Abstract
Bronchopulmonary dysplasia (BPD) is characterized by arrested alveolar development and complicated by pulmonary hypertension (PH). Nitric oxide (NO) promotes alveolar growth. Inhaled NO (iNO) ameliorates the BPD phenotype in experimental models and in some premature infants. Arginosuccinate synthetase (ASS) and arginosuccinate lyase (ASL) convert L-citrulline to L-arginine; L-citrulline is regenerated during NO synthesis from L-arginine. Plasma levels of these NO precursors are low in PH. We hypothesized that L-citrulline prevents experimental O2-induced BPD in newborn rats. Rat pups were assigned from birth through postnatal day 14 (P14) to room air (RA), RA+L-citrulline, 95% hyperoxia (BPD model), and 95%O2+L-citrulline. Rat pups exposed to hyperoxia had fewer and enlarged air spaces and decreased capillary density, mimicking human BPD. This was associated with decreased plasma L-arginine and L-citrulline concentrations on P7. L-Citrulline treatment significantly increased plasma L-arginine and L-citrulline concentrations and increased ASL protein expression in hyperoxia. L-Citrulline preserved alveolar and vascular growth in O2-exposed pups and decreased pulmonary arterial medial wall thickness and right ventricular hypertrophy. Increased lung arginase activity in O2-exposed pups was reversed by L-citrulline treatment. L-Citrulline supplementation prevents hyperoxia-induced lung injury and PH in newborn rats. L-citrulline may represent a novel therapeutic alternative to iNO for prevention of BPD.
Introduction
Bronchopulmonary dysplasia (BPD), the chronic lung disease that follows acute respiratory failure after premature birth, is the most common complication in premature infants born<28 weeks gestation, with an incidence of 30–50%(1). BPD currently lacks specific treatment or prevention strategies. BPD interrupts normal lung development and results in arrested alveolar and vascular growth(2, 3). The extent to which the disruption of lung growth leads to an earlier or more severe decline in respiratory function in later life is unknown(4). Case reports are emerging describing arrested alveolar growth in older children(5) and early onset emphysema in young adults(6) who were diagnosed with BPD.
Evidence suggests that NO promotes lung growth (7, 8). Lungs of endothelial NO synthase (eNOS)-deficient mice have a paucity of distal arteries and reduced alveolarization(9), and are more susceptible to disrupted lung growth after exposure to mild hypoxia and hyperoxia(10). Arrested alveolar growth in newborn rats after VEGF inhibition and in the chronically ventilated premature sheep and baboon models of BPD are associated with decreased lung eNOS protein and NO production. Inhaled NO (iNO) improves vascular and alveolar growth in these animal models(11–13). These data suggest that NO deficiency contributes to the decreased alveolarization in experimental BPD. Most recent randomized human trials suggest that iNO decreases the incidence of BPD in subsets of premature babies(14–16). Inconsistent efficacy, complexity of iNO delivery in non-intubated patients and high cost provide rationales for efficacious alternatives to iNO.
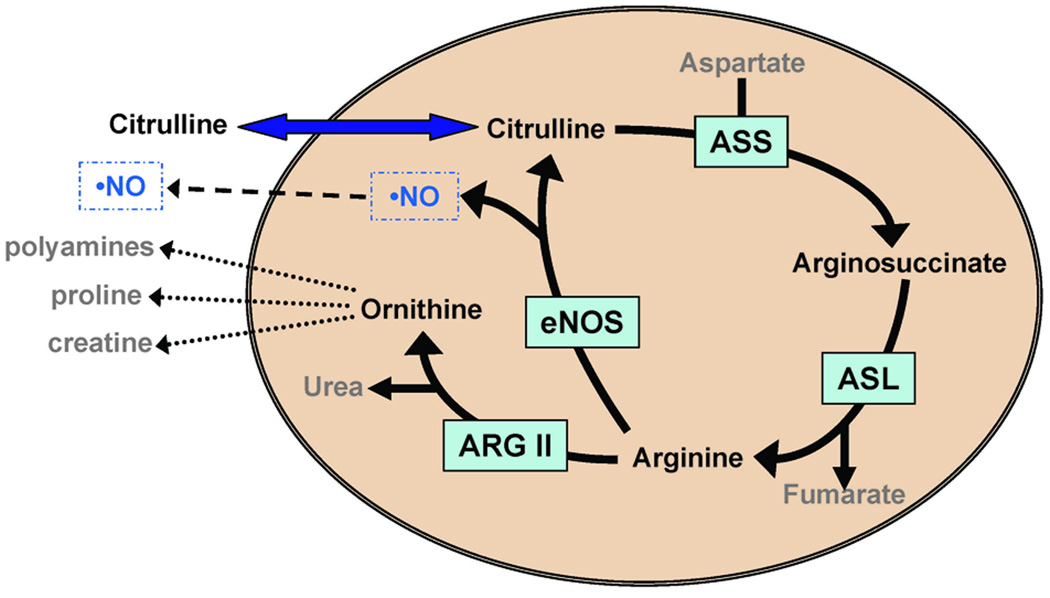
Endogenous NO is produced from the metabolism of L-arginine to L-citrulline, two amino acids generated by the urea cycle (17). L-Citrulline to L-arginine recycling in endothelial cells by the enzymes arginosuccinate synthetase (ASS) and arginosuccinate lyase (ASL) is proposed to be the principal mechanism for sustaining local L-arginine availability for eNOS-catalyzed NO production(18) (Figure 1). Polymorphisms in the gene encoding carbamoyl-phosphate synthetase 1, the mitochondrial enzyme catalyzing the rate-limiting step in hepatic L-citrulline formation via the urea cycle, influences NO metabolite concentrations and NO-mediated vasodilation in humans(19). Infants with persistent pulmonary hypertension of the newborn (PPHN) or after bypass surgery for congenital heart disease have low plasma concentrations of arginine, citrulline and NO metabolites(20, 21) and this can be reversed by oral citrulline supplementation(22, 23). In addition to NO production, an adequate supply of precursor molecule is necessary to maintain the NOS complex in its “coupled” state(24). Uncoupling of NOS results in free radical oxygen production which may potentiate vascular damage and dysfunction(24). Thus, decreased concentrations of NO precursors and breakdown products suggest that inadequate NO production contributes to PPHN and abnormal vascular function.
Figure 1. Schematic of the citrulline-arginine-NO pathway in endothelial cells.
This cartoon depicts the intracellular enzymes that convert citrulline to arginine (ASS and ASL) as well as the enzymes eNOS that convert arginine to NO and citrulline, and ARG II, that converts arginine to ornithine and urea.
We hypothesized that L-citrulline supplementation preserves alveolar growth and prevents pulmonary hypertension (PH) in a model of chronic oxygen-induced BPD in newborn rats.
Materials and Methods
All procedures and protocols were approved by the Animal Health Care Committee of the University of Alberta. Expanded methods are available in the online-Supplement.
Animal Model
Experimental BPD was induced as previously described(25, 26). Sprague-Dawley rats (Charles River, Saint Constant, QC, Canada) were exposed to normoxia (21% O2; n=90) or hyperoxia (95% O2, BPD model; n=90) from birth to postnatal day (P) 7, 10 or 14 in sealed plexiglass chambers with continuous O2 monitoring (BioSpherix, Redfield, NY) (Supplemental methods, http://links.lww.com/PDR/XXX).
Experimental Protocol
Newborn rat pups were randomized to four groups: 1-normoxia (21%, control group; n=45), 2-normoxia+L-citrulline (n=45), 3-hyperoxia (95% O2, BPD model; n=45), and 4-hyperoxia+L-citrulline (n=45). L-citrulline was administered daily from P4 to P14 via subcutaneous injection. The dose (8 g/m2/day) was based on the effective L-citrulline dosage reported in the literature(22). Controls received normal saline vehicle. From P14-P21, rat pups were allowed to recover in 21% oxygen. Amongst animals housed in hyperoxia for 14 days, 26 of the 30 untreated rats in the O2-induced BPD group survived; all 30 citrulline treated rats in the O2-induced BPD group survived the study protocol.
Plasma amino acid levels
Amino acids (citrulline, arginine, ornithine) were analyzed using a Hitachi 8800 (Hitachi USA) amino acid analyzer and NO metabolites (NOx) were measured using a Sievers 280i instrument (GE Analytical Instruments, USA) as previously described (Supplemental methods, http://links.lww.com/PDR/XXX).(23).
Lung morphometry
Lungs were inflated and fixed via the trachea with a 4% formaldehyde solution at a constant pressure of 20 cm H2O(25, 26). Lungs were paraffin embedded, cut in 4 µm thick serial sections and lung sections were stained with hematoxylin and eosin. Alveolar structures were quantified using the mean linear intercept as described (Supplemental methods, http://links.lww.com/PDR/XXX).(26).
Barium-Gelatin Angiograms
Barium was instilled into the pulmonary vasculature as previously described (Supplemental methods, http://links.lww.com/PDR/XXX).(25, 26). Barium-filled pulmonary arteries were counted per high-powered field (×100 magnification). Lungs of five animals/group, five sections/lung, and 10 high-power fields/section were counted.
Right Ventricular Hypertrophy (RVH) and Pulmonary Artery Remodeling
Right and left ventricles including the septum were weighed separately to determine the right ventricle to left ventricle+septum ratio (RV/LV+S) as an index of RVH(25). To assess pulmonary artery remodeling, the percent medial wall thickness (MWT) was calculated as (2 × wall thickness/external diameter) ×100%(25).
Western blot analysis
Whole rat lungs were used to determine eNOS, ASS, ASL and arginase II (ARG II) protein expression by western blot using a modification of our previously published methods (Supplemental methods, http://links.lww.com/PDR/XXX) (27).
Arginase activity
Arginase activity in frozen lung tissue was measured by determining the amount of urea generated by the enzyme (Supplemental methods, http://links.lww.com/PDR/XXX) (28).
Statistics
Values are expressed as the mean±SEM. Statistical comparisons were made with the use of ANOVA. Post hoc analysis used a Fisher’s probable least significant difference test (Statview 5.1, Abacus Concepts). A value of P<0.05 was considered statistically significant.
Results
Plasma concentrations of NO precursors are decreased in O2-induced BPD in newborn rats
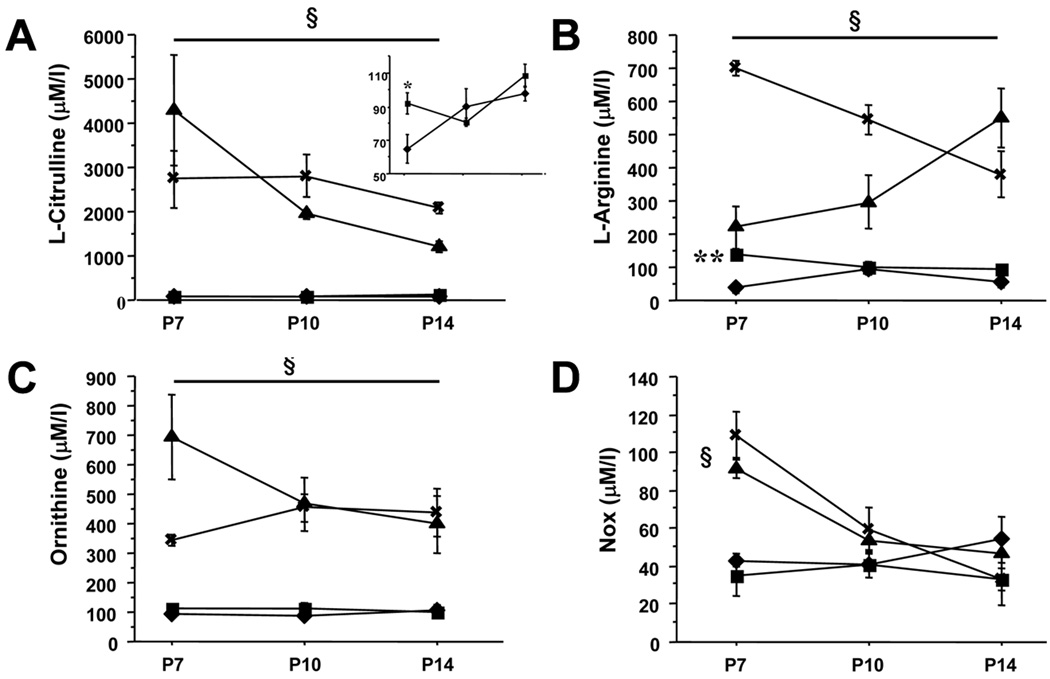
L-Citrulline and L-arginine plasma levels were significantly decreased in hyperoxic-exposed animals on P7 compared to room air controls (Figure 2A inset and Figure 2B). L-Citrulline supplementation significantly increased the plasma amino acids concentrations (Figure 2A–C). Plasma NOx levels were not significantly different between untreated room air and hyperoxic-exposed animals. L-Citrulline supplementation significantly increased P7 plasma NOx levels in both normoxia- and hyperoxia-exposed rat pups (Figure 2D).
