Abstract
Acetylcholine is a neurotransmitter that plays a major role in the function of the insulin secreting pancreatic beta cell1,2. Parasympathetic innervation of the endocrine pancreas, the islets of Langerhans, has been shown to provide cholinergic input to the beta cell in several species1,3,4, but the role of autonomic innervation in human beta cell function is at present unclear. Here we show that, in contrast to mouse islets, cholinergic innervation of human islets is sparse. Instead, we find that the alpha cells of the human islet provide paracrine cholinergic input to surrounding endocrine cells. Human alpha cells express the vesicular acetylcholine transporter and release acetylcholine when stimulated with kainate or a lowering in glucose concentration. Acetylcholine secretion by alpha cells in turn sensitizes the beta cell response to increases in glucose concentration. Our results demonstrate that in human islets acetylcholine is a paracrine signal that primes the beta cell to respond optimally to subsequent increases in glucose concentration. We anticipate these results to revise models about neural input and cholinergic signaling in the endocrine pancreas. Cholinergic signaling within the islet represents a potential therapeutic target in diabetes5, highlighting the relevance of this advance to future drug development.
Acetylcholine is crucial for pancreatic beta cell function. Acetylcholine stimulates insulin secretion by increasing the cytoplasmic free Ca2+ concentration, [Ca2+]i, via inositol phosphate production and enhancing the effects of Ca2+ on exocytosis via protein kinase C in beta cells1 (Supplementary Fig. 1). Muscarinic receptors found in pancreatic beta cells are essential for maintaining proper insulin secretion and glucose homeostasis in mice2. Cholinergic agonists have been reported to restore defective glucose-stimulated insulin secretion6,7. In humans, variations in the gene that encodes the muscarinic receptor M3 are associated with increased risk for early-onset type 2 diabetes8. It is generally believed that acetylcholine is released during feeding from parasympathetic nerve endings in the pancreatic islet1,3. The consensus is that the endocrine pancreas is richly innervated by the autonomic nervous system1,3,4,9, with studies based on the cholinesterase technique revealing dense parasympathetic innervation in cat, rat, rabbit, and human islets10–13. Human pancreatic islets, however, have not been examined for the presence of prototypical cholinergic markers such as vesicular acetylcholine transporter (vAChT) or choline acetyltransferase (ChAT).
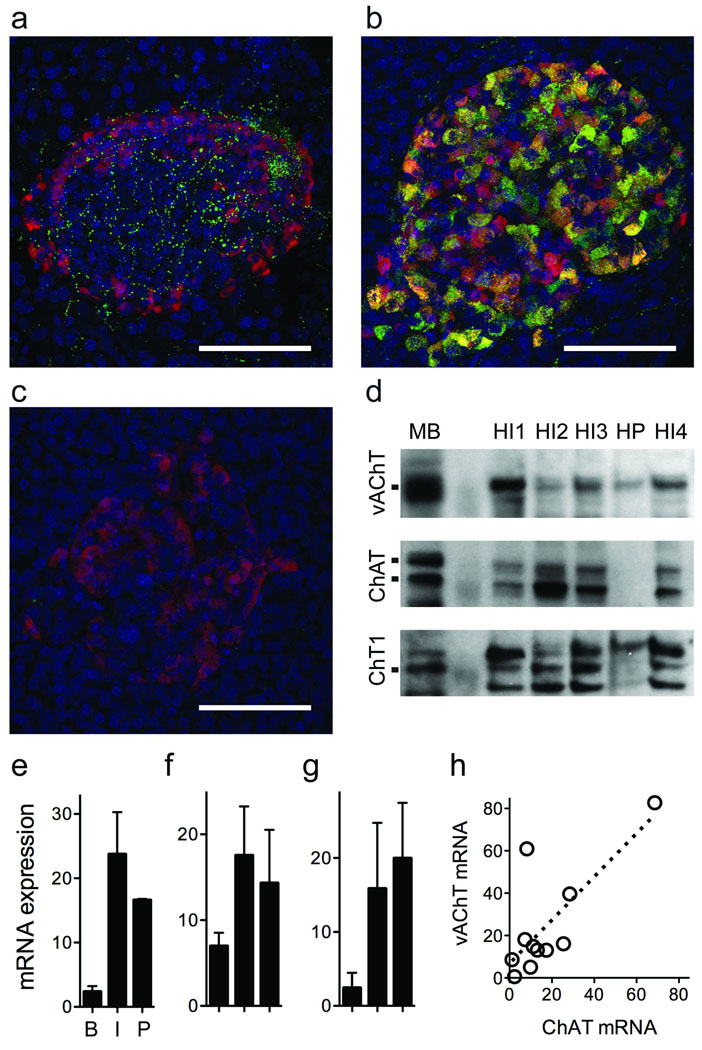
Cells and nerve fibers capable of vesicular release of acetylcholine express vAChT14. We performed immunohistochemistry on mouse and human pancreatic sections and found that mouse islets were densely innervated by vAChT-immunoreactive nerve fibers. These fibers formed a plexus with numerous axonal varicosities predominantly innervating beta cells (Fig. 1a, Supplementary Fig. 2, and Supplementary Movie 1). By contrast, although many nerve fibers were immunostained for vAChT in the exocrine regions of the human pancreas, few if any fibers could be seen inside human islets (Fig 1b). Instead, many endocrine cells were strongly vAChT immunoreactive (Fig. 1b and Supplementary Movie 2). Western blots confirmed the specificity of the vAChT staining and further showed that human islets express ChAT and choline transporter 1 (ChT, Fig 1d). Because our experiments were conducted with isolated islets in which severed nerve fibers had degenerated after ≥ 2 days in culture15,16, contribution of neuronal elements to the Western blots and to the physiological experiments described below were ruled out. Furthermore, we consistently found vAChT, ChAT, and ChT mRNA expression in human islets that was comparable to or higher than that in the brain (Fig. 1e–g). vAChT mRNA levels correlated with ChAT mRNA levels, as expected for gene products that share a common gene locus and regulatory elements for gene transcription17 (Fig. 1h). We thus conclude that human islet cells express the defining components of the cholinergic phenotype.
