Iranmanesh A, Veldhuis JD, Johnson ML, Lizarralde G. 24-hour pulsatile and circadian patterns of cortisol secretion in alcoholic men. J Androl. 1989;10(1):54–63. doi: 10.1002/j.1939-4640.1989.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Job MO, Ramachandra V, Anders S, Low MJ, Gonzales RA. Reduced basal and ethanol stimulation of striatal extracellular dopamine concentrations in dopamine D2 receptor knockout mice. Alcohol Clin Exp Res. 2003;27:1573–1582. doi: 10.1002/syn.20283. [DOI] [PubMed] [Google Scholar]
- Johnson RF, Morin LP, Moore RY. Retinohypothalamic projections in the hamster and rat demonstrated using cholera toxin. Brain Res. 1988;462(2):301–312. doi: 10.1016/0006-8993(88)90558-6. [DOI] [PubMed] [Google Scholar]
- Knoch ME, Gobes SM, Pavlovska I, Su C, Mistlberger RE, Glass JD. Short-term exposure to constant light promotes strong circadian phase-resetting responses to nonphotic stimuli in Syrian hamsters. Eur J Neurosci. 2004;19(10):2779–2790. doi: 10.1111/j.0953-816X.2004.03371.x. [DOI] [PubMed] [Google Scholar]
- Kolb JE, Trettel J, Levine ES. BDNF enhancement of postsynaptic NMDA receptors is blocked by ethanol. Synapse. 2005;55(1):52–57. doi: 10.1002/syn.20090. [DOI] [PubMed] [Google Scholar]
- Kornhauser JM, Nelson DE, Mayo KE, Takahashi JS. Photic and circadian regulation of c-fos gene expression in the hamster suprachiasmatic nucleus. Neuron. 1990;5(2):127–134. doi: 10.1016/0896-6273(90)90303-w. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D’Souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther. 2003;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Kühlwein E, Hauger RL, Irwin MR. Abnormal nocturnal melatonin secretion and disordered sleep in abstinent alcoholics. Biol Psychiatry. 2003;54:1437–1443. doi: 10.1016/s0006-3223(03)00005-2. [DOI] [PubMed] [Google Scholar]
- LeMarquand D, Pihl PO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: findings of animal studies. Biol Psychiatry. 1994;36(6):395–421. doi: 10.1016/0006-3223(94)91215-7. [DOI] [PubMed] [Google Scholar]
- Meijer JH, van der Zee EA, Dietz M. Glutamate phase shifts circadian activity rhythms in hamsters. Neurosci Lett. 1988;86(2):177–183. doi: 10.1016/0304-3940(88)90567-8. [DOI] [PubMed] [Google Scholar]
- Meyer-Bernstein EL, Blanchard JH, Morin LP. The serotonergic projection from the median raphe nucleus to the suprachiasmatic nucleus modulates activity phase onset, but not other circadian rhythm parameters. Brain Res. 1997;755(1):112–120. doi: 10.1016/s0006-8993(97)00111-x. [DOI] [PubMed] [Google Scholar]
- Mintz EM, Marvel CL, Gillespie CF, Price KM, Albers HE. Activation of NMDA receptors in the suprachiasmatic nucleus produces light-like phase shifts of the circadian clock in vivo. J Neurosci. 1999;19(12):5124–5130. doi: 10.1523/JNEUROSCI.19-12-05124.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE, Antle MC. Behavioral inhibition of light-induced circadian phase resetting is phase and serotonin dependent. Brain Res. 1998;786(1–2):31–38. doi: 10.1016/s0006-8993(97)01269-9. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Antle MC, Glass JD. Behavioral and serotonergic regulation of circadian rhythms. Biol Rhythm Res. 2000;31(3):240–283. [Google Scholar]
- Mrosovsky N. Phase response curves for social entrainment. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1988;162(1):35–46. doi: 10.1007/BF01342701. [DOI] [PubMed] [Google Scholar]
- Mukai M, Uchimura N, Hirano T, Ohshima H, Ohshima M, Nakamura J. Circadian rhythms of hormone concentrations in alcohol withdrawal. Psychiatry Clin Neurosci. 1998;52:238–240. doi: 10.1111/j.1440-1819.1998.tb01051.x. [DOI] [PubMed] [Google Scholar]
- Prosser RA. Glutamate blocks serotonergic phase advances of the mammalian circadian pacemaker through AMPA and NMDA receptors. J Neurosci. 2001;21(19):7815–7822. doi: 10.1523/JNEUROSCI.21-19-07815.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA. Serotonin phase-shifts the mouse suprachiasmatic circadian clock in vitro. Brain Res. 2003;966(1):110–115. doi: 10.1016/s0006-8993(02)04206-3. [DOI] [PubMed] [Google Scholar]
- Prosser RA, Dean RR, Edgar DM, Heller HC, Miller JD. Serotonin and the mammalian circadian system: I. in vitro phase shifts by serotonergic agonists and antagonists. J Biol Rhythms. 1993;8:1–16. doi: 10.1177/074873049300800101. [DOI] [PubMed] [Google Scholar]
