Abstract
There are many reports of relations between age and cognitive variables and of relations between age and variables representing different aspects of brain structure, and a few reports of relations between brain structure variables and cognitive variables. These findings have sometimes led to inferences that the age-related brain changes cause the age-related cognitive changes. Although this conclusion may well be true, it is widely recognized that simple correlations are not sufficient to warrant causal conclusions, and other types of correlational information, such as mediation and correlations between longitudinal brain changes and longitudinal cognitive changes, also have limitations with respect to causal inferences. These issues are discussed, and the existing results on relations of regional volume, white matter hyperintensities, and DTI measures of white matter integrity to age and to measures of cognitive functioning are reviewed. It is concluded that at the current time the evidence that these aspects of brain structure are neuroanatomical substrates of age-related cognitive decline is weak. The final section contains several suggestions concerned with measurement and methodology that may lead to stronger conclusions in the future.
The primary question addressed in this article is the extent to which relations of age with measures of cognitive functioning are attributable to relations of age with measures of brain structure. The article is organized in five sections. The first section briefly describes the primary phenomenon to be explained, namely, age-related differences and age-related changes in cognitive functioning. The second section consists of a discussion of analytical methods that can be used to examine hypothesized causal relationships among sets of variables. The third section contains a brief rationale for a focus on aspects of brain structure rather than functional activation, and the fourth section is a review of empirical research on the interrelations of age, brain structure variables, and cognitive variables based on the framework outlined in the second section. The final section summarizes the major conclusions, discusses limitations of current research, and offers suggestions for future research.
Age-related differences and changes in cognitive functioning
Age-cognition relations are well-established in cross-sectional comparisons, and are becoming better established in longitudinal comparisons. Two broad trends are typically found; an increase until about 60 years of age followed by a decrease for measures reflecting acquired knowledge, and a nearly linear decline from early adulthood for measures of the efficiency or effectiveness of processing at the time of assessment (for reviews see Craik & Salthouse, 2008; Hedden & Gabrieli, 2004; Salthouse, 2010d).
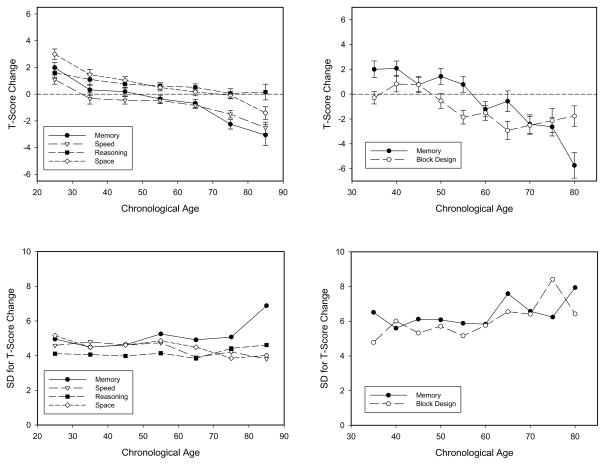
Although longitudinal studies involving adults with mean ages of 60 or older are not uncommon (e.g., see Hofer & Piccinin, 2007, for a recent review), only a limited number of longitudinal studies have compared adults across the entire range of adulthood on measures of cognitive functioning. Nevertheless, the available studies are consistent in finding that increased age is associated with more negative cognitive change (e.g., Giambra et al., 1995; Huppert & Whittington, 1993; Schaie, 2005; Zelinski & Burnight, 1997; also see Figure 2. 2 in Salthouse, 2010d).
Figure 2.
Means (and standard errors) of longitudinal changes in different cognitive variables (top panel), and standard deviations corresponding to the means (bottom panel) as a function of age in two projects. The panels on the left portray data from projects by Salthouse (2010a, 2010b, 2010c), and those on the right portray data from Ronnlund et al. (2005, 2006).
The patterns with variables reflecting processing efficiency and effectiveness can be illustrated with results from two projects in which both cross-sectional and longitudinal data are available from the same individuals across a wide range of ages. In order to facilitate comparisons across variables and projects, all scores are expressed in T-score units which have a mean of 50 and a standard deviation of 10. Except for the Block Design measure, each variable is represented as a composite score based on three or more separate test scores.
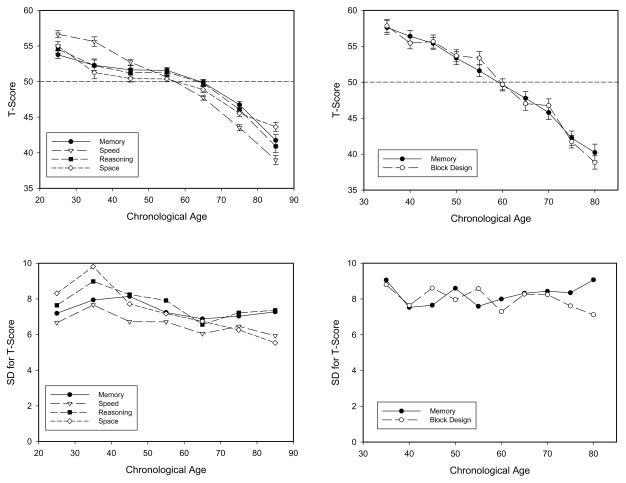
The left panels in Figures 1 and 2 contain data from a project by Salthouse (e.g., 2010a, b, c), that involved over 1,500 individuals, and an average retest interval of 2.5 years. The right panels contain data from a project by Ronnlund and colleagues (2005 2006), that involved an initial sample of 1,000 adults and a retest interval of 5 years. The top panels in each figure contain means, and the bottom panels contain between-person standard deviations for the same data used to compute the corresponding means.
Figure 1.
Means (and standard errors) in different cognitive variables (top panel), and standard deviations corresponding to the means (bottom panel) as a function of age in cross-sectional comparisons in two projects. The panels on the left portray data from projects by Salthouse (2010a, 2010b, 2010c), and those on the right portray data from Ronnlund et al. (2005, 2006).
Figure 1 portrays the cross-sectional results. Notice that there are large age-related differences in the mean level of performance for all six variables. (Age correlations for the abilities in the left panels were −.46 for reasoning, −.45 for space, −.44 for memory, and −.63 for speed.) Although age-related increases in between-person variability are sometimes found (e.g., Christensen et al., 1999; Nelson & Dannefer, 1992; Rabbitt, 1993), the bottom panels in Figure 1 reveal that this is not the case in these data. Moreover, this finding is not specific to these two projects because nearly constant between-person variability has been found in cognitive variables from different standardized test batteries (see Figures 1.12 to 1.15 in Salthouse, 2010d). Age-related increases in variance may be more likely when the measures are in units of time in which there is typically a strong positive relation between mean and variance, or when the sample contains individuals with various pathological conditions. However, the results in Figure 1 and elsewhere clearly indicate that age-related differences in mean performance can occur without concomitant increases in between-person variability.
Longitudinal results for the same variables and individuals illustrated in Figure 1 are portrayed in Figure 2. The results in the top panels indicate that the longitudinal changes (across a span of up to 5 years) are small relative to the cross-sectional differences (across a span of about 60 years), as the changes have a range of about 8 T-score units compared to a range of about 20 T-score units for the differences. However, the cross-sectional and longitudinal data are similar in other respects. For example, the longitudinal changes are systematically related to age, but with positive values at young ages, and negative values at older ages. (Age correlations for the longitudinal changes in the left panel were −.11 for reasoning, −.21 for space, −.25 for memory, and −.17 for speed.) At least some of the positive values for adults under about 50 years of age are likely attributable to retest effects associated with prior performance of the tests (cf. Salthouse, 2010c). Additional analyses revealed that the quadratic age relations in the Salthouse data were not significantly different from zero, and because there is no evidence of a discrete step between a period of stability and a period of negative change, it does not appear to be the case that change begins only at middle age or later. Furthermore, as with the cross-sectional differences, there was little relation between age and between-person variability as the individual differences in change were as large at age 30 as at age 70. That is, despite differences in the direction and magnitude of longitudinal change across different periods of adulthood, the magnitude of individual differences in change in these studies was nearly the same at all ages. There was also little indication of a relation of age to the individual differences in longitudinal change in other age-heterogeneous studies containing information about the variability of change (e.g., Alder et al., 1990; Giambra et al., 1995; Hertzog & Schaie, 1986; 1988; Huppert & Whittington, 1993; also see Finkel et al., 1996; 1998).
To summarize, nearly linear age-related declines in both cross-sectional and longitudinal comparisons have been reported in several major cognitive abilities. Although prior research has primarily focused on mean values, between-person variability is actually more important when examining relations with other variables because it sets limits on the magnitude of possible correlations the variable can have with other variables. The results summarized above are therefore noteworthy in indicating that not only is the magnitude of variability considerable in both the levels (cross-sectional) and the changes (longitudinal) in cognitive performance, but that variability does not inevitably increase with advancing age. Of particular relevance for the current review is that these results imply that there is no statistical reason why correlations involving cognitive variables would necessarily be weaker among young adults than among middle-aged or old adults.
A key question to be addressed in the remaining sections of this article is the role of neurobiological factors in these age-cognition relations. Although different mechanisms may be operating at different ages, it is important to recognize that the phenomenon to be explained is not merely relations of age in one ability within a narrow age range, but rather the nearly continuous relations of age to a wide variety of cognitive variables across nearly all of adulthood.
Analytical methods
Although most cognitive neuroscientists probably assume that individual differences in cognitive functioning have a neural basis, there is still considerable uncertainty about the role of specific brain structure characteristics on the age differences and age changes in cognitive functioning. Inferences that age-related cognitive declines are attributable to age-related changes in brain structure are sometimes based on correlations between a brain variable and a cognitive variable, on correlations of age with both the brain variable and the cognitive variable, and occasionally on correlations between longitudinal changes in a brain variable and longitudinal changes in a cognitive variable. As will be described below, all of these correlations are limited with respect to the information they provide about causal relations.
It is well-accepted that the ideal procedure for investigating causality is an experimental study in which individuals are randomly assigned either to a control group or to an experimental group, and differences between groups are examined in a relevant outcome variable. Furthermore, when the primary outcome of interest concerns effects on aging, long-term monitoring is needed to examine rates of aging in the target measures of cognitive functioning. However, even if it were ethical, it is difficult to manipulate specific aspects of brain structure, and it is seldom feasible to follow individuals long enough to observe effects on rates of aging. Correlational data are therefore the primary means of investigating interrelations of age, brain structure, and cognitive functioning in research on humans.
One type of correlational evidence simply consists of the relations of age with the relevant brain and cognitive variables. For example, as noted in several early studies (e.g., Bigler et al., 1995; Raz et al., 1993), a discovery of similar age trends in brain variables and cognitive variables could lead to speculations that the age-related influences on the brain variables are causally related to the age-related influences on the cognitive variables. However, a very large number of variables are related to age, and therefore additional information is needed to determine which variables might be causally related to one another, and which ones have no causal relation.
Several analytical procedures have been proposed to investigate causal relations based on the principle that although correlation does not imply causation, causation does imply correlation. That is, although causal hypotheses cannot be directly investigated with correlational procedures, implications of causal hypotheses can be examined with correlational data. One of the first systematic outlines of correlation-based procedures involving mediation and moderation was published by Baron and Kenny (1986), and the fact that the article has become one of the most cited articles in psychology is an indication of the high level of interest in these procedures. Applications of these procedures in research concerned with the interrelations of age, brain variables, and cognitive variables have been discussed by a number of authors (e.g., Fjell & Walhovd, 2010; Madden, Bennett et al., 2009; Rabbitt et al., 2007), but their assessments of the issues, and of possible solutions, differ from those described here.
Mediation
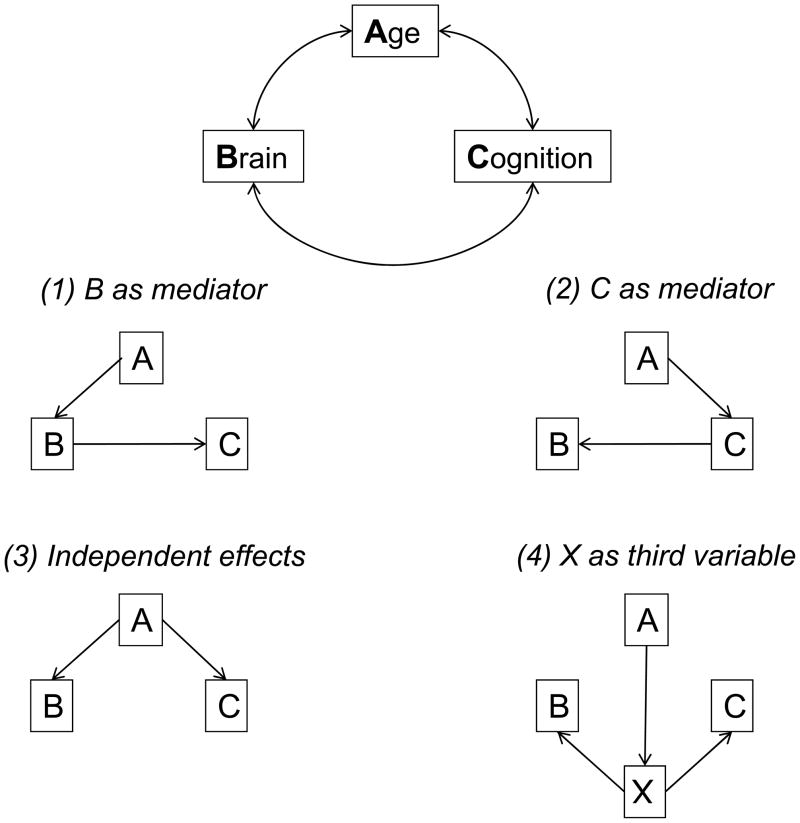
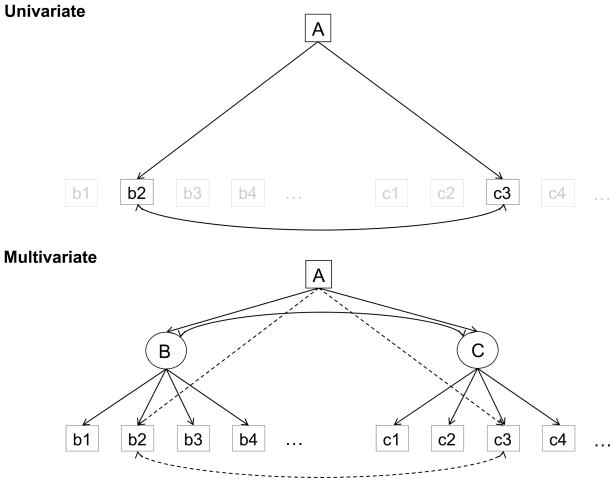
The simplest versions of mediation procedures are based on relations among three variables. In the current context, the age variable will be designated A, brain structure variables will be designated B, and cognitive variables will be designated C. The top panel in Figure 3 indicates that correlations are frequently observed among the A, B, and C variables, usually in the direction of lower cognitive performance and less intact brain structure with increased age, and lower levels of cognitive performance with greater structural degradation. The remaining panels in the figure portray alternative models that could produce these types of correlations among the variables. Researchers interested in neural correlates of age differences in cognition are often most interested in Model 1, in which a brain variable is hypothesized to mediate the age-related influences on a cognitive variable. However, the other models in Figure 3 represent alternative conceptualizations of the relations among the three variables. Additional models could also be specified, such as one in which A, B, and C are all reflections of a single common factor, but the four in Figure 3 are the most frequently discussed models of the relations among these variables.
Figure 3.
Schematic illustration of correlations among age, brain, and cognitive variables (top), and four models of the relations among the variables which could produce the correlations.
Models 1 and 2 differ in that the hypothesized causal direction is from brain structure to cognitive functioning in Model 1, whereas the causal direction between these two variables is reversed in Model 2. Model 3 is an independence model in which B and C are postulated to be related to each other only because of the common influence of A on both variables. One way to evaluate the plausibility of these models involves examining implications of specific relations under the assumption that a particular model is valid. For example, if Model 1 is valid then statistical control of the variation in B should reduce the magnitude of the A–C relation because much of the influence of A on C is postulated to be mediated through B. In a similar manner, if Model 2 is valid then statistical control of the variation in C should reduce the A–B relation. Because Model 3 specifies that B and C are actually independent, and only related to each other because of their relations to A, an implication of this model is that control of the variation in A should reduce or eliminate the B–C relation.
Model 4 postulates that some other variable, which could reflect health status, aspects of lifestyle, genetic profile, etc., is related to age, and to both the brain and cognitive variables. Unless all other relevant variables are known and represented in the model, it is difficult to rule out variants of Model 4 in which some unspecified variable X is involved in the relations among A, B, and C. Although seldom definitively rejected, interpretations based on Model 4 can gradually be rendered less plausible as different candidates for X are examined across a series of studies, and are found to have minimal effects on the relations among A, B, and C.
A considerable number of researchers have conducted mediation analyses by postulating a particular model, such as Model 1, and concluding that the mediation interpretation is confirmed if the A–C relation is reduced after controlling the variation in B. Although seemingly straightforward, two important issues need to be considered in the application and interpretation of this strategy.
One issue is how the difference in the relevant relations before and after statistical control is evaluated. Unless no other factors are assumed to be operating, it may be unrealistic to expect complete elimination of a relation after variability in a third variable is controlled. The question therefore arises as to how much reduction of a relation should be considered meaningful, and interpreted as supporting the prediction. One possibility is to rely on statistical significance of the residual (partialled) relation, or of the indirect (mediated) path, but this has the disadvantage that significance depends on sample size. Because correlations are measures of effect size, a potentially more desirable alternative is to indicate the magnitude of the alteration in the relation by comparison of correlations. For example, expanding on Cohen’s (1992) convention, correlation differences less than .1 might be considered small, those between .1 and .3 might be considered of medium size, and those greater than .5 might be considered large, and most consistent with the prediction.
A second issue to be considered in mediation analyses is that the outcomes are asymmetric because although a failure to support the prediction could be interpreted as falsifying the model, support for the prediction would merely be consistent with that model, as well as with other possible models (e.g., Edwards & Lambert, 2007; Kraemer et al., 2001; Kraemer et al., 2008; Lindenberger & Potter, 1998; Penke & Deary, 2010; Stone-Romero & Rosopa, 2008). A more informative approach than focusing exclusively on a single model might therefore involve examining the implications of multiple models, such as Models 1, 2, and 3, with the same data. That is, in addition to determining whether the data are consistent with Model 1, the data could also be examined to determine whether they are inconsistent with alternative models, in which case confidence in the target model would be enhanced relative to the alternative models.
If one assumes that increased age is associated with a decrease in some measure of brain structure, which in turn contributes to a decrease in cognitive functioning, then it is reasonable to expect that the relation between age and cognitive functioning would be smaller if there were no variation among the research participants in the brain structure measure. Mediation analyses are useful in revealing the extent to which this is the case. Nevertheless, it is important to emphasize that the results of these types of statistical control analyses do not provide a direct test of causal relations, and instead merely provide an opportunity for implications of particular hypotheses of causal relations to be disconfirmed. However, because alternative models can often be postulated to account for relations among the variables, confidence in the plausibility of the hypothesized model can be increased if the data are found to be inconsistent with alternative models of the relations.
Correlated Changes
A major limitation of analyses with cross-sectional data is that all of the observations are collected at the same point in time, and thus models with different directions of causal relations are not easily distinguished (e.g., Muller et al., 2005; Penke & Deary, 2010; Selig & Preacher, 2009; Shrout & Bolger, 2002). For example, in terms of the models in Figure 3, the absence of definitive temporal ordering means that Model 1, with B as the mediator, may not be distinguished from Model 2, with C as the mediator, and neither may be distinguished from Model 3 in which there is no mediation.
It is sometimes assumed that the availability of longitudinal data solves the problem of temporal precedence among variables, and therefore can help distinguish among Models 1, 2, and 3. Moreover, correlations between longitudinal changes in two variables are occasionally interpreted as implying the existence of a causal relation between the variables (e.g., Van Den Heuvel et al., 2007). Although it is true that longitudinal comparisons involve observations at different points in time, longitudinal data also have limitations when used to investigate causal relations (e.g., MacKinnon et al., 2007; Selig & Preacher, 2009). One of the most serious limitations is that it may not be reasonable to assume an instantaneous causal influence, with a zero lag between changes in the relevant variables (i.e., Gollub & Reichardt, 1987). That is, in most cases a temporal lag probably exists between the changes in two sets of variables, and analyses of lagged changes are only meaningful if the longitudinal interval between observations matches the interval between early change in the presumed causal variable and later change in the presumed effect variable (e.g., Collins, 2006; Hofer & Piccinin, 2007). For example, the relation between the two changes would likely be missed with a single longitudinal interval of one year if effects do not occur until at least three years after changes begin in the causal variable.
Unfortunately, relatively little is currently known about the dynamics of changes in either brain or cognitive variables, or about the lags between changes in the two types of variables, and therefore two-occasion longitudinal information (and information from related cross-lagged panel analyses) may be of limited value for distinguishing temporal order among variables (cf. Raz et al., 2010). Increasing the number of longitudinal assessments can help address this concern because a researcher could then determine whether early changes in the brain variable are associated with later changes in the cognitive variable. However, even these types of lead-lag analyses may not eliminate the problem if the total observation interval, or the spacing of observations within the intervals, does not match the timing of critical events.
Although they have limitations, there is at least one respect in which longitudinal data are more informative than cross-sectional data. This is that with correlations among changes one can be confident that the relevant individual differences are manifested during the period between measurements. That is, correlations based on cross-sectional data (i.e., B–C) reflect relations between influences that could have occurred any time prior to the measurements, whereas correlations of longitudinal changes (i.e., ΔB–ΔC) reflect coupling of changes that occur during the interval between measurements. For example, a cross-sectional correlation between memory performance and volume of the medial temporal lobe (MTL) region could be due to factors operating at any time from conception until the period of measurement. In contrast, a discovery that people with the greatest declines in memory over a given interval also have the greatest reductions in MTL volume indicates that the relation between the two variables is evident during that period of time. We may not know why the changes in either variable occurred, or when they first started, but a significant correlation indicates that people who change the most in one of the variables over the specified interval also have a great deal of change in the other variable. Stated somewhat differently, although the influences on one or both variables could have originated at any time in the past, which limits inferences about causal order, a discovery that longitudinal changes are correlated indicates that there is a relation between the manifestations of these influences during the interval between successive observations.
