Abstract
Objective
To determine the regional cell density distribution and basal oxygen consumption rates (based on tissue volume and cell number) of temporomandibular joint (TMJ) discs and further examine the impact of oxygen tension on these rates.
Design
TMJ discs from pigs aged 6–8 months were divided into five regions: anterior, intermediate, posterior, lateral and medial. The cell density was determined using confocal laser scanning microscopy. The change in oxygen tension was recorded while TMJ disc explants were cultured in sealed metabolism chambers. The volume based oxygen consumption rate of explants was determined by theoretical curve fitting of the recoded oxygen tension data with the Michaelis-Menten equation. The rate on a per-cell basis was calculated based on the cell density measurements and volume based rate measured in another group of discs.
Results
The overall cell density (mean, 95% CI) was 51.3(21.3–81.3)×106cells/mL wet tissue. Along the anteroposterior axis, the anterior band had 25.5% higher cell density than the intermediate zone (p<0.02) and 29.1% higher than the posterior band (p<0.008). Along the mediolateral axes, the medial region had 26.2% higher cell density than the intermediate zone (p<0.04) and 25.4% higher than the lateral region (p<0.045). The overall volume and cell based maximum oxygen consumption rates were 1.44(0.44–2.44) μmol/mL wet tissue/hr and 28.7(12.2–45.2) nmol/106 cells/hr, respectively. The central regions (intermediate, lateral, and medial) had significantly higher volume based (p<0.02) and cell based (p<0.005) oxygen consumption rates than the anterior and posterior bands. At high oxygen tension, the oxygen consumption rate remained constant, but dropped as oxygen tension fell below 5%.
Conclusions
The TMJ disc had higher cell density and oxygen consumption rates than articular cartilage reported in the literature. These results suggest that a steeper oxygen gradient may exist in the TMJ disc and may be vulnerable to pathological events that impede nutrient supply.
Keywords: Temporomandibular joint (TMJ) disc, Cell density distribution, Oxygen consumption rate, Metabolism
INTRODUCTION
The temporomandibular joint (TMJ) is a synovial, bilateral joint with unique morphology and function. The TMJ disc, a fibrocartilaginous tissue, is a major component of jaw function by providing stress distribution and lubrication in the joint1–2. Disc derangement (e.g., dislocation of the disc) is a common clinical finding in patients with TMJ disorders (affecting more than 10 million Americans)3. It has been suggested that degenerative processes predispose the disc to displacement and result in significant changes in disc morphology, biochemistry, material properties, and function3–4.
The normal adult human TMJ disc is a large avascular structure 5–7, so the nutrients required by disc cells for maintaining disc health are supplied by synovial fluid at the margins of the disc as well as through nearby blood vessels at the connection to the posterior bilaminar zone8. The transport of small nutrients (i.e., oxygen and glucose) within the TMJ disc mainly depends on diffusion. The balance between the rate of nutrient diffusion through the matrix and the rate of consumption by disc cells potentially establishes a concentration gradient within the disc. In articular cartilage, these gradients can profoundly affect chondrocyte viability, energy metabolism, matrix synthesis, and response to inflammatory factors9–16. Recent studies have shown that hypoxia with inflammation modulates gene expressions of tenascin-C and matrix metalloproteinases in TMJ disc cells17–18. Understanding these gradients is therefore important for studying TMJ disc physiology and pathophysiology. Due to the difficulty of measuring these gradients in vivo, mathematical models have been used to predict the nutrient environment inside cartilaginous tissues. Most of these models have primarily studied oxygen as it is regarded as an important factor directly affecting cell biological activity14. Oxygen gradients have been modeled for growth cartilage19, articular cartilage20, engineered cartilage21–22 and the intervertebral disc (IVD)23–24. In order to obtain a realistic prediction of in vivo nutrient distribution, metabolic rates of cells have to be taken into account in the theoretical model. Therefore, measuring the oxygen consumption rate of TMJ disc cells is crucial for precise theoretical analyses of nutrient transport in the TMJ disc. Moreover, oxygen consumption data will provide useful information for understanding the mechanism of the energy metabolism of TMJ disc cells.
On a per-cell basis, the oxygen consumption rate of articular cartilage and IVD are remarkably lower than vascularized tissues (~ 2–5% of liver or kidney tissue rates)25 since articular chondrocytes14, 26 and IVD cells27 obtain their energy primarily through glycolysis. The rates of oxygen consumption in articular cartilage20, 28 and IVD29–30 depend on the local oxygen tension. The consumption of oxygen decreases as oxygen tension decreases and is regionally dependent. The deep zone articular chondrocytes had higher oxygen consumption rates than superficial zone cells28. In IVD, the nucleus pulposus cells have a higher rate than annulus fibrosus cells30–32. Compared to articular cartilage and other fibrocartilaginous tissues (e.g., IVD or knee meniscus), the TMJ disc has a unique matrix composition and cell phenotype33–35. Differences in biochemical composition and structure distinguish three regions of the TMJ disc: anterior band, intermediate zone, and posterior band5. Based on the cell morphological studies it appears that the TMJ disc contains an inhomogeneous distribution of a mixed cell population of fibroblast-like cells and chondrocyte-like cells, which are distinct from chondrocytes from hyaline cartilage36. These differences imply that the nutrient consumption rate in the TMJ disc may be region-dependent and different from the rates of articular cartilage. However, to our knowledge the oxygen consumption rate of the TMJ disc has not been investigated.
The objective of this paper was to determine basal oxygen consumption rates in each porcine TMJ disc region and further examine the impact of oxygen tension on these rates. The oxygen consumption in a tissue depends on the cell density and oxygen consumption rate per cell, so both were experimentally determined in this study. The volume based in situ TMJ disc cell density distribution was established using confocal laser scanning microscopy. Next, oxygen consumption rates (on a per tissue volume basis) were determined at various oxygen tensions for TMJ disc explants. The oxygen consumption rates on a per-cell basis were finally calculated based on the independently measured cell density and volume based tissue oxygen consumption rate.
MATERIALS AND METHOD
Specimen preparation
A total of nine pig heads (American Yorkshire, male, aged ~ 6–8 months) were collected from a local abattoir within 2 hours of slaughter. The entire TMJ with capsule intact was removed en bloc. Joints were opened under a sterile dissection hood; TMJ discs were then removed and washed with 5–6 changes of phosphate buffered saline. Five TMJ discs of the left joints were used to determine cell density distribution via confocal laser scanning microscopy. Five TMJ discs of the corresponding right joints were used to determine DNA content to validate cell density measurements. Eight discs from both left and right joints were then used to measure the oxygen consumption rate of tissue explants.