- Prosser RA, Glass JD. The mammalian circadian clock exhibits acute tolerance to ethanol. Alcohol Clin Exp Res. 2009;33:2088–2093. doi: 10.1111/j.1530-0277.2009.01048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA, Lee HM, Wehner A. Serotonergic pre-treatments block in vitro serotonergic phase shifts of the mouse suprachiasmatic nucleus circadian clock. Neuroscience. 2006;142:547–555. doi: 10.1016/j.neuroscience.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Prosser RA, Mangrum CA, Glass JD. Acute ethanol modulates glutamatergic and serotonergic phase shifts of the mouse circadian clock in vitro. Neuroscience. 2008;152:837–848. doi: 10.1016/j.neuroscience.2007.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea MA, Glass JD, Colwell CS. Serotonin modulates photic responses in the hamster suprachiasmatic nuclei. J Neurosci. 1994;14(6):3635–3642. doi: 10.1523/JNEUROSCI.14-06-03635.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reebs SG, Mrosovsky N. Effects of induced wheel running on the circadian activity rhythms of syrian hamsters: entrainment and phase response curve. J Biol Rhythms. 1989;4:39–48. doi: 10.1177/074873048900400103. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Roth T. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med Rev. 2001;5:287–297. doi: 10.1053/smrv.2001.0162. [DOI] [PubMed] [Google Scholar]
- Röjdmark S, Wikner J, Adner N, Andersson DE, Wetterberg L. Inhibition of melatonin secretion by ethanol in man. Metabolism. 1993;42:1047–1051. doi: 10.1016/0026-0495(93)90021-f. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM. Alcohol, antidepressants, and circadian rhythms: human and animal models. Alcohol Res Health. 2001;25(2):126–135. [PMC free article] [PubMed] [Google Scholar]
- Ruby CL, Brager AJ, DePaul MA, Prosser RA, Glass JD. Chronic ethanol disrupts circadian behavior and photic phase-resetting in the hamster. Am J Physiol. 2009b;298:R729–R737. doi: 10.1152/ajpregu.00268.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby CL, Prosser RA, DePaul MA, Roberts RJ, Glass JD. Acute ethanol impairs photic and nonphotic circadian phase resetting in the Syrian hamster. Am J Physiol. 2009a;296:R411–R418. doi: 10.1152/ajpregu.90782.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, Wang Y-M, Bachtell RK, Kinney AE, Grubb MC, Mark GP. Cocaine- and alcohol-mediated expression of inducible transcription factors is blocked by pentobarbital anesthesia. Brain Res. 2000;877:251–261. doi: 10.1016/s0006-8993(00)02681-0. [DOI] [PubMed] [Google Scholar]
- Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP, Vitiello MV, Zhdanova IV. Circadian rhythm sleep disorders: part I, basic principles, shift work, and jet lag disorders. Sleep. 2007;30(11):1460–1483. doi: 10.1093/sleep/30.11.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyan P, Golden HB, Miranda RC. Competing interactions between micro-RNAs determine neural progenitor survival and proliferation after ethanol exposure: evidence from an ex vivo model of the fetal cerebral cortical neuroepithelium. J Neurosci. 2007;27:8546–8557. doi: 10.1523/JNEUROSCI.1269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seggio JA, Fixaris MC, Reed JD, Logan RW, Rosenwasser AM. Chronic ethanol intake alters circadian phase shifting and free-running period in mice. J Biol Rhythms. 2009;24:304–312. doi: 10.1177/0748730409338449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seggio JA, Logan RW, Rossenwasser AM. Chronic ethanol intake modulates photic and non-photic circadian phase responses in the Syrian hamster. Pharmacol Biochem Behav. 2007;3:297–305. doi: 10.1016/j.pbb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Tsuneyoshi A, Hamada T, Tominaga K, Watanabe S. Phase-resetting effect of 8-OH-DPAT, a serotonin1A receptor agonist, on the circadian rhythm of firing rate in the rat suprachiasmatic nuclei in vitro. Brain Res. 1992;582(2):353–356. doi: 10.1016/0006-8993(92)90156-4. [DOI] [PubMed] [Google Scholar]
- Smart RG. Drinking problems among employed, unemployed and shift workers. J Occup Med. 1979;21(11):731–736. doi: 10.1097/00043764-197911000-00005. [DOI] [PubMed] [Google Scholar]
- Sookoian S, Gemma C, Gianotti T, Fernandez-Burgueno A, Alvarez A, Gonzalez CD, Pirola CJ. Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. J Intern Med. 2007;261(3):285–292. doi: 10.1111/j.1365-2796.2007.01766.x. [DOI] [PubMed] [Google Scholar]
- Thiele TH, Navarro M, Sparta DR, Fee JR, Knapp DJ, Cubero I. Alcoholism and obesity: overlapping neuropeptide pathways? Neuropeptides. 2003;37(6):321–337. doi: 10.1016/j.npep.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Trinkoff AM, Storr CL. Work schedule characteristics and substance use in nurses. Am J Ind Med. 1998;34(3):266–271. doi: 10.1002/(sici)1097-0274(199809)34:3<266::aid-ajim9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Wang LM, Schroeder A, Loh A, Smith D, Lin K, Han JH, Michel S, Hummer DL, Ehlen JC, Albers HE, Colwell CS. Role for the NR2B subunit of the NMDA receptor in mediating light input to the circadian system. Eur J Neurosci. 2008;27(7):1771–1779. doi: 10.1111/j.1460-9568.2008.06144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasielewski JA, Holloway FA. Alcohol's interactions with circadian rhythms. Alcohol Res Health. 2001;25:94–100. [PMC free article] [PubMed] [Google Scholar]
- Yan QS. Extracellular dopamine and serotonin after ethanol monitored with 5-minute microdialysis. Alcohol. 1999;19(1):1–7. doi: 10.1016/s0741-8329(99)00006-3. [DOI] [PubMed] [Google Scholar]