In some cases longitudinal information is available for only one of the variables, such that the correlation is between either the initial or final value of one variable (e.g., B) and the subsequent or preceding change in the other variable (e.g., ΔC). Although a correlation of this type does not allow coupled changes to be investigated, it is still potentially more informative than a cross-sectional correlation because relevant change is occurring during the observation period for at least one variable.
To summarize, even correlations between longitudinal changes do not necessarily imply causality, and interpretations of the relations between changes can be complicated when the time courses of critical changes are not known. Nevertheless, correlations among changes can be informative because they indicate that people who change the most in one of the variables also have large changes in the other variable, and that the relevant changes are occurring in the period between the successive observations rather than at some unknown time in the past.
Moderation
Although there are many reports of correlations between brain variables and cognitive variables, several meditational analyses, and a few reports of correlations between brain changes and cognitive changes, only a small number of studies have investigated moderation by determining whether the relations between brain variables and cognitive variables vary as a function of age. This neglect is unfortunate because in some respects results of moderation analyses can be considered to provide the most valuable type of information about the role of age on the relations between brain structure differences and changes and cognitive differences and changes.
Salthouse (2010d) used a metaphor of an avalanche to describe the difficulty of inferring the causal, or triggering, variable (i.e., the first rock to move in an avalanche) on the basis of observations late in the sequence (i.e., the positions of rocks near the bottom of the mountain). However, information about temporal, and potentially causal, relations might be available by comparing relations between positions, and changes in positions, of rocks at several different locations on the mountain. An important implication of this perspective is that even longitudinal data may be of limited value if they are only available at some unknown point after the critical relations originated.
The avalanche metaphor of cognitive aging is obviously limited in many respects, but to the extent that cognitive aging is conceptualized as a long-term, dynamic phenomenon, examination of brain-cognition relations at different ages could provide valuable information about sequential relations among the variables. Unfortunately, many studies concerned with age and brain-cognition relations have focused only on the period of old age, which may be analogous to observing rock positions and motions at the bottom of the mountain, when many of the important precipitating factors occurred much earlier. Of course, if the phenomenon of interest occurs during a relatively brief period late in life, then restricting observations to the period of old age would be justified. However, because the results in Figures 1 and 2 indicate that cross-sectional differences and longitudinal changes in cognitive functioning occur continuously across adulthood, conclusions based on observations from a very restricted age range could be misleading with respect to the origin, and causal nature, of relations among variables.
Age moderation of brain-cognition relations might be expected in at least three different types of situations. First, moderation might occur if there are different determinants of C at different ages. For example, retest-related influences on cognitive variables might be stronger at younger ages than at older ages (Salthouse, 2010c), or adults of different ages might employ qualitatively different strategies to perform the tasks. Neuroanatomical substrate involvement could also vary with age, as suggested by the discovery of age differences in the regional distribution of activation in functional neuroimaging studies (e.g., Dennis & Cabeza, 2008; Grady, 2009).
Second, moderation might be expected if there are different determinants of B at different ages. For example, weaker relations might be expected at younger ages if the B–C relations are at least partially attributable to preclinical pathology, and the pathology is less likely at younger ages.
And third, moderation could occur if there is a shift in either total variance, or the proportion of variance that is reliable, as a function of age. That is, because the key factor affecting the magnitude of relations with other variables is not the level of the variable, but rather the magnitude of the individual differences in the variable, the B–C or ΔB–ΔC relations might shift with age because of shifts in variance. For example, differences in variance might be expected when there are non-linear age trajectories for either the B or C variables, such that age-related variance is pronounced only after a particular age.
The question of the similarity of B–C relations at different ages can be investigated with versions of moderation analysis in which the focus is on interactions, or conditional relations. That is, moderation implies that the A–C relation varies as a function of the value of B (e.g., little relation of age to cognition at high values of brain volume, but more negative relations at lower values of brain volume), or equivalently, that the B–C relation varies as a function of age (e.g., weak relation of brain volume to cognition at young ages, but a stronger relation at older ages). Similar analyses could be carried out with correlations of changes to determine if the ΔB–ΔC correlations remain constant with age, or are stronger at either young or old ages.
Assuming adequate statistical power, lack of evidence of moderation would suggest that the B–C (or the ΔB–ΔC) relation is quantitatively similar at all ages, and would imply that a given value (or change) in B would be associated with a similar value (or change) in C at all ages. In contrast, evidence of moderation would imply that the B–C (or the ΔB–ΔC) relation differs as a function of age, possibly because of an age-related shift in the neural substrates of cognitive functioning. It is also important to note that discovery of a shift in the B–C (or ΔB–ΔC) relation with age would complicate interpretation of mediation analyses because it implies that control of the B variable would have different meaning at different ages (cf. MacKinnon et al., 2007).
In summary, simple correlations are not sufficient to infer causal relations between brain aging and cognitive aging, but correlation-based procedures can be informative. In particular, if the causal relation is as hypothesized, the critical relation would be expected to be reduced when variability in the relevant third variable is controlled, whereas implications of alternative models would be expected to receive less support. In addition, confidence in the hypothesized brain-cognition linkage would be strengthened if there were evidence that the relations occur across time within the same individuals in longitudinal data, and not just across individuals of different ages at a single point in time, as in cross-sectional data. Finally, moderation analyses are informative to determine whether the brain-cognition relations are invariant across all of adulthood, or whether there are shifts in magnitude, or direction, of the relations as a function of increasing age.
Brain structure versus functional activation
A considerable amount of functional neuroimaging research has been conducted in which activity in different brain regions is examined during the performance of a cognitive task (e.g., for reviews of age-comparative research see Dennis & Cabeza, 2008; Grady, 2008; and Park & Reuter-Lorenz, 2009). Because it allows brain activity to be linked to cognitive performance at the time the task is being performed, functional neuroimaging provides unique and valuable information. Nevertheless, there are several complications associated with the interpretation of functional neuroimaging measures in research on aging.
One important issue is that increased age is often associated with lower performance in a wide variety of cognitive tasks, and consequently there could be many different patterns of age differences in functional activation. Furthermore, when only a single task is administered it is difficult to distinguish task-specific contributions to the activation from contributions associated with the construct of primary interest. One possible approach to this concern is to focus on the activation common across multiple tasks to identify aspects of shared activation (as in Collette et al., 2007; Grady et al., 2006; Nyberg et al., 2003; and Ranganath et al., 2003), but relatively few studies have collected functional neuroimaging data from multiple tasks in adults of different ages.
A second issue is that increased age is often associated with lower levels of performance, and functional activation can vary according to the level of performance in the cognitive task. The conditions of the task could be manipulated to equate the average level of performance when studying age differences in activation, but this introduces a confounding between age and task condition which can make it difficult to interpret differences in activation patterns.
A third complicating issue is that activation in functional neuroimaging may be dependent on structural characteristics, and yet direct investigations of structure-function relations are rare (cf. Dennis & Cabeza, 2008; Raz, 2000). A few studies have reported that the strength of activation is related to structural characteristics, such as brain volume (e.g., Andrews-Hanna et al., 2008; Brodtmann et al., 2003; 2009; Nordahl et al., 2006; Rosen et al., 2005; Thomsen et al., 2004; Tyler et al., 2010; Venkatraman et al., 2010), but no relations have been found in other studies (e.g., Berlingeri et al., 2010; Hazlett et al., 1998; Langennecker et al., 2007). Furthermore, relatively little is currently known about age-related influences on vascular reactivity and properties of blood oxygenation that are relevant to fMRI activation based on the BOLD signal. The inconsistent pattern regarding structure-function linkages led Grady (2008, p. 140) to conclude that: “…the influence of structural changes is far from clear and will need further work to determine whether there is an influence, which structural measure (e.g., white matter or grey matter) is most closely related to activation, and what form the influence will take (i.e., leading to an increase or a decrease of activity).”
For the preceding reasons, the current review will be limited to structural factors, and to only a subset of three of these, namely, regional volume, white matter pathology, and white matter integrity. These three characteristics have the largest amount of relevant research with concurrent brain and cognitive measurements on the same individuals across a range of ages, but it is acknowledged that they are not necessarily the most fundamental, or informative, structural characteristics. Although other structural (and physiological) characteristics such as cortical thickness, cerebral blood flow, concentration of brain metabolites, quantity of neurotransmitters or receptor sites, number of neurons, density of synapses or spines, etc. will not be considered, analytical methods similar to those described here could be applied with these measures when appropriate data become available.
Review of research
Relevant research studies were identified by searching databases (e.g., PsychInfo, Web of Science) with “brain aging,” “brain cognition,” “structural brain changes,” “brain cognition correlations,” and related terms, and manual inspection of relevant journals. In addition, potentially relevant citations from the reference lists in the retrieved articles were also examined whenever possible.
Two important limitations of the literature review need to be acknowledged at the outset. The first is that there may be some publication bias in the literature. That is, positive relations may be over-represented in the review because negative findings may not have been published, or conversely, they may be under-represented because they might have been considered mere replications of well-established results. It is difficult to estimate the magnitude of either type of publication bias, but it should be noted that many of the analyses to be reported lead to questions about the positive findings that have been published. In other words, regardless whether the proportion of positive and negative findings in the published literature is representative of the true state of affairs, concerns are raised about the interpretation of the findings that have been assumed to provide support for the hypotheses.
A second limitation of the literature review is that many published articles contain overlapping samples, often with little information about the actual degree of overlap. Although most serious when different reports focus on the same combination of brain and cognitive variables, this is also a concern across reports involving different variables from the same individuals unless there is evidence that the variables are independent of one another. As with the first limitation, the unknown degree of overlap of samples makes it difficult to derive quantitative estimates of effect sizes, but it is unlikely to have a major impact on the overall conclusions.
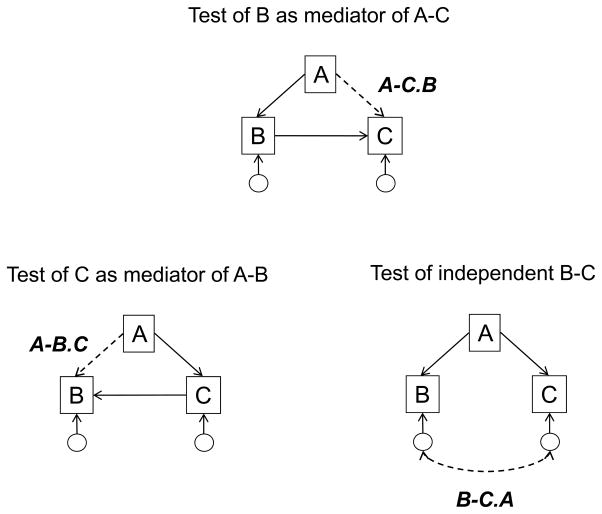
Relations among A (age), B (brain), and C (cognition) will be examined in cross-sectional data with the three models represented in Figure 4. The models are expressed in the form of path analyses, with the dotted line in each model representing the critical relation in the path analysis which can be compared with the simple correlation between the relevant two variables. The models are not exhaustive, in part because variants of Model 4 are not considered, and they are not mutually exclusive, because mixtures of different types of effects could occur simultaneously. Nevertheless, the three models in Figure 4 represent some of the simplest models of the relations among the A, B, and C variables, and they reflect quite different causal hypotheses.
Figure 4.
Schematic illustration of three models of the relations among the A, B, and C variables, and the relation (dotted line) hypothesized to be small or non-existent according to the model.
Model 1, in which the brain variable is assumed to mediate the relations between age and cognition, is probably the most commonly assumed model. The relationship between brain and cognition is reversed in Model 2 as cognitive functioning is postulated to affect aspects of brain structure. This causal direction has become more plausible in recent years because of studies reporting changes in brain variables as a function of cognitively-demanding activities such as intensive study (e.g., Ceccarelli et al., 2009; Draganski et al., 2006), memory training (Envig et al., 2010), skill acquisition (Boyke et al., 2008; Ilg et al., 2008), and second-language learning (e.g., Mechelli et al., 2009). Model 3 portrays the possibility that the brain and cognition variables are only related to one another because they are each related to age. This is an important model to consider in age-heterogeneous samples because many brain and cognitive variables are related to age, and thus it is possible that at least some of their relations with one another are spurious, and attributable to their common relation with age.
Because confirmation of a prediction provides relatively weak evidence, whereas a pattern in which the results are not only consistent with implications of the target model but also inconsistent with implications of alternative models is more convincing, results with all three models will be considered when evaluating the plausibility of the models. However, it is important to note that simultaneous consideration of multiple models is not atheoretical or exploratory, but instead can be considered to provide a stronger test of the plausibility of a particular model. That is, because results consistent with a particular model merely indicate that the model survived an opportunity for falsification, the plausibility of the model relative to alternative models would be increased if the results were found to be inconsistent with the implications of the other models. For example, Model 1 would be considered most plausible if the reduction implied by Model 1 (i.e., A–C > A–C.B, which means that the correlation between A and C is greater than the correlation between A and C after partialling or controlling B) is greater than the reduction implied by Model 2 (i.e., A–B > A–B.C), and the reduction implied by Model 3 (i.e., B–C > B–C.A).
The analyses require that correlations among the three variables, or R2 values that can be used to derive the relevant correlations, were reported in the research article. Additional criteria to ensure meaningfulness of the results were that the sample size was at least 25, that the age range in a continuous sample was at least 25 years, and that cognition was assessed by sensitive performance tests rather than ratings, or with a global dementia screening test such as the Mini-Mental Status Exam (MMSE; Folstein et al., 1975). In some cases the results reported in the original article are slightly different than those reported here because the current estimates are derived from path analyses based on correlations with only three variables, whereas some studies included many brain and cognitive variables in the same analyses, some merely reported that paths from A to B and from B to C were both significantly different from zero, and some reported regression analyses and focused on ratios or proportions of variance before and after statistical control, rather than differences between correlations.
Results will be reported with all available combinations of brain variables and cognitive variables, rather than only those based on hypotheses derived from the past literature. One reason for this inclusive approach is that some hypotheses are based on the literature on focal lesions, and they may not be applicable to the gradual, and possibly diffuse, patterns associated with aging (cf. Raz & Kennedy, 2009). Another reason is that consideration of multiple combinations of brain and cognitive variables helps establish the baselines for differences between relations when there is little reason to expect a particular model to be correct.
Five questions relevant to the causal role of B on A–C relations will be examined on the basis of the available data on interrelations among age (A), measures of brain structure (B) based on volume, white-matter hyperintensities and diffusion tensor imaging, and various cognitive (C) variables.
are the results of mediation analyses consistent with Model 1, in which B is a mediator of the A–C relation (is the correlation between age and cognition [A–C] reduced when the variability in the brain variable is held constant [A–C.B]; i.e., A–C > A–C.B?)?
are the results of reverse mediation analyses consistent with Model 2, in which C is a mediator of the A–B relation (i.e., A–B > A–B.C?)?
are the relations between brain and cognitive variables consistent with Model 3, such that the B–C relation is attenuated after control of age (i.e., is B–C > B–C.A?)?
are the longitudinal changes in brain structure over a given interval correlated with the longitudinal changes in cognition (i.e., [ΔB–ΔC] > 0), or are the values of B at one point in time correlated with longitudinal changes in C (i.e., [B–ΔC] > 0?)?
does the strength of the B–C relation, or of the ΔB–ΔC relation, vary as a function of age?
None of the information is definitive by itself, but inferences about causal relations among A, B, and C become more plausible as answers are obtained to each question, and supportive evidence accumulates. For example, the plausibility of Model 1 would be increased if the results are consistent with positive answers to questions 1 and 4, and negative answers to questions 2 and 3. As noted earlier, question 5 is frequently neglected, and yet it is valuable in indicating whether there is a shift with age in either the B–C or ΔB–ΔC relation. A discovery that the relations are moderated by age would be consistent with the assumption that there are different neuroanatomical substrates of cognitive functioning at different ages. In contrast, lack of evidence of moderation would suggest that there may be nothing special about the period of older adulthood with respect to the qualitative nature of the B–C, or ΔB–ΔC, relations.
Regional Volume
Although shrinkage of brain volume with increased age is well-documented, reasons for age-related decreases in brain volume are not fully understood. Contrary to early views, loss of neurons does not appear to be the major factor contributing to the volume reduction (e.g., Fjell & Walhovd, 2010; Freeman et al., 2008; Morrison & Hof, 1997), but there could be shrinkage of the dendritic arbor and of cell bodies, decrease in synaptic density, loss of glial cells, reduction of myelination, and possibly decreases in vascularization. In part because of the uncertainties about the nature and causes of brain volume reduction, Van Petten (2004, p. 1395) characterized brain volume as “the crudest of neurobiological metrics”. Raz and Kennedy (2009) also pointed out that the relationship between volume and cognitive performance can be difficult to interpret because “… if smaller volume means atrophy and loss of valuable neural elements, then it should predict poorer performance on cognitive tests. However, if increased regional volume reflects pathological processes, such as gliosis or failure to dispose of the unnecessary elements of the neural networks, then decreased volume is expected to go with better cognitive status (p. 51).” Despite the uncertainty about its nature, relations between brain volume measures and either age or measures of cognitive functioning have been reported in many studies.
Age Relations (AB)
Negative cross-sectional age relations with whole brain volume have been reported in a large number of studies (e.g., Abe et al., 2008; Adamson et al., 2010; Blatter et al., 1995; Carlesimo et al., 2010; Carne et al., 2006; Chee et al., 2009; Courchesne et al., 2000; DeCarli et al., 1999; 2005; Fotenos et al., 2005; 2008; Good et al., 2001; Hasan et al., 2007; Hutton et al., 2009; LeMaitre et al., in press; Marcus et al., 2007; Narr et al., 2007; Pieperhoff et al., 2008; Resnick et al., 2000; Schretlen et al., 2000; Seshadri et al., 2004; Taki et al., 2004; Van Petten et al., 2004; Wahlvold et al., 2005; Zimmerman et al., 2006; see Fjell & Walhovd, 2010, for a recent review). Global estimates of age differences across the entire period of adulthood range from about 0.2% to 0.5% per year, although there are clear regional variations. For example, volume reduction seems to be largest in the frontal and parietal lobes and least in the occipital lobe (e.g., Carne et al., 2006; Chee et al., 2009; 2010; DeCarli et al., 2005; Fjell et al., 2010; Fjell & Walhovd, 2010; Gonoi et al., 2010; Jernigan et al., 2001; Kalpouzos et al., 2009; Kennedy, Erickson et al., 2009; Lemaitre et al., in press; Raz et al., 2005; Sowell et al., 2003; Tisserand et al., 2002; Walhovd et al., 2005), and may be small in the medial temporal region and hippocampus until middle or late adulthood (e.g., Allen et al., 2005; Carlesimo et al., 2010; Fjell et al., 2010; Good, 2001; Grieve et al., 2005; Raz, et al, 2004; Sakamoto et al., 2007). Age relations may also be relatively small in the corpus callosum (e.g., Driesen & Raz, 1995; Fillipini et al., 2009; Head et al., 2005; Muller-Oehring et al., 2007; Sullivan et al., 2002).
There have also been reports of volume declines in longitudinal comparisons (e.g., Driscoll et al., 2009; Du et al., 2006; Enzinger et al., 2005; Firbank et al., 2007; Fjell, Walhovd et al., 2009; Walhovd et al., 2010; Fotenos et al., 2005; Jack et al., 2005; 2008; Lawrie et al., 2002; Lieberman et al., 2001; Liu et al., 2003; Pfefferbaum et al., 1998; Raz et al., 2004; 2005; 2008; 2010; Resnick et al., 2003; Rettmann et al., 2006; Rodrigue & Raz, 2004; Scahill et al., 2003; Schmidt et al., 2005; Sullivan et al., 2002). Moreover, a few studies have reported greater shrinkage in longitudinal comparisons than in cross-sectional comparisons (e.g., Du et al., 2006; Raz et al., 2005; Taki et al., 2009).
Although the negative age relations are nearly linear from early adulthood in gray matter volume (e.g., Abe et al., 2008; Allen et al., 2005; Bartzokis et al., 2001; Courchesne et al., 2000; Fotenos et al., 2005; Good et al., 2001; Grieve et al., 2005; Hasan et al., 2007; Kalpouzos et al., 2009; Michielse et al., 2010; Pieperhoff et al., 2005; Sowell et al., 2003; Taki et al., 2004; Terribilli et al., 2011), there may be little difference, or possibly even an increase, in white matter volume until the 40s or 50s, albeit with some across-region variation (e.g., Abe et al., 2008; Allen et al., 2005; Courchesne et al., 2000; Fotenos et al., 2005; Good et al., 2001; Grieve et al., 2005; Guttmann et al., 1998; Hasan et al., 2007; Michielse et al., 2010; Salat, Greve et al., 2009; Salat, Lee et al., 2009; Sowell et al., 2003; Sullivan et al., 2000; Taki et al., 2004; Walhvold et al., 2005; Westlye et al., 2010). A recent integrative analysis by Walhovd et al. (2009) on data combined across multiple samples involving a large number of adults across a wide age range (i.e., N > 880, ages 18 to 90+) revealed both linear and non-linear age trends, usually with accelerated shrinkage at older ages.
Cognition Relations (B–C)
Some reviews of the literature on volume-cognition relations have stressed the inconsistencies, and have suggested that it is not yet possible to reach definitive conclusions about the relations between brain volume and cognitive performance (e.g., Raz, 2000; Van Petten, 2004). Nevertheless, many studies have reported positive correlations (i.e., better cognitive performance associated with larger volumes) between performance in various cognitive tests and regional volume. For example, two recent meta-analyses have reported correlations of .33 (McDaniel, 2005) and .40 (Rushton & Ankney, 2009) between overall brain size and measures of general cognitive ability. Some studies have relied on crude measures based on CSF volume (e.g., Cook et al., 2002; Ikram et al., 2008; Rabbitt et al., 2006; Seshardi et al., 2004; van der Werf et al., 2001; Wickett et al., 2000; Willerman et al., 1991), or other indices of whole brain volumes (e.g., Aggarwal et al., 2010; Muller et al., 2009; Posthuma et al., 2002). Other studies have reported relations between regional volume measures and either general or specific measures of cognition (e.g., Adamson et al., 2010; Andreasen et al., 1993; Colom et al., 2006a; 2006b; 2009; Eckert et al., 2010; Flashman et al., 1997; Frangou et al., 2004; Haier et al., 2004; 2009; Hulshoff Pol et al., 2006; Ikram et al., 2010; Johnson et al., 2008; Jung & Haier, 2007; Kennedy, Rodrigue et al., 2009; Luders et al., 2009; MacLullich et al., 2002; Moffat et al., 2007; Oosterman et al., 2008; Paul et al., 2005; 2009; Schwartz et al., 2007; Soderlund et al., 2004; 2006; Staff et al., 2006; Thompson et al., 2001; Ullen et al., 2008; Wickett et al., 2000). There are also quite a few reports of relations between frontal lobe volume of either gray or white matter and measures of fluid intelligence or executive functioning (e.g., Chee et al., 2009; Colcombe et al., 2005; Duarte et al., 2006; Fine et al., 2009; Gong et al., 2005; Kramer et al., 2007; Nestor et al., 2010; Newman et al., 2007; Raz et al., 1998; 2008; Schretlen et al., 2000; Ziegler et al., 2008; Zimmerman et al., 2006). Volume-cognition relations have also been reported with other combinations of variables, including memory and medial temporal lobe volume (e.g., Andreasen et al., 1993; Brickman et al., 2006; Cardenas et al., 2009; Carey et al., 2008; Chen et al., 2010; Cohen et al., 2006; Hackert et al., 2002; Kalpouzos et al., 2009; Lye et al., 2006; Mungas et al., 2005; O’Brien et al., 1997; Oosterman et al., 2008; Yonelinas et al., 2007; Ystad, et. al, 2009), general information with frontal and temporal volume (Flashman et al., 1997), vocabulary knowledge with temporal lobe volume (Colom et al., 2009) and inferior parietal volume (Lee et al., 2007), and digit symbol speed with parietal volume (Flashman et al., 1997).