Cell density measurement
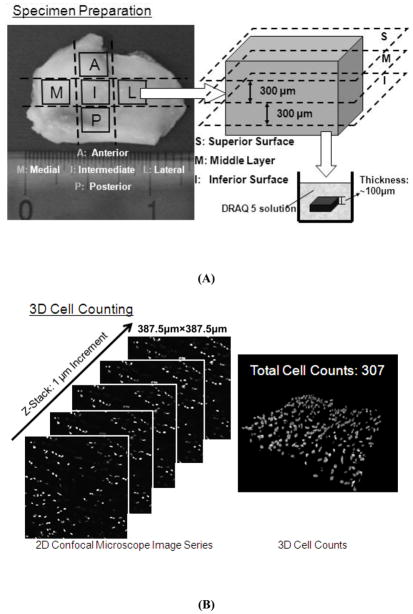
A confocal microscopy based technique was developed to determine the in situ surface-regional cell distribution of the TMJ disc. The volume-based cell density measurements were accomplished by counting cell numbers in specific volumes from reconstructed three-dimensional (3D) images. Each porcine TMJ disc was divided into five regions: anterior, intermediate, posterior, lateral and medial [Fig. 1(A)]. These specimens were then sectioned into three layers (100μm each) along the superior-inferior axis using a microtome (SM2400, Leica Microsystems GmbH, Wetzlar, Germany). The nuclei of samples were stained with DRAQ5™ (Biostatus Limited, Leicestershire, UK) and all samples then were scanned with a Leica TCS SP5 Confocal Microscope System (Leica Microsystems, Inc., Exton, PA). 2D image series were acquired by Z-stack scanning with a 1μm step in the Z-direction and a Field of View in the X-Y plane of 387.5μm×387.5μm. 3D images of stained cells were reconstructed in the image processing software based on the Z-stack image series, and cell density measurements were obtained from these by counting cell numbers in observed tissue volumes [Fig. 1(B)].
Figure 1.
(A) Representation of the regions investigated for determining cell density and oxygen consumption rate. The TMJ disc was divided into five regions: anterior, intermediate, posterior, lateral and medial. From each region, sections (100μm) were further taken from 3 layers for cell density measurement. (B) Schematic of 3D cell counting using a confocal microscopy technique.
DNA content measurement
The above technique was validated by measuring the DNA content of corresponding disc tissues from the same animals. Tissue plugs were punched from five regions of the right side discs, and the volume of each specimen was determined in PBS using a density determination kit (Sartorius YDK01, Germany) and an analytical balance based on the Archimedes’ principle37. The specimens were lyophilized for 2 days and then digested in 1 mL of papain solution (125 μg/ml papain; Worthington Biomedical Corporation), 100 mM phosphate buffer, 10 mM cysteine and 10 mM EDTA, at pH 6.3 and 60°C for 36 hours. The DNA content was determined using a DNA Quantitation Kit, Fluorescence Assay (Sigma, St. Louis, MO, USA). A conversion factor of 7.7 pg DNA per TMJ disc cell was used38. The cell density of the specimen was determined by taking the ratio of cell number to tissue volume.
Oxygen consumption rate measurement
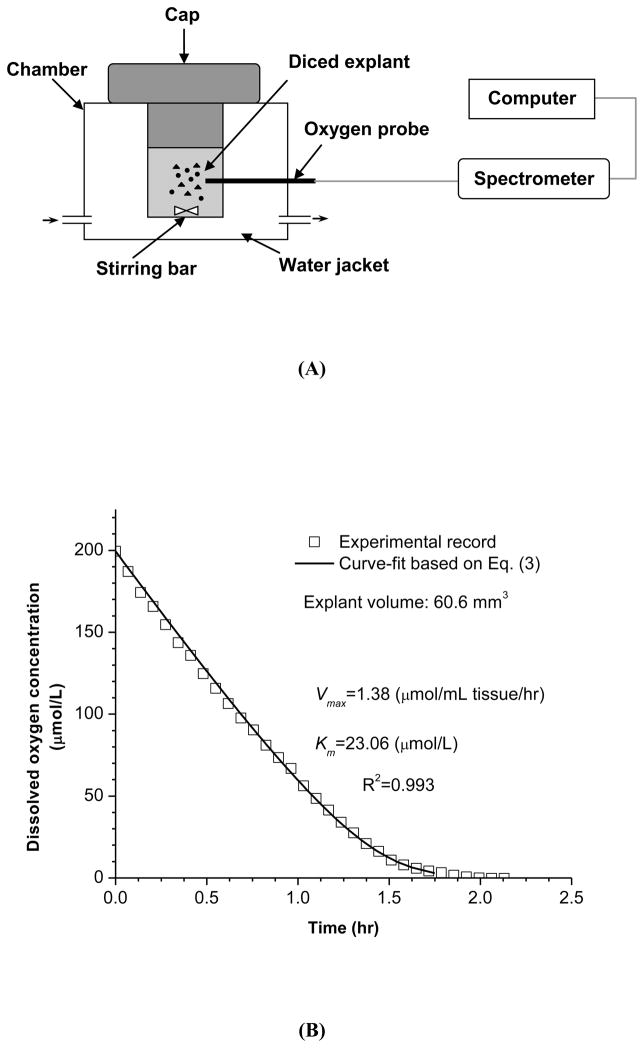
Fresh TMJ disc explants (~0.075g wet weight/explant) were harvested from 5 regions and the tissue volume of each explant was determined in PBS based on the Archimedes’ principle using the previously described method. Explants were immediately diced into small pieces to minimize the concentration gradient of oxygen within the explant and then placed into a metabolism chamber containing DMEM with 5mM glucose at pH 7.4. This glucose concentration is considered physiological in synovial fluid26 and was selected for measuring basal oxygen consumption rate of the TMJ disc in this study. The medium used in the metabolism chamber had been preheated to 37 °C and stirred in air for 10 minutes to establish constant initial dissolved oxygen concentration. The concentration of dissolved oxygen in the culture medium at 37°C and atmospheric pressure was 200 μmol/L (or 6.4 mg/L). The oxygen consumption rates of TMJ disc explants were measured in a stirred, water-jacketed chamber maintained at 37°C (Instech Laboratories, Plymouth Meeting, PA) [Fig. 2(A)]. After the chamber was sealed, real time dissolved oxygen concentration in the medium was recorded by a fiber optic oxygen sensor (Ocean Optics, Dunedin, FL) until the oxygen concentrations fell to 0.95 μmol/L (0.1% oxygen). At the end of the experiments, the glucose concentration and pH of the culture medium were measured. The decrease in glucose concentration and the change of pH were found to be minimal. In addition, the explant pieces were fully digested and the cell viability was examined via trypan blue exclusion (greater than 90% viability).