Figure 2. Effect of L-citrulline administration from postnatal days (P) 4 to 14 on amino acid plasma levels and NOx levels in normoxic control rats and O2-induced BPD rats.
Plasma levels of L-citrulline (A), L-arginine (B), ornithine (C) and NOx (D) were measured on P7, 10 and 14 (N=5/group, *P<0.05 and **P<0.0005, normoxia vs hyperoxia, §P<0.001, normoxia (■) and hyperoxia (◆) vs normoxia+L-Citrulline (⨯) and hyperoxia+L-Citrulline (▲). Inset shows plasma levels of L-Citrulline in normoxia (■) and hyperoxia (◆) only
L-Citrulline prevents alveolar simplification in O2-induced BPD in newborn rats
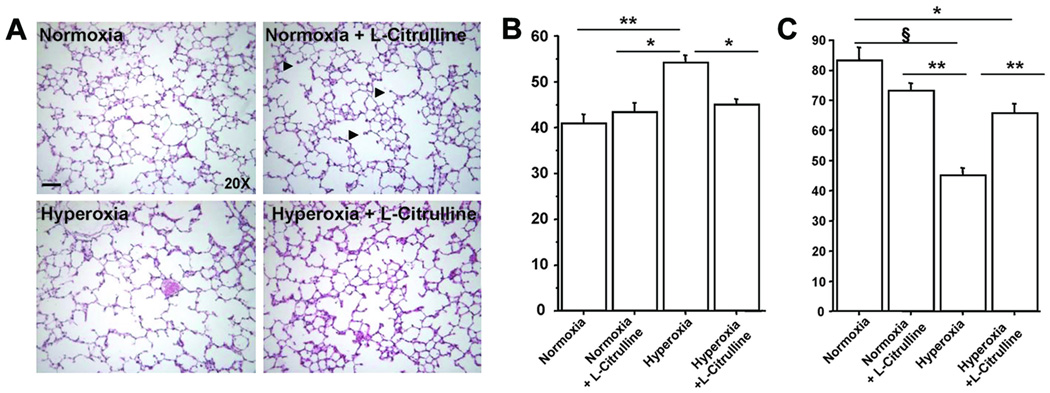
Hyperoxic-exposed rat pups displayed the characteristic features of alveolar simplification, with larger and fewer alveoli and decreased septation compared with normoxic animals (Figure 3A). L-Citrulline preserved alveolar growth in hyperoxic rats as quantified by the mean linear intercept and septal counts (Figure 3B) measured on P21. No effect of L-citrulline was observed in control rats.
Figure 3. L-Citrulline prevents alveolar simplification in O2-induced BPD in newborn rats.
Representative H&E–stained lung sections show larger and fewer alveoli in hyperoxia-exposed lungs (A), resulting in a significantly higher mean linear intercept (Lm) (B) and lower septal counts than in control (normoxia) animals (C). Treatment with L-citrulline preserved alveolar structure and significantly improved Lm and septal counts as compared with the experimental BPD model. N=5/group, **P<0.001 hyperoxia vs normoxia, *P<0.01 hyperoxia vs normoxia+L-Citrulline and hyperoxia+L-Citrulline for Lm. For septal counts, §P<0.0001 hyperoxia vs normoxia, **P<0.001 hyperoxia vs normoxia+L-Citrulline and hyperoxia vs L-Citrulline, *P<0.01 normoxia vs hyperoxia+L-Citrulline.
L-Citrulline preserves lung vascular growth in O2-induced BPD in newborn rats
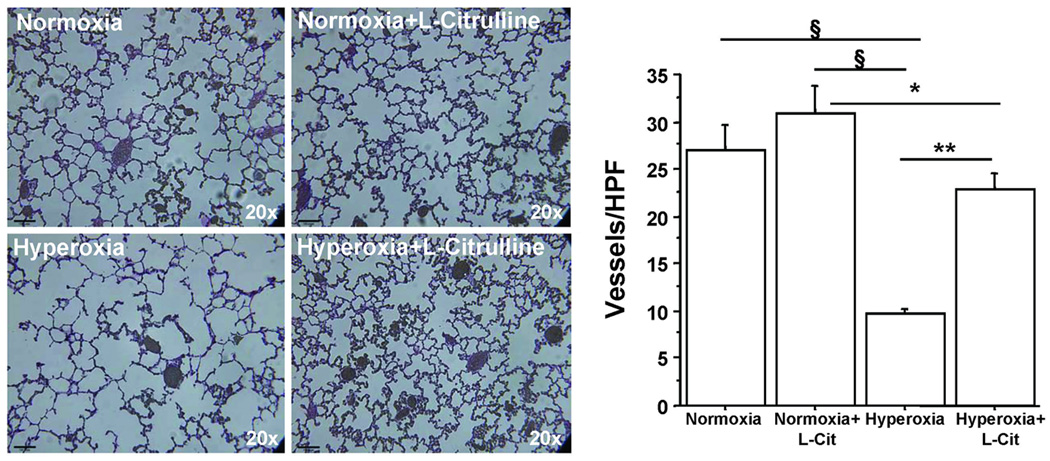
Hyperoxic-exposed rat pups showed a significant decrease in pulmonary arterial density compared to normoxic rat pups as demonstrated by barium angiograms performed on P21 (Figure 4). Hyperoxia-exposed rat pups treated with L-citrulline exhibited increased arterial density when compared to untreated hyperoxic rat pups (Figure 4). L-Citrulline had no effect on normoxic rats.
Figure 4. L-Citrulline improves lung angiogenesis in O2-induced BPD in newborn rats.
Capillary density, as quantified by the number of barium-filled pulmonary arteries counted per high-power field, was significantly decreased in hyperoxia-exposed lungs compared with room air controls. Treatment with L-citrulline preserved lung capillary density in experimental BPD. N=5/group, §P<0.0001 hyperoxia vs normoxia and normoxia+L-Citrulline, **P<0.001 hyperoxia vs hyperoxia+L-Citrulline, *P<0.02 normoxia+L-Citrulline vs hyperoxia+L-Citrulline.
L-Citrulline prevents PH in O2-induced BPD in newborn rats
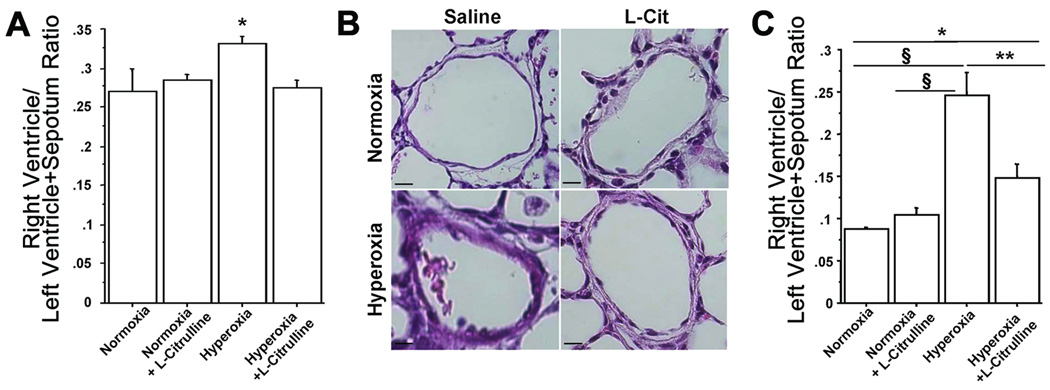
Chronic exposure to hyperoxia for 14 days was associated with a significant increase in RVH (Figure 5A) and %MWT of small pulmonary arteries measured on P21 (Figure 5B). L-Citrulline attenuated these structural features of PH as indicated by a reduction in RV/LV+S and %MWT (Figures 5A and 5B).
Figure 5. L-Citrulline prevents pulmonary hypertension in O2-induced BPD in newborn rats.
A. Hyperoxia-exposed rats had significant RVH as indicated by the increase in RV/LV+S ratio compared with normoxic controls. L-Citrulline reduced RVH. N=5/group, *P<0.05 hyperoxia vs other groups. (B) Representative H&E stained lung sections showing pulmonary arteries displaying a thickened medial arterial wall in hyperoxic rat lungs compared with normoxic controls (scale bar is 65μm, original magnification is 4×). (C) L-Citrulline significantly reduced the %MWT compared with pulmonary arteries from untreated hyperoxic rat lungs. N=5/group, §P<0.0001 hyperoxia vs normoxia and normoxia+L-Citrulline, **P<0.002 hyperoxia vs hyperoxia+L-Citrulline, *P<0.05 normoxia vs hyperoxia+L-Citrulline.
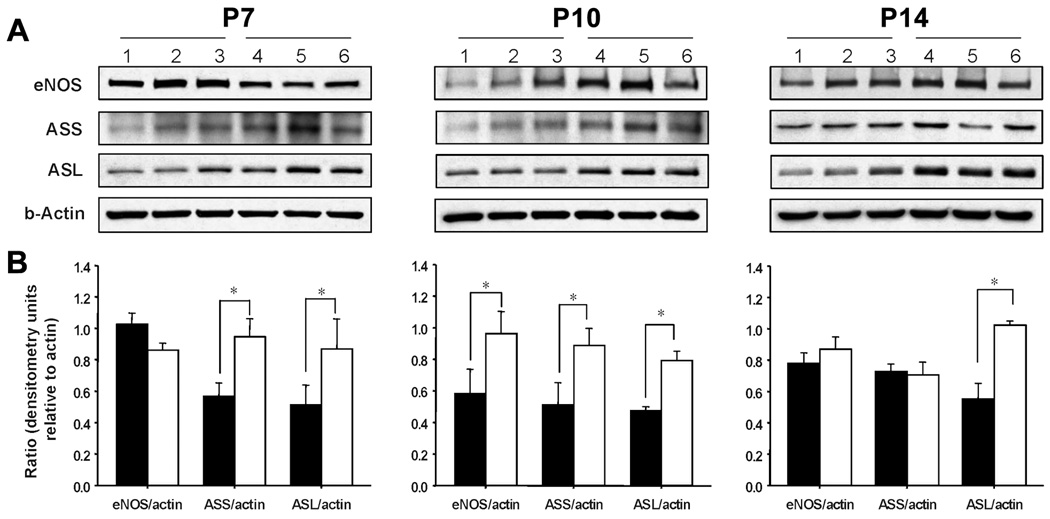
Hyperoxia alters the expression of proteins involved in the citrulline-arginine-NO cycle in a postnatal age-dependent manner
eNOS expression was not statistically different between control and hyperoxic rat lungs on P7 or P14 but was transiently increased on P10 in lungs from hyperoxia-exposed rats. There was a significant increase in protein abundance of ASL throughout the 14 days exposure to hyperoxia; ASS protein expression was also significantly increased in hypoxic rat lungs on P7 and P10, but not on P14 (Figures 6A–B).
Figure 6. Effects of hyperoxia and postnatal age on protein abundance in rat lung homogenates.
(A) Representative Western blots demonstrating the abundance of eNOS, ASL, ASS and β-Actin in protein lysates from normoxic (lanes 1–3) and hyperoxic (lanes 4–6) rat lungs on P7, P10 and P14. Blots are representative of two separate studies performed on a total of 6–8 normoxic and hyperoxic neonatal rat lungs at each postnatal time point.(B). Summary densitometry data showing the effects of hyperoxia (□) on eNOS, ASS and ASL protein expression relative to β-actin at each of three postnatal ages. *P<0.05 different from age-matched normoxic control (■) group.
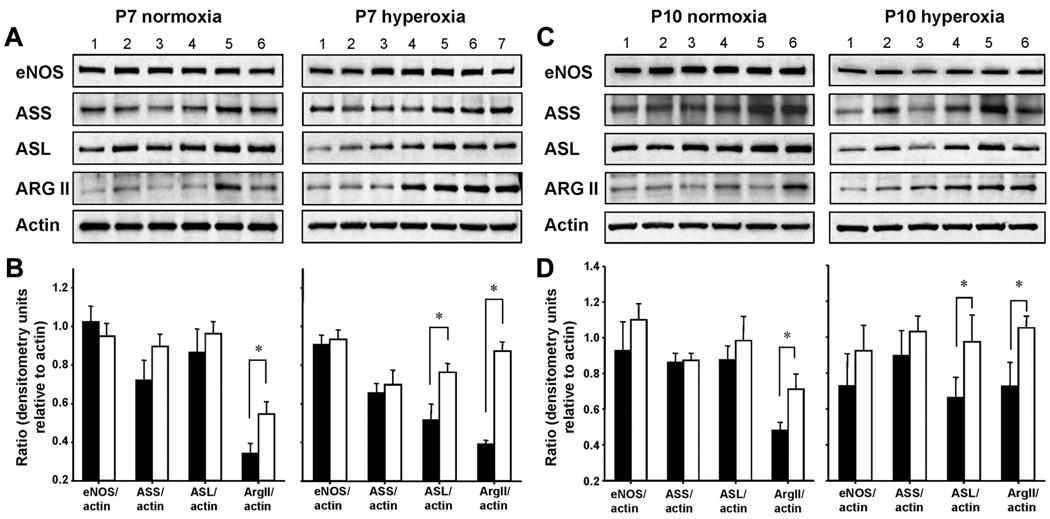
L-Citrulline increases protein expression of ASL in hyperoxia-exposed rat lungs
L-citrulline treatment did not alter eNOS or ASS lung protein abundances in normoxia- or hyperoxia-exposed rat lungs on P7 or P10 (Figure 7). However, under hyperoxic conditions, L-citrulline increased ASL protein expression at both time points. Unexpecedly, L-citrulline treatment induced arginase II expression in normoxic and hyperoxic lungs on P7 and P10 (Figures 7A–7D).
Figure 7. Effects of L-citrulline treatment on the abundance of proteins in the citrulline, arginine, NO regenerating pathway on P7 and P10.
(A and C) Representative western blots demonstrating the abundance of eNOS, ASS, ASL, Arg II and β-actin in protein lysates from L-citrulline-treated and untreated normoxic (left panel, Lane 1–3: normoxia; Lane 4–6: normoxia+L-Cit) and hyperoxic (right panel, Lane 1–3: hyperoxia; Lane 4–7: hyperoxia+L-Cit) rat lungs on P7 (7A) and P10 (7C). Blots are representative of two separate studies performed on a total of 6–8 untreated and citrulline-treated normoxic and hyperoxic neonatal rat lungs. (B and D) Summary densitometry showing the effect of L-citrulline (□) on lung protein abundance of eNOS, ASS, ASL and Arg II on P7 (7B) and P10 (7D). *P<0.05 different from untreated normoxic or hyperoxic control (■) group.
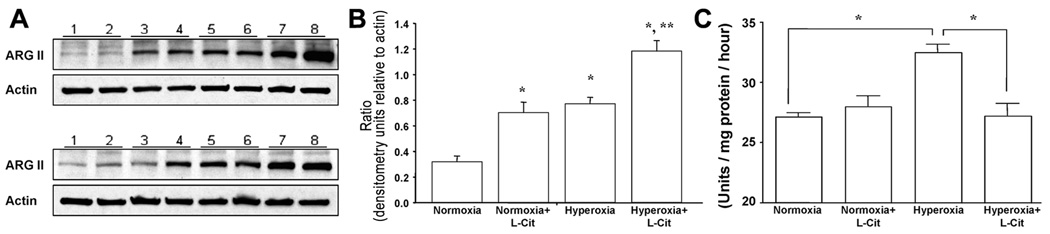
Hyperoxia and L-citrulline treatment synergistically increase arginase II protein expression in newborn rat lungs
Chronic exposure to hyperoxia caused a significant increase in pulmonary arginase II expression (Figure 8A, B); the elevated arginase II protein abundance in hyperoxic lungs was further increased by L-citrulline therapy (Figures 8A, B).
Figure 8. Effects of hyperoxia and L-citrulline treatment on protein abundance of arginase II and arginase activity in P7 rat lung homogenates.
(A) Representative western blots of three separate studies performed on a total of 6 untreated (lane 1–2) and citrulline-treated (lane 3–4) normoxic and untreated (lane 5–6) and citrulline-treated (lane 7–8) hyperoxic neonatal rat lungs. (B) Summary densitometry showing that both hyperoxia and L-citrulline independently and synergistically increased arginase II expression in neonatal rat lungs. *P<0.05 different than normoxia; **P<0.05 different from all other groups. (C) L-Citrulline decreases pulmonary arginase activity in O2-induced BPD in newborn rats. Hyperoxic exposed rats had significantly increased lung arginase activity on postnatal day 7 (P7). L-Citrulline normalized lung arginase activity in hyperoxic exposed rats. Arginase activity was unchanged in L-citrulline treated control rats. N=5/group, *P<0.05.
Chronic exposure to hyperoxia was associated with a significant increase in lung arginase activity on P7 (Figure 8C). L-Citrulline administration normalized lung arginase activity.
Discussion
We demonstrate that L-citrulline preserves alveolar and lung vascular development and prevents PH in an experimental O2-induced BPD model in newborn rats. Our results suggest a pivotal role for the L-citrulline-NO pathway in the developing lung. This pathway may be an effective target to protect the lung from impaired alveolar development.
The availability of intracellular arginine is potentially a rate-limiting factor in the production of endogenous NO. Oral supplementation with L-arginine has been used in a variety of clinical conditions to improve NO-mediated vascular function(29). Experimental and human studies suggest that after oral administration, L-arginine is extensively metabolized by arginase in the gut wall and liver(30, 31). This may limit its bioavailability as a substrate for NOS and subsequent effects on vascular function. In addition, supplemental L-arginine enhances arginase expression and activity, thus reducing the effectiveness of L-arginine therapy. In contrast, L-citrulline is not metabolized in the intestine or liver and does not induce tissue arginase I, but rather inhibits its activity and is more effective in maintaining plasma L-arginine concentrations than L-arginine itself in healthy volunteers(32). The physical relationship of the enzymes in cycling citrulline, arginine and producing NO (ASS, ASL, eNOS) may also make L-citrulline a more effective extracellular supplement to improve NO production(33–36).
L-citrulline has no recognized toxicity and is used clinically as replacement therapy for children with certain types of urea cycle defects(37). Furthermore, oral L-citrulline, as a precursor to L-arginine and NO, improves sickle cell disease symptoms in children(38) and decreases PH following surgery for congenital heart disease(22, 23).
Our data suggest that plasma L-citrulline and L-arginine concentrations can be markedly increased by supplemental L-citrulline (Figure 2). This limitation in metabolic precursors could contributeto low NO production and thus to arrested alveolar growth and PH in the O2-induced BPD model. Our data parallel findings in human infants with PPHN and in infants and children with PH after congenital heart disease surgery(20, 21). Our data also suggest that L-Citrulline increases expression of ASL in the O2-injured lungs (Figure 7). L-Citrulline exhibits good bioavailability with ease of movement across cellular membranes. The cytosolic portion of the urea cycle which includes ASS and ASL enables localized, intracellular production of L-arginine from citrulline within the pulmonary endothelium. This recycling pathway might be important in sustaining the production of NO in endothelial cells, especially when availability of L-arginine for NO synthesis becomes limiting. Although total eNOS protein expression did not change with citrulline treatment, the data suggest that eNOS function was enhanced. This is supported by the finding of increased NO metabolites (NOx) on day 7 in normoxic and hyperoxic rats (Fig 2D) which is a reflection of increased NOS activity and NO production. Although plasma levels of NOx were only transiently increased by L-citrulline treatment, local pulmonary production of NO may be inferred from the improved lung architecture and attenuated PH. L-citrulline’s efficacy in this study mirrored those obtained with inhaled NO in experimental BPD models(11–13).
PH contributes to morbidity and mortality in human BPD(39). The hyperoxic BPD model in rats also exhibits PH characterized by RVH and pulmonary artery medial wall thickening. L-citrulline attenuated RVH and pulmonary artery remodeling in this model (Figures 4 and 5). These findings are consistent with a recent study of L-citrulline administration in a neonatal piglet model of chronic hypoxia-induced PH(40).
Amongst other mechanisms, increased arginase activity may account for abnormal airway and vascular remodeling in the hyperoxia model of BPD in newborn rats. Arginases mediate the conversion of L-arginine to urea and ornithine(41) (Figure 1) and compete with NOS for L-arginine as substrate. Increased arginase activity in PH and other lung diseases, including asthma and cystic fibrosis, is believed to result in decreased availability of L-arginine for constitutive NOSs and consequently diminished NO production(42–44). Interestingly, lung arginase expression and activity in the rat is developmentally regulated, and expressed at the highest levels in the fetus and newborn(45), suggesting that arginase contributes to the maintenance of high pulmonary vascular resistance during fetal life. Hyperoxia upregulates arginase expression in the adult rat lung(46). Here we show that both arginase II expression and arginase activity are increased in chronic O2-exposed neonatal rat lungs (Figures 7–8). This may limit arginine availability to NOS and contribute to decreased NO production in this model(47). Unexpectedly, we found that L-citrulline increased expression of arginase II, the isoform expressed in non-hepatic tissue (Figures 7–8). It had been previously reported that L-citrulline, unlike L-arginine, does not induce tissue arginase(32). Notably, and despite the increase in protein expression, L-citrulline normalized lung arginase activity in neonatal oxygen-induced lung injury (Figure 8). An interesting link between increased arginase activity and BPD has been proposed recently. Ornithine, the downstream product of arginase activity, is further metabolized into polyamines that are involved in tissue repair and growth, as well as proline, the precursor for collagen formation(41) (Figure 1). In bleomycin-induced lung fibrosis, arginase expression and activity are also increased(48, 49), suggesting that these enzymes contribute to lung remodeling and thus represent a therapeutic target.
In conclusion, this is the first report on the potential therapeutic benefit of L-citrulline in preventing O2-induced alveolar damage and altered angiogenesis in the developing lung. The bioavailability, safety and efficacy of L-citrulline, combined with its low cost and ease of administration, make it an attractive therapeutic option that warrants further studies.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Sowndramalingam Sankaralingam for technical assistance.
B.T. is supported by the Canadian Institutes of Health Research (CIHR), Alberta Heritage Foundation for Medical Research (AHFMR), Canada Foundation for Innovation (CFI), and a Canada Research Chair. A.V. and G.J.R.-P are supported by a stipend from the Maternal Fetal Neonatal Health Training Program (MFN) sponsored by CIHR-IHDCYH. G.J.R.-P is also supported by a stipend from AHFMR. J.LA. is supported by NIH R01 HL75511. M.S. is supported by NIH U54RR019453.
Abbreviations
- ARG
Arginase
- ASL
Arginosuccinate lyase
- ASS
Arginosuccinate synthetase
- BPD
Bronchopulmonary dysplasia
- eNOS
endothelial NO synthase
- iNO
inhaled nitric oxide
- MWT
Medial wall thickness
- NOx
NO metabolites
- P
postnatal day
- PH
Pulmonary hypertension
- PPHN
Persistent pulmonary hypertension of the newborn
- RVH
Right Ventricular Hypertrophy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.pedresearch.org).
References
- 1.Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet. 2006;367:1421–1431. doi: 10.1016/S0140-6736(06)68615-7. [DOI] [PubMed] [Google Scholar]
- 2.Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol. 2005;67:623–661. doi: 10.1146/annurev.physiol.67.040403.102229. [DOI] [PubMed] [Google Scholar]
- 3.Thébaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med. 2007;175:978–985. doi: 10.1164/rccm.200611-1660PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stocks J, Coates A, Bush A. Lung function in infants and young children with chronic lung disease of infancy: the next steps? Pediatr Pulmonol. 2007;42:3–9. doi: 10.1002/ppul.20520. [DOI] [PubMed] [Google Scholar]
- 5.Cutz E, Chiasson D. Chronic lung disease after premature birth. N Engl J Med. 2008;358:743–745. doi: 10.1056/NEJMc073362. author reply 745–746. [DOI] [PubMed] [Google Scholar]
- 6.Wong PM, Lees AN, Louw J, Lee FY, French N, Gain K, Murray CP, Wilson A, Chambers DC. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur Respir J. 2008;32:321–328. doi: 10.1183/09031936.00127107. [DOI] [PubMed] [Google Scholar]
- 7.Young SL, Evans K, Eu JP. Nitric oxide modulates branching morphogenesis in fetal rat lung explants. Am J Physiol Lung Cell Mol Physiol. 2002;282:L379–L385. doi: 10.1152/ajplung.00462.2000. [DOI] [PubMed] [Google Scholar]
- 8.Balasubramaniam V, Tang JR, Maxey A, Plopper CG, Abman SH. Mild hypoxia impairs alveolarization in the endothelial nitric oxide synthase-deficient mouse. Am J Physiol Lung Cell Mol Physiol. 2003;284:L964–L971. doi: 10.1152/ajplung.00421.2002. [DOI] [PubMed] [Google Scholar]
- 9.Han RN, Babaei S, Robb M, Lee T, Ridsdale R, Ackerley C, Post M, Stewart DJ. Defective lung vascular development and fatal respiratory distress in endothelial NO synthase-deficient mice: a model of alveolar capillary dysplasia? Circ Res. 2004;94:1115–1123. doi: 10.1161/01.RES.0000125624.85852.1E. [DOI] [PubMed] [Google Scholar]
- 10.Balasubramaniam V, Tang JR, Maxey A, Plopper CG, Abman SH. Mild hypoxia impairs alveolarization in the endothelial nitric oxide synthase (eNOS) deficient mouse. Am J Physiol Lung Cell Mol Physiol. 2003;284:L964–L971. doi: 10.1152/ajplung.00421.2002. [DOI] [PubMed] [Google Scholar]
- 11.Balasubramaniam V, Maxey AM, Morgan DB, Markham NE, Abman SH. Inhaled NO restores lung structure in eNOS-deficient mice recovering from neonatal hypoxia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L119–L127. doi: 10.1152/ajplung.00395.2005. [DOI] [PubMed] [Google Scholar]
- 12.Bland RD, Albertine KH, Carlton DP, MacRitchie AJ. Inhaled nitric oxide effects on lung structure and function in chronically ventilated preterm lambs. Am J Respir Crit Care Med. 2005;172:899–906. doi: 10.1164/rccm.200503-384OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCurnin DC, Pierce RA, Chang LY, Gibson LL, Osborne-Lawrence S, Yoder BA, Kerecman JD, Albertine KH, Winter VT, Coalson JJ, Crapo JD, Grubb PH, Shaul PW. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2005;288:L450–L459. doi: 10.1152/ajplung.00347.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, Walsh MC, Durand DJ, Mayock DE, Eichenwald EC, Null DR, Hudak ML, Puri AR, Golombek SG, Courtney SE, Stewart DL, Welty SE, Phibbs RH, Hibbs AM, Luan X, Wadlinger SR, Asselin JM, Coburn CE. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med. 2006;355:343–353. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 15.Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, Sekar KC, Auten RL, Bhutani VK, Gerdes JS, George TN, Southgate WM, Carriedo H, Couser RJ, Mammel MC, Hall DC, Pappagallo M, Sardesai S, Strain JD, Baier M, Abman SH. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med. 2006;355:354–364. doi: 10.1056/NEJMoa060442. [DOI] [PubMed] [Google Scholar]
- 16.Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med. 2003;349:2099–2107. doi: 10.1056/NEJMoa031154. [DOI] [PubMed] [Google Scholar]
- 17.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 18.Goodwin BL, Solomonson LP, Eichler DC. Argininosuccinate synthase expression is required to maintain nitric oxide production and cell viability in aortic endothelial cells. J Biol Chem. 2004;279:18353–18360. doi: 10.1074/jbc.M308160200. [DOI] [PubMed] [Google Scholar]
- 19.Summar ML, Gainer JV, Pretorius M, Malave H, Harris S, Hall LD, Weisberg A, Vaughan DE, Christman BW, Brown NJ. Relationship between carbamoyl-phosphate synthetase genotype and systemic vascular function. Hypertension. 2004;43:186–191. doi: 10.1161/01.HYP.0000112424.06921.52. [DOI] [PubMed] [Google Scholar]
- 20.Barr FE, Beverley H, VanHook K, Cermak E, Christian K, Drinkwater D, Dyer K, Raggio NT, Moore JH, Christman B, Summar M. Effect of cardiopulmonary bypass on urea cycle intermediates and nitric oxide levels after congenital heart surgery. J Pediatr. 2003;142:26–30. doi: 10.1067/mpd.2003.mpd0311. [DOI] [PubMed] [Google Scholar]
- 21.Pearson DL, Dawling S, Walsh WF, Haines JL, Christman BW, Bazyk A, Scott N, Summar ML. Neonatal pulmonary hypertension--urea-cycle intermediates, nitric oxide production, and carbamoyl-phosphate synthetase function. N Engl J Med. 2001;344:1832–1838. doi: 10.1056/NEJM200106143442404. [DOI] [PubMed] [Google Scholar]
- 22.Barr FE, Tirona RG, Taylor MB, Rice G, Arnold J, Cunningham G, Smith HA, Campbell A, Canter JA, Christian KG, Drinkwater DC, Scholl F, Kavanaugh-McHugh A, Summar ML. Pharmacokinetics and safety of intravenously administered citrulline in children undergoing congenital heart surgery: potential therapy for postoperative pulmonary hypertension. J Thorac Cardiovasc Surg. 2007;134:319–326. doi: 10.1016/j.jtcvs.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 23.Smith HA, Canter JA, Christian KG, Drinkwater DC, Scholl FG, Christman BW, Rice GD, Barr FE, Summar ML. Nitric oxide precursors and congenital heart surgery: a randomized controlled trial of oral citrulline. J Thorac Cardiovasc Surg. 2006;132:58–65. doi: 10.1016/j.jtcvs.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Förstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 25.Ladha F, Bonnet S, Eaton F, Hashimoto K, Korbutt G, Thebaud B. Sildenafil improves alveolar growth and pulmonary hypertension in hyperoxia-induced lung injury. Am J Respir Crit Care Med. 2005;172:750–756. doi: 10.1164/rccm.200503-510OC. [DOI] [PubMed] [Google Scholar]
- 26.Thébaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, Archer SL. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation. 2005;112:2477–2486. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- 27.Aschner JL, Zeng H, Kaplowitz MR, Zhang Y, Slaughter JC, Fike CD. Heat shock protein 90-eNOS interactions mature with postnatal age in the pulmonary circulation of the piglet. Am J Physiol Lung Cell Mol Physiol. 2009;296:L555–L564. doi: 10.1152/ajplung.90456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corraliza IM, Campo ML, Soler G, Modolell M. Determination of arginase activity in macrophages: a micromethod. J Immunol Methods. 1994;174:231–235. doi: 10.1016/0022-1759(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 29.Böger RH. L-Arginine therapy in cardiovascular pathologies: beneficial or dangerous? Curr Opin Clin Nutr Metab Care. 2008;11:55–61. doi: 10.1097/MCO.0b013e3282f2b0c3. [DOI] [PubMed] [Google Scholar]
- 30.Castillo L, deRojas TC, Chapman TE, Vogt J, Burke JF, Tannenbaum SR, Young VR. Splanchnic metabolism of dietary arginine in relation to nitric oxide synthesis in normal adult man. Proc Natl Acad Sci USA. 1993;90:193–197. doi: 10.1073/pnas.90.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris SM., Jr Enzymes of arginine metabolism. J Nutr. 2004;134:2743S–2747S. doi: 10.1093/jn/134.10.2743S. [DOI] [PubMed] [Google Scholar]
- 32.Romero MJ, Platt DH, Caldwell RB, Caldwell RW. Therapeutic use of citrulline in cardiovascular disease. Cardiovasc Drug Rev. 2006;24:275–290. doi: 10.1111/j.1527-3466.2006.00275.x. [DOI] [PubMed] [Google Scholar]
- 33.Flam BR, Hartmann PJ, Harrell-Booth M, Solomonson LP, Eichler DC. Caveolar localization of arginine regeneration enzymes, argininosuccinate synthase, and lyase, with endothelial nitric oxide synthase. Nitric Oxide. 2001;5:187–197. doi: 10.1006/niox.2001.0340. [DOI] [PubMed] [Google Scholar]
- 34.Isayama H, Nakamura H, Kanemaru H, Kobayashi K, Emson PC, Kawabuchi M, Tashiro N. Distribution and co-localization of nitric oxide synthase and argininosuccinate synthetase in the cat hypothalamus. Arch Histol Cytol. 1997;60:477–492. doi: 10.1679/aohc.60.477. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan JC, Pollock JS. NOS 3 subcellular localization in the regulation of nitric oxide production. Acta Physiol Scand. 2003;179:115–122. doi: 10.1046/j.1365-201X.2003.01181.x. [DOI] [PubMed] [Google Scholar]
- 36.Yu JG, O'Brien WE, Lee TJ. Morphologic evidence for L-citrulline conversion to L-arginine via the argininosuccinate pathway in porcine cerebral perivascular nerves. J Cereb Blood Flow Metab. 1997;17:884–893. doi: 10.1097/00004647-199708000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Tuchman M, Lee B, Lichter-Konecki U, Summar ML, Yudkoff M, Cederbaum SD, Kerr DS, Diaz GA, Seashore MR, Lee HS, McCarter RJ, Krischer JP, Batshaw ML. Cross-sectional multicenter study of patients with urea cycle disorders in the United States. Mol Genet Metab. 2008;94:397–402. doi: 10.1016/j.ymgme.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waugh WH, Daeschner CW, 3rd, Files BA, McConnell ME, Strandjord SE. Oral citrulline as arginine precursor may be beneficial in sickle cell disease: early phase two results. J Natl Med Assoc. 2001;93:363–371. [PMC free article] [PubMed] [Google Scholar]
- 39.Mourani PM, Sontag MK, Younoszai A, Ivy DD, Abman SH. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics. 2008;121:317–325. doi: 10.1542/peds.2007-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ananthakrishnan M, Barr FE, Summar ML, Smith HA, Kaplowitz M, Cunningham G, Magarik J, Zhang Y, Fike CD. L-Citrulline ameliorates chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol. 2009;297:L506–L511. doi: 10.1152/ajplung.00017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mori M, Gotoh T. Arginine metabolic enzymes, nitric oxide and infection. J Nutr. 2004;134:2820S–2825S. doi: 10.1093/jn/134.10.2820S. [DOI] [PubMed] [Google Scholar]
- 42.Grasemann H, Schwiertz R, Matthiesen S, Racke K, Ratjen F. Increased arginase activity in cystic fibrosis airways. Am J Respir Crit Care Med. 2005;172:1523–1528. doi: 10.1164/rccm.200502-253OC. [DOI] [PubMed] [Google Scholar]
- 43.Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM, Jr, Gladwin MT. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morris CR, Poljakovic M, Lavrisha L, Machado L, Kuypers FA, Morris SM., Jr Decreased arginine bioavailability and increased serum arginase activity in asthma. Am J Respir Crit Care Med. 2004;170:148–153. doi: 10.1164/rccm.200309-1304OC. [DOI] [PubMed] [Google Scholar]
- 45.Belik J, Shehnaz D, Pan J, Grasemann H. Developmental changes in arginase expression and activity in the lung. Am J Physiol Lung Cell Mol Physiol. 2008;294:L498–L504. doi: 10.1152/ajplung.00242.2007. [DOI] [PubMed] [Google Scholar]
- 46.Que LG, Kantrow SP, Jenkinson CP, Piantadosi CA, Huang YC. Induction of arginase isoforms in the lung during hyperoxia. Am J Physiol. 1998;275:L96–L102. doi: 10.1152/ajplung.1998.275.1.L96. [DOI] [PubMed] [Google Scholar]
- 47.Belik J, Jankov RP, Pan J, Yi M, Chaudhry I, Tanswell AK. Chronic O2 exposure in the newborn rat results in decreased pulmonary arterial nitric oxide release and altered smooth muscle response to isoprostane. J Appl Physiol. 2004;96:725–730. doi: 10.1152/japplphysiol.00825.2003. [DOI] [PubMed] [Google Scholar]
- 48.Endo M, Oyadomari S, Terasaki Y, Takeya M, Suga M, Mori M, Gotoh T. Induction of arginase I and II in bleomycin-induced fibrosis of mouse lung. Am J Physiol Lung Cell Mol Physiol. 2003;285:L313–L321. doi: 10.1152/ajplung.00434.2002. [DOI] [PubMed] [Google Scholar]
- 49.Kitowska K, Zakrzewicz D, Konigshoff M, Chrobak I, Grimminger F, Seeger W, Bulau P, Eickelberg O. Functional role and species-specific contribution of arginases in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L34–L45. doi: 10.1152/ajplung.00007.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.