Nearly all of these relations have been in the direction of bigger volumes associated with better performance. However, there have been some exceptions, as negative volume-cognition relations have also been reported (e.g., Salat et al., 2002; van Petten, 2004; van Petten et al., 2004). Unfortunately, the reasons for these inconsistencies are not yet understood.
The most detailed specification of relations between regional volumes and cognitive performance at the current time is the Parieto-Frontal Integration Theory (P-FIT) model by Jung and Haier (2007). This model postulates a distributed pattern of regional influence, with frontal, parietal, and temporal volumes related to fluid intelligence. It has also been reported that the regional involvement is more extensive (i.e., more gray matter voxels) when the tests have higher loadings on a general factor (e.g., Colom et al., 2006a, 2006b).
Mediation (A–C.B)
A relatively large number of studies have reported mediation analyses with global or regional brain volume as the hypothesized mediator. Summary results for the three models in Figure 4 for the studies with relevant information are reported in Table 1.
Table 1.
Analyses with Volume as the Brain Structure Variable
| C | B | A–C | A–C.B | A–B | A–B.C | B–C | B–C.A |
|---|---|---|---|---|---|---|---|
| Raz et al. (1993), N = 29, Ages 18 to 78 | |||||||
| Cattell Culture Fair IQ | Total intracranial volume | −.72 | −.65 | −.40 | −.19 | .43 | .22 |
| Dorso-lateral prefrontal | −.72 | −.61 | −.43 | −.13 | .51 | .32 | |
| Primary Somato-Sensory | −.72 | −.74 | −.09 | −.47 | −.19 | −.37 | |
| Inferior Parietal Lobule | −.72 | −.72 | −.22 | −.23 | .15 | −.01 | |
| Prefrontal White Matter | −.72 | −.70 | −.36 | −.27 | .32 | .09 | |
| Hippocampus | −.72 | −.72 | −.03 | .03 | .06 | .06 | |
| Vocabulary | Total intracranial volume | .26 | .36 | −.40 | −.46 | .10 | .23 |
| Dorso-lateral prefrontal | .26 | .34 | −.43 | −.47 | .03 | .16 | |
| Primary Somato-Sensory | .26 | .24 | −.09 | −.04 | −.20 | −.18 | |
| Inferior Parietal Lobule | .26 | .32 | −.22 | −.30 | .21 | .28 | |
| Prefrontal White Matter | .26 | .30 | −.36 | −.38 | −.01 | .09 | |
| Hippocampus | .26 | .26 | −.03 | .04 | .03 | .04 | |
| Raz et al. (1998), N = 95, Ages 18 to 77 | |||||||
| WCST (Perseverations) | Prefrontal cortex | .44 | .31 | −.51 | −.40 | −.42 | −.25 |
| Limbic cortex | .44 | .41 | −.18 | −.10 | −.23 | −.17 | |
| Visual cortices | .44 | .39 | −.35 | −.28 | −.29 | −.16 | |
| Inferior parietal lobule | .44 | .45 | −.15 | −.19 | .01 | .09 | |
| Verbal Memory | Prefrontal cortex | −.39 | −.41 | −.51 | −.52 | .17 | −.04 |
| Limbic cortex | −.39 | −.38 | −.18 | −.15 | .13 | .07 | |
| Visual cortices | −.39 | −.41 | −.35 | −.38 | .08 | −.07 | |
| Inferior parietal lobule | −.39 | −.40 | −.15 | −.18 | −.01 | −.08 | |
| Nonverbal Memory | Prefrontal cortex | −.38 | −.38 | −.51 | −.51 | .20 | .01 |
| Limbic cortex | −.38 | −.37 | −.18 | −.15 | .14 | .08 | |
| Visual cortices | −.38 | −.35 | −.35 | −.32 | .21 | .09 | |
| Inferior parietal lobule | −.38 | −.38 | −.15 | −.16 | .04 | −.02 | |
| Raz et al. (1998), N = 95, Ages 18 to 77 | |||||||
| Priming | Prefrontal cortex | −.10 | −.16 | −.51 | −.52 | −.04 | −.11 |
| Limbic cortex | −.10 | −.10 | −.18 | −.18 | .01 | −.01 | |
| Visual cortices | −.10 | −.15 | −.35 | −.36 | −.09 | −.13 | |
| Inferior parietal lobule | −.10 | −.11 | −.15 | −.15 | −.02 | −.04 | |
| Working Memory (Verbal) | Prefrontal cortex | −.39 | −.34 | −.51 | −.48 | .27 | .09 |
| Limbic cortex | −.39 | −.36 | −.18 | −.12 | .21 | .15 | |
| Visual cortices | −.39 | −.40 | −.35 | −.36 | .11 | −.03 | |
| Inferior parietal lobule | −.39 | −.37 | −.15 | −.09 | .20 | .16 | |
| Working Memory (NonVerbal) | Prefrontal cortex | −.35 | −.27 | −.51 | −.47 | .29 | .14 |
| Limbic cortex | −.35 | −.32 | −.18 | −.12 | .21 | .16 | |
| Visual cortices | −.35 | −.27 | −.35 | −.27 | .33 | .24 | |
| Inferior parietal lobule | −.35 | −.34 | −.15 | −.13 | .11 | .06 | |
| Raz. et al. (2000), N = 68, Ages 22 to 80 | |||||||
| Working Memory (Verbal) | Dorso-lateral prefrontal | −.47 | −.55 | −.64 | −.68 | .23 | −.10 |
| Hippocampus | −.47 | −.46 | −.31 | −.29 | .18 | .04 | |
| Cerebellum | −.47 | −.41 | −.29 | −.17 | .34 | .24 | |
| Caudate | −.47 | −.46 | −.08 | −.04 | .11 | .08 | |
| Putamen | −.47 | −.40 | −.32 | −.21 | .34 | .23 | |
| Working Memory (NonVerbal) | Dorso-lateral prefrontal | −.28 | −.16 | −.64 | −.61 | .29 | .15 |
| Hippocampus | −.28 | −.23 | −.31 | −.26 | .24 | .17 | |
| Cerebellum | −.28 | −.20 | −.29 | −.22 | .32 | .26 | |
| Caudate | −.28 | −.27 | −.08 | −.02 | .21 | .20 | |
| Putamen | −.28 | −.23 | −.32 | −.25 | .35 | .14 | |
| Pursuit Rotor, Day 1 | Dorso-lateral prefrontal | −.26 | −.19 | −.64 | −.62 | .23 | .09 |
| Hippocampus | −.26 | −.20 | −.31 | −.26 | .25 | .19 | |
| Cerebellum | −.26 | −.16 | −.29 | −.20 | .39 | .34 | |
| Caudate | −.26 | −.25 | −.08 | −.05 | .12 | .10 | |
| Putamen | −.26 | −.17 | −.32 | −.25 | .35 | .29 | |
| Head et al. (2002), N = 68, Ages 22 to 80 | |||||||
| Working Memory (Verbal) | Lateral Prefrontal | −.50 | −.54 | −.64 | −.67 | .28 | −.06 |
| Caudate | −.50 | −.49 | −.17 | −.13 | .15 | .08 | |
| Putamen | −.50 | −.46 | −.31 | −.23 | .27 | .14 | |
| Cerebellum | −.50 | −.47 | −.29 | −.22 | .25 | .13 | |
| Hippocampus | −.50 | −.53 | −.20 | −.28 | −.02 | −.14 | |
| Visual Cortex | −.50 | −.50 | .03 | .07 | .05 | .08 | |
| Working Memory (NonVerbal) | Lateral Prefrontal | −.24 | −.04 | −.64 | −.59 | .34 | .25 |
| Caudate | −.24 | −.20 | −.17 | −.10 | .30 | .27 | |
| Putamen | −.24 | −.18 | −.31 | −.27 | .24 | .18 | |
| Cerebellum | −.24 | −.22 | −.29 | −.28 | .12 | .05 | |
| Hippocampus | −.24 | −.20 | −.20 | −.16 | .22 | .18 | |
| Visual Cortex | −.24 | −.24 | .03 | .05 | .07 | .08 | |
| WCST Composite (Perseverations) | Lateral Prefrontal | .45 | .30 | −.64 | −.56 | −.43 | −.21 |
| Caudate | .45 | .42 | −.17 | −.07 | −.25 | −.20 | |
| Putamen | .45 | .40 | −.31 | −.23 | −.29 | −.18 | |
| Cerebellum | .45 | .43 | −.29 | −.25 | −.20 | −.08 | |
| Hippocampus | .45 | .43 | −.20 | −.16 | −.17 | −.09 | |
| Visual Cortex | .45 | .45 | .03 | .09 | −.10 | −.13 | |
| Tower Hanoi, Time/Move Blk1 | Lateral Prefrontal | .57 | .66 | −.64 | −.71 | −.28 | .13 |
| Caudate | .57 | .56 | −.17 | −.14 | −.13 | −.04 | |
| Putamen | .57 | .56 | −.31 | −.27 | −.22 | −.06 | |
| Cerebellum | .57 | .60 | −.29 | −.36 | −.08 | .11 | |
| Hippocampus | .57 | .54 | −.20 | −.06 | −.28 | −.21 | |
| Visual Cortex | .57 | .57 | .03 | .12 | −.09 | −.13 | |
| Tower Hanoi, Time/Move Blk 2 | Lateral Prefrontal | .51 | .44 | −.64 | −.60 | −.39 | −.06 |
| Caudate | .51 | .49 | −.17 | −.10 | −.19 | −.12 | |
| Putamen | .51 | .47 | −.31 | −.23 | −.27 | −.14 | |
| Cerebellum | .51 | .52 | −.29 | −.30 | −.13 | .02 | |
| Hippocampus | .51 | .52 | −.20 | −.24 | −.04 | .07 | |
| Visual Cortex | .51 | .51 | .03 | .08 | −.05 | −.08 | |
| Head et al. (2002), N = 68, Ages 22 to 80 | |||||||
| Tower Hanoi, Moves/Blk, Blk 1 | Lateral Prefrontal | .33 | .15 | −.64 | −.58 | −.38 | −.23 |
| Caudate | .33 | .32 | −.17 | −.15 | −.10 | −.05 | |
| Putamen | .33 | .33 | −.31 | −.31 | −.11 | −.01 | |
| Cerebellum | .33 | .34 | −.29 | −.30 | −.08 | .02 | |
| Hippocampus | .33 | .28 | −.20 | −.12 | −.29 | −.24 | |
| Visual Cortex | .33 | .33 | .03 | .01 | .06 | .05 | |
| Tower Hanoi, Moves/Blk, Blk 2 | Lateral Prefrontal | .11 | −.01 | −.64 | −.63 | −.18 | −.14 |
| Caudate | .11 | .11 | −.17 | −.17 | −.03 | −.01 | |
| Putamen | .11 | .08 | −.31 | −.30 | −.12 | −.09 | |
| Cerebellum | .11 | .12 | −.29 | −.29 | −.01 | .02 | |
| Hippocampus | .11 | .15 | −.20 | −.22 | .15 | .18 | |
| Visual Cortex | .11 | .11 | .03 | .04 | −.09 | −.09 | |
| Gunning-Dixon & Raz (2003), N = 139, Ages 50 to 81 | |||||||
| Working Memory (Verbal) | Prefrontal | −.27 | −.19 | −.29 | −.22 | .33 | .27 |
| Fusiform gyrus | −.27 | −.21 | −.20 | −.12 | .33 | .29 | |
| WCST (Perseverations) | Prefrontal | .27 | .20 | −.29 | −.23 | −.30 | −.24 |
| Fusiform gyrus | .27 | .22 | −.20 | −.13 | −.30 | −.26 | |
| Kennedy, Rodrigue et al. (2009), N = 169, Ages 18 to 80 | |||||||
| Working Memory (Verbal) | Lateral Prefrontal cortex | −.37 | −.34 | −.58 | −.57 | .25 | .05 |
| Orbital frontal | −.37 | −.33 | −.35 | −.31 | .22 | .10 | |
| Prefrontal white matter | −.37 | −.32 | −.33 | −.28 | .25 | .15 | |
| Hippocampus | −.37 | −.35 | −.30 | −.27 | .18 | .08 | |
| Caudate | −.37 | −.36 | −.29 | −.27 | .15 | .05 | |
| Fusiform gyrus | −.37 | −.36 | −.41 | −.40 | .18 | .03 | |
| Visual cortex | −.37 | −.36 | −.12 | −.08 | .15 | .11 | |
| Kennedy, Rodrigue et al. (2009), N = 169, Ages 18 to 80 | |||||||
| Working Memory (NonVerbal) | Lateral Prefrontal cortex | −.34 | −.26 | −.58 | −.54 | .29 | .12 |
| Orbital frontal | −.34 | −.29 | −.35 | −.30 | .25 | .15 | |
| Prefrontal white matter | −.34 | −.28 | −.33 | −.27 | .28 | .19 | |
| Hippocampus | −.34 | −.29 | −.30 | −.24 | .25 | .17 | |
| Caudate | −.34 | −.32 | −.29 | −.27 | .15 | .06 | |
| Fusiform gyrus | −.34 | −.30 | −.41 | −.38 | .23 | .11 | |
| Visual cortex | −.34 | −.33 | −.12 | −.07 | .16 | .13 | |
| Fluid Intelligence | Lateral Prefrontal cortex | −.54 | −.48 | −.58 | −.53 | .38 | .10 |
| Orbital frontal | −.54 | −.49 | −.35 | −.26 | .31 | .15 | |
| Prefrontal white matter | −.54 | −.48 | −.33 | −.21 | .33 | .19 | |
| Hippocampus | −.54 | −.51 | −.30 | −.23 | .26 | .12 | |
| Caudate | −.54 | −.53 | −.29 | −.26 | .20 | .05 | |
| Fusiform gyrus | −.54 | −.48 | −.41 | −.32 | .34 | .15 | |
| Visual cortex | −.54 | −.52 | −.12 | −.02 | .20 | .16 | |
| Priming Training | Lateral Prefrontal cortex | .38 | .35 | −.58 | −.57 | −.25 | −.04 |
| Orbital frontal | .38 | .39 | −.35 | −.36 | −.12 | .02 | |
| Prefrontal white matter | .38 | .39 | −.33 | −.34 | −.11 | .02 | |
| Hippocampus | .38 | .37 | −.30 | −.28 | −.15 | −.04 | |
| Caudate | .38 | .38 | −.29 | −.30 | −.10 | .01 | |
| Fusiform gyrus | .38 | .33 | −.41 | −.37 | −.25 | −.11 | |
| Visual cortex | .38 | .37 | −.12 | −.10 | −.10 | −.06 | |
| Priming Repeated | Lateral Prefrontal cortex | .50 | .48 | −.58 | −.57 | −.31 | −.03 |
| Orbital frontal | .50 | .52 | −.35 | −.39 | −.12 | .07 | |
| Prefrontal white matter | .50 | .52 | −.33 | −.37 | −.11 | .07 | |
| Hippocampus | .50 | .50 | −.30 | −.29 | −.16 | −.01 | |
| Caudate | .50 | .49 | −.29 | −.27 | −.18 | −.04 | |
| Fusiform gyrus | .50 | .48 | −.41 | −.38 | −.25 | −.06 | |
| Visual cortex | .50 | .48 | −.12 | −.03 | −.19 | −.15 | |
| Priming Novel | Lateral Prefrontal cortex | .47 | .46 | −.58 | −.57 | −.29 | −.02 |
| Orbital frontal | .47 | .49 | −.35 | −.38 | −.12 | .05 | |
| Prefrontal white matter | .47 | .48 | −.33 | −.34 | −.14 | .02 | |
| Hippocampus | .47 | .46 | −.30 | −.28 | −.18 | −.05 | |
| Caudate | .47 | .49 | −.29 | −.32 | −.09 | .06 | |
| Fusiform gyrus | .47 | .44 | −.41 | −.37 | −.26 | −.08 | |
| Visual cortex | .47 | .46 | −.12 | −.06 | −.16 | −.12 | |
| Schretlen et al. (2000), N = 112, Ages 20 to 92 | |||||||
| Fluid Intelligence | Frontal volume | −.52 | −.45 | −.28 | −.12 | .37 | .27 |
| Tisserand et al. (2000), N = 61, Ages 21 to 81 | |||||||
| Word Recall Immediate | Total brain volume | −.33 | −.07 | −.78 | −.73 | .39 | .22 |
| Third ventricle | −.33 | −.27 | .61 | .59 | −.26 | −.08 | |
| Hippocampus | −.33 | −.37 | −.32 | −.36 | .00 | −.12 | |
| Parahippocampal gyrus | −.33 | −.28 | −.42 | −.38 | .24 | .12 | |
| Word Recall Delayed | Total brain volume | −.28 | −.06 | −.78 | −.75 | .33 | .19 |
| Third ventricle | −.28 | −.19 | .61 | .58 | −.26 | −.12 | |
| Hippocampus | −.28 | −.31 | −.32 | −.35 | .00 | −.10 | |
| Parahippocampal gyrus | −.28 | −.24 | −.42 | −.40 | .20 | .10 | |
| Stroop Control Time | Total brain volume | .41 | .27 | −.78 | −.75 | −.39 | −.12 |
| Third ventricle | .41 | .33 | .61 | .57 | .33 | .11 | |
| Hippocampus | .41 | .33 | −.32 | −.21 | −.36 | −.27 | |
| Parahippocampal gyrus | .41 | .31 | −.42 | −.32 | −.37 | −.24 | |
| Stroop Conflict Time | Total brain volume | .58 | .49 | −.78 | −.74 | −.50 | −.09 |
| Third ventricle | .58 | .49 | .61 | .53 | 45 | .15 | |
| Hippocampus | .58 | .58 | −.32 | −.31 | −.20 | −.02 | |
| Parahippocampal gyrus | .58 | .58 | −.42 | −.42 | −.24 | .01 | |
| Memory Scanning 1 item | Total brain volume | .81 | .69 | −.78 | −.64 | −.69 | −.16 |
| Third ventricle | .81 | .75 | .61 | .46 | .56 | .14 | |
| Hippocampus | .81 | .77 | −.32 | −.06 | −.37 | −.20 | |
| Parahippocampal gyrus | .81 | .75 | −.42 | −.14 | −.46 | −.23 | |
| Memory Scanning 3 items | Total brain volume | .53 | .24 | −.78 | −.67 | −.56 | −.28 |
| Third ventricle | .53 | .49 | .61 | .58 | .36 | .06 | |
| Hippocampus | .53 | .50 | −.32 | −.25 | −.26 | −.11 | |
| Parahippocampal gyrus | .53 | .46 | −.42 | −.32 | −.36 | −.18 | |
| Cook et al. (2002), N = 43, Ages 60 to 83 | |||||||
| Trail Making B | Global atrophy | .57 | .42 | .47 | .27 | .51 | .33 |
| Shipley Abstraction | Global atrophy | −.57 | −.54 | .47 | .43 | −.32 | −.07 |
| Walhovd et al. (2004), N = 54, Ages 20 to 88 | |||||||
| Memory - 5 minutes recall | Cortical volume | −.63 | −.62 | −.85 | −.85 | .54 | .01 |
| White matter volume | −.63 | −.62 | −.52 | −.51 | .34 | .02 | |
| Hippocampus | −.63 | −.53 | −.50 | −.34 | .47 | .23 | |
| Memory – 30 minutes recall | Cortical volume | −.64 | −.65 | −.85 | −.85 | .54 | −.01 |
| White matter volume | −.64 | −.64 | −.52 | −.51 | .34 | .01 | |
| Hippocampus | −.64 | −.56 | −.50 | −.37 | .44 | .18 | |
| Memory – multi-week recall | Cortical volume | −.57 | −.37 | −.85 | −.80 | .55 | .15 |
| White matter volume | −.57 | −.52 | −.52 | −.46 | .37 | .11 | |
| Hippocampus | −.57 | −.38 | −.50 | −.26 | .57 | .40 | |
| Walhovd et al. (2005), N = 71, Ages 21 to 88 | |||||||
| Performance IQ | Cortical volume | −.74 | −.57 | −.65 | −.41 | .63 | .29 |
| Verbal IQ | Cortical volume | −.15 | −.06 | −.65 | −.64 | .18 | .11 |
| Walhovd & Fjell (2007), N = 71, Ages 20 to 88 | |||||||
| Performance IQ | Cortical gray matter | −.67 | −.52 | −.78 | −.69 | .60 | .17 |
| White matter | −.67 | −.62 | −.50 | −.41 | .41 | .12 | |
| Verbal IQ | Cortical gray matter | −.14 | −.06 | −.78 | −.77 | .15 | .07 |
| White matter | −.14 | −.13 | −.50 | −.50 | .08 | .01 | |
| Mean Reaction Time | Cortical gray matter | .15 | −.12 | −.78 | −.76 | −.25 | −.22 |
| White matter | .15 | .10 | −.50 | −.49 | −.15 | −.09 | |
| Brickman et al. (2006), N = 199, Ages 21 to 79 | |||||||
| Choice Reaction Time | L. frontal rel. white matter | .42 | .42 | −.18 | −.17 | −.09 | −.02 |
| R. frontal rel. white matter | .42 | .37 | −.35 | −.28 | −.28 | −.16 | |
| L. temporal rel. white matter | .42 | .43 | −.18 | −.21 | −.02 | .06 | |
| R. temporal rel. white matter | .42 | .43 | −.22 | −.23 | −.07 | .03 | |
| Digits Forward | L. frontal rel. white matter | −.20 | −.20 | −.18 | −.18 | .05 | .02 |
| R. frontal rel. white matter | −.20 | −.20 | −.35 | −.35 | .07 | .00 | |
| L. temporal rel. white matter | −.20 | −.20 | −.18 | −.18 | .04 | .00 | |
| R. temporal rel. white matter | −.20 | −.20 | −.22 | −.22 | .06 | .02 | |
| Digits Backward | L. frontal rel. white matter | −.33 | −.33 | −.18 | −.17 | .08 | .02 |
| R. frontal rel. white matter | −.33 | −.31 | −.35 | −.33 | .16 | .05 | |
| L. temporal rel. white matter | −.33 | −.34 | −.18 | −.18 | .00 | −.06 | |
| R. temporal rel. white matter | −.33 | −.32 | −.22 | −.20 | .12 | .05 | |
| List learning | L. frontal rel. white matter | −.48 | −.43 | −.18 | −.02 | .34 | .29 |
| R. frontal rel. white matter | −.48 | −.37 | −.35 | −.18 | .44 | .33 | |
| L. temporal rel. white matter | −.48 | −.47 | −.18 | −.14 | .15 | .07 | |
| R. temporal rel. white matter | −.48 | −.46 | −.22 | −.21 | .20 | .11 | |
| Verbal interference | L. frontal rel. white matter | −.62 | −.62 | −.18 | −.15 | .14 | .04 |
| R. frontal rel. white matter | −.62 | −.59 | −.35 | −.28 | .29 | .10 | |
| L. temporal rel. white matter | −.62 | −.62 | −.18 | −.17 | .12 | .01 | |
| R. temporal rel. white matter | −.62 | −.62 | −.22 | −.21 | .15 | .02 | |
| Fluency (FAS) | L. frontal rel. white matter | −.10 | −.10 | −.18 | −.18 | .02 | .00 |
| R. frontal rel. white matter | −.10 | −.05 | −.35 | −.34 | .17 | .15 | |
| L. temporal rel. white matter | −.10 | −.10 | −.18 | −.18 | .03 | .01 | |
| R. temporal rel. white matter | −.10 | −.07 | −.22 | −.21 | .17 | .15 | |
| Fluency (Animals) | L. frontal rel. white matter | −.51 | −.51 | −.18 | −.17 | .10 | .01 |
| R. frontal rel. white matter | −.51 | −.49 | −.35 | −.31 | .24 | .08 | |
| L. temporal rel. white matter | −.51 | −.49 | −.18 | −.12 | .18 | .10 | |
| R. temporal rel. white matter | −.51 | −.48 | −.22 | −.13 | .24 | .15 | |
| Attention Switching 1 | L. frontal rel. white matter | .48 | .48 | −.18 | −.19 | −.07 | .02 |
| R. frontal rel. white matter | .48 | .47 | −.35 | −.33 | −.20 | −.04 | |
| L. temporal rel. white matter | .48 | .51 | −.18 | −.27 | .05 | .16 | |
| R. temporal rel. white matter | .48 | .47 | −.22 | −.19 | −.15 | .05 | |
| Attention Switching 2 | L. frontal rel. white matter | .57 | .59 | −.18 | −.28 | .02 | .15 |
| R. frontal rel. white matter | .57 | .55 | −.35 | −.31 | −.25 | −.07 | |
| L. temporal rel. white matter | .57 | .59 | −.18 | −.25 | −.02 | .10 | |
| R. temporal rel. white matter | .57 | .58 | −.22 | −.27 | −.07 | .07 | |
| Muller-Oehring et al. (2007), N = 37, Ages 26 to 79 | |||||||
| Precedence in Global Local RT | Corpus callosum genu | .32 | .21 | −.43 | −.36 | −.34 | −.24 |
| Interference in Global Local RT | Corpus callosum genu | .28 | .09 | −.43 | −.30 | −.48 | −.42 |
| Resp. Conflict in Global Local RT | Corpus callosum genu | .31 | .15 | −.43 | −.33 | −.43 | −.35 |
| Rabbitt et al. (2006), N = 62, Ages 69 to 85 | |||||||
| Culture Fair IQ | CSF Volume | −.45 | −.42 | NA | NA | −.28 | −.05 |
| AH4-1 IQ | CSF Volume | −.46 | −.35 | NA | NA | −.40 | −.22 |
| AH4-2 IQ | CSF Volume | −.48 | −.45 | NA | NA | −.30 | −.06 |
| Memory for People | CSF Volume | −.39 | −.30 | NA | NA | −.33 | −.18 |
| Memory for Objects in a Circle | CSF Volume | −.61 | −.46 | NA | NA | −.37 | −.12 |
| Visual Search | CSF Volume | −.37 | −.25 | NA | NA | −.35 | −.23 |
| Letter-Digit Substitution | CSF Volume | −.42 | −.23 | NA | NA | −.46 | −.35 |
| Category fluency | CSF Volume | −.35 | −.34 | NA | NA | −.42 | −.34 |
| Brixton | CSF Volume | .40 | .28 | NA | NA | .37 | .23 |
| Rey-Osterrieth Figure | CSF Volume | −.52 | −.44 | NA | NA | −.39 | −.16 |
| Charlton et al. (2010), N = 104, Ages 50 to 90 | |||||||
| Immediate memory | Hippocampus | −.57 | −.56 | −.19 | −.15 | .16 | .06 |
| Whole brain volume | −.57 | −.65 | −.53 | −.63 | .19 | −.16 | |
| Chee et al. (2009), N = 248, Ages 55 to 86 | |||||||
| Attention | Right white matter | −.21 | −.17 | −.41 | −.39 | .17 | .09 |