Figure 2.
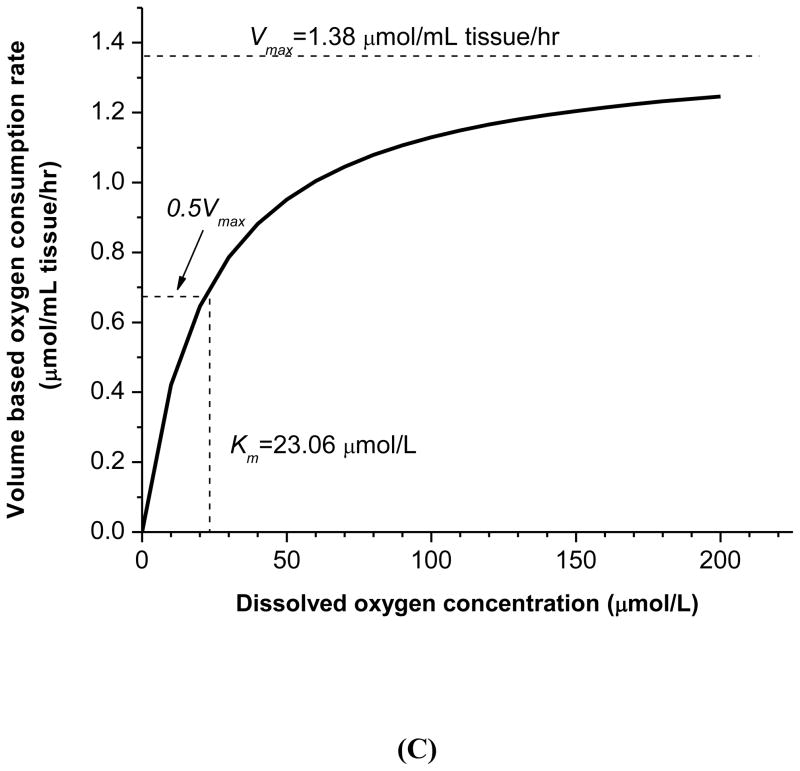
(A) Schematic of the experimental setup for oxygen consumption rate measurements. TMJ disc explants were cultured in a sealed water-jacketed metabolism chamber. (B) Typical record of dissolved oxygen concentration in the chamber over time. The experimental data were curve fit to Equation (3). (C) The oxygen consumption rate was plotted based on the Michaelis-Menten equation with determined parameters Vmax and Km.
A typical plot of dissolved oxygen concentration over time is shown in Fig. 2(B). The rate of oxygen consumption in the TMJ disc cells enclosed in the metabolism chamber can be calculated from the recorded decrease in oxygen concentration versus time. Based on our pilot study, the relationship between the oxygen consumption rate and oxygen concentration can be expressed using the Michaelis-Menten equation:
| (1) |
where R is oxygen consumption rate (μmol/mL tissue/hr), Vmax is the maximum oxygen consumption rate (μmol/mL tissue/hr), Km is the Michaelis-Menten constant (μmol/L), and C is the oxygen concentration in the chamber (μmol/L). Based on the conservation of mass, the time rate of oxygen concentration change (dC/dt) in the sealed chamber is given by:
| (2) |
where Voltissue is the tissue volume of the explant (mL), Volchamber is the volume of the metabolism chamber (mL). In this study, the chamber volume is 0.5mL. Integrating Equation 2, we can determine the oxygen concentration in the chamber over the time:
| (3) |
where C0 is the initial (t=0) oxygen concentration in the chamber. Curve-fitting the recorded oxygen concentration data to Equation 3, we determined the kinetic coefficients Vmax and Km to establish the functional relationship between the oxygen consumption rate R and the oxygen concentration C. Each measured volume based Vmax was then normalized by the mean values for cell density in the 5 disc regions obtained from the confocal experiment to calculate the cell based Vmax.
Statistical analysis
The measurements were presented as mean with 95% confidence interval. The cell density (n=5), tissue volume based oxygen consumption rate (n=4), and cell based oxygen consumption rate (n=4) were examined for significant differences between the five TMJ disc regions using SPSS statistical software. For the volume/cell based oxygen consumption rate, note that the mean value of the left and right TMJ discs was used to represent a single animal, resulting in 4 independent observations. One-way ANOVA and Tukey’s post hoc tests were performed to determine if significant differences existed between regions and were reported at p-values < 0.05.
RESULTS
Distribution of cell density
The surface-regional distribution of the volume based cell density in porcine TMJ discs was determined in situ using confocal microscopy techniques. Confocal assessment yielded an overall cell density (mean, 95% CI) of 51.3(21.3–81.3)×106cells/mL in wet tissue. Surface-regional variations in cell density along the superoinferior, anteroposterior, and mediolateral axes are depicted in Table 1. Along the 3 axes, statistically significant differences in total cell numbers were observed only along the anteroposterior and mediolateral axes, with no statistical difference between layers along the superoinferior axis. Although there was no statistically significant difference along the superoinferior axis, the cell density in the middle layer was lower than in the superior and inferior layers. Along the anteroposterior axis, the anterior band had 25.5% higher cell density than the intermediate zone (p<0.02) and 29.1% higher than the posterior band (p<0.008), with no significant difference between the intermediate and posterior band. Along the mediolateral axes, the medial region had 26.2% higher cell density than the intermediate zone (p<0.04) and 25.4% higher than the lateral region (p<0.045), although there was no significant difference between the intermediate zone and lateral region.
Table 1.