Figure 1.
Endocrine cells in human pancreatic islets express cholinergic markers. (a) Z-stack of confocal images of a mouse pancreatic section showing an islet immunostained for vesicular acetylcholine transporter (vAChT, green) and glucagon (red). Intense vAChT staining is present in nerve fibers and fiber varicosities in the islet but not in islet cells. (b) Z-stack of confocal images of a human pancreatic section showing strong vAChT immunostaining in islet cells. Merge of glucagon and vAChT immunostaining appears yellow. (c) Preincubation with control peptide abolishes vAChT staining in human islets. Scale bar = 50 µm (a–c). (d) Western blotting analyses of lysates from four separate human islet preparations (HI1–4) and human pancreatic exocrine tissue (HP), with mouse brain (MB) as a positive control. Specific bands were seen in human islet lysates for vAChT (~70 kDa; upper), for choline acetyltransferase (ChAT; ~63 kDA and ~68 kDa; middle), and for choline transporter 1 (ChT1; ~68 kDa; lower). Notice the reduced expression of these cholinergic markers in exocrine tissue. A molecular marker was run in parallel (second lane). (e–g) vAChT (e), ChAT (f), and ChT1 (g) mRNA expression in brain (B, n = 4), human islets (I, n = 12), and human pancreas (P, n = 3). (h) vAChT mRNA levels were associated with ChAT mRNA levels (r2 = 0.57; slope significantly different from 0, P < 0.01).
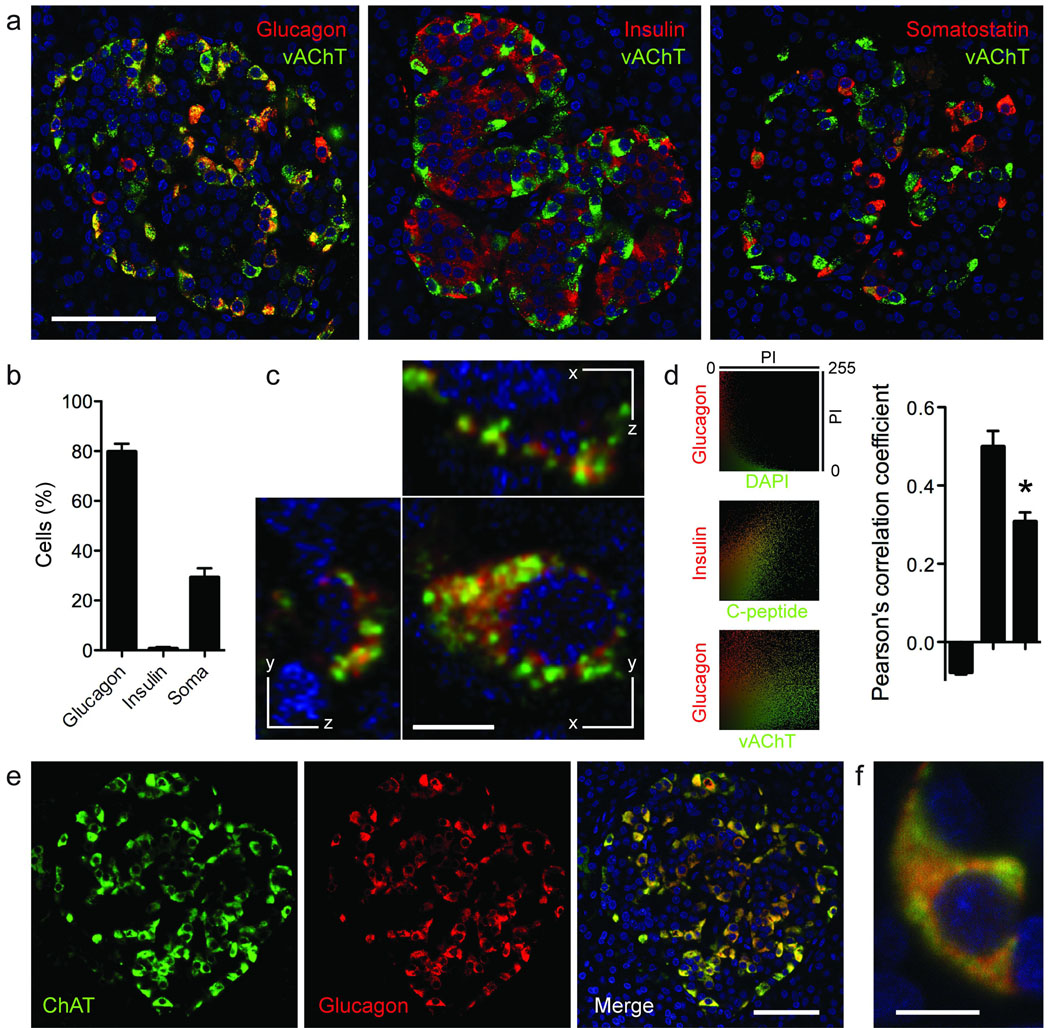
To localize the expression of vAChT to particular cell types within the human islet we performed multiple immunostaining on human pancreatic sections (Fig. 2). We found that most (~80%) vAChT-labeled cells were immunoreactive for glucagon (Fig. 2b). Few vAChT cells expressed somatostatin or insulin. More than 60% of glucagon-labeled alpha cells were strongly immunoreactive for vAChT. Most alpha cells (> 90%) were also immunoreactive for ChAT (Fig. 2). Within the alpha cell, vAChT staining did not overlap with glucagon staining and appeared confined to distinct compartments (Fig. 2c). We examined colocalization of vAChT and glucagon immunofluorescence18 and found a Pearson’s correlation coefficient significantly smaller than that of C-peptide and insulin colocalization and closer to that of the clearly segregated nuclear DAPI and glucagon staining (Fig. 2d). This is in concurrence with studies showing that in neuro-endocrine cells, vAChT localizes preferentially to synaptic-like microvesicles and is excluded from hormone granules19,20. Furthermore, human alpha cells have been reported to possess secretory vesicles of different sizes21. To determine that acetylcholine and glucagon are stored in different secretory granules, however, would require EM studies.
Figure 2.
Human alpha cells express vAChT and ChAT. (a) Confocal images of human pancreatic sections showing that vAChT immunostaining (green) colocalized with glucagon immunostaining (red, left) in many human alpha cells, with somatostatin in some delta cells (right), but not with insulin immunostaining in beta cells (middle). Colocalization appears yellow. (b) Quantification shows the percentage of vAChT immunostained cells also labeled for glucagon, insulin, or somatostatin (n = 3 human pancreata). Percentages do not add exactly to 100% because analyses were performed on different sections. (c) At high magnification, glucagon (red) and vAChT immunostaining (green) appear localized to different regions in alpha cells. Shown are three optical planes through an alpha cell. (d) Fluorescence colocalization in alpha and beta cells showing strong colocalization of insulin and C-peptide in beta cells and lack of colocalization of DAPI and glucagon in alpha cells. The degree of glucagon and vAChT Colocalization was significantly lower than that of C-peptide and insulin (ANOVA followed by multiple comparison, *P < 0.05). Shown are scatter plots of pixel intensities (PI) in the specified channels (left) and the respective thresholded Pearson’s correlation coefficients (right, n = 12 cells). (e) ChAT immunostaining (green, left) was present in glucagon-labeled alpha cells (red, middle). Colocalization appears yellow (merge, right). (f) High magnification confocal image of an alpha cell stained for glucagon (red) and ChAT (green). Scale bars, 50 µm (a, e) and 5 µm (c, f).
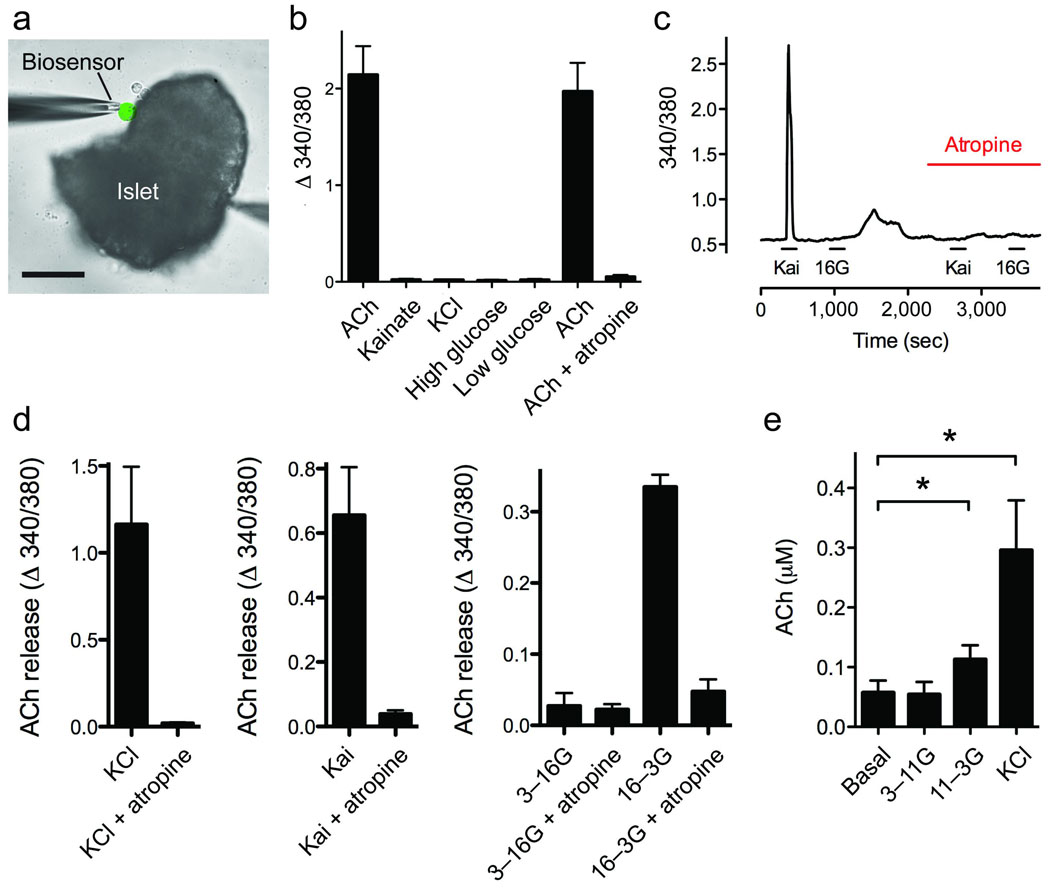
Our immunohistochemical results suggested that in human alpha cells, acetylcholine is packaged in secretory vesicles for exocytotic release. We therefore examined human islets for acetylcholine secretion using cellular biosensors, namely CHO cells expressing the muscarinic receptor M3 (Fig. 3). Acetylcholine secretion from human islets was monitored in real time by recording [Ca2+]i responses from biosensors loaded with the [Ca2+]i indicator Fura-2 and placed in apposition to isolated human islets (Fig. 3a). Biosensors showed large responses to KCl depolarization (25 mM) of human islets, indicating that acetylcholine release was induced from excitable islet cells and ruling out a contribution from exocrine tissue. Stimulation with kainate (100 µM) or lowering the glucose concentration from 16 mM to 3 mM, which are both alpha cell-specific stimuli16,22,23 (Supplementary Fig. 3), induced strong acetylcholine release as measured by large [Ca2+]i responses in the biosensors (Fig. 3). By contrast, increases in the glucose concentration from 3 mM to 16 mM did not elicit acetylcholine secretion. Biosensor responses could be blocked by the muscarinic antagonist atropine (5 µM), and none of the stimuli used, including KCl depolarization, induced responses in biosensors in the absence of human islets (Fig. 3b). This confirmed that the [Ca2+]i responses were elicited by acetylcholine released from islet cells. We obtained similar results using an enzymatic assay to detect acetylcholine release (Fig. 3e). Because acetylcholine was released in response to treatments known to specifically stimulate alpha cells and not in response to increased glucose concentration, which stimulates beta and delta cells, we conclude that human alpha cells secrete acetylcholine.
Figure 3.
Isolated human islets secrete acetylcholine (ACh) in response to alpha cell-specific stimuli. (a) Photomicrograph of an ACh biosensor (colorized green) apposed to an isolated human islet to monitor ACh secretion evoked by stimulation of islet cells. Responses in the biosensor were recorded by loading biosensors with fura-2 and imaging cytoplasmic [Ca2+]. Scale bar, 50 µm. (b) In the absence of human islets, biosensors responded to direct application of ACh (10 µM), but not to kainate (100 µM), KCl (25 mM), or a change in glucose concentration. Responses to ACh were inhibited by the muscarinic antagonist atropine (5 µM). (c) Trace shows stimulus-induced secretion of ACh from endocrine cells in a human islet, measured with an ACh biosensor positioned against the islet as in a. Kainate (100 µM) and a decrease in glucose concentration (return from 16 mM to 3 mM) evoked responses in the biosensor. Biosensor responses were blocked by atropine 5 µM). Bars denote drug applications. (d), Summary of data from experiments such as those shown in c. Bars show means ± SEM for ACh biosensor signals in response to stimulation of islets. KCl (25 mM) depolarization (n = 8 experiments), kainate (100 µM, n = 11) and decreases in glucose concentration (from 16 mM to 3 mM, n = 4) induced ACh secretion. Biosensor responses were blocked by atropine (5 µM). (e) ACh release was stimulated by lowering the glucose concentration from 11 mM to 3 mM or by depolarizing with KCl (25 mM), as determined with a fluorescent enzymatic assay (see Methods; n = 6 islet preparations; ANOVA followed by multiple comparison, *P < 0.05).
We further used the biosensor assay to detect acetylcholine release from mouse islets. Mouse islets for these experiments were cultured for 4 days after isolation to eliminate neural elements, the same time as for human islets. Acetylcholine secretion could not be recorded from mouse islets stimulated with 25 mM KCl or 100 µM kainate (0 out of 26 mouse islets responded versus 17 out of 75 human islets; P = 0.0052, Fisher’s exact test), consistent with the lack of vAChT immunostaining in mouse endocrine cells (Fig. 1a).
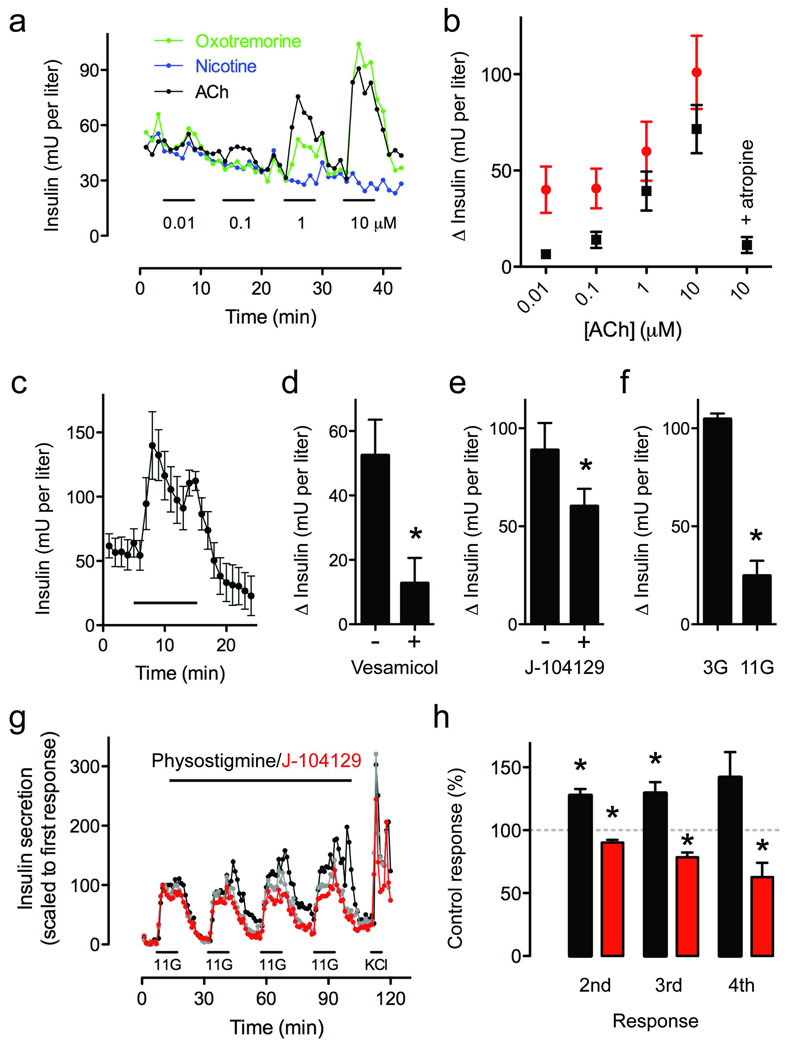
What is the role of alpha cell-derived acetylcholine for islet function? Studies have shown that exposure to cholinergic agonists sensitizes beta cells to subsequent stimuli, increasing insulin secretion1,24. Given that in human islets most beta cells are closely associated with alpha cells25 we hypothesized that alpha cells release acetylcholine to prime neighboring beta cells. To test this hypothesis we first examined if cholinergic agonists induce insulin responses in human beta cells. At low glucose concentration (3 mM), acetylcholine and the muscarinic agonist oxotremorine elicited concentration-dependent insulin release from isolated human islets, indicating that activation of muscarinic receptors can induce insulin secretion at basal glucose concentrations (3 mM; Fig. 4a,b).
Figure 4.
Endogenously released ACh amplifies glucose-induced insulin secretion in human islets. (a) ACh (black trace) and the muscarinic agonist oxotremorine (green trace), but not nicotine (blue trace), elicited concentration-dependent insulin release from human islets. Bars denote stimulus application. Representative traces of n = 3 islet preparations. (b) Summary of data from experiments similar to those shown in a, but conducted in the presence of low (3 mM; black symbols) and high (11 mM; red symbols) glucose (n = 3 preparations). (c) The acetylcholinesterase inhibitor physostigmine (30 µM) increased insulin secretion at 3 mM glucose (n = 5 human islet preparations). (d–f) Physostigmine-induced increases in insulin secretion were significantly inhibited by the vAChT blocker vesamicol (10 µM, d), by the M3 receptor antagonist J-104129 (50 nM, e), or when the experiment was performed at a higher glucose concentration (11 mM, f; Student’s t-test, P < 0.05). (g) Insulin secretion induced by repeatedly raising glucose from 3 mM to 11 mM was increased in the presence of physostigmine (30 µM; black symbols) and reduced in the presence of J-104129 (50 nM; red symbols; representative traces of four experiments). A control experiment with untreated islets was run in parallel (grey symbols). Bar denotes drug application. 11G indicates 15 min of elevated glucose (11 mM). Islets were stimulated four times with glucose followed by 25 mM KCl. (h) Summary of data from experiments such as those shown in c. J-104129 significantly reduced glucose-induced insulin secretion (red bars; n = 4 preparations), whereas the cholinesterase inhibitor physostigmine (30 µM) increased insulin secretion (black bars; n = 5 preparations). Responses are expressed as percentage of the respective insulin response of control islets (100%, grey dashed line). One sample t-tests were used to compare the actual mean to a theoretical mean of 100% (control; *P < 0.05).
To infer the role of acetylcholine as a paracrine signal we manipulated endogenous levels of acetylcholine. Applying the acetylcholinesterase inhibitor physostigmine (30 µM) at 3 mM glucose increased insulin secretion in isolated islets cultured for 4 days (Fig. 4c). These insulin responses to physostigmine were strongly inhibited by vesamicol, a selective inhibitor of vAChT that blocks acetylcholine transport into vesicles and depletes cells of releasable acetylcholine (Fig. 4d). We also found that physostigmine-induced increases in insulin secretion were partially inhibited by the M3 antagonist J-104129 (Fig. 4e). This antagonist by itself reduced insulin secretion at 3 mM glucose, and this effect was negligible at 11 mM glucose. Physostigmine-induced increases in insulin secretion were reduced at 11 mM glucose (Fig. 4f). These experiments demonstrate that acetylcholine is endogenously released at low glucose concentrations to stimulate insulin secretion and that acetylcholine secretion requires vesicular mechanisms. These results are consistent with our immunohistochemical results showing the presence of vAChT in alpha cells.
In vivo, the secretion of insulin and glucagon fluctuates constantly with periods of approximately 10 minutes26,27. We hypothesized that these fluctuations allow alpha cells to increase acetylcholine secretion and influence beta cells. We reproduced these hormonal fluctuations in vitro by subjecting isolated human islets to an experimental protocol in which beta cells and alpha cells were stimulated intermittently while modulating cholinergic signaling (Fig. 4g,h and Supplementary Fig. 3). When acetylcholine degradation was inhibited with physostigmine (30 µM), insulin release increased during repeated exposure to high glucose (11 mM). Blocking muscarinic receptors with the general antagonist atropine (10 µM) produced variable results, most likely because multiple receptors on different cells were activated. By contrast, adding the M3 receptor-specific antagonist J-104129 (50 nM) consistently reduced insulin responses (Fig. 4g,h). These results show that endogenously released acetylcholine contributed to the enhanced beta cell response by activating M3 receptors. Thus, in the absence of any influence from the autonomic nervous system, endogenously released acetylcholine in human islets is able to sensitize the beta cell to subsequent increases in glucose concentration.
Based on our results we propose that acetylcholine is a paracrine signal secreted by alpha cells in human islets. Our findings showing that alpha cells express vAChT and that alpha-specific stimuli induce acetylcholine secretion indicate that acetylcholine is stored in alpha cells for exocytotic release. In our model, alpha cells release acetylcholine when activated by lowering glucose concentration to prime the beta cell response to a subsequent increase in glucose concentration. While additional paracrine effects of acetylcholine on other cells within the human islet (e.g. delta cells) remain to be investigated, our results suggest that acetylcholine serves as feed-forward signal to keep the beta cell responsive to future challenges, thus limiting plasma glucose fluctuations. Moreover, the intracellular signaling pathways activated by acetylcholine may promote long-term survival of beta cells28. Thus, this model not only explains how beta cell responses are optimized to counter persistent physiological fluctuations in blood glucose concentration in humans but it also suggests that alpha cell-derived acetylcholine acts as a trophic factor to enhance beta cell survival.
This paracrine interaction is only possible because of the unique cytoarchitecture of the human islet, where most beta cells are closely associated with alpha cells25,29,30. With beta cells comprising 64% and alpha cells most of the remaining volume in the human islet31, there is a high probability for a beta cell to be close to an alpha cell. Indeed, most beta cells (70–80%) face alpha cells25,30 and maintain a strong association even after dispersion of the islet30. This cellular arrangement is compatible with the notion that paracrine interactions occur via the interstitial space between endocrine cells, although the vascular route may also be used32. Thus, in human islets, alpha cells seem optimally placed to influence beta cells.
Although we cannot rule out a contribution of parasympathetic nervous input, our results suggest that cholinergic innervation of human islets may be sparse. This is consistent with studies showing that the influence of neural input on insulin secretion occurring before the actual absorption of nutrients (cephalic phase) plays a relatively minor role in humans33,34. Along these lines, vagotomized patients have normal postprandial serum insulin levels35 and patients with type 1 diabetes who have undergone pancreas transplantation (and thus have denervated islets) remain euglycemic without therapy36–38. Furthermore, it is possible that the reported parasympathetic influence on islet function in human beings may be mediated by peptidergic axons12. The human islet may thus be self-reliant in terms of cholinergic input. That acetylcholine is a paracrine signal, and not only a neural signal as in rodents, further implies that cholinergic signaling in human islets is activated under circumstances that cannot be modeled with rodent studies, highlighting the importance of species divergence in the pancreatic islet. Because cholinergic signaling pathways have been proposed as intervention points to promote beta cell function and survival5 our study has important implications for therapies in diabetes mellitus.
Methods
Pancreatic islets
We obtained human pancreatic islets from the Human Islet Cell Processing Facility at the Diabetes Research Institute, University of Miami Miller School of Medicine, or from the Islet Cell Resource basic science islet distribution program, Islet Cell Resource Centers (ICRs) Consortium, Division of Clinical Research, National Center for Research Resources, National Institutes of Health.
Determination of acetylcholine secretion with biosensor cells
We adapted real time measurements of acetylcholine secretion from Huang et al.39. We used Fura-2 loaded CHO cells stably expressing muscarinic M3 receptors40.
Determination of acetylcholine secretion with Amplex assay
We measured acetylcholine secretion with the Amplex red Acetylcholine Assay Kit (Invitrogen, Carlsbad, CA, USA).
Insulin and glucagon secretion
We measured insulin and glucagon secretion as described16,22,23. We purchased Kainate, oxotremorine, physostigmine hemisulfate, vesamicol hydrochloride, and J 104129 fumarate from Tocris Bioscience (Ellisville, MO, USA), atropine sulfate and nicotine from Sigma (St Louis, MO, USA).
Immunohistochemistry
We performed immunostaining as described16,23,25 in sections of > 20 human pancreata. Antibodies used included rabbit antibody to vAChT (Synaptic Systems, 139103; control peptide 139-1P), rabbit antibody to vAChT (Sigma, V5387), rabbit antibody to ChT1 (Chemicon, AB5966), mouse antibody to somatostatin (Chemicon, MAB354), mouse antibody to glucagon (Sigma, G2654), guinea pig antibody to insulin (Dako, A0564), and rabbit antibody to C-peptide (GeneTex, GTX14181). In control experiments, we incubated primary antibodies with corresponding control peptide at a ratio of 50 µg antigenic peptide/1 µg antibody at room temperature for 5 h.
For ChAT immunostaining we used rabbit antibody to ChAT (Chemicon, AB143), goat antibody to ChAT (Chemicon, AB144P), or rabbit antibody to ChAT (Pierce, OSC00008W) antibodies. Only antibody AB144P gave reliable results, but it required signal amplification (ABC method followed by tyramide signal amplification). The strong signal obtained after amplification may explain why the proportion of ChAT stained alpha cells (90%) was higher than that of vAChT stained alpha cells (60%).
Colocalization studies
We quantified the degree of association of glucagon and vAChT staining within alpha cells with the colocalization macros of Volocity software (Perkin Elmer) as described18.
Western blotting
We carried out immunoblot analysis by standard methods with the antibodies used for immunohistochemistry (1:1000).