| Speed | Right white matter | −.41 | −.36 | −.41 | −.36 | .27 | .12 |
| Verbal Memory | Right white matter | −.22 | −.15 | −.41 | −.38 | .23 | .16 |
| Visual Memory | Right white matter | −.22 | −.17 | −.41 | −.39 | .19 | .11 |
| Executive functions | Right white matter | −.30 | −.22 | −.41 | −.36 | .29 | .19 |
| Language | Right white matter | −.22 | −.19 | −.41 | −.40 | .15 | .07 |
| Chen et al. (2010), N = 147, Ages 55 to 83 | |||||||
| Memory – RAVLT Immediate | L Hippocampus | −.15 | −.15 | −.31 | −.31 | .06 | .01 |
| R Hippocampus | −.15 | −.11 | −.36 | −.34 | .16 | .12 | |
| Memory – RAVLT Delayed | L Hippocampus | −.05 | −.07 | −.31 | −.31 | −.05 | −.07 |
| R Hippocampus | −.05 | −.05 | −.36 | −.36 | .03 | .01 | |
| Memory – Visual Repr. Immed. | L Hippocampus | −.32 | −.30 | −.31 | −.29 | .15 | .06 |
| R Hippocampus | −.32 | −.29 | −.36 | −.33 | .19 | .09 | |
| Memory – Visual Repr. Delayed | L Hippocampus | −.21 | −.19 | −.31 | −.30 | .13 | .07 |
| R Hippocampus | −.21 | −.20 | −.36 | −.36 | .10 | .03 | |
| Mazes Efficiency | L Hippocampus | −.26 | −.24 | −.31 | −.29 | .14 | .07 |
| R Hippocampus | −.26 | −.23 | −.36 | −.34 | .17 | .09 | |
| Mazes Errors | L Hippocampus | .23 | .27 | −.31 | −.34 | .03 | .11 |
| R Hippocampus | .23 | .29 | −.36 | −.39 | .05 | .15 | |
| Simple Reaction Time | L Hippocampus | .27 | .29 | −.31 | −.32 | −.04 | .05 |
| R Hippocampus | .27 | .31 | −.36 | −.39 | −.01 | .10 | |
Note: All of the B–C combinations within a given study presumably involved the same research participants. It is likely that there was at least some overlap in the samples reported in different articles by the same research team. However, the degree of overlap was not always stated, and because results were not reported separately for the new and old participants, the data were not suitable for meta-analyses.
Entries in bold represent combinations that were most consistent with the mediation model in that the reduction in A–C after control of B was larger than both the reduction of A–B after control of C and the reduction of B–C after control of A.
NA indicates that the estimates could not be computed because the relevant correlations were not available.
Perhaps the most noteworthy characteristic of the results in Table 1 is that many of the results are more consistent with alternative models than with the meditation model that was the focus in the majority of the studies. For example, Raz et al. (1998) stated that “shrinkage of the prefrontal cortex mediates age-related increases in perseveration (p. 95)”. However, the pattern in Table 1 indicates that the A–C reduction implied by Model 1 was .13, whereas the B–C reduction implied by Model 3 was .17, which is more consistent with a model postulating that the brain and cognitive variables are independently related to age. A similar pattern was evident in Head et al. (2002) as the A–C reduction of .15 was smaller than the B–C reduction of .22.
Head et al. (2002) also concluded that volume of the lateral pre-frontal cortex (PFC) mediates the relation between age and verbal working memory. Table 1 indicates that the reductions in the relevant relations were .20 for A–C, .06 for A–B, and .09 for B–C, which is consistent with the B-mediation interpretation. However, these results are apparently not very robust because the pattern was not replicated in similar comparisons reported in Raz et al. (1998) and Raz et al. (2000).
Brickman et al. (2006) claimed that age-related decline in neuropsychological functioning is in part mediated by an age-related reduction in relative frontal white matter. However, the entries in Table 1 indicate that the combination of left frontal white matter (B) and the switch 2 cognitive variable (C) had a larger, rather than smaller, age relation after control of B, which is inconsistent with mediation. Furthermore, when the list learning variable served as C and right frontal white matter as B, the A–C reduction of .09 was smaller than both the A–B (.17) and B–C (.11) reductions, and thus the results are more consistent with either reverse mediation or independence than with B mediation.
Schretlen et al. (2000) also suggested that their results were consistent with mediation of the relation of age on fluid intelligence through frontal lobe volume, but the results are actually more consistent with reverse mediation as the A–C reduction was .07 compared to .16 for the AB reduction, and .10 for the B–C reduction. Finally, Rabbitt et al. (2006) claimed that brain shrinkage accounted for large proportions of the age-related variance in a variety of cognitive tests, but the results in Table 1 indicate that with many variables the results were more compatible with the independent influences model. (The article did not report A–B correlations, and thus it was not possible to examine reverse mediation in this study.) Another study by Rabbitt and colleagues (Rabbitt, Mogapi et al., 2007) reported mediation analyses with brain volume as one indicator of a “neuro” factor, with measures of cerebral blood flow and white matter lesions as the other indicators, but correlations were not reported to allow the plausibility of alternative models to be determined.
Mediation analyses have also been reported with extreme groups of young and old adults in a number of studies. For example, Head et al. (2008, 2009) summarized their results with path analysis models in which mediation was implied. However, none of these studies considered alternative models of the relations among the variables, and therefore the results are not easily interpreted.
The overall impression from the results in Table 1 is that there does not appear to be much support for the hypothesis that age-related reductions in brain volume mediate the age differences in various cognitive variables. This interpretation is reinforced by a more detailed examination of the 12 combinations (out of the 254 entries in Table 1) in which the results were consistent with the mediation model. The results with corpus callosum volume as the B variable and measures derived from reaction time tasks as the C variable are plausible because of the importance of the corpus callosum for rapid interregional communication. However, two of the combinations involved cerebellum volume as the B variable, and although those with the pursuit rotor task might be expected because of the motor requirement in the task, those with nonverbal working memory are not easily interpreted. Several B–C combinations involved total brain volume, or CSF volume, as the B variable, but there was little coherence among the C variables as they included measures of word memory, memory scanning, and verbal ability. A few combinations consistent with the mediation model involved measures of prefrontal volume as B, and measures of working memory or perseverations as C. However, as noted above, the Head et al. (2002) pattern with perseverations was not replicated in two other studies. The other B–C combinations with prefrontal measures as B were also not replicated as the pattern reported by Head et al. (2002) with a measure of non-verbal working memory as C was not found in studies by Kennedy, Rodrigue et al. (2009) or Raz et al. (2000), and the pattern reported by Gunning-Dixon and Raz (2003) with verbal working memory as C was not replicated in Brickman et al. (2006), Head et al. (2002), Kennedy, Rodrigue et al. (2009), or Raz et al. (2000).
Correlated Change (ΔB–ΔC)
Relatively few studies have been reported with longitudinal B and C data, and the correlated change results have been inconsistent. Significant correlations between the two types of change have been reported in at least four studies. For example, Kramer et al. (2007) found significant relations between changes in hippocampal volume and changes in episodic memory, and between changes in cortical gray matter and changes in executive functioning. Sullivan et al. (2002) also reported a correlation between decline in corpus callosum size and change in Stroop word reading time, and Schmidt et al. (2005) found that longitudinal change in normalized brain volume was correlated with longitudinal changes in memory and attention/speed. Furthermore, Murphy et al. (2010) recently found that shrinkage of the MTL regions over a 6-month period was associated with decline in various measures of memory over a 2-year period.
However, other studies did not find significant correlations, either between hippocampal volume and memory or other cognitive variables (e.g., Cohen et al., 2001; Du et al., 2006; Ylikoski et al., 2000), or between whole brain volume and working memory (Charlton et al., 2010). Although longitudinal data were available with both brain volume and cognitive functioning variables in a project by Raz et al. (2008), no correlations between brain changes and cognitive changes were reported because the individual differences in the change in the cognitive measures were not significantly different from zero.
Partial correlated change (B–ΔC)
Several studies have reported that smaller baseline hippocampus volume was associated with greater subsequent (e.g., Cardenas et al., 2009; Golomb et al., 1996; Tupler et al., 2007; Woodard et al., 2010), or prior, memory decline (Borghesani et al., in press; Persson et al., 2006). In addition, Prins et al. (2005) found that greater generalized brain shrinkage at baseline was associated with steeper subsequent decline in a composite measure of cognitive functioning, and Swan et al. (1998) and Tisserand et al. (2004) found relations between prior cognitive decline and brain volume or gray matter density at the second occasion. Finally, at least three studies found ΔB–C correlations between the magnitude of longitudinal change in the hippocampus or entorhinal cortex regions and memory or fluid cognition (e.g., Cohen et al., 2006; Raz et al., 2008; Rodrigue & Raz, 2004).
Moderation
It is often claimed that relations between regional volumes and cognitive functioning are stronger at older ages (e.g., Gunning-Dixon et al., 2009; Hedden & Gabrieli, 2004; Raz & Rodrigue, 2006; Van Petten, 2004). Indeed, there does appear to be a shift with age in the nature of the relation between medial temporal lobe volume or hippocampal volume and memory functioning. That is, at least two studies with young adults have found a negative relation, with larger volumes associated with poorer memory (i.e., Chantome et al., 1999; Foster et al., 1999; but see Rajah et al., 2010, for a contradictory finding), a large study with middle-aged adults found little relation (i.e., Cherbuin et al., 2009), but a positive correlation between memory and hippocampus volume has been reported in several studies with older adults (e.g., Adamson et al., 2010; Cardenas et al., 2009; Carey et al., 2008; Dickerson et al., 2004; Golomb et al., 1996; Hackert et al., 2002; Lye et al., 2006; Mungas et al., 2001, 2005; O’Brien et al., 1997; Yonelinas et al., 2007; Ystad et al., 2009). Raz et al. (1998) also noted that there was no correlation between memory and hippocampal volume among their total sample of adults from 18 to 77, but that the correlation was positive for the subset of adults between 60 and 77 years of age. The age moderation in this case may reflect qualitative differences in the B–C relations, as some of the positive relations at older ages may be a reflection of prodomal dementia status, whereas negative relations at young ages may be related to inefficient pruning of redundant neurons during development.
Moderation results with other B–C combinations have been less consistent. Zimmerman et al. (2006) found a stronger positive relation between lateral frontal volume and executive function performance at older ages than at younger ages. In contrast, an opposite pattern was reported by Kennedy and Raz (2009), in which several interactions were in the direction of stronger brain-cognition relations at younger ages. An earlier study by these same researchers (Kennedy & Raz, 2005) did not report direct interaction tests, but did mention that separate analyses in each age group revealed a PFC - mirror tracing relation only in older adults, and not in young and middle aged adults. Finally, Moffat et al (2007) reported an interaction of age and hippocampal volume on a spatial navigation task, with a significant relation only among the younger participants.
Only a few of the longitudinal studies have involved adults under about 60 years of age, and none of them reported analyses of the relation between age and the magnitude of the correlations between brain changes and cognitive changes.
Summary of Volume Relations
Many reports have been published of simple correlations among age, performance in cognitive and neuropsychological tests, and regional or total brain volume, and the robustness of these relations may have tempted researchers to infer the existence of causal relations among the variables. However, the analyses summarized in Table 1 revealed weak evidence for the mediation pattern, with no B–C combinations exhibiting the strongest pattern of complete reduction of the A–C relation after control of B, together with no reduction in the A–B relation after control of C, and no reduction of the B–C relation after control of A. Some results have been interpreted as support for mediation, but in many cases the results were at least as consistent with alternative models of the relations among the variables. Furthermore, in a few cases in which the pattern was most consistent with the mediation pattern, the results were not replicated in different samples. The patterns with correlated changes were also inconsistent, although there do appear to be replicable relations between B at one point in time, and prior or subsequent change in C.
Although it has sometimes been assumed that the volume-cognition relations are stronger at older ages, the evidence for this pattern was weak, and primarily limited to relations between MTL volume and memory. At the present time the available evidence does not permit strong conclusions about the role of global or regional brain shrinkage as a cause of age-related declines in cognitive functioning.
White matter hyperintensities
Age-related white matter degradation has been of considerable interest because it might affect communication efficiency across different brain regions (e.g., Bartsokis, 2004; O’Sullivan et al., 2001). That is, because myelinated white matter pathways are critical for connections across different regions of the brain, and because myelin is associated with faster conduction velocity, the presence of white matter abnormalities could have important consequences for cognition by reducing effective connectivity.
White matter hyperintensities (WMH) are abnormal clusters of white matter manifested as increased signal intensity in T2-weighted MR images, and are assumed to reflect white matter damage (cf. Gunning-Dixon et al., 2009; Mayda et al., 2009). It has sometimes been suggested that the relations with age and with measures of cognition differ according to the location of the WMH. However, the results are not very consistent as more pronounced relations with age and cognitive measures are sometimes found in deep white matter lesions (e.g., Delano-Wood et al., 2008), and sometimes in peri-ventricular regions (DeGroot et al., 2002; Van Den Heuvel et al., 2007). Furthermore, WMH lesions in the two regions are often highly correlated (e.g., DeCarli, Fletcher et al., 2005; Vannorsdall et al., 2009).
Age Relations (A–B)
Increased age has been found to be associated with higher ratings of white matter hyperintensities, or greater white matter hyperintensity volume, in both cross-sectional (e.g., DeCarli et al., 1995; 1999; Gunning-Dixon & Raz, 2003; Hentschel et al., 2007; Kochunov et al., 2008; Rovaris et al., 2003; Schaivone et al., 2009; Vannorsdall et al., 2009; Ylikoski et al., 1993), and longitudinal comparisons (e.g., Chen et al., 2006; Firbank et al., 2007; Kramer et al., 2007; Raz et al., 2007; Sachdev et al., 2007; Schmidt et al., 2005). Although some measures of white matter hyperintensities have skewed distributions, nearly linear relations with age have been found either after eliminating data from individuals with very high values (e.g., DeCarli et al., 1995), or after transforming the data to minimize skew (Gunning-Dixon & Raz, 2003).
Cognition Relations (B–C)
Reviews of relations between white matter hyperintensities and cognition have been published by Ferro and Madureira (2002), Gunning-Dixon and Raz (2000), Gunning-Dixon et al. (2009), and Mayda et al. (2009). The reviews all note that while there are some negative findings, there are also many reports of significant relations with cognition. In particular, more white matter damage has been associated with poorer performance on speed tasks (e.g., Aggarawal et al., 2010; Bunce et al., 2010; Burns et al., 2005; De Groot et al., 2000; Dufouil et al., 2003; Eckert et al., 2010; Longstreth et al., 1996; Nebes et al., 2006; Prins et al., 2005; Rabbitt et al., 2007; Saczynski et al., 2008; Soderlund et al., 2006; Ylikoski et al., 1993), and on a variety of executive functioning tasks (e.g., Aggarawal et al., 2010; Au et al., 2006; Bunce et al., 2010; Carey et al., 2008; Cook et al., 2002; Delano-Wood et al., 2008; Gunning-Dixon & Raz, 2003; Kramer et al., 2007; Oosterman et al., 2008; Paul et al., 2005; Prins et al., 2005; Raz et al., 2007; Tullberg et al., 2004). Relations have also been reported between white matter lesions and measures of other types of cognition (e.g., Au et al., 2006; Burns et al., 2005; DeCarli et al., 1995; de Groot et al., 2000; 2002; Kramer et al., 2007; Nordahl et al., 2006; O’Brien et al., 2002; Petkov et al., 2004; Prins et al., 2005; Schiavone et al., 2009; Van Petten et al., 2004; Vannorsdall et al., 2009; Wright et al., 2008).
Mediation (A–C.B)
Results from studies with relevant correlations based on the models in Figure 4 are contained in Table 2. It can be seen that the overall pattern was mixed, although several B–C combinations were consistent with the mediation model (Model 1). For example, Gunning-Dixon and Raz (2003) concluded that that frontal WMH mediates the relation between age and perseverations, and the results of the analyses in Table 2 are consistent with this interpretation. That is, the reduction in the A–C relation after control of B of .11 was greater than both the A–B (.07) and B–C (.08) reductions. However, it should be noted that there were no other reports with the same combination of B and C variables, and thus the replicability of this result cannot be determined at this time.
Table 2.