Surface-regional distribution of cell density (mean, 95% CI): superoinferior direction (n=5, p=0.415), anteroposterior direction (n=5, p<0.01), and mediolateral direction (n=5, p<0.04).
| Surface/Region | Cell density (×106cells/mL) | |
|---|---|---|
| Superoinferior | Superior | 53.6(20.1–87.1) |
| Middle | 48.1(22.8–73.4) | |
| Inferior | 52.6(21.6–83.6) | |
| Anteroposterior | Anterior | 58.5(31.8–85.2)* |
| Intermediate | 46.6(23.9–69.3) | |
| Posterior | 45.3(22.8–67.8) | |
| Mediolateral | Medial | 58.8(24.5–93.1)* |
| Intermediate | 46.6(23.9–69.3) | |
| Lateral | 46.9(14.2–79.6) |
The symbol (*) indicates significance for the post hoc test (p<0.05) in each direction.
Cell density validation using DNA assays
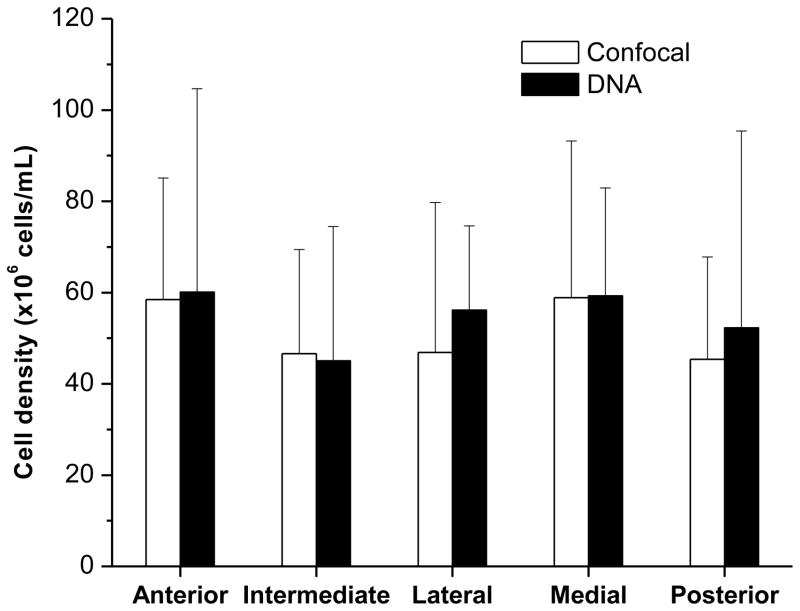
The total DNA content in the TMJ disc was 0.42(0.32–0.518)mg/mL in wet tissue. Correspondingly, the cell density across the disc was calculated as 54.6(42.4–66.7)×106cells/mL wet tissue. The comparison of cell density between the confocal microscopy technique and DNA assay is shown in Fig. 3. The cell densities measured by the DNA assay was comparable to the measurements of the confocal technique. There were no significant differences between the two methods for cell density measurements in any of the five regions.
Figure 3.
Comparison of cell density between the confocal microscopy technique (n=5) and DNA assay (n=5). The error bar is a 95% confidence interval. No significant differences were detected in each disc region.
Oxygen consumption rate
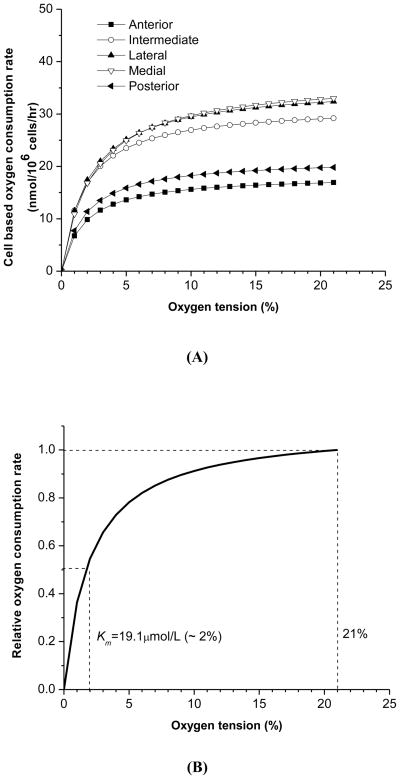
A typical curve-fit of the recorded oxygen concentration data with Equation 3 is shown in Fig. 2(B). Good agreement was found between the experimental data and theoretical curve-fitting with R2=0.989±0.007 (n=40), indicating that the relationship between the oxygen consumption rate and oxygen concentration can be well expressed using the Michaelis-Menten equation with the two parameters Vmax and Km. The Vmax is the maximum oxygen consumption rate at high oxygen tension, and the Km is the oxygen tension at which the oxygen consumption rate decreases to 50% of the Vmax [Fig. 2(C)]. The overall tissue volume based Vmax was 1.44(0.44–2.44) μmol/mL wet tissue/hr. One-way ANOVA showed that the volume based Vmax was significantly region-dependent (p<0.02). The medial region had the highest, while the anterior had the lowest regional consumption rate (Table 2). There was no significant difference between the medial, intermediate, and lateral regions, as well as between the anterior and posterior bands. However, the averaged volume based Vmax of central regions [1.69(0.55–2.83)μmol/mL wet tissue/hr], including intermediate, lateral, and medial, was 76% higher than the averaged value of the anterior and posterior bands [0.96(0.39–1.53)μmol/mL wet tissue/hr]. The overall Km was 19.1(13.6–24.6) μmol/L with no significant regional differences found.
Table 2.
Regional distribution of oxygen consumption rate (n=4): volume based Vmax (significantly region-dependent, p<0.02), cell based Vmax (significantly region-dependent, p<0.005), and kinetic constant Km (not significantly region-dependent, p=0.965).
| Volume based Vmax (μmol/mL tissue/hr) | Cell based Vmax (nmol/106 cells/hr) | Km (μmol/L) | |
|---|---|---|---|
| Anterior | 0.95(0.28–1.62) | 18.3(5.4–31.2) | 17.2(5.6–28.8) |
| Intermediate | 1.47(0.19–2.75) | 31.6(0.6–62.6) | 17.3(4.6–30.0) |
| Lateral | 1.67(0.42–2.92) | 35.6(8.4–62.8)* | 20.9(4.4–37.4) |
| Medial | 2.16(0.38–3.94)* | 36.7(6.5–66.9)* | 23.6(13.2–34.0) |
| Posterior | 0.98(0.45–1.51) | 21.5(9.9–33.1) | 17.8(10.4–25.2) |
The symbol (*) indicates significance compared to anterior/posterior bands for the post hoc test (p<0.05).