RT-PCR
We extracted RNA from human brain, total human pancreas, or from human islets using RiboPure™ Kit (Ambion, Austin, TX) and we prepared cDNA using High-Capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). We ran PCR reactions in duplicate using Taqman gene expression assays (Applied Biosystems, Foster City, CA) in a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA). We performed relative quatification (RQ) of gene expression based on the equation RQ=2−ΔCt×10,000, where ΔCt is the difference between the Ct value (number of cycles at which amplification for a gene reaches a threshold) of the target gene and the Ct value of the ubiquitous housekeeping gene GAPDH.
Statistical analyses
For statistical comparisons we used Student’s t-test, ANOVA followed by multiple comparisons (Bonferroni), or Fisher’s exact test. Throughout the manuscript we presented data as average ± s.e.m.
Supplementary Material
Acknowledgements
We thank Alexander Formoso for help with data analyses, Kevin Johnson for histological work, and Mayrin Correa-Medina for assistance with RT-PCR. This work was funded by the Diabetes Research Institute Foundation (DRIF), NIH grants R56DK084321 (A.C.), R01DK084321 (A.C.), R01DC000374 (S.D.R.), and R01DC007630 (S.D.R.), the Juvenile Diabetes Research Foundation, the Swedish Research Council, the Novo Nordisk Foundation, the Swedish Diabetes Association and The Family Erling-Persson Foundation, the World Class University program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (R31-2008-000-10105-0).
Footnotes
Contributions
R.R.D., M.C.J.S., A.F. and J.M. performed hormone assay experiments and ELISAs; R.D. performed experiments with biosensor cells to detect acetylcholine secretion; R.R.D. and M.C.J.S. conducted Amplex assays to measure acetylcholine secretion; R.R.D. collected, analyzed, and quantified immunohistochemical data; R.R.D. performed Western blottings. R.R.D., R.D., S.D.R., P.O.B. and A.C. designed the study, analyzed data, and wrote the paper. R.R.D. and R.D. contributed equally to the study. All authors discussed the results and commented on the manuscript.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Gilon P, Henquin J. Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr Rev. 2001;22:565–604. doi: 10.1210/edrv.22.5.0440. [DOI] [PubMed] [Google Scholar]
- 2.Gautam D, et al. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 2006;3:449–461. doi: 10.1016/j.cmet.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Ahrén B. Autonomic regulation of islet hormone secretion--implications for health and disease. Diabetologia. 2000;43:393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- 4.Conn PM, Goodman HM, Kostyo JL. The endocrine system. New York: Published for the American Physiological Society by Oxford University Press; 1998. [Google Scholar]
- 5.Gautam D, et al. Role of the M3 muscarinic acetylcholine receptor in beta-cell function and glucose homeostasis. Diabetes Obes Metab. 2007;9(Suppl 2):158–169. doi: 10.1111/j.1463-1326.2007.00781.x. [DOI] [PubMed] [Google Scholar]
- 6.Guenifi A, Simonsson E, Karlsson S, Ahrén B, Abdel-Halim S. Carbachol restores insulin release in diabetic GK rat islets by mechanisms largely involving hydrolysis of diacylglycerol and direct interaction with the exocytotic machinery. Pancreas. 2001;22:164–171. doi: 10.1097/00006676-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Doliba N, et al. Restitution of defective glucose-stimulated insulin release of sulfonylurea type 1 receptor knockout mice by acetylcholine. Am J Physiol Endocrinol Metab. 2004;286:E834–E843. doi: 10.1152/ajpendo.00292.2003. [DOI] [PubMed] [Google Scholar]
- 8.Guo Y, et al. CHRM3 gene variation is associated with decreased acute insulin secretion and increased risk for early-onset type 2 diabetes in Pima Indians. Diabetes. 2006;55:3625–3629. doi: 10.2337/db06-0379. [DOI] [PubMed] [Google Scholar]
- 9.Woods S, Porte DJ. Neural control of the endocrine pancreas. Physiol Rev. 1974;54:596–619. doi: 10.1152/physrev.1974.54.3.596. [DOI] [PubMed] [Google Scholar]
- 10.Coupland R. The innervation of pan creas of the rat, cat and rabbit as revealed by the cholinesterase technique. J Anat. 1958;92:143–149. [PMC free article] [PubMed] [Google Scholar]
- 11.Amenta F, Cavallotti C, de Rossi M, Tonelli F, Vatrella F. The cholinergic innervation of human pancreatic islets. Acta Histochem. 1983;73:273–278. doi: 10.1016/S0065-1281(83)80038-5. [DOI] [PubMed] [Google Scholar]
- 12.Ahrén B, Taborsky GJ, Porte DJ. Neuropeptidergic versus cholinergic and adrenergic regulation of islet hormone secretion. Diabetologia. 1986;29:827–836. doi: 10.1007/BF00870137. [DOI] [PubMed] [Google Scholar]
- 13.Brunicardi F, Shavelle D, Andersen D. Neural regulation of the endocrine pancreas. Int J Pancreatol. 1995;18:177–195. doi: 10.1007/BF02784941. [DOI] [PubMed] [Google Scholar]
- 14.Weihe E, Tao-Cheng J, Schäfer M, Erickson J, Eiden L. Visualization of the vesicular acetylcholine transporter in cholinergic nerve terminals and its targeting to a specific population of small synaptic vesicles. Proc Natl Acad Sci U S A. 1996;93:3547–3552. doi: 10.1073/pnas.93.8.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karlsson S, Myrsén U, Nieuwenhuizen A, Sundler F, Ahrén B. Presynaptic sympathetic mechanism in the insulinostatic effect of epinephrine in mouse pancreatic islets. Am J Physiol. 1997;272:R1371–R1378. doi: 10.1152/ajpregu.1997.272.5.R1371. [DOI] [PubMed] [Google Scholar]
- 16.Cabrera O, et al. Glutamate is a positive autocrine signal for glucagon release. Cell Metabolism. 2008;7:545–554. doi: 10.1016/j.cmet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eiden LE. The cholinergic gene locus. J Neurochem. 1998;70:2227–2240. doi: 10.1046/j.1471-4159.1998.70062227.x. [DOI] [PubMed] [Google Scholar]
- 18.Barlow AL, Macleod A, Noppen S, Sanderson J, Guérin CJ. Colocalization analysis in fluorescence micrographs: verification of a more accurate calculation of pearson's correlation coefficient. Microsc Microanal. 2010;16:710–724. doi: 10.1017/S143192761009389X. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Edwards R. Differential localization of vesicular acetylcholine and monoamine transporters in PC12 cells but not CHO cells. J Cell Biol. 1997;139:907–916. doi: 10.1083/jcb.139.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krantz D, et al. A phosphorylation site regulates sorting of the vesicular acetylcholine transporter to dense core vesicles. J Cell Biol. 2000;149:379–396. doi: 10.1083/jcb.149.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greider M, Bencosme S, Lechago J. The human pancreatic islet cells and their tumors. I. The normal pancreatic islets. Lab Invest. 1970;22:344–354. [PubMed] [Google Scholar]
- 22.Cabrera O, et al. Automated, high-throughput assays for evaluation of human pancreatic islet function. Cell Transplant. 2008;16:1039–1048. [PMC free article] [PubMed] [Google Scholar]
- 23.Jacques-Silva M, et al. ATP-gated P2X3 receptors constitute a positive autocrine signal for insulin release in the human pancreatic beta cell. Proc Natl Acad Sci U S A. 2010;107:6465–6470. doi: 10.1073/pnas.0908935107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zawalich W, Zawalich K, Rasmussen H. Cholinergic agonists prime the beta-cell to glucose stimulation. Endocrinology. 1989;125:2400–2406. doi: 10.1210/endo-125-5-2400. [DOI] [PubMed] [Google Scholar]
- 25.Cabrera O, et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stagner JI, Samols E, Weir GC. Sustained oscillations of insulin, glucagon, and somatostatin from the isolated canine pancreas during exposure to a constant glucose concentration. J Clin Invest. 1980;65:939–942. doi: 10.1172/JCI109750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang DA, Matthews DR, Peto J, Turner RC. Cyclic oscillations of basal plasma glucose and insulin concentrations in human beings. N Engl J Med. 1979;301:1023–1027. doi: 10.1056/NEJM197911083011903. [DOI] [PubMed] [Google Scholar]
- 28.Wessler I, Kirkpatrick C. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol. 2008;154:1558–1571. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brissova M, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53:1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- 30.Bosco D, et al. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes. 2010;59:1202–1210. doi: 10.2337/db09-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pisania A, et al. Lab Invest. Vol. 90. United States: 2010. Quantitative analysis of cell composition and purity of human pancreatic islet preparations; pp. 1661–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stagner JI, Samols E. The vascular order of islet cellular perfusion in the human pancreas. Diabetes. 1992;41:93–97. doi: 10.2337/diab.41.1.93. [DOI] [PubMed] [Google Scholar]
- 33.Taylor I, Feldman M. Effect of cephalic-vagal stimulation on insulin, gastric inhibitory polypeptide, and pancreatic polypeptide release in humans. J Clin Endocrinol Metab. 1982;55:1114–1117. doi: 10.1210/jcem-55-6-1114. [DOI] [PubMed] [Google Scholar]
- 34.Teff K, Mattes R, Engelman K, Mattern J. Cephalic-phase insulin in obese and normal-weight men: relation to postprandial insulin. Metabolism. 1993;42:1600–1608. doi: 10.1016/0026-0495(93)90157-j. [DOI] [PubMed] [Google Scholar]
- 35.Becker H, Börger H, Schafmayer A. Effect of vagotomy on gastrointestinal hormones. World J Surg. 1979;3:615–622. doi: 10.1007/BF01654771. [DOI] [PubMed] [Google Scholar]
- 36.Pozza G, et al. Metabolic control of type I (insulin dependent) diabetes after pancreas transplantation. Br Med J (Clin Res Ed) 1985;291:510–513. doi: 10.1136/bmj.291.6494.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diem P, et al. Glucagon, catecholamine and pancreatic polypeptide secretion in type I diabetic recipients of pancreas allografts. J Clin Invest. 1990;86:2008–2013. doi: 10.1172/JCI114936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blackman J, et al. Insulin secretory profiles and C-peptide clearance kinetics at 6 months and 2 years after kidney-pancreas transplantation. Diabetes. 1992;41:1346–1354. doi: 10.2337/diab.41.10.1346. [DOI] [PubMed] [Google Scholar]
- 39.Huang Y, et al. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci. 2005;25:843–847. doi: 10.1523/JNEUROSCI.4446-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buck M, Fraser C. Muscarinic acetylcholine receptor subtypes which selectively couple to phospholipase C: pharmacological and biochemical properties. Biochem Biophys Res Commun. 1990;173:666–672. doi: 10.1016/s0006-291x(05)80087-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.