Analyses with White Matter Hyperintensities (WMH) as the Brain Structure Variable
| C | B | A–C | A–C.B | A–B | A–B.C | B–C | B–C.A |
|---|---|---|---|---|---|---|---|
| Cook et al. (2002), N = 43, Ages 60 to 93 | |||||||
| Trail Making B | Peri-ventricular WMH | .57 | .53 | .47 | .42 | .33 | .09 |
| Deep WMH | .57 | .53 | .22 | .07 | .30 | .22 | |
| Shipley Abstraction | Peri-ventricular WMH | −.57 | −.53 | .47 | .42 | −.33 | −.09 |
| Deep WMH | −.57 | −.55 | .22 | .15 | −.21 | −.11 | |
| Gunning-Dixon & Raz (2003), N = 139, Ages 50 to 81 | |||||||
| Working Memory (Verbal) | Frontal WMH | −.27 | −.22 | .40 | .37 | −.22 | −.13 |
| Temporal WMH | −.27 | −.23 | .48 | .46 | −.19 | −.07 | |
| WCST (Perseverations) | Frontal WMH | .27 | .16 | .40 | .33 | .34 | .26 |
| Temporal WMH | .27 | .25 | .48 | .47 | .17 | .05 | |
| Gootjes et al. (2007), N = 36, Ages 50 to 81 | |||||||
| Dichotic listening | Deep WMH | −.59 | −.57 | .67 | .66 | −.41 | −.03 |
| Charlton et al. (2010), N = 104, Ages 50 to 93 | |||||||
| Immediate memory | WMH | −.57 | −.55 | .48 | .46 | −.30 | −.04 |
| Rabbitt et al. (2007), N = 65, Ages 64 to 85 | |||||||
| Culture Fair IQ | WMH | −.41 | −.40 | .38 | .37 | −.18 | −.03 |
| AH4-1 IQ | WMH | −.41 | −.36 | .38 | .32 | −.27 | −.14 |
| AH4-2 IQ | WMH | −.48 | −.47 | .38 | .37 | −.20 | −.02 |
| WAIS Vocabulary | WMH | −.21 | −.12 | .38 | .34 | −.28 | −.22 |
| Mill Hill A Vocabulary | WMH | −.16 | −.13 | .38 | .37 | −.13 | −.08 |
| Mill Hill B Vocabulary | WMH | −.16 | −.09 | .38 | .36 | −.21 | −.16 |
| Forward Digit Span | WMH | −.21 | −.15 | .38 | .35 | −.21 | −.14 |
| Backward Digit Span | WMH | −.19 | −.07 | .38 | .33 | −.34 | −.30 |
| Word Span | WMH | −.17 | −.09 | .38 | .35 | −.24 | −.19 |
| Memory for People | WMH | −.29 | −.20 | .38 | .32 | −.31 | −.23 |
| Memory for Objects in a Circle | WMH | −.52 | −.46 | .38 | .28 | −.34 | −.18 |
| Visual Search | WMH | −.31 | −.19 | .38 | .29 | −.38 | −.30 |
| Letter-Digit Substitution | WMH | −.33 | −.19 | .38 | .26 | −.44 | −.36 |
| Phonemic fluency | WMH | −.22 | −.16 | .38 | .35 | −.22 | −.15 |
| Category fluency | WMH | −.35 | −.24 | .38 | .28 | −.38 | −.29 |
| Brixton | WMH | .37 | .34 | .38 | .35 | .22 | .09 |
| Rey-Osterrieth Figure | WMH | −.56 | −.51 | .38 | .28 | −.33 | −.15 |
Note: All of the B–C combinations within a given study presumably involved the same research participants. It is likely that there was at least some overlap in the samples reported in different articles by the same research team. However, the degree of overlap was not always stated, and because results were not reported separately for the new and old participants, the data were not suitable for meta-analyses.
Entries in bold represent combinations that were most consistent with the mediation model in that the reduction in A–C after control of B was larger than both the reduction of A–B after control of C and the reduction of B–C after control of A.
NA indicates that the estimates could not be computed because the relevant correlations were not available.
Although Rabbitt, Scott et al. (2007) titled their article “White matter lesions account for all age-related declines in speed but not in intelligence,” the results in Table 2 indicate that many different types of cognitive tests had similar relations, including speed, fluency, memory span, and vocabulary. These findings suggest that WMH influences are apparently not limited to particular types of cognitive tests.
Other B–C combinations have also been examined, but have yielded mixed results, and were often more consistent with independent age relations than with B mediation. This was also true in an earlier study with only incomplete correlation information as Ylikoski et al. (1993) found reduction of the B–C relation after control of age, with decreases from −.30 to −.07 for Block Design, .41 to .23 for Trail Making A, and .42 to .27 for Stroop Word Reading Time.
Correlated Change (ΔB–ΔC)
Several studies have reported that increases in WMH were correlated with decreases in cognition. However, the results are apparently not very specific to a particular type of cognition because correlations have been reported for quite different types of cognitive variables. For example, correlations with WMH changes have been reported with measures of executive functioning (e.g., Kramer et al., 2007; Vannorsdall et al., 2009), speed (Longstreth et al., 2005; Schmidt et al., 2005), memory (e.g., Schmidt et al., 2005; Vannorsdall et al., 2009), fluid intelligence (Raz et al., 2007), and crystallized (verbal) intelligence (e.g., Garde et al., 2005; Vannorsdall et al., 2009). Only one study could be found with a negative finding of no significant change correlation, in this case between change in WMH and change in working memory (Charlton et al., 2010).
Partial correlated change (B–ΔC)
At least five studies have reported correlations between WMH at one point in time and cognitive change. For example, Garde et al. (2000) reported a correlation with WAIS IQ, Swan et al. (1998) reported correlations with digit symbol, verbal fluency, and Benton Visual Retention, Prins et al. (2005) reported correlations with speed and executive functioning, and Van Den Heuvel et al. (2007) reported a correlation with measures of speed. In a sample of adults between 55 and 90 years of age with cognitive complaints at baseline, Jacobs et al. (in press) found that greater WMH at baseline was associated with more negative change in Stroop and Trail Making performance. However, very few significant correlations between change in white matter hyperintensities and cognition at a second assessment were found by Cook et al. (2004).
Moderation
A meta-analysis of WMH-cognition relations by Gunning-Dixon and Raz (2000) found little or no differences in the relations as a function of age. However, two studies have reported stronger WMH-cognition relations over age 60 (Vannorsdall et al., 2009), or age 65 (Au et al., 2006). No reports could be found in which relations of age were examined on ΔB–ΔC correlations, or B–ΔC correlations.
Summary of WMH Relations
As with measures of brain volume as the B variable, many simple correlations between A–B and B–C have been reported in which WMH was the B variable. Only a limited number of mediation analyses have been reported, and few of them have yielded results most consistent with the mediation pattern. Several studies have reported correlated changes in B and C, or correlations of B at one point in time with change in C. Because the cognitive variables involved in these relations have been quite diverse, relations with WMH may be relatively general. Finally, very few moderation studies have been reported, and the results have been inconsistent. The overall pattern of results from the different types of evidence could be viewed as consistent with the possibility that age-related increases in white matter lesions are contributing to at least some of the age differences and changes in cognitive functioning, but this conclusion should be considered tentative at the current time.
White matter integrity with DTI
Measures derived from diffusion tensor imaging (DTI), such as fractional anisotropy (FA) and mean diffusivity (MD), are potentially more sensitive indicators of white matter integrity than measures based on white matter lesions because they may reflect micro-structural differences that occur prior to actual lesion formation. Several DTI measures are available, and all reflect aspects of water diffusion. Water molecules normally spread (diffuse) in a direction parallel to the axon and myelin sheath, and thus the flow is generally anisotropic. However, with degradation in the fibers, and in the boundaries that constrain diffusion of water molecules, the diffusion becomes more heterogeneous (mean diffusivity increases), and the degree of anisotropy decreases (becomes more isotropic). DTI measures are affected by the organization of the fiber tracts, but within a tract higher values of mean diffusivity (MD) have been interpreted as indicating lower white matter integrity, whereas higher values of fractional anisotropy (FA), which is the fraction of total diffusion that is anisotropic, have been interpreted as reflecting higher white matter integrity. Axial diffusivity is postulated to be more related to axonal damage or changes in intracellular space, whereas radial diffusivity is postulated to be more related to myelin or glial cell damage, and it is noteworthy that several studies found age effects, and strong relations with cognition, on radial diffusivity (e.g., Burzynska et al., 2010; Davis et al., 2009; Georgio et al., 2010; Hasan et al., 2009; Madden, Spaniol et al., 2009; Michielse et al., 2010; Sullivan et al., 2006; Sullivan & Pfefferbaum, 2006; Westlye et al., 2010).
Age Relations (A–B)
Several studies have reported nearly linear negative cross-sectional age relations in white matter integrity assessed with DTI (e.g., Abe et al., 2008; Ardekani et al., 2007; Bendlin et al., 2010; Carlesimo et al., 2010; Charlton et al., 2006; 2008; Georgio et al., 2010; Grieve et al., 2007; Hasan et al., 2007; 2009; Hsu et al., 2008; Hugenschmidt et al., 2008; Kennedy & Raz, 2009; Kochunov et al., 2008; Lebel et al., 2010; McLaughlin et al., 2007; Michielse et al., 2010; Perry et al., 2009; Pfefferbaum et al., 2005; Pfefferbaum & Sullivan, 2003; Rovaris et al., 2003; Salat, Tuch, Greve et al., 2005; Schiavone et al., 2009; Stadlbauer et al., 2008; Sullivan et al., 2010; Voineskos et al., in press; Westlye et al., 2010; Yoon et al., 2008; Zahr et al., 2008; see reviews in Madden, Bennett et al., 2009, and Sullivan & Pfefferbaum, 2006). A number of studies have reported that the age effects are primarily, or largest, in frontal regions (e.g., Bennett et al., 2009; Burzynska et al., 2010; Damoiseaux et al., 2009; Davis et al., 2009; Gold et al., 2010; Head et al., 2004; Lebel et al., 2010; Lehmbeck et al., 2006; Madden et al., 2004; Madden, Spaniol et al., 2009; Michielse et al., 2010; O’Sullivan et al., 2001; Pfefferbaum et al., 2005; Salat, Tuch, Greve et al., 2005; Salat, Tuch, Hevelone et al., 2005; Schiavone et al., 2009; Sullivan et al., 2006; Vernooij et al., 2008; Voineskos et al., in press), although Kennedy and Raz (2009) reported nearly identical correlations between age and FA in the frontal lobe (−.60) and in the occipital lobe (−.59), Westlye et al. (2010) also found similar age correlations in different brain regions, and Salat et al. (2005) reported negative age relations in posterior peri-ventricular regions. Longitudinal decline over two years in white matter integrity has been reported with the MD measure (Charlton et al., 2010), and the FA measure (Barrick et al., 2010).
Cognition Relations (B–C)
As with other structural variables, some negative results regarding white matter integrity and cognition have been reported, but there are also many positive results (for recent reviews see Kennedy & Raz, 2009; and Madden, Bennett et al., 2009). For example, significant correlations of DTI variables have been reported with measures of general intelligence, executive functioning, working memory, speed, and various types of memory (e.g., Begre et al., 2009; Bendlin et al., 2010; Bohr et al., 2007; Bucor et al., 2008; Charlton et al., 2006, 2008, 2010; Chiang et al., 2009; Davis et al., 2009; Deary et al., 2006; Gold et al., 2007; 2010; Goldstein et al., 2009; Grieve et al., 2007; Haut et al., 2007; Madden et al., 2004; O’Sullivan et al., 2001; Sasson et al., 2010; Schiavone et al., 2009; Sullivan et al., 2006; 2010; Tuch et al., 2005; Turken et al., 2008; Vernooij et al., 2008; Yu et al., 2008; Zahr et al., 2008; Ziegler et al., 2010). Kennedy and Raz (2009) also noted some region-specific associations, as FA in anterior regions was more closely related to speed and working memory, FA in posterior regions with executive functions, and FA in the medial-temporal lobes with memory. However, somewhat different regional associations were reported in Davis et al. (2009), who found anterior FA related to executive functioning measures and posterior FA related to memory measures.
Mediation (A–C.B)
Table 3 contains results of comparisons of the models in Figure 4 from studies with all relevant correlations. It can be seen that only a few B–C combinations were available, and in none of them was the pattern consistent with that expected from Model 1. Charlton et al. (2008) claimed that MD was a mediator of age differences in working memory. However, the results in Table 3 indicate that although there was a reduction in A–C after control of B (i.e., .17), the reduction in B–C after control of A (i.e., .21) was actually larger, and therefore the results are at least as consistent with a model postulating independent relations of age with white matter integrity and cognition as with a model in which white matter integrity mediates age-cognition relations.
Table 3.
Analyses with DTI White Matter Integrity as the Brain Structure Variable
| C | B | A–C | A–C.B | A–B | A–B.C | B–C | B–C.A |
|---|---|---|---|---|---|---|---|
| Charlton et al. (2008), N = 118, Ages 50 to 90 | |||||||
| Working Memory | Mean diffusivity | −.35 | −.18 | .77 | .73 | −.36 | −.15 |
| Flexibility | Mean diffusivity | −.54 | −.60 | .77 | .79 | −.39 | .05 |
| Speed | Mean diffusivity | −.55 | −.53 | .77 | .77 | −.43 | −.02 |
| Fluid Intelligence | Mean diffusivity | −.42 | −.48 | .77 | .79 | −.29 | .05 |
| Charlton et al. (2009), N = 106, Ages 50 to 90 | |||||||
| Theory of Mind | Mean diffusivity | −.30 | −.29 | .78 | .78 | −.24 | −.01 |
| Charlton et al. (2010), N = 104, Ages 50 to 90 | |||||||
| Episodic memory | Mean diffusivity | −.57 | −.69 | .78 | .84 | −.38 | −.12 |
| Carlesimo et al. (2010), N = 76, Ages 20 to 80 | |||||||
| Word memory, delayed | L. Hippocampal diffusivity | −.42 | −.57 | .75 | .79 | −.23 | .14 |
| R. Hippocampal diffusivity | −.42 | −.45 | .70 | .71 | −.27 | .04 | |
| L. Hippocampal FA | −.42 | −.73 | −.60 | −.77 | −.08 | −.46 | |
| R. Hippocampal FA | −.42 | −.74 | −.67 | −.80 | .02 | −.39 | |
| Rey Visual Figure Memory | L. Hippocampal diffusivity | −.52 | −.91 | .75 | .91 | −.16 | .41 |
| R. Hippocampal diffusivity | −.52 | −.73 | .70 | .81 | −.21 | .25 | |
| L. Hippocampal FA | −.52 | −.63 | −.60 | −.69 | .19 | −.18 | |
| R. Hippocampal FA | −.52 | −.75 | −.67 | −.80 | .16 | −.30 | |
Note: All of the B–C combinations within a given study presumably involved the same research participants. It is likely that there was at least some overlap in the samples reported in different articles by the same research team. However, the degree of overlap was not always stated, and because results were not reported separately for the new and old participants, the data were not suitable for meta-analyses.
Entries in bold represent combinations that were most consistent with the mediation model in that the reduction in A–C after control of B was larger than both the reduction of A–B after control of C and the reduction of B–C after control of A.
NA indicates that the estimates could not be computed because the relevant correlations were not available.
A few studies have reported reduction of A–C after control of B. For example, Sullivan et al. (2001) found that age was no longer a significant predictor of finger tapping speed when FA from posterior regions was included in the analysis as a simultaneous predictor. In samples of only young and old adults, Gold et al. (2010), Madden, Spaniol et al. (2009) and Zahr et al. (2009) all found a reduction of the age-related variance in several cognitive measures when FA measures were controlled. However, the reported information in these studies was insufficient to determine whether the results were also consistent with alternative models of the relations among the variables. Voineskos et al. (in press) described a structural equation model implying FA mediation of age-related differences in several cognitive measures, but again there was insufficient information to determine whether the results were also consistent with alternative models.
Correlated Change (ΔB–ΔC)
Only one study was found in which correlations of longitudinal changes were reported in DTI measures. Charlton et al. (2010) found that change in working memory was correlated −.33 with change in mean diffusivity even after controlling the variation associated with age. However, no correlations were found with the FA measure often used in other DTI studies.
Partial correlated change (B–ΔC)
The one study that could be located reporting partial correlated change, Persson et al. (2006), found lower DTI-derived measures at a second time point for participants who had declined the most in memory.
Moderation
In a study involving young adults and older adults, Madden et al. (2004) reported a significant interaction in which young and old adults had different relations of FA to reaction time (RT). However, a similar study by Gold et al. (2010) did not find an interaction of age on the FA-RT relations.
Several studies did not report direct tests of the interactions, but did mention that the DTI-cognition relations were stronger at older ages (e.g., Bucor et al., 2008; Carlesimo et al., 2010; Davis et al., 2009; Vannorsdall et al., 2009). Direct interaction tests were conducted by Kennedy and Raz (2009), but surprisingly, most of the significant interactions were in the direction of stronger relations at younger ages (e.g., higher internal capsule FA – WM correlation at younger ages, higher temporal FA – delayed recall at young and middle ages).
Summary of DTI Relations
As with the other brain structure variables, when B is represented with DTI measures assumed to reflect white matter integrity, many simple correlations exist between A–B and B–C, but the results from mediation analyses were not very consistent. Only a single study was located reporting correlated change, and although there was a significant correlation with the MD measure, it was not significant with the more common FA measure. Results of moderation analyses have been inconsistent, with some reports of stronger relations at older ages, but other reports of stronger relations at younger ages, and very few direct tests of interactions involving age. Based on these results, the evidence for a causal role of DTI on age-cognition relations must be considered relatively weak at the current time.
Conclusions
The literature reviewed above reveals considerable evidence of A–C, A–B and B–C relations with B represented by global or regional volume, white matter hyperintensities, or DTI measures postulated to reflect white matter integrity, and with C represented by a variety of different cognitive variables. Statistical mediation analyses were reported in some studies, and a number of them found a reduction in the A–C relation after control of the variation in B. However, alternative models were seldom examined to rule out other interpretations, and the systematic analyses of the three models in Figure 4 revealed very few combinations in which the B-as-mediator model was more consistent with the results than alternative models. Correlated change has been examined in a very limited number of studies, and because most involved only older adults for whom both types of changes may have been occurring for many years, they may not be very informative about causal relations. Results of moderation analyses in which B–C relations are examined as a function of age have been mixed. Although there are several reasons to expect stronger B–C relations at older ages, this pattern has not always been found, and the results have occasionally been in the opposite direction. There have apparently been no studies in which the ΔB–ΔC relations were examined as a function of age.
In an extensive review of the relevant literature, Raz and Kennedy (2009) recently concluded that: “The search for the neuroanatomical basis of cognitive aging has so far yielded limited and somewhat contradictory results (p. 59).” A similar conclusion is implied by the results reviewed above as some relevant comparisons have very little data, and the available results have not been very consistent. In light of the inconclusive results, it is worth considering how stronger inferences might eventually be possible. The following paragraphs therefore describe suggestions for future research based on limitations of current studies with respect to samples, variables, and analytical methods.
Several suggestions concern relatively basic aspects of methodology that, if neglected, can weaken the contribution of the research. For example, the samples in many studies have been relatively small, which means that confidence intervals around the correlations are large, and the power to detect differences in simple correlations and partial correlations is weak. Power depends on the values of all relevant correlations, but if A–C = .5 and B–C = .5, then detection of an A–C.B correlation of .3, corresponding to difference of .2 correlation units from the A–C correlation, will have power of only .17 with a sample of 20, .36 with a sample of 50, .62 with a sample of 100, and reaches .80 only with a sample of 150 (Calculations based on G-Power 3.1, Faul, Erdfelder,Buchner & Lang, 2009). Because neuroimaging is expensive, it is understandable that many studies have had small samples, but the cost of low power also needs to be recognized (see Fjell & Walhovd, 2010, for a similar observation).
Another concern is that the samples in many studies had a narrow age range, which precludes tests of age-related moderation of the B–C relations. Furthermore, when the samples consist of only older adults interpretations of the B–C relations may also be limited by the fact that both sets of variables could have been declining for decades prior to the period of observation, and may no longer reflect causal relations. In other words, relations between two variables after an extended period of change could be quite different from those apparent when one or both variables are just beginning to change. A related point is that some studies only compared extreme groups of young and old adults instead of adults throughout the entire range of adulthood. The use of extreme groups is more efficient for the detection of age differences than a continuous sample, but this practice inflates estimates of the age relations because variance associated with middle-aged adults is omitted, and it precludes analyses of the age at which possible shifts in B–C relations occur.
Four other methodological limitations are not as widely recognized as those just mentioned. First, in many studies cognitive functioning was assessed with a single variable from one task, and therefore the variable can be assumed to reflect a mixture of task-specific influences, influences of the construct of primary interest, and influences of measurement error. Because individual variables seldom exclusively and exhaustively reflect a single theoretical construct, it can be difficult to determine how much of a relation is attributable to influences of the relevant constructs, and how much is attributable to task-specific influences. Furthermore, the presence of measurement error in individual variables means that they often have low reliability, which may attenuate relations of the variable with other variables.
Some researchers have attempted to deal with these problems by analyzing composite scores postulated to represent broader cognitive constructs. Aggregation of variables typically results in an increase in reliability, but composites can be formed from any combination of variables, and the constituent variables do not necessarily represent a single meaningful construct. An alternative approach to the problem is to investigate both convergent and discriminant validity with a measurement model based on factor analysis, and then use factor scores defined by the reliable shared variance among the variables in subsequent analyses. An advantage of a procedure such as this is that one can be confident that the variables represent a common construct that is distinct from other constructs. Unfortunately, this approach requires moderately large samples of individuals and a relatively large number of variables, which are not always feasible. Nevertheless, whenever variables are combined, it is important to be sensitive to the possibility that they may not all represent the same construct, and to a similar extent at every age.
A second limitation of prior studies is that different types of structural variables have been considered separately even though there is evidence that they are not independent. Anatomical regions are often considered separately for a given type of brain variable because of the well-documented regional specialization. However, moderate correlations have often been reported between the same type of measure in different regions (e.g., Alexander et al., 2006; Bergfield et al., 2010; Brickman et al., 2007; 2008; DeCarli et al., 2005; Gunning-Dixon & Raz, 2003), which raises the possibility that it may be informative to consider both general and specific relations of brain structure, as in Ecker et al. (2009) with cortical thickness measures, and Penke et al. (2010) with FA measures of white matter integrity.
Figure 5 portrays the contrast between the typical approach (top panel), involving a relation between a brain structure characteristic in a single anatomical region and a single cognitive variable, and an alternative multivariate approach (bottom panel), involving relations between higher-order constructs which represent many variables, as well as relations between specific brain regions and cognitive variables. Data from a project by Kennedy, Rodrigue et al. (2009) can be used to illustrate the two approaches. The simple correlation between lateral prefrontal volume and fluid intelligence in this study was .38, which is similar to values reported in other studies (e.g., Raz et al., 1993; Schretlen et al., 2000). However, volumes of six other regions were also analyzed in this study, and each was correlated with the other volume measures and with various cognitive measures. Furthermore, a re-analysis of the data based on the reported correlations revealed that a latent brain volume construct had significant loadings from each regional volume measure, and that a latent cognitive construct had significant loadings from the fluid intelligence, verbal working memory, and nonverbal working memory variables. Of greatest interest in the current context was that the correlation between the two latent constructs was .44, and that none of the relations between specific regions and cognitive variables (including the relation between lateral prefrontal volume and fluid intelligence) was significant after taking the relation between the two constructs into consideration. At least in the Kennedy, Rodrigue et al. (2009) project, therefore, it appears that all of the brain-cognition relations were operating at a relatively general level, with no evidence of specific relations after taking the general relation into consideration. Penke and Deary (2010) recently made a similar point regarding the desirability of considering higher-order factors based on re-analyses of data from a study by Charlton et al. (2008).