The cell based Vmax was calculated by normalizing each volume based Vmax with the corresponding regional mean value for cell density obtained from confocal microscopy. The overall cell based Vmax was 28.7(12.2–45.2) nmol/106 cells/hr. Compared to the volume based Vmax, region-dependency was further enhanced in the cell based Vmax (p<0.005) with similar trends of regional distribution (Table 2). The average cell based Vmax of central regions [34.4(13.6–55.2) nmol/106 cells/hr], including intermediate, lateral, and medial, was 72% higher than the averaged value of anterior and posterior bands [20.0(7.8–32.2) nmol/106 cells/hr].
The relationships between the oxygen consumption rate and oxygen concentration in the five disc regions are plotted in Fig. 4(A) using the Michaelis-Menten equation with averaged Vmax and Km. The oxygen consumption rate was relatively constant and fairly independent of oxygen tension until the oxygen tension fell below 5%. Below 5% oxygen, the rate fell in a highly concentration-dependent manner. Based on the Michaelis-Menten equation, the sensitivity of the oxygen consumption rate to the oxygen tension is solely controlled by the parameter Km. Using the averaged Km (19.1 μmol/L) of this study, the oxygen consumption rate relative to that at 21% oxygen tension (1% oxygen = 9.5 μmol/L) was shown in Fig. 4(B). The relative oxygen consumption rate was 0.91 at 10% oxygen, 0.78 at 5% oxygen, 0.55 at 2% oxygen, and 0.36 at 1% oxygen.
Figure 4.
(A) Relationship between the oxygen consumption rate and the oxygen tension based on the Michaelis-Menten equation with averaged cell based Vmax and Km in five disc regions. (B) Predicted relationship between the relative oxygen consumption rate and the oxygen tension. The averaged Km across the TMJ disc was 19.1 μmol/L. The oxygen consumption rate was normalized by the rate at 21% oxygen.
DISCUSSION
Since the normal adult human TMJ disc is avascular5–7, the consumption rate of the embedded cell population will be a key determinant of nutrient concentrations within the tissue. The objective of this paper was to determine the basal oxygen consumption rate in porcine TMJ discs using tissue explants and further examine the effects of disc region and oxygen tension on those rates. Recent studies have shown that the oxygen consumption of isolated articular chondrocytes increases with in vitro culture duration39. Oxygen consumption increased exponentially in that previous study within the first week and had doubled within the first 24 hours. The increase in oxygen consumption capacity could not be negated by culturing the cells under reduced oxygen atmospheres (2% and 5% O2), thought to fall within the physiological range of oxygen tensions40. Therefore, in this study, fresh TMJ disc explants were used to determine baseline oxygen consumption rates at the physiological glucose concentration of 5mM.
In using tissue explants, it became necessary to measure the distribution of volume based cell density to determine the oxygen consumption rate on a per-cell basis. Conventional histology slices can only provide cell numbers per unit area36. Although enzymatic cell isolation can determine cell numbers per tissue volume, the sequential enzymatic digestion may lose a significant amount of cells41. Therefore in this study, a confocal microscopy based technique was developed to determine the in situ surface-regional cell distribution of the TMJ disc. The confocal measurements were performed in three layers (i.e., superior, middle, and inferior) for each disc region. Multiple cell layers were found in each 3D confocal imaging data set. Therefore, the confocal measurement did provide real volume based cell number for each disc region. The confocal assessment yielded an overall cell density of 51.3(21.3–81.3)×106cells/mL wet tissue in porcine TMJ disc. Our validation assessment using DNA content yielded the overall cell density of 54.6(42.4–66.7)×106cells/mL. Those values are comparable to the cell density of 50×106cells/mL for porcine TMJ discs using a DNA assay in the literature38. However, complete enzymatic digestion of the bovine TMJ disc yielded a cell density of 20×106cells/mL wet tissue (assuming wet tissue density=1.08g/mL)33. While there is no layer dependency, our results showed that porcine TMJ disc has higher cell density in the anterior and medial regions. This is consistent with the DNA distribution of the porcine TMJ disc in the literature38. Previous qualitative studies have also demonstrated that cells were more numerous in the anterior band compared with the intermediate zone in rabbit and primate TMJ discs42–43. One possible explanation is that the peripheral regions have higher cellularity due to a better nutrient supply from surrounding tissues. It is also likely due to an inhomogeneous mechanical strain distribution within the TMJ disc during jaw function. In contrast, a recent study quantitatively indicated that the anterior band has fewer cells than the intermediate zone and posterior band36. Note that the cell density in that study was measured by counting cells on histological slides which cannot be translated for 3D tissue volume based cell density.
The tissue thickness and cell density of the TMJ discs from this study were compared to those of knee joint cartilage in Table 3. Stockwell25 showed that, in general, thinner cartilage has higher cell density than thicker cartilage due to the limitation of nutrient diffusion. The TMJ disc has a bi-concave shape and the thickness of the disc in the superior and inferior direction varies across the surface between 2–4mm. Considering the larger thickness of the TMJ disc, the cell density of TMJ discs is apparently higher than in knee cartilage. This implies that the TMJ disc might have greater demand of nutrients.
Table 3.
Comparison of tissue thickness and cell density between knee articular cartilage and the TMJ disc.
The oxygen consumption rate of the TMJ disc was measured in a sealed metabolism chamber. This approach has been used to investigate the effect of oxygen tension on the oxygen consumption rate of isolated articular chondrocytes20 and IVD cells30. Those studies have shown that the relationship between the oxygen consumption rate and oxygen tension can be modeled by the Michaelis-Menten equation with the two parameters Vmax and Km. Our results revealed that the kinetics of the oxygen consumption rate of TMJ disc explants can also be well expressed by this equation. Due to small Km, the oxygen consumption rate of the TMJ disc was relatively constant until the oxygen tension fell below 5%. Below 5% oxygen, the rate fell in a concentration-dependent manner. This finding is similar to the association between the oxygen consumption rate and oxygen concentration for articular cartilage20, 28 and IVD30.
The oxygen consumption rates of tissue explants are usually determined at 21% O2. It is apparent that the tissue volume based oxygen consumption rate of the TMJ disc is the highest among the cartilaginous tissues listed in Table 4A. Although the TMJ disc has a high cell density compared to other cartilage, the maximum cell based oxygen consumption rate (Vmax) of TMJ disc cells is still about 3 times higher than for articular chondrocytes and IVD cells (Table 4B). Both chondrocytes and IVD cells obtain their energy primarily through Embden-Meyerhof-Parnas (EMP) pathway glycolysis, even in the presence of high oxygen tension14, 26–27. Therefore, the oxygen consumption rate of those cells is exceptionally low. It has even been reported that the mitochondria of chondrocytes in situ lack certain cytochromes that are required for a fully functional electron transport chain44. Cell morphological studies using electron microscopy have shown that the porcine TMJ disc contains an inhomogeneous distribution of a mixed cell population of fibroblast-like cells and chondrocyte-like cells, which are distinct from hyaline cartilage chondrocytes36. The chondrocyte-like cells in the TMJ disc do not appear to exhibit the distinct pericellular capsule typical of articular chondrocytes34. Moreover, there are significant differences in organelle content between articular chondrocytes and chondrocyte-like cells in TMJ disc, which likely suggest differences in cellular behavior. The chondrocyte-like cells in TMJ discs have a greater number of mitochondria, suggesting a higher metabolic activity than articular chondrocytes36. The overall higher oxygen consumption rate determined in this study might be related to some extent of oxidative phosphorylation in TMJ disc cells. Detamore et al. reported that the distributions of cell subpopulations in TMJ disc are significantly region-dependent36. This might lead to the region-dependent oxygen consumption rate determined in this study. For example, the intermediate zone of the TMJ disc had a higher oxygen consumption rate possibly due to the relatively higher number of chondrocyte-like cells in this region.
Table 4A.
Comparison of tissue volume based oxygen consumption rate in the TMJ disc and other cartilaginous tissues.
| Type of joint and species | Medium glucose (mM) | Volume based oxygen consumption rate (μmol/mL tissue/hr) | Reference |
|---|---|---|---|
| Bovine Knee cartilage | 0 | 0.11 | [46] |
| 10 | 0.21 | [46] | |
| Porcine knee cartilage | 0 | 0.94 | [23] |
| 5 | 0.45 | [23] | |
| 10 | 0.4 | [23] | |
| Bovine AF | n/a | 0.68 | [29] |
| Bovine NP | n/a | 0.75 | [29] |
| Porcine TMJ disc | 5 | 1.44 | Present study |
Table 4B.
Comparison of cell based oxygen consumption rate between the TMJ disc and other cartilaginous tissues.
| Type of joint and species | Subpopulation | Cell based Vmax consumption rate (nmol/106 cells/hr) | Km (μmol/L) | Reference |
|---|---|---|---|---|
| Bovine articular chondrocytes | Superficial | 3.2 | 68 | [25] |
| Deep | 6.6 | 63 | [25] | |
| Porcine IVD | AF | 6.0 | 35.7 | [27] |
| NP | 11.5 | 6.8 | [27] | |
| Porcine TMJ disc | Anterior | 18.3 | 17.2 | Present study |
| Central | 34.4 | 20.6 | Present study | |
| Posterior | 21.5 | 17.8 | Present study |
Due to the difficulty of measuring nutrient concentrations in vivo, mathematical models have been used to evaluate them in cartilaginous tissues. The results of these calculations indicate that there is a steep gradient of oxygen in normal cartilage and the concentration can be as low as 1%20. The normal adult human TMJ disc is a large avascular structure5–7, although some research has shown vasculature in young animal36 and human discs45, as well as degenerated human discs46. Considering the higher cell density and oxygen consumption rate of TMJ disc cells, a steeper oxygen gradient potentially exists in the normal TMJ disc. Such a steep oxygen gradient would make this tissue uniquely vulnerable to any pathological event which impedes nutrient supply, such as sustained joint loading due to jaw clenching47. To precisely predict the nutrient environment using a mathematical model, it is crucial to determine quantitative relationships between the nutrient consumption rates and the local nutrient concentrations. In this study, the relationship between the oxygen consumption rate and oxygen tension was established for the TMJ disc. Studies on articular cartilage and IVD have shown that nutrient consumption rates are dependent not only on a single substrate but also on other nutrients. For instance, stimulation of oxygen uptake at low glucose concentrations (the Crabtree effect) was observed in articular cartilage26, 48–50. The present study only determined the oxygen consumption rate of TMJ disc at 5mM glucose. Therefore, it is necessary to investigate the effect of glucose on the oxygen consumption rate in a future study. It will also be valuable to study the coupling of oxygen consumption, glucose consumption, and lactate production to fully understand the energy metabolism in the TMJ disc.
In summary, the distributions of cell density and basal oxygen consumption rate in five TMJ disc regions were determined using porcine tissue explants. The impact of the oxygen tension on the oxygen consumption rate was investigated and a quantitative relationship between them was established. The TMJ disc had a higher cellularity compared to artilcular cartilage. The cell density of the TMJ disc was region-dependent, and the anterior and medial regions had higher values compared to intermediate, lateral, and posterior regions. Compared to articular cartilage and IVD, the TMJ disc had a higher oxygen consumption rate on a tissue volume basis, as well as on a per-cell basis. The central regions, including intermediate, lateral, and medial, had a higher average oxygen consumption rate than anterior and posterior bands. The oxygen consumption rate was also dependent on the oxygen tension. At high oxygen tension, the oxygen consumption rate remained constant, and only dropped significantly as oxygen tension fell below 5%. This relationship can be well expressed by the Michaelis-Menten equation. Considering the higher cell density and oxygen consumption rate of TMJ disc cells, a steeper oxygen gradient potentially exists in the normal TMJ disc. Such an oxygen gradient will likely be very vulnerable to any pathological event that can impede nutrient supply, and ultimately result in tissue degeneration.
Acknowledgments
This project was supported by NIH grants DE018741, RR017696, and AR055775, a NSF RII grant fellowship (EPS-00903795) to CS, and a NIH F31 training grant DE020230 to JK.
Footnotes
AUTHOR CONTRIBITIONS
The authors made substantial contributions in designing the study (JK, MJK, HY), gathering and analyzing the data (JK, CS, SC, LZ, HY), and drafting the article (JK, MJK, HY).
CONFLICT OF INTEREST
None of the authors of this paper have a conflict of interest that might be construed as affecting the conduct or reporting of the work presented.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nickel JC, McLachlan KR. In vitro measurement of the stress-distribution properties of the pig temporomandibular joint disc. Arch Oral Biol. 1994;39:439–48. doi: 10.1016/0003-9969(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 2.Nickel JC, McLachlan KR. In vitro measurement of the frictional properties of the temporomandibular joint disc. Arch Oral Biol. 1994;39:323–31. doi: 10.1016/0003-9969(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 3.Stegenga B. Osteoarthritis of the temporomandibular joint organ and its relationship to disc displacement. J Orofac Pain. 2001;15:193–205. [PubMed] [Google Scholar]
- 4.Stegenga B, de Bont LG, Boering G. Osteoarthrosis as the cause of craniomandibular pain and dysfunction: a unifying concept. J Oral Maxillofac Surg. 1989;47:249–56. doi: 10.1016/0278-2391(89)90227-9. [DOI] [PubMed] [Google Scholar]
- 5.Rees LA. The structure and function of the mandibular joint. Br Dent J. 1954;96:125–33. [Google Scholar]
- 6.Leonardi R, Lo ML, Bernasconi G, Caltabiano C, Piacentini C, Caltabiano M. Expression of vascular endothelial growth factor in human dysfunctional temporomandibular joint discs. Arch Oral Biol. 2003;48:185–92. doi: 10.1016/s0003-9969(02)00207-8. [DOI] [PubMed] [Google Scholar]
- 7.Detamore MS, Athanasiou KA. Motivation, characterization, and strategy for tissue engineering the temporomandibular joint disc. Tissue Eng. 2003;9:1065–87. doi: 10.1089/10763270360727991. [DOI] [PubMed] [Google Scholar]
- 8.Detamore MS, Athanasiou KA. Structure and function of the temporomandibular joint disc: implications for tissue engineering. J Oral Maxillofac Surg. 2003;61:494–506. doi: 10.1053/joms.2003.50096. [DOI] [PubMed] [Google Scholar]
- 9.Cernanec J, Guilak F, Weinberg JB, Pisetsky DS, Fermor B. Influence of hypoxia and reoxygenation on cytokine-induced production of proinflammatory mediators in articular cartilage. Arthritis Rheum. 2002;46:968–75. doi: 10.1002/art.10213. [DOI] [PubMed] [Google Scholar]
- 10.Gray ML, Pizzanelli AM, Grodzinsky AJ, Lee RC. Mechanical and physiochemical determinants of the chondrocyte biosynthetic response. J Orthop Res. 1988;6:777–92. doi: 10.1002/jor.1100060602. [DOI] [PubMed] [Google Scholar]
- 11.Grimshaw MJ, Mason RM. Bovine articular chondrocyte function in vitro depends upon oxygen tension. Osteoarthritis Cartilage. 2000;8:386–92. doi: 10.1053/joca.1999.0314. [DOI] [PubMed] [Google Scholar]
- 12.Grimshaw MJ, Mason RM. Modulation of bovine articular chondrocyte gene expression in vitro by oxygen tension. Osteoarthritis Cartilage. 2001;9:357–64. doi: 10.1053/joca.2000.0396. [DOI] [PubMed] [Google Scholar]
- 13.Henrotin Y, Kurz B, Aigner T. Oxygen and reactive oxygen species in cartilage degradation: friends or foes? Osteoarthritis. Cartilage. 2005;13:643–54. doi: 10.1016/j.joca.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Lee RB, Urban JP. Evidence for a negative Pasteur effect in articular cartilage. Biochem J. 1997;321 ( Pt 1):95–102. doi: 10.1042/bj3210095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin G, Andriamanalijaona R, Grassel S, Dreier R, Mathy-Hartert M, Bogdanowicz P, et al. Effect of hypoxia and reoxygenation on gene expression and response to interleukin-1 in cultured articular chondrocytes. Arthritis Rheum. 2004;50:3549–60. doi: 10.1002/art.20596. [DOI] [PubMed] [Google Scholar]
- 16.Ysart GE, Mason RM. Responses of articular cartilage explant cultures to different oxygen tensions. Biochim Biophys Acta. 1994;1221:15–20. doi: 10.1016/0167-4889(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 17.Tojyo I, Yamaguchi A, Nitta T, Yoshida H, Fujita S, Yoshida T. Effect of hypoxia and interleukin-1beta on expression of tenascin-C in temporomandibular joint. Oral Dis. 2008;14:45–50. doi: 10.1111/j.1601-0825.2006.01344.x. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi A, Tojyo I, Yoshida H, Fujita S. Role of hypoxia and interleukin-1beta in gene expressions of matrix metalloproteinases in temporomandibular joint disc cells. Arch Oral Biol. 2005;50:81–87. doi: 10.1016/j.archoralbio.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Haselgrove JC, Shapiro IM, Silverton SF. Computer modeling of the oxygen supply and demand of cells of the avian growth cartilage. Am J Physiol. 1993;265:C497–C506. doi: 10.1152/ajpcell.1993.265.2.C497. [DOI] [PubMed] [Google Scholar]
- 20.Zhou S, Cui Z, Urban JP. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage-bone interface: a modeling study. Arthritis Rheum. 2004;50:3915–24. doi: 10.1002/art.20675. [DOI] [PubMed] [Google Scholar]
- 21.Zhou S, Cui Z, Urban JP. Nutrient gradients in engineered cartilage: metabolic kinetics measurement and mass transfer modeling. Biotechnol Bioeng. 2008;101:408–21. doi: 10.1002/bit.21887. [DOI] [PubMed] [Google Scholar]
- 22.Malda J, Rouwkema J, Martens DE, Le Comte EP, Kooy FK, Tramper J, et al. Oxygen gradients in tissue-engineered PEGT/PBT cartilaginous constructs: measurement and modeling. Biotechnol Bioeng. 2004;86:9–18. doi: 10.1002/bit.20038. [DOI] [PubMed] [Google Scholar]
- 23.Stairmand JW, Holm S, Urban JP. Factors influencing oxygen concentration gradients in the intervertebral disc. A theoretical analysis. Spine. 1991;16:444–49. doi: 10.1097/00007632-199104000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Huang CY, Gu WY. Effects of mechanical compression on metabolism and distribution of oxygen and lactate in intervertebral disc. J Biomech. 2008;41:1184–96. doi: 10.1016/j.jbiomech.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stockwell RA. Biology of Cartilage Cells. Cambridge, UK: Cambridge University Press; 1979. [Google Scholar]
- 26.Otte P. Basic cell metabolism of articular cartilage. Manometric studies. Z Rheumatol. 1991;50:304–12. [PubMed] [Google Scholar]
- 27.Holm S, Maroudas A, Urban JP, Selstam G, Nachemson A. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8:101–19. doi: 10.3109/03008208109152130. [DOI] [PubMed] [Google Scholar]
- 28.Heywood HK, Knight MM, Lee DA. Both superficial and deep zone articular chondrocyte subpopulations exhibit the Crabtree effect but have different basal oxygen consumption rates. J Cell Physiol. 2010;223:630–39. doi: 10.1002/jcp.22061. [DOI] [PubMed] [Google Scholar]
- 29.Bibby SR, Jones DA, Ripley RM, Urban JP. Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine (Phila Pa 1976) 2005;30:487–96. doi: 10.1097/01.brs.0000154619.38122.47. [DOI] [PubMed] [Google Scholar]
- 30.Huang CY, Yuan TY, Jackson AR, Hazbun L, Fraker C, Gu WY. Effects of low glucose concentrations on oxygen consumption rates of intervertebral disc cells. Spine (Phila Pa 1976) 2007;32:2063–69. doi: 10.1097/BRS.0b013e318145a521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guehring T, Wilde G, Sumner M, Grunhagen T, Karney GB, Tirlapur UK, et al. Notochordal intervertebral disc cells: sensitivity to nutrient deprivation. Arthritis Rheum. 2009;60:1026–34. doi: 10.1002/art.24407. [DOI] [PubMed] [Google Scholar]
- 32.Ishihara H, Urban JP. Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. J Orthop Res. 1999;17:829–35. doi: 10.1002/jor.1100170607. [DOI] [PubMed] [Google Scholar]
- 33.Landesberg R, Takeuchi E, Puzas JE. Cellular, biochemical and molecular characterization of the bovine temporomandibular joint disc. Arch Oral Biol. 1996;41:761–67. doi: 10.1016/s0003-9969(96)00068-4. [DOI] [PubMed] [Google Scholar]
- 34.Berkovitz BK, Pacy J. Ultrastructure of the human intra-articular disc of the temporomandibular joint. Eur J Orthod. 2002;24:151–58. doi: 10.1093/ejo/24.2.151. [DOI] [PubMed] [Google Scholar]
- 35.Detamore MS, Orfanos JG, Almarza AJ, French MM, Wong ME, Athanasiou KA. Quantitative analysis and comparative regional investigation of the extracellular matrix of the porcine temporomandibular joint disc. Matrix Biol. 2005;24:45–57. doi: 10.1016/j.matbio.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Detamore MS, Hegde JN, Wagle RR, Almarza AJ, Montufar-Solis D, Duke PJ, et al. Cell type and distribution in the porcine temporomandibular joint disc. J Oral Maxillofac Surg. 2006;64:243–48. doi: 10.1016/j.joms.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao H, Justiz MA, Flagler D, Gu WY. Effects of Swelling Pressure and Hydraulic Permeability on Dynamic Compressive Behavior of Lumbar Annulus Fibrosus. Annals of Biomed Engng. 2002;30:1234–41. doi: 10.1114/1.1523920. [DOI] [PubMed] [Google Scholar]
- 38.Almarza AJ, Bean AC, Baggett LS, Athanasiou KA. Biochemical analysis of the porcine temporomandibular joint disc. Br J Oral Maxillofac Surg. 2006;44:124–28. doi: 10.1016/j.bjoms.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Heywood HK, Lee DA. Monolayer expansion induces an oxidative metabolism and ROS in chondrocytes. Biochem Biophys Res Commun. 2008;373:224–29. doi: 10.1016/j.bbrc.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Heywood HK, Lee DA. Low oxygen reduces the modulation to an oxidative phenotype in monolayer-expanded chondrocytes. J Cell Physiol. 2010;222:248–53. doi: 10.1002/jcp.21946. [DOI] [PubMed] [Google Scholar]
- 41.Berkovitz BK, Pacy J. The ultrastructure of the intra-articular disc of the temporomandibular joint, with special reference to fibrocartilage. Bull Group Int Rech Sci Stomatol Odontol. 1999;41:2–13. [PubMed] [Google Scholar]
- 42.Mills DK, Fiandaca DJ, Scapino RP. Morphologic, microscopic, and immunohistochemical investigations into the function of the primate TMJ disc. J Orofac Pain. 1994;8:136–54. [PubMed] [Google Scholar]
- 43.Scapino RP, Canham PB, Finlay HM, Mills DK. The behaviour of collagen fibres in stress relaxation and stress distribution in the jaw-joint disc of rabbits. Arch Oral Biol. 1996;41:1039–52. doi: 10.1016/s0003-9969(96)00079-9. [DOI] [PubMed] [Google Scholar]
- 44.Mignotte F, Champagne AM, Froger-Gaillard B, Benel L, Gueride M, Adolphe M, et al. Mitochondrial biogenesis in rabbit articular chondrocytes transferred to culture. Biol Cell. 1991;71:67–72. doi: 10.1016/0248-4900(91)90052-o. [DOI] [PubMed] [Google Scholar]
- 45.Mah J. Histochemistry of the foetal human temporomandibular joint articular disc. Eur J Orthod. 2004;26:359–65. doi: 10.1093/ejo/26.4.359. [DOI] [PubMed] [Google Scholar]
- 46.Yoshida H, Fujita S, Nishida M, Iizuka T. Localization of lymph capillaries and blood capillaries in human temporomandibular joint discs. J Oral Rehabil. 1999;26:600–7. doi: 10.1046/j.1365-2842.1999.00402.x. [DOI] [PubMed] [Google Scholar]
- 47.Milam SB. Pathogenesis of degenerative temporomandibular joint arthritides. Odontology. 2005;93:7–15. doi: 10.1007/s10266-005-0056-7. [DOI] [PubMed] [Google Scholar]
- 48.Bywaters EGL. The metabolism of joint tissues. J Pathol Bacterial. 1937;44:247–68. [Google Scholar]
- 49.Rosenthal O, Bowei MA, Wagoner G. Studies in the metabolism of articular cartilage I. Respiration and glycolysis of cartilage in relation to its age. J Cell Physiol. 1941;17:221–33. [Google Scholar]
- 50.Heywood HK, Bader DL, Lee DA. Rate of oxygen consumption by isolated articular chondrocytes is sensitive to medium glucose concentration. J Cell Physiol. 2006;206:402–10. doi: 10.1002/jcp.20491. [DOI] [PubMed] [Google Scholar]