Figure 5.
Illustration of univariate (top panel) and multivariate (bottom panel) analyses of the relations among A, B, and C variables. Note that in the bottom panel both specific (dotted lines) and general (involving the circled B and C variables) relations are examined.
Because multiple-level analyses allow both specific and general relations to be examined, analytical methods based on models such as that portrayed in Figure 5 are likely to be more informative than the bivariate analyses that have dominated earlier studies. Some researchers adopt a narrow focus because they are primarily interested in a particular brain region, or in measures from a cognitive task designed to isolate critical cognitive processes. However, unless the brain-cognition relations are examined in a broader context it is impossible to determine if those relations are truly specific, or are merely another manifestation of a broader phenomenon.
Third, many of the measures of longitudinal changes are not very reliable, which limits the magnitude of correlations with other variables, and can lead to weak correlations of changes for statistical, rather than substantive, reasons. Longitudinal change is sometimes assessed with a simple difference or residual score, which tend to have low reliability because reliability of difference scores is inversely related to the (frequently high) correlation between the scores across the two occasions. A more desirable alternative approach is to use methods based on latent difference score or latent growth curve models which minimize measurement error when assessing change (cf. Ferrer & McArdle, 2010; Raz et al., 2005, 2008, 2010; Salthouse, 2010b).
And finally, as discussed earlier in this article, inferences about causal relations are often based on correlations, but simple correlations are very limited with respect to causal information. Moreover, when mediation results have been reported the importance of examining alternative models, and establishing that the results are replicable, has often been neglected. In addition, very few studies have involved longitudinal data to examine correlated changes, or have examined whether the brain-cognition relations vary as a function of age, and yet both types of information are relevant to the causal role of brain changes in cognitive changes. Because every methodological approach has limitations, inferences will generally be stronger when they are based on converging results from several different analytical methods rather than those from a single procedure.
Acknowledgments
This research was supported by NIA Grant R37AG024270. I would like to thank David Madden and Jason Steffener for their constructive comments on earlier drafts of this manuscript.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bul
References
- Abe O, Yamasue H, Aoki S, Suga M, Yamada H, Kasai K, Masutani Y, Kato N, Kato N, Ohtomo K. Aging in the CNS: Comparison of gray/white matter volume and diffusion tensor data. Neurobiology of Aging. 2008;29:102–116. doi: 10.1016/j.neurobiolaging.2006.09.003. http://dx.doi.org/10.1016/j.neurobiolaging.2006.09.003. [DOI] [PubMed]
- Adamson MH, Samarina V, Xiangyan X, Huynh V, Kennedy Q, Weiner M, Yesavage J, Taylor JL. The impact of brain size on pilot performance varies with aviation training and years of education. Journal of the International Neuropsychological Society. 2010;16:412–423. doi: 10.1017/S1355617710000111. http://dx.doi.org/10.1017/S1355617710000111. [DOI] [PMC free article] [PubMed]
- Adamson MH, Landy KM, Duong S, Fox-Bosetti S, Ashford JW, Murphy GM, Weinder M, Taylor JL. Apolioprotein E e4 influences on episodic recall and brain structures in aging pilots. Neurobiology of Aging. 2010;31:1059–1063. doi: 10.1016/j.neurobiolaging.2008.07.017. http://dx.doi.org/10.1016/j.neurobiolaging.2008.07.017. [DOI] [PMC free article] [PubMed]
- Aggarwal NT, Wilson RS, Bienias JL, DeJager PL, Bennett DA, Evans DA, DeCarli C. The association of magnetic resonance imaging measures with cognitive function in a biracial population sample. Archives of Neurology. 2010;67:475–482. doi: 10.1001/archneurol.2010.42. http://dx.doi.org/10.1001/archneurol.2010.42. [DOI] [PMC free article] [PubMed]
- Alder AG, Adam J, Arenberg D. Individual differences assessment of the relationship between change in and initial level of adult cognitive functioning. Psychology and Aging. 1990;5:560–568. doi: 10.1037//0882-7974.5.4.560. http://dx.doi.org/10.1037//0882-7974.5.4.560. [DOI] [PubMed]
- Alexander GE, Chen K, Merkley TL, Reiman EM, Caselli RJ, Aschenbrenner M, Santerre-Lemmon L, Lewis DJ, Pietrini P, Teipel SJ, Hampel H, Rapoport SI, Moeller JR. Regional network of magnetic resonance imaging gray matter volume in healthy aging. NeuroReport. 2006;17:951–956. doi: 10.1097/01.wnr.0000220135.16844.b6. http://dx.doi.org/10.1097/01.wnr.0000220135.16844.b6. [DOI] [PubMed]
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: The major lobes and a parcellation of the temporal region. Neurobiology of Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. http://dx.doi.org/10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed]
- Andreasen NC, Flaum M, Swayze V, O’Leary DS, Alliger R, Cohen G, Ehrhardt J, Yuh WTC. Intelligence and brain structure in normal individuals. American Journal of Psychiatry. 1993;150:130–134. doi: 10.1176/ajp.150.1.130. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. http://dx.doi.org/10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed]
- Ardekani S, Kumar A, Bartzokis G, Sinha U. Exploratory voxel-based analysis of diffusion indices and hemispheric asymmetry in normal aging. Magnetic Resonance Imaging. 2007;25:154–167. doi: 10.1016/j.mri.2006.09.045. http://dx.doi.org/10.1016/j.mri.2006.09.045. [DOI] [PubMed]
- Au R, Massaro JM, Wolf PA, Young ME, Beiser A, Seshadri S, D’Agostino RB, DeCarli C. Association of white matter hyperintensity volume with decreased cognitive functioning. Archives of Neurology. 2006;63:246–250. doi: 10.1001/archneur.63.2.246. http://dx.doi.org/10.1001/archneur.63.2.246. [DOI] [PubMed]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. http://dx.doi.org/10.1037//0022-3514.51.6.1173. [DOI] [PubMed]
- Barrick TR, Charlton RA, Clark CA, Markus HS. White matter structural decline in normal ageing: A prospective longitudinal study using tract-based spatial statistics. NeuroImage. 2010;51:565–577. doi: 10.1016/j.neuroimage.2010.02.033. http://dx.doi.org/10.1016/j.neuroimage.2010.02.033. [DOI] [PubMed]
- Bartzokis G. Age-related myelin breakdown: A developmental model of cognitive decline and Alzheimer’s disease. Neurobiology of Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. http://dx.doi.org/10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men. Archives of General Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. http://dx.doi.org/10.1001/archpsyc.58.5.461. [DOI] [PubMed]
- Begre S, Kiefer C, von Kanel R, Frommer A, Federspiel A. Rey visual design learning test performance correlates with white matter structure. Acta Neuropsychiatrica. 2009;21:67–74. doi: 10.1111/j.1601-5215.2009.00361.x. http://dx.doi.org/10.1111/j.1601-5215.2009.00361.x. [DOI] [PubMed]
- Bendlin BB, Fitzgerald ME, Ries ML, Xu G, Kastman EK, Thiel BW, Rowley HA, Lazar M, Alexander AL, Johnson SC. White matter in aging and cognition: A cross-sectional study of microstructure in adults aged eighteen to eighty-three. Developmental Neuropsychology. 2010;35:257–277. doi: 10.1080/87565641003696775. http://dx.doi.org/10.1080/87565641003696775. [DOI] [PMC free article] [PubMed]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH. Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Human Brain Mapping. 2009 doi: 10.1002/hbm.20872. (Advance Publication). http://dx.doi.org/10.1002/hbm.20872. [DOI] [PMC free article] [PubMed]
- Berlingeri M, Bottini G, Danelli L, Ferri F, Traficante D, Sacheli L, Colombo N, Sberna M, Sterzi R, Scialfa G, Paulesu E. With time on our side? Task-dependent compensatory processes in graceful aging. Experimental Brain Research. 2010;205:307–324. doi: 10.1007/s00221-010-2363-7. http://dx.doi.org/10.1007/s00221-010-2363-7. [DOI] [PubMed]
- Bigler ED, Johnson SC, Jackson C, Blatter DD. Aging, brain size, and IQ. Intelligence. 1995;21:109–119. http://dx.doi.org/10.1016/0160-2896(95)90041-1.
- Blatter DD, Bigler ED, Gale SD, Johnson SC, Anderson CV, Burnett BM, Parker N, Kurth S, Horn SD. Quantitative volumetric analysis of Brain MRI: Normative database spanning 5 decades of life. American Journal of Neuroradiology. 1995;16:241–251. [PMC free article] [PubMed] [Google Scholar]
- Bohr S, Gullmar D, Knab R, Reichenbach JR, Witte OW, Haueisen J. Fractional anisotropy correlates with auditory simple reaction time performance. Brain Research. 2007;1186:194–202. doi: 10.1016/j.brainres.2007.10.013. http://dx.doi.org/10.1016/j.brainres.2007.10.013. [DOI] [PubMed]
- Borghesani PR, Weaver KE, Aylward EH, Richards AL, Madhyastha TM, Kahn AR, Liang O, Ellenbogen RL, Beg MF, Schaie KW, Willis SL. Midlife memory improvement predicts preservation of hippocampal volume in old age. Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2010.09.026. in press. http://dx.doi.org/10.1016/j.neurobiolaging.2010.09.026. [DOI] [PMC free article] [PubMed]
- Boyke J, Driemeyer J, Gaser C, Buchel C, May A. Training-induced brain structure changes in the elderly. The Journal of Neuroscience. 2008;28:7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. http://dx.doi.org/10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed]
- Brickman AM, Habeck C, Zarahn E, Flynn J, Stern Y. Structural MRI covariance patterns associated with normal aging and neuropsychological functioning. Neurobiology of Aging. 2007;28:284–295. doi: 10.1016/j.neurobiolaging.2005.12.016. http://dx.doi.org/10.1016/j.neurobiolaging.2005.12.016. [DOI] [PubMed]
- Brickman AM, Habeck C, Ramos MA, Scarmeas N, Stern Y. A forward application of age associated gray and white matter networks. Human Brain Mapping. 2008;29:1139–1146. doi: 10.1002/hbm.20452. http://dx.doi.org/10.1002/hbm.20452. [DOI] [PMC free article] [PubMed]
- Brickman AM, Zimmerman ME, Paul RH, Grieve SM, Tate DF, Cohen RA, Williams LM, Clark CR, Gordon E. Regional white matter and neuropsychological functioning across the adult lifespan. Biological Psychiatry. 2006;60:444–453. doi: 10.1016/j.biopsych.2006.01.011. http://dx.doi.org/10.1016/j.biopsych.2006.01.011. [DOI] [PubMed]
- Brodtmann A, Puce A, Darby D, Donnan G. Regional fMRI brain activation does correlate with global brain volume. Brain Research. 2009;1259:17–25. doi: 10.1016/j.brainres.2008.12.044. http://dx.doi.org/10.1016/j.brainres.2008.12.044. [DOI] [PubMed]
- Brodtmann A, Puce A, Syngeniotis A, Darby D, Donnan G. The functional magnetic resonance imaging hemodynamic response to faces remains stable until the ninth decade. NeuroImage. 2003;20:520–528. doi: 10.1016/s1053-8119(03)00237-4. http://dx.doi.org/10.1016/S1053-8119(03)00237-4. [DOI] [PubMed]
- Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, Huettel SA. Age-related slowing of memory retrieval: Contributions of perceptual speed and cerebral white matter integrity. Neurobiology of Aging. 2008;29:1070–1079. doi: 10.1016/j.neurobiolaging.2007.02.008. http://dx.doi.org/10.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed]
- Bunce D, Anstey KJ, Cherbuin N, Burns R, Christensen H, Wen W, Sachdev PS. Cognitive deficits are associated with frontal and temporal lobe white matter lesions in middle-aged adults living in the community. PLoS ONE. 2010;5(10):e13567. doi: 10.1371/journal.pone.0013567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JM, Church JA, Johnson DK, Xiong C, Marcus D, Fotenos AF, Snyder AZ, Morris JC, Buckner RL. White matter lesions are prevalent but differentially related with cognition in aging and early Alzheimer disease. Archives of Neurology. 2005;62:1870–1876. doi: 10.1001/archneur.62.12.1870. http://dx.doi.org/10.1001/archneur.62.12.1870. [DOI] [PubMed]
- Burzynska AZ, Preuschhof C, Backman L, Nyberg L, Li S-C, Lindenberger U, Heekeren HR. Age-related differences in white matter microstructure: Region-specific patterns of diffusivity. NeuroImage. 2010;49:2104–2112. doi: 10.1016/j.neuroimage.2009.09.041. http://dx.doi.org/10.1016/j.neuroimage.2009.09.041. [DOI] [PubMed]
- Cardenas VA, Chao LL, Studholme C, Yaffe K, Miller BL, Madison C, Buckley ST, Mungas D, Schuff N, Weiner MW. Brain atrophy associated with baseline and longitudinal measures of cognition. Neurobiology of Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.04.011. (Advance Publication). http://dx.doi.org/10.1016/j.neurobiolaging.2009.04.011. [DOI] [PMC free article] [PubMed]
- Carey CL, Kramer JH, Josephson SA, Mungas D, Reed BR, Schuff N, Weiner MW, Chui HC. Subcortical lacunes are associated with executive dysfunction in cognitively normal elderly. Stroke. 2008;38:397–402. doi: 10.1161/STROKEAHA.107.491795. http://dx.doi.org/10.1161/STROKEAHA.107.491795. [DOI] [PMC free article] [PubMed]
- Carlesimo GA, Cherubini A, Caltagirone C, Spalletta G. Hippocampal mean diffusivity and memory in healthy elderly individuals: A cross-sectional study. Neurology. 2010;74:194–200. doi: 10.1212/WNL.0b013e3181cb3e39. http://dx.doi.org/10.1212/WNL.0b013e3181cb3e39. [DOI] [PubMed]
- Carne RP, Vogrin S, Litewka L, Cook MJ. Cerebral cortex: An MRI-based study of volume and variance with age and sex. Journal of Clinical Neuroscience. 2006;13:60–72. doi: 10.1016/j.jocn.2005.02.013. http://dx.doi.org/10.1016/j.jocn.2005.02.013. [DOI] [PubMed]
- Ceccarelli A, Rocca MA, Pagani E, Falini A, Comi G, Filippi M. Cognitive learning is associated with gray matter changes in healthy human individuals: A tensor-based morphometry study. NeuroImage. 2009;48:585–589. doi: 10.1016/j.neuroimage.2009.07.009. http://dx.doi.org/10.1016/j.neuroimage.2009.07.009. [DOI] [PubMed]
- Chantome M, Perruchet P, Hasboun D, Dermont D, Sahel M, Sourour N, Zouaoui A, Marsault C, Duyme M. Is there a negative correlation between explicit memory and hippocampal volume? NeuroImage. 1999;10:589–595. doi: 10.1006/nimg.1999.0486. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O’Sullivan M, Howe FA, Clark CA, Morris RG, Markus HS. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66:217–222. doi: 10.1212/01.wnl.0000194256.15247.83. http://dx.doi.org/10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed]
- Charlton RA, Landau S, Schiavone F, Barrick TR, Clark CA, Markus HS, Morris RG. A structural equation modeling investigation of age-related variance in executive function and DTI measured white matter damage. Neurobiology of Aging. 2008;29:1547–1555. doi: 10.1016/j.neurobiolaging.2007.03.017. http://dx.doi.org/10.1016/j.neurobiolaging.2007.03.017. [DOI] [PubMed]
- Charlton RA, Schiavone F, Barrick TR, Morris RG, Markus HS. Diffusion tensor imaging detects age-related white matter change over a 2 year follow-up which is associated with working memory decline. Journal of Neurology, Neurosurgery, and Psychiatry. 2010;81:13–19. doi: 10.1136/jnnp.2008.167288. http://dx.doi.org/10.1136/jnnp.2008.167288. [DOI] [PubMed]
- Chee MWL, Chen KHM, Zheng H, Chan KPL, Isaac V, Sim SKY, Chuah LYM, Schuchinsky M, Fischl B, Pin Ng T. Cognitive function and brain structure correlations in healthy elderly East Asians. NeuroImage. 2009;46:257–269. doi: 10.1016/j.neuroimage.2009.01.036. http://dx.doi.org/10.1016/S1053-8119(09)71904-4. [DOI] [PubMed]
- Chee MWL, Zheng H, Goh JOS, Park D. Brain structure in young and old East Asians and Westerners: Comparisons of structural volume and cortical thickness. Journal of Cognitive Neuroscience. 2010;X:XX–YY. doi: 10.1162/jocn.2010.21513. http://dx.doi.org/10.1162/jocn.2010.21513. [DOI] [PMC free article] [PubMed]
- Chen KHM, Chuah LYM, Sim SKY, Chee MWL. Hippocampal region-specific contributions to memory performance in normal elderly. Brain and Cognition. 2010;72:400–407. doi: 10.1016/j.bandc.2009.11.007. http://dx.doi.org/10.1016/j.bandc.2009.11.007. [DOI] [PubMed]
- Chen PS, Mcquoid DR, Payne ME, Steffens DC. White matter and subcortical gray matter lesion volume changes and late-life depression outcome: A 4-year magnetic resonance imaging study. International Psychogeriatrics. 2006;18:445–456. doi: 10.1017/S1041610205002796. http://dx.doi.org/10.1017/S1041610205002796. [DOI] [PubMed]
- Cherbuin N, Anstey KJ, Reglade-Meslin C, Sachdev PS. In vivo hippocampal measurement and memory: A comparison of manual tracing and automated segmentation in a large community-based sample. PLoS One. 2009;4:e5265. doi: 10.1371/journal.pone.0005265. http://dx.doi.org/10.1016/S1053-8119(09)70154-5. [DOI] [PMC free article] [PubMed]
- Chiang M-C, Barysheva M, Shattuck DW, Lee AD, Madsen SK, Avedissian C, Klunder AD, Toga AW, McMahon KL, de Zubicaray GI, Wright MJ, Srivastava A, Balov N, Thompson PM. Genetics of brain fiber architecture and intellectual performance. Journal of Neuroscience. 2009;29:2212–2224. doi: 10.1523/JNEUROSCI.4184-08.2009. http://dx.doi.org/10.1523/JNEUROSCI.4184-08.2009. [DOI] [PMC free article] [PubMed]
- Christensen H, Mackinnon AJ, Korten AE, Jorm AF, Henderson AS, Jacomb P, Ridgers B. An analysis of diversity in the cognitive performance of elderly community dwellers: Individual differences in change scores as a function of age. Psychology and Aging. 1999;14:365–379. doi: 10.1037//0882-7974.14.3.365. http://dx.doi.org/10.1037//0882-7974.14.3.365. [DOI] [PubMed]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. http://dx.doi.org/10.1037/0033-2909.112.1.155. [DOI] [PubMed]
- Cohen RM, Small C, Lalonde F, Friz J, Sunderland T. Effect of apolipoprotein E genotype on hippocampal volume loss in aging healthy women. Neurology. 2001;57:2223–2228. doi: 10.1212/wnl.57.12.2223. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Szczepanik J, McManus M, Mirza N, Putnam K, Levy J, Sunderland T. Hippocampal atrophy in the healthy is initially linear and independent of age. Neurobiology of Aging. 2006;27:1385–1394. doi: 10.1016/j.neurobiolaging.2005.07.018. http://dx.doi.org/10.1016/j.neurobiolaging.2005.07.018. [DOI] [PubMed]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P. The implications of cortical recruitment and brain morphology for individual differences in inhibitory function in aging humans. Psychology and Aging. 2005;20:363–375. doi: 10.1037/0882-7974.20.3.363. http://dx.doi.org/10.1037/0882-7974.20.3.363. [DOI] [PubMed]
- Collette F, van der Linden M, Laureys S, Arigoni F, Delfiore G, Degueldre C, Luxen A, Salmon E. Mapping the updating process: Common and specific brain activations across different versions of the running span task. Cortex. 2007;43:146–158. doi: 10.1016/s0010-9452(08)70452-0. http://dx.doi.org/10.1016/S0010-9452(08)70452-0. [DOI] [PubMed]
- Collins LM. Analysis of longitudinal data: The integration of theoretical model, temporal design, and statistical model. Annual Review of Psychology. 2006;57:505–528. doi: 10.1146/annurev.psych.57.102904.190146. http://dx.doi.org/10.1146/annurev.psych.57.102904.190146. [DOI] [PubMed]
- Colom R, Haier RJ, Head K, Alvarez-Linera J, Quiroga MA, Shih PC, Jung RE. Gray matter correlates of fluid, crystallized, and spatial intelligence: Testing the P-FIT model. Intelligence. 2009;37:124–135. http://dx.doi.org/10.1016/j.intell.2008.07.007.
- Colom R, Jung RE, Haier RJ. Distributed brain sites for the g-factor of intelligence. NeuroImage. 2006a;31:1359–1365. doi: 10.1016/j.neuroimage.2006.01.006. http://dx.doi.org/10.1016/j.neuroimage.2006.01.006. [DOI] [PubMed]
- Colom R, Jung RE, Haier RJ. Finding the g-factor in brain structure using the method of correlated vectors. Intelligence. 2006b;34:561–570. http://dx.doi.org/10.1016/j.intell.2006.03.006.
- Cook IA, Leuchter AF, Morgan ML, Conlee EW, David S, Lufkin R, Babai A, Dunkin JJ, O’Hara R, Simon S, Lightner A, Thomas S, Broumandi D, Badjatia N, Mickes L, Mody RK, Arora S, Zheng Z, Abrams M, Rosenberg-Thompson S. Cognitive and physiologic correlates of subclinical structural brain disease in elderly healthy control subjects. Archives of Neurology. 2002;59:1612–1620. doi: 10.1001/archneur.59.10.1612. http://dx.doi.org/10.1001/archneur.59.10.1612. [DOI] [PubMed]
- Cook IA, Leuchter AF, Morgan ML, Dunkin JJ, Witte E, David S, Mickes L, O’Hara R, Simon S, Lufkin R, Abrams M, Rosenberg S. Longitudinal progression of subclinical structural brain disease in normal aging. American Journal of Geriatric Psychiatry. 2004;12:190–200. http://dx.doi.org/10.1097/00019442-200403000-00010. [PubMed]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: Quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Smith SM, Witter MP, Sanz-Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Zarei M, Rombouts SARB. White matter tract integrity in aging and Alzheimer’s Disease. Human Brain Mapping. 2009;30:1051–1059. doi: 10.1002/hbm.20563. http://dx.doi.org/10.1002/hbm.20563. [DOI] [PMC free article] [PubMed]
- Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. NeuroImage. 2009;46:530–541. doi: 10.1016/j.neuroimage.2009.01.068. http://dx.doi.org/10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed]
- Deary IJ, Bastin ME, Pattie A, Clayden JD, Whalley LJ, Starr JM, Wardlaw JM. White matter integrity and cognition in childhood and old age. Neurology. 2006;66:505–512. doi: 10.1212/01.wnl.0000199954.81900.e2. http://dx.doi.org/10.1212/01.wnl.0000199954.81900.e2. [DOI] [PubMed]
- DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): Exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005;36:50–55. doi: 10.1161/01.STR.0000150668.58689.f2. http://dx.doi.org/10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed]
- DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R, Wolf PA. Measures of brain morphology and infarction in the Framingham heart study: Establishing what is normal. Neurobiology of Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. http://dx.doi.org/10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed]
- DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Garner J, Jack L, Carmelli D. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999;30:529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Murphy DGM, Tranh M, Grady CK, Haxby JV, Gillette JA, Salerno JA, Gonzales-Aviles A, Horwitz B, Rapoport SI, Schapiro MB. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45:2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- De Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, Breteler MMB. Cerebral white matter lesions and cognitive function: The Rotterdam Scan Study. Annals of Neurology. 2000;47:145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- De Groot JC, de Leeuw F-E, Oudkerk M, van Gijn J, Hofman A, Jolles J, Breteler MMB. Periventricular cerebral white matter lesions predict rate of cognitive decline. Annals of Neurology. 2002;52:335–341. doi: 10.1002/ana.10294. http://dx.doi.org/10.1002/ana.10294. [DOI] [PubMed]
- Delano-Wood L, Abeles N, Sacco JM, Wierenga CE, Horne NR, Bozoki A. Regional white matter pathology in mild cognitive impairment: Differential influence of lesion type on neuropsychological functioning. Stroke. 2008;39:794–799. doi: 10.1161/STROKEAHA.107.502534. http://dx.doi.org/10.1161/STROKEAHA.107.502534. [DOI] [PubMed]
- Dennis NA, Cabeza R. Neuroimaging of healthy cognitive aging. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. New York: Psychology Press; 2008. pp. 1–54. [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, Sperling RA. Medial temporal lobe function and structure in mild cognitive impairment. Annals of Neurology. 2004;56:27–35. doi: 10.1002/ana.20163. http://dx.doi.org/10.1002/ana.20163. [DOI] [PMC free article] [PubMed]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, May A. Temporal and spatial dynamics of brain structure changes during extensive learning. The Journal of Neuroscience. 2006;26:6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. http://dx.doi.org/10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed]
- Driesen NR, Raz N. The influence of sex, age, and handedness on corpus callosum morphology: A meta-analysis. Psychobiology. 1995;23:240–247. [Google Scholar]
- Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, Resnick SM. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72:1906–1913. doi: 10.1212/WNL.0b013e3181a82634. http://dx.doi.org/10.1212/WNL.0b013e3181a82634. [DOI] [PMC free article] [PubMed]
- Du A-T, Schuff N, Chao LL, Kornak J, Jagust WJ, Kramer JH, Reed BR, Miller BL, Norman D, Chui HC, Weiner MW. Age effects on atrophy rates of entorhinal cortex and hippocampus. Neurobiology of Aging. 2006;27:733–740. doi: 10.1016/j.neurobiolaging.2005.03.021. http://dx.doi.org/10.1016/j.neurobiolaging.2005.03.021. [DOI] [PMC free article] [PubMed]
- Duarte A, Hayasaka S, Du A, Schuff N, Jahng G-H, Kramer J, Miller B, Weiner M. Volumetric correlates of memory and executive function in normal elderly, mild cognitive impairment and Alzheimer’s disease. Neuroscience Letters. 2006;406:60–65. doi: 10.1016/j.neulet.2006.07.029. http://dx.doi.org/10.1016/j.neulet.2006.07.029. [DOI] [PMC free article] [PubMed]
- Dufouil C, Alperovitch A, Tzourio C. Influence of education on the relationship between white matter lesions and cognition. Neurology. 2003;60:831–836. doi: 10.1212/01.wnl.0000049456.33231.96. http://dx.doi.org/10.1002/ana.10440. [DOI] [PubMed]
- Ecker C, Stahl D, Daly E, Johnston P, Thomson A, Murphy DGM. Is there a common underlying mechanism for age-related decline in cortical thickness? NeuroReport. 2009;20:1155–1160. doi: 10.1097/WNR.0b013e32832ec181. http://dx.doi.org/10.1097/WNR.0b013e32832ec181. [DOI] [PubMed]
- Eckert MA, Keren NI, Roberts DR, Calhoun VD, Harris KC. Age-related changes in processing speed: Unique contributions of cerebellar and prefrontal cortex. Frontiers in Human Neuroscience. 2010;4:Article 10. doi: 10.3389/neuro.09.010.2010. http://dx.doi.org/10.3389/neuro.09.010.2010. [DOI] [PMC free article] [PubMed]
- Edwards JR, Lambert LS. Methods for integrating moderation and mediation: A general analytical framework using moderated path analysis. Psychological Methods. 2007;12:1–22. doi: 10.1037/1082-989X.12.1.1. http://dx.doi.org/10.1037/1082-989X.12.1.1. [DOI] [PubMed]
- Envig A, Fjell AM, Westlye LT, Moberget T, Sundseth O, Larsen VA, Walhovd KB. Effects of memory training on cortical thickness in the elderly. NeuroImage. 2010;52:1667–1676. doi: 10.1016/j.neuroimage.2010.05.041. [DOI] [PubMed] [Google Scholar]
- Enzinger C, Fazekas F, Matthews PM, Ropele S, Schmidt H, Smith S, Schmidt R. Risk factors for progression of brain atrophy in aging: Six-year follow-up of normal subjects. Neurology. 2005;64:1704–1711. doi: 10.1212/01.WNL.0000161871.83614.BB. http://dx.doi.org/10.1212/01.WNL.0000161871.83614.BB. [DOI] [PubMed]
- Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. http://dx.doi.org/10.3758/BRM.41.4.1149. [DOI] [PubMed]
- Ferrer E, McArdle JJ. Longitudinal modeling of developmental changes in psychological research. Current Directions in Psychological Science. 2010;19:149–154. http://dx.doi.org/10.1177/0963721410370300.
- Ferro JM, Madureira S. Age-related white matter changes and cognitive impairment. Journal of the Neurological Sciences. 2002;203:221–225. doi: 10.1016/s0022-510x(02)00295-2. http://dx.doi.org/10.1016/S0022-510X(02)00295-2. [DOI] [PubMed]
- Filippini N, Zarei M, Beckmann CF, Galluzzi S, Borsci G, Testa C, Bonetti M, Beltramello A, Ghidoni R, Benussi L, Binetti G, Frisoni GB. Regional atrophy of transcallosal prefrontal connections in cognitively normal APOE e4 carriers. Journal of Magnetic Resonance Imaging. 2009;29:1021–1026. doi: 10.1002/jmri.21757. http://dx.doi.org/10.1002/jmri.21757. [DOI] [PubMed]
- Fine EM, Delis DC, Dean D, Beckman V, Miller BL, Rosen HJ, Kramer JH. Left frontal lobe contributions to concept formation: A quantitative MRI study of performance on the Delis-Kaplan Executive Function System Sorting Test. Journal of Clinical and Experimental Neuropsychology. 2009;31:624–631. doi: 10.1080/13803390802419017. http://dx.doi.org/10.1080/13803390802419017. [DOI] [PMC free article] [PubMed]
- Finkel D, Pedersen NL, McClearn GE, Plomin R, Berg S. Cross-sequential analysis of genetic influences on cognitive ability in the Swedish Adoption/Twin Study of Aging. Aging, Neuropsychology and Cognition. 1996;3:84–99. http://dx.doi.org/10.1080/13825589608256614.
- Finkel D, Pedersen NL, Plomin R, McClearn GE. Longitudinal and cross-sectional twin data on cognitive abilities in adulthood: The Swedish Adoption/Twin Study of Aging. Developmental Psychology. 1998;34:1400–1413. doi: 10.1037//0012-1649.34.6.1400. http://dx.doi.org/10.1037//0012-1649.34.6.1400. [DOI] [PubMed]
- Firbank MJ, Wiseman RM, Burton EJ, Saxby BK, O’Brien JT, Ford GA. Brain atrophy and white matter hyperintensity change in older adults and relationship to blood pressure. Journal of Neurology. 2007;254:713–721. doi: 10.1007/s00415-006-0238-4. http://dx.doi.org/10.1007/s00415-006-0238-4. [DOI] [PubMed]
- Fjell AM, Walhovd KB. Structural brain changes in aging: Courses, causes and cognitive consequences. Reviews in the Neurosciences. 2010;21:187–221. doi: 10.1515/revneuro.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM. One-year brain atrophy evident in healthy aging. Journal of Neuroscience. 2009;29:15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009. http://dx.doi.org/10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed]
- Fjell AM, Westlye LT, Espeseth T, Reinvang I, Dale AM, Holland D, Walhovd KB. Cortical gray matter atrophy in healthy aging cannot be explained by undetected incipient cognitive disorders: A comment on Burgmans et al. (2009) Neuropsychology. 2010;24:258–263. doi: 10.1037/a0018827. http://dx.doi.org/10.1037/a0018827. [DOI] [PMC free article] [PubMed]
- Flashman LA, Andreasen NC, Flaum M, Swayze VW. Intelligence and regional brain volumes in normal controls. Intelligence. 1997;25:149–160. http://dx.doi.org/10.1016/S0160-2896(97)90039-8.
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. http://dx.doi.org/10.1016/0022-3956(75)90026-6. [DOI] [PubMed]
- Foster JK, Meikle A, Goodson G, Mayes AR, Howard M, Sunram SI, Cezayirli E, Roberts N. The hippocampus and delayed recall: Bigger is not necessarily better? Memory. 1999;7:715–732. doi: 10.1080/096582199387823. http://dx.doi.org/10.1080/096582199387823. [DOI] [PubMed]
- Fotenos AF, Mintun MA, Snyder AZ, Morris JC, Buckner RL. Brain volume decline in aging: Evidence for a relation between socioeconomic status, preclinical Alzheimer Disease and reserve. Archives of Neurology. 2008;65:113–120. doi: 10.1001/archneurol.2007.27. http://dx.doi.org/10.1001/archneurol.2007.27. [DOI] [PubMed]
- Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64:1032–1039. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- Francou S, Chitins X, Williams SCR. Mapping IQ and gray matter density in healthy young people. NeuroImage. 2004;23:800–805. doi: 10.1016/j.neuroimage.2004.05.027. http://dx.doi.org/10.1016/j.neuroimage.2004.05.027. [DOI] [PubMed]
- Freeman SH, Kandel R, Cruz L, Rozkalne A, Newell K, Frosch MP, Hedley-Whyte T, Locascio JJ, Lipsitz LA, Hyman BT. Preservation of neuronal number despite age-related cortical atrophy in elderly subjects without Alzheimer Disease. Journal of Neuropathology and Experimental Neurology. 2008;67:1205–1212. doi: 10.1097/NEN.0b013e31818fc72f. http://dx.doi.org/10.1097/NEN.0b013e31818fc72f. [DOI] [PMC free article] [PubMed]
- Garde E, Mortensen EL, Krabbe K, Rostrup E, Larsson HBW. Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenerians: A longitudinal study. Lancet. 2000;356:628–634. doi: 10.1016/S0140-6736(00)02604-0. http://dx.doi.org/10.1016/S0140-6736(00)02604-0. [DOI] [PubMed]
- Garde E, Mortensen EL, Rostrup E, Paulson OB. Decline in intelligence is associated with progression in white matter hyperintensity volume. Journal of Neurology, Neurosurgery and Psychiatry. 2005;76:1289–1291. doi: 10.1136/jnnp.2004.055905. http://dx.doi.org/10.1136/jnnp.2004.055905. [DOI] [PMC free article] [PubMed]
- Giambra LM, Arenberg D, Kawas C, Zonderman AB, Costa PT. Adult life span changes in immediate visual memory and verbal intelligence. Psychology and Aging. 1995;10:123–139. doi: 10.1037//0882-7974.10.1.123. http://dx.doi.org/10.1037//0882-7974.10.1.123. [DOI] [PubMed]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, de Stefano N, Johansen-Berg H. Age-related changes in grey and white matter structure throughout adulthood. NeuroImage. 2010;51:943–951. doi: 10.1016/j.neuroimage.2010.03.004. http://dx.doi.org/10.1016/j.neuroimage.2010.03.004. [DOI] [PMC free article] [PubMed]
- Gold BT, Powell DK, Xuan L, Jiang Y, Hardy PA. Speed of lexical decision correlates with diffusion anisotropy in left parietal and frontal white matter: Evidence from diffusion tensor imaging. Neuropsychologia. 2007;45:2439–2446. doi: 10.1016/j.neuropsychologia.2007.04.011. http://dx.doi.org/10.1016/j.neuropsychologia.2007.04.01. [DOI] [PMC free article] [PubMed]
- Gold BT, Powell DK, Xuan L, Jicha GA, Smith CD. Age-related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiology of Aging. 2010;31:512–522. doi: 10.1016/j.neurobiolaging.2008.04.005. http://dx.doi.org/10.1016/j.neurobiolaging.2008.04.005. [DOI] [PMC free article] [PubMed]
- Goldstein FC, Mao H, Wang L, Ni C, Lah JJ, Levey AI. White matter integrity and episodic memory performance in mild cognitive impairment: A diffusion tensor imaging study. Brain Imaging and Behavior. 2009;3:132–141. doi: 10.1007/s11682-008-9055-y. http://dx.doi.org/10.1007/s11682-008-9055-y. [DOI] [PMC free article] [PubMed]
- Gollob HF, Reichard CS. Taking account of time lags in causal models. Child Development. 1987;58:80–92. http://dx.doi.org/10.2307/1130293. [PubMed]
- Golomb J, Kluger A, de Leon MJ, Ferris SH, Mittelman M, Cohen J, George AE. Hippocampal formation size predicts declining memory performance in normal aging. Neurology. 1996;47:810–813. doi: 10.1212/wnl.47.3.810. [DOI] [PubMed] [Google Scholar]
- Gong Q-Y, Sluming V, Mayes A, Keller S, Barrick T, Cezayirli E, Roberts N. Voxel-based morphometry and stereology provide convergent evidence of the importance of medial prefrontal cortex for fluid intelligence in healthy adults. NeuroImage. 2005;25:1175–1186. doi: 10.1016/j.neuroimage.2004.12.044. http://dx.doi.org/10.1016/j.neuroimage.2004.12.044. [DOI] [PubMed]
- Gonoi W, Abe O, Yamasue H, Yamada H, Masutani Y, Takao H, Kasai K, Aoki S, Ohtomo K. Age-related changes in regional brain volume evaluated by atlas-based method. Neuroradiology. 2010;52:865–873. doi: 10.1007/s00234-009-0641-5. http://dx.doi.org/10.1007/s00234-009-0641-5. [DOI] [PubMed]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. A voxel-based morphometric study of ageing in 465 normal adult brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. http://dx.doi.org/10.1109/SSBI.2002.1233974. [DOI] [PubMed]
- Gootjes L, Scheltens P, van Strien JW, Bouma A. Subcortical white matter pathology as a mediating factor for age-related decreased performance in dichotic listening. Neuropsychologia. 2007;45:2322–2332. doi: 10.1016/j.neuropsychologia.2007.02.014. http://dx.doi.org/10.1016/j.neuropsychologia.2007.02.014. [DOI] [PubMed]
- Grady CL. Cognitive neuroscience of aging. Annals of New York Academy of Sciences. 2008;1124:127–144. doi: 10.1196/annals.1440.009. http://dx.doi.org/10.1196/annals.1440.009. [DOI] [PubMed]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. Journal of Cognitive Neuroscience. 2006;182:227–241. doi: 10.1162/089892906775783705. http://dx.doi.org/10.1162/089892906775783705. [DOI] [PubMed]
- Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Human Brain Mapping. 2005;25:391–401. doi: 10.1002/hbm.20115. http://dx.doi.org/10.1002/hbm.20115. [DOI] [PMC free article] [PubMed]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: A diffusion tensor MR imaging study. American Journal of Neuroradiology. 2007;28:226–235. [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: A review of MRI findings. International Journal of Geriatric Psychiatry. 2009;24:109–117. doi: 10.1002/gps.2087. http://dx.doi.org/10.1002/gps.2087. [DOI] [PMC free article] [PubMed]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: A quantitative review. Neuropsychology. 2000;14:224–232. doi: 10.1037//0894-4105.14.2.224. http://dx.doi.org/10.1037/0894-4105.14.2.224. [DOI] [PubMed]
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults. A prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. http://dx.doi.org/10.1016/S0028-3932(03)00129-5. [DOI] [PubMed]
- Guttmann CRG, Jolesz FA, Kikinis R, Killiany RJ, Moss MB, Sandor T, Albert MS. White matter changes with normal aging. Neurology. 1998;50:972–978. doi: 10.1212/wnl.50.4.972. [DOI] [PubMed] [Google Scholar]
- Hackert VH, den Heijer T, Oudkerk M, Koudstaal PJ, Hofman A, Breteler MMB. Hippocampal head size associated with verbal memory performance in nondemented elderly. NeuroImage. 2002;17:1365–1372. doi: 10.1006/nimg.2002.1248. http://dx.doi.org/10.1006/nimg.2002.1248. [DOI] [PubMed]
- Haier RJ, Colom R, Schroeder DH, Condon CA, Tang C, Eaves E, Head K. Gray matter and intelligence factors: Is there a neuro-g? Intelligence. 2009;37:136–144. http://dx.doi.org/10.1016/j.intell.2008.10.011.
- Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. Structural brain variation and general intelligence. NeuroImage. 2004;23:425–433. doi: 10.1016/j.neuroimage.2004.04.025. http://dx.doi.org/10.1016/j.neuroimage.2004.04.025. [DOI] [PubMed]
- Hasan KM, Kamali A, Iftikhar A, Kramer LA, Papanicolaou AC, Fletcher JM, Ewing-Cobbs L. Diffusion tensor tractography quantification of the human corpus callosum fiber pathways across the lifespan. Brain Research. 2009;1249:91–100. doi: 10.1016/j.brainres.2008.10.026. http://dx.doi.org/10.1016/j.brainres.2008.10.026. [DOI] [PMC free article] [PubMed]
- Hasan KM, Sankar A, Halphen C, Kramer LA, Brandt ME, Juranek J, Cirino PT, Fletcher JM, Papanicolaou AC, Ewing-Cobbs L. Development and organization of the human brain tissue compartments across the lifespan using diffusion tensor imaging. NeuroReport. 2007;18:1735–1739. doi: 10.1097/WNR.0b013e3282f0d40c. http://dx.doi.org/10.1097/WNR.0b013e3282f0d40c. [DOI] [PubMed]
- Haut MW, Moran MT, Lancaster MA, Kuwabara H, Parsons MW, Puce A. White matter correlates of cognitive capacity studied with diffusion tensor imaging: Implications for cognitive reserve. Brain Imaging and Behavior. 2007;1:83–92. http://dx.doi.org/10.1007/s11682-007-9008-x.
- Hazlett EA, Buchsbaum MS, Mohs RC, Spiegel-Cohen J, Wei TC, Azueta R, Haznedar MM, Singer MB, Shihabuddin L, Luu-Hsia C, Harvey PD. Age-related shift in brain region activity during successful memory performance. Neurobiology of Aging. 1998;19:437–445. doi: 10.1016/s0197-4580(98)00075-x. http://dx.doi.org/10.1016/S0197-4580(98)00075-X. [DOI] [PubMed]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: Evidence from diffusion tensor imaging. Cerebral Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. http://dx.doi.org/10.1093/cercor/bhh003. [DOI] [PubMed]
- Head D, Kennedy KM, Rodrigue KM, Raz N. Age differences in perseveration: Cognitive and neuroanatomical mediators of performance on the Wisconsin Card Sorting Test. Neuropsychologia. 2009;47:1200–1203. doi: 10.1016/j.neuropsychologia.2009.01.003. http://dx.doi.org/10.1016/j.neuropsychologia.2009.01.003. [DOI] [PMC free article] [PubMed]
- Head D, Raz N, Gunning-Dixon F, Williamson A, Acker JD. Age-related differences in the course of cognitive skill acquisition: The role of regional cortical shrinkage and cognitive resources. Psychology and Aging. 2002;17:72–84. doi: 10.1037//0882-7974.17.1.72. http://dx.doi.org/10.1037//0882-7974.17.1.72. [DOI] [PubMed]
- Head D, Rodrigue KM, Kennedy KM, Raz N. Neuroanatomical and cognitive mediators of age-related differences in episodic memory. Neuropsychology. 2008;22:491–507. doi: 10.1037/0894-4105.22.4.491. http://dx.doi.org/10.1037/0894-4105.22.4.491. [DOI] [PMC free article] [PubMed]
- Head D, Snyder AZ, Girton LE, Morris JC, Buckner RL. Frontal-hippocampal double dissociation between normal aging and Alzheimer’s Disease. Cerebral Cortex. 2005;15:732–739. doi: 10.1093/cercor/bhh174. http://dx.doi.org/10.1093/cercor/bhh174. [DOI] [PubMed]
- Hedden T, Gabrieli JDE. Insights into the ageing mind: A view from cognitive neuroscience. Nature Neuroscience Reviews. 2004;5:87–97. doi: 10.1038/nrn1323. http://dx.doi.org/10.1038/nrn1323. [DOI] [PubMed]
- Hentschel F, Damian M, Krumm B, Froelich L. White matter lesions – age-adjusted values for cognitively healthy and demented subjects. Acta Neurologica Scandinavia. 2007;115:174–180. doi: 10.1111/j.1600-0404.2006.00762.x. http://dx.doi.org/10.1111/j.1600-0404.2006.00762.x. [DOI] [PubMed]
- Hertzog C, Schaie KW. Stability and change in adult intelligence: 1. Analysis of longitudinal covariance structures. Psychology and Aging. 1986;1:159–171. doi: 10.1037//0882-7974.1.2.159. http://dx.doi.org/10.1037//0882-7974.1.2.159. [DOI] [PubMed]
- Hertzog C, Schaie KW. Stability and change in adult intelligence: 2. Simultaneous analysis of longitudinal means and covariance structures. Psychology and Aging. 1988;3:122–130. doi: 10.1037//0882-7974.3.2.122. http://dx.doi.org/10.1037//0882-7974.3.2.122. [DOI] [PubMed]
- Hofer SM, Piccinin AM. Longitudinal studies. In: BIrren JE, editor. Encylclopedia of Gerontology. 2. San Diego, CA: Elsevier; 2007. pp. 107–115. http://dx.doi.org/10.1016/B0-12-370870-2/00117-7. [Google Scholar]
- Hsu J-L, Leemans A, Bai C-H, Lee C-H, Tsai Y-F, Chiu H-C, Chen W-H. Gender differences and age-related white matter changes of the human brain: A diffusion tensor imaging study. NeuroImage. 2008;39:566–577. doi: 10.1016/j.neuroimage.2007.09.017. http://dx.doi.org/10.1016/j.neuroimage.2007.09.017. [DOI] [PubMed]
- Hugenschmidt CE, Peiffer AM, Kraft RA, Casanova R, Deibler AR, Burdette JH, Maldjian JA, Laurenti PJ. Relating imaging indices of white matter integrity and volume in healthy older adults. Cerebral Cortex. 2008;18:433–442. doi: 10.1093/cercor/bhm080. http://dx.doi.org/10.1093/cercor/bhm080. [DOI] [PubMed]
- Hulshoff Pol HE, Schnack HG, Posthuma D, Mandl RCW, Baare WF, van Oel C, Van Haren NE, Collins DL, Evans AC, Amunts K, Burgel U, Zilles K, de Geus E, Boomsma DI, Kahn RS. Genetic contributions to human brain morphology and intelligence. Journal of Neuroscience. 2006;26:10235–10242. doi: 10.1523/JNEUROSCI.1312-06.2006. http://dx.doi.org/10.1523/JNEUROSCI.1312-06.2006. [DOI] [PMC free article] [PubMed]
- Huppert FA, Whittington JE. Longitudinal changes in mental state and personality measures. In: Cox BD, Huppert FA, Whichelow MJ, editors. The Health and Lifestyle Survey: Seven Years On. Aldershot, England: Dartmouth Publishing Company; 1993. pp. 133–172. [Google Scholar]
- Hutton C, Draganski B, Ashburner J, Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. NeuroImage. 2009;48:371–380. doi: 10.1016/j.neuroimage.2009.06.043. http://dx.doi.org/10.1016/j.neuroimage.2009.06.043. [DOI] [PMC free article] [PubMed]
- Ikram MA, Vrooman HA, Vernooij MW, den Heijer T, Hofman A, Niessen WJ, van der Lugt A, Koudstaal PJ, Breteler MMB. Brain tissue volumes in relation to cognitive function and risk of dementia. Neurobiology of Aging. 2010;31:378–386. doi: 10.1016/j.neurobiolaging.2008.04.008. http://dx.doi.org/10.1016/j.neurobiolaging.2008.04.008. [DOI] [PubMed]
- Ikram MA, Vrooman HA, Vernooij MW, van der Lijn F, Hofman A, van der Lugt A, Niessen WJ, Breteler MMB. Brain tissue volumes in the general elderly population: The Rotterdam Scan Study. Neurobiology of Aging. 2008;29:882–890. doi: 10.1016/j.neurobiolaging.2006.12.012. http://dx.doi.org/10.1016/j.neurobiolaging.2006.12.012. [DOI] [PubMed]
- Ilg R, Wohlschlager AM, Gaser C, Liebau Y, Dauner R, Woller A, Zimmer C, Zihl J, Muhlau M. Gray matter increase induced by practice correlates with task-specific activation: A combined functional and morphometric magnetic resonance imaging study. The Journal of Neuroscience. 2008;28:4210–4215. doi: 10.1523/JNEUROSCI.5722-07.2008. http://dx.doi.org/10.1523/JNEUROSCI.5722-07.2008. [DOI] [PMC free article] [PubMed]
- Jack CR, Shiung MM, Weigand SD, O’Brien PC, Gunter JL, Boeve BF, Knopman DS, Smith GE, Ivnik RJ, Tangalos EG, Petersen RC. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. http://dx.doi.org/10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed]
- Jack CR, Weigand SD, Shiung MM, Przybelksi SA, O’Brien PC, Gunter JL, Knopman DS, Boeve BF, Smith GE, Petersen RC. Atrophy rates accelerate in amnestic mild cognitive impairment. Neurology. 2008;70:1740–1752. doi: 10.1212/01.wnl.0000281688.77598.35. http://dx.doi.org/10.1212/01.wnl.0000281688.77598.35. [DOI] [PMC free article] [PubMed]
- Jacobs HIL, Visser PJ, van Boxtel MPJ, Frisoni GB, Tsolaki M, Papapostolou P, Nobili F, Wahlund L-O, Minthon L, Frolich L, Hampel H, Soininen H, van de Pol L, Scheltens P, Tan FES, Jolles J, Verhey FRJ. The association between white matter hyperintensities and executive decline in mild cognitive impairment is network dependent. Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2010.07.015. in press. http://dx.doi.org/10.1016/j.neurobiolaging.2010.07.015. [DOI] [PubMed]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. http://dx.doi.org/10.1016/S0197-4580(01)00217-2. [DOI] [PubMed]
- Johnson W, Jung RE, Colom R, Haier RJ. Cognitive abilities independent of IQ correlate with regional brain structure. Intelligence. 2008;36:18–28. http://dx.doi.org/10.1016/j.intell.2007.01.005.
- Jung RE, Haier RJ. The parieto-frontal integration theory (P-FIT) of intelligence: Converging neuroimaging evidence. Behavioral and Brain Sciences. 2007;30:135–187. doi: 10.1017/S0140525X07001185. http://dx.doi.org/10.1017/S0140525X07001185. [DOI] [PubMed]
- Kalpouzos G, Chetelat G, Baron J-C, Landeau B, Mevel K, Godeau C, Barre L, Constans J-M, Viader F, Eustache F, Desgranges B. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiology of Aging. 2009;30:112–124. doi: 10.1016/j.neurobiolaging.2007.05.019. http://dx.doi.org/10.1016/j.neurobiolaging.2007.05.019. [DOI] [PubMed]
- Kennedy KM, Erickson KI, Rodrigue KM, Voss MW, Colcombe SJ, Kramer AF, Acker JD, Raz N. Age-related differences in regional brain volumes: A comparison of optimized voxel-based morphometry to manual volumetry. Neurobiology of Aging. 2009;30:1657–1676. doi: 10.1016/j.neurobiolaging.2007.12.020. http://dx.doi.org/10.1016/j.neurobiolaging.2007.12.020. [DOI] [PMC free article] [PubMed]
- Kennedy KM, Raz N. Age, sex and regional brain volumes predict perceptual-motor acquisition. Cortex. 2005;41:560–569. doi: 10.1016/s0010-9452(08)70196-5. http://dx.doi.org/10.1016/S0010-9452(08)70196-5. [DOI] [PubMed]
- Kennedy KM, Raz N. Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47:816–927. doi: 10.1016/j.neuropsychologia.2009.01.001. http://dx.doi.org/10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed]
- Kennedy KM, Rodrigue KM, Gunning-Dixon F, Head D, Raz N. Neuroanatomical and cognitive mediators of age-related differences in perceptual priming and learning. Neuropsychology. 2009;23:475–491. doi: 10.1037/a0015377. http://dx.doi.org/10.1037/a0015377. [DOI] [PMC free article] [PubMed]
- Kochunov P, Thompson PM, Coyle TR, Lancaster JL, Kochunov V, Royall D, Mangin J-F, Riviere D, Fox PT. Relationship among neuroimaging indices of cerebral health during normal aging. Human Brain Mapping. 2008;29:36–45. doi: 10.1002/hbm.20369. http://dx.doi.org/10.1002/hbm.20369. [DOI] [PMC free article] [PubMed]
- Kraemer HC, Kiernan M, Essex M, Kupfer DJ. How and why criteria defining moderators and mediators differ between the Baron & Kenny and MacArthur approaches. Health Psychology. 2008;27:S101–S108. doi: 10.1037/0278-6133.27.2(Suppl.).S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. American Journal of Psychiatry. 2001;158:848–856. doi: 10.1176/appi.ajp.158.6.848. http://dx.doi.org/10.1176/appi.ajp.158.987.848. [DOI] [PubMed]
- Kramer JH, Mungas D, Reed BR, Wetzel ME, Burnett MM, Miller BL, Weinder MW, Chui HC. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007;21:412–418. doi: 10.1037/0894-4105.21.4.412. http://dx.doi.org/10.1037/0894-4105.21.4.412. [DOI] [PMC free article] [PubMed]
- Langenecker SA, Briceno EM, Hamid NM, Nielson KA. An evaluation of distinct volumetric and functional MRI contributions toward understanding age and task performance: A study in the basal ganglia. Brain Research. 2007;1135:58–68. doi: 10.1016/j.brainres.2006.11.068. http://dx.doi.org/10.1016/j.brainres.2006.11.068. [DOI] [PMC free article] [PubMed]
- Lawrie SM, Whalley HC, Abukmeil SS, Kestelman JN, Miller P, Best JJK, Owens DGC, Johnstone EC. Temporal lobe volume changes in people at high risk of schizophrenia with psychotic symptoms. British Journal of Psychiatry. 2002;181:138–143. doi: 10.1017/s0007125000161860. [DOI] [PubMed] [Google Scholar]
- Lebel C, Caverhill-Godkewitsch S, Beaulieu C. Age-related regional variations of the corpus callosum identified by diffusion tensor tractography. NeuroImage. 2010;52:20–31. doi: 10.1016/j.neuroimage.2010.03.072. http://dx.doi.org/10.1016/j.neuroimage.2010.03.072. [DOI] [PubMed]
- Lee H, Devlin JT, Shakeshaft C, Stewart LH, Brennan A, Glensman J, Pitcher K, Crinion J, Mechelli A, Frakcowiak RSJ, Green DW, Price CJ. Anatomical traces of vocabulary acquisition in the adolescent brain. Journal of Neuroscience. 2007;27:1184–1189. doi: 10.1523/JNEUROSCI.4442-06.2007. http://dx.doi.org/10.1523/JNEUROSCI.4442-06.2007. [DOI] [PMC free article] [PubMed]
- Lehmbeck JT, Brassen S, Weber-Fahr W, Braus DF. Combining voxel-based morphometry and diffusion tensor imaging to detect age-related brain changes. NeuroReport. 2006;17:467–470. doi: 10.1097/01.wnr.0000209012.24341.7f. http://dx.doi.org/10.1097/01.wnr.0000209012.24341.7f. [DOI] [PubMed]
- Lemaitre H, Goldman AL, Sambataro F, Verchinski BA, Meyer-Lindenberg A, Weinberger DR, Mattay VS. Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2010.07.013. in press. http://dx.doi.org/10.1016/j.neurobiolaging.2010.07.013. [DOI] [PMC free article] [PubMed]
- Lieberman J, Chakos M, Wu H, Alvir J, Hoffman E, Robinson D, Bilder R. Longitudinal study of brain morphology in first episode schizophrenia. Biological Psychiatry. 2001;49:487–499. doi: 10.1016/s0006-3223(01)01067-8. http://dx.doi.org/10.1016/S0006-3223(01)01067-8. [DOI] [PubMed]
- Lindenberger U, Potter U. The complex nature of unique and shared effects in hierarchical linear regression: Implications for developmental psychology. Psychological Methods. 1998;3:218–230. http://dx.doi.org/10.1037/1082-989X.3.2.218.
- Liu RSN, Lemieux L, Bell GS, Sisodiya SM, Shorvon SD, Sander JWAS, Duncan JS. A longitudinal study of brain morphometrics using quantitative magnetic resonance imaging and difference image analysis. NeuroImage. 2003;20:22–33. doi: 10.1016/s1053-8119(03)00219-2. http://dx.doi.org/10.1016/S1053-8119(03)00219-2. [DOI] [PubMed]
- Longstreth WT, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O’Leary D, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Toga AW. Neuroanatomical correlates of intelligence. Intelligence. 2009;37:156–163. doi: 10.1016/j.intell.2008.07.002. http://dx.doi.org/10.1016/j.intell.2008.07.002. [DOI] [PMC free article] [PubMed]
- Lye TC, Grayson DA, Creasey H, Piguet O, Bennett HP, Ridley LJ, Kril JJ, Broe GA. Predicting memory performance in normal ageing using different measures of hippocampal size. Neuroradiology. 2006;48:90–99. doi: 10.1007/s00234-005-0032-5. http://dx.doi.org/10.1007/s00234-005-0032-5. [DOI] [PubMed]
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annual Review of Psychology. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. http://dx.doi.org/10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed]
- MacLullich AMJ, Ferguson KJ, Deary IJ, Seckl JR, Starr JM, Wardlaw JM. Intracranial capacity and brain volumes are associated with cognition in healthy elderly men. Neurology. 2002;59:169–174. doi: 10.1212/wnl.59.2.169. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: Contributions from diffusion tensor imaging. Neuropsychology Review. 2009;19:415–435. doi: 10.1007/s11065-009-9113-2. http://dx.doi.org/10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, Huettel SA. Cerebral white matter integrity mediates adult age differences in cognitive performance. Journal of Cognitive Neuroscience. 2009;21:289–302. doi: 10.1162/jocn.2009.21047. http://dx.doi.org/10.1162/jocn.2009.21047. [DOI] [PMC free article] [PubMed]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: Relation to response time. NeuroImage. 2004;21:1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. http://dx.doi.org/10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed]
- Marcus DS, Wang TH, Parker J, Csernansky JG, Morris JC, Buckner RL. Open access series of imaging studies (OASIS): Cross-sectional MRI data in young, middle aged, nondemented, and demented older adults. Journal of Cognitive Neuroscience. 2007;19:1498–1507. doi: 10.1162/jocn.2007.19.9.1498. http://dx.doi.org/10.1162/jocn.2007.19.9.1498. [DOI] [PubMed]
- Mayda A, Yoshita M, DeCarli C. White matter hyperintensities in aging and dementia. In: Jagust W, D’Esposito M, editors. Imaging the aging brain. New York: Oxford University Press; 2009. pp. 273–291. http://dx.doi.org/10.1093/acprof:oso/9780195328875.003.0017. [Google Scholar]
- McDaniel MA. Big-brained people are smarter; A meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence. 2005;33:337–346. http://dx.doi.org/10.1016/j.intell.2004.11.005.
- McLaughlin NCR, Paul RH, Grieve SM, Williams LM, Laidlaw D, DiCarlo M, Clark CR, Whelihan W, Cohen RA, Whitford TJ, Gordon E. Diffusion tensor imaging of the corpus callosum: A cross-sectional study across the lifespan. International Journal of Developmental Neuroscience. 2007;25:215–221. doi: 10.1016/j.ijdevneu.2007.03.008. http://dx.doi.org/10.1016/j.ijdevneu.2007.03.008. [DOI] [PubMed]
- Mechelli A, Crinion JT, Noppeney U, O’Doherty J, Ashburner J, Frackowiak RS, Price CJ. Structural plasticity in the bilingual brain: Proficiency in a second language and age at acquisition affect grey-matter density. Nature. 2004;431:757. doi: 10.1038/431757a. http://dx.doi.org/10.1038/431757a. [DOI] [PubMed]
- Michielse S, Coupland N, Camicioli R, Carter R, Seres P, Sabino J, Malykhin N. Selective effects of aging on brain white matter microstructure: A diffusion tensor imaging tractography study. NeuroImage. 2010;52:1190–1201. doi: 10.1016/j.neuroimage.2010.05.019. http://dx.doi.org/10.1016/j.neuroimage.2010.05.019. [DOI] [PubMed]
- Moffat SD, Kennedy KM, Rodrigue KM, Raz N. Extrahippocampal contributions to age differences in human spatial navigation. Cerebral Cortex. 2007;17:1274–1282. doi: 10.1093/cercor/bhl036. http://dx.doi.org/10.1093/cercor/bhl036. [DOI] [PubMed]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. http://dx.doi.org/10.1126/science.278.5337.412. [DOI] [PubMed]
- Muller-Oehring EM, Schulte T, Raassi C, Pfefferbaum A, Sullivan EV. Local-global interference is modulated by age, sex and anterior corpus callosum size. Brain Research. 2007;1142:189–205. doi: 10.1016/j.brainres.2007.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D, Judd CM, Yzerbyt VY. When moderation is mediated and mediation is moderated. Journal of Personality and Social Psychology. 2005;89:852–863. doi: 10.1037/0022-3514.89.6.852. http://dx.doi.org/10.1037/0022-3514.89.6.852. [DOI] [PubMed]
- Muller M, Appelman APA, van der Graaf Y, Vincken KL, Mali WPThM, Geerlings MI. Brain atrophy and cognition: Interaction with vascular pathology? Neurobiology of Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.05.005. (Advance Publication). http://dx.doi.org/10.1016/j.neurobiolaging.2009.05.005. [DOI] [PubMed]
- Mungas D, Harvey D, Reed BR, Jagust WJ, DeCarli C, Beckett L, Mack WJ, Kramer JH, Weiner MW, Schuff N, Chui HC. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology. 2005;65:565–571. doi: 10.1212/01.wnl.0000172913.88973.0d. http://dx.doi.org/10.1212/01.wnl.0000172913.88973.0d. [DOI] [PMC free article] [PubMed]
- Mungas D, Jagust WJ, Reed BR, Kramer JH, Weiner MW, Schuff N, Norman D, Mack WJ, Willis L, Chui HC. MRI predictors of cognition in subcortical ischemic vascular disease and Alzheimer’s disease. Neurology. 2001;57:2229–2235. doi: 10.1212/wnl.57.12.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy EA, Holland D, Donohue M, McEvoy LK, Hagler DJ, Dale AM, Brewer JB. Six-month atrophy in MTL structures is associated with subsequent memory decline in elderly controls. NeuroImage. 2010;53:1310–1317. doi: 10.1016/j.neuroimage.2010.07.016. http://dx.doi.org/10.1016/j.neuroimage.2010.07.016. [DOI] [PMC free article] [PubMed]
- Narr KL, Woods RP, Thompson PM, Szesko P, Robinson D, Dimtcheva T, Gurbani M, Toga AW, Bilder RM. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cerebral Cortex. 2007;17:2163–2171. doi: 10.1093/cercor/bhl125. http://dx.doi.org/10.1093/cercor/bhl125. [DOI] [PubMed]
- Nebes RD, Meltzer CC, Whyte WM, Scanlon JM, Halligan EM, Saxton JA, Houck PR, Boada FE, DeKosky ST. The relation of white matter hyperintensities to cognitive performance in normal old: Education matters. Aging, Neuropsychology and Cognition. 2006;13:326–340. doi: 10.1080/138255890969294. http://dx.doi.org/10.1080/138255890969294. [DOI] [PubMed]
- Nelson EA, Dannefer D. Aged heterogeneity: Fact or fiction? The fate of diversity in gerontological research. Gerontologist. 1992;32:17–23. doi: 10.1093/geront/32.1.17. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Nakamrua M, Niznikiewicz M, McCarley RW, Shenton ME. Comparing prefrontal gray and white matter contributions to intelligence and decision making in schizophrenia and healthy controls. Neuropsychology. 2010;24:121–129. doi: 10.1037/a0016981. http://dx.doi.org/10.1037/a0016981. [DOI] [PMC free article] [PubMed]
- Newman LM, Trivedi MA, Bendlin BB, Ries ML, Johnson SC. The relationship between gray matter morphometry and neuropsychological performance in a large sample of cognitively healthy adults. Brain Imaging and Behavior. 2007;1:3–10. doi: 10.1007/s11682-007-9000-5. http://dx.doi.org/10.1007/s11682-007-9000-5. [DOI] [PMC free article] [PubMed]
- Nordahl CW, Ranganath C, Yonelinas AP, DeCarli C, Fletcher E, Jagust WJ. White matter changes compromise prefrontal cortex function in healthy elderly individuals. Journal of Cognitive Neuroscience. 2006;18:418–429. doi: 10.1162/089892906775990552. http://dx.doi.org/10.1162/089892906775990552. [DOI] [PMC free article] [PubMed]
- Nyberg L, Marklund P, Persson J, Cabeza R, Forkstam C, Petersson KM, Ingvar M. Common prefrontal activations during working memory, episodic memory, and semantic memory. Neuropsychologia. 2003;41:371–377. doi: 10.1016/s0028-3932(02)00168-9. http://dx.doi.org/10.1016/S0028-3932(02)00168-9. [DOI] [PubMed]
- O’Brien JT, Desmond P, Ames D, Schweitzer I, Tress B. Magnetic resonance imaging correlates of memory impairment in the healthy elderly: Association with medial temporal lobe atrophy but not white matter lesions. International Journal of Geriatric Psychiatry. 1997;12:369–374. http://dx.doi.org/10.1002/(SICI)1099-1166(199703)12:3<369::AID-GPS516>3.3.CO;2-M. [PubMed]
- O’Brien JT, Wiseman R, Burton EJ, Barber B, Wesnes K, Saxby B, Ford GA. Cognitive associations of subcortical white matter lesions in older people. Annals of New York Academy of Science. 2002;977:436–444. doi: 10.1111/j.1749-6632.2002.tb04849.x. http://dx.doi.org/10.1111/j.1749-6632.2002.tb04849.x. [DOI] [PubMed]
- Oosterman JM, Vogels RLC, van Harten B, Gouw AA, Scheltens P, Poggesi A, Weinstein HC, Scherder EJA. The role of white matter hyperintensities and medial temporal lobe atrophy in age-related executive dysfunctioning. Brain and Cognition. 2008;68:128–133. doi: 10.1016/j.bandc.2008.03.006. http://dx.doi.org/10.1016/j.bandc.2008.03.006. [DOI] [PubMed]
- O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SCR, Markus HS. Evidence for cortical “disconnection” as a mechanism for age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: Aging and neurocognitive scaffolding. Annual Review of Psychology. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. http://dx.doi.org/10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed]
- Paul R, Grieve SM, Chaudary B, Gordon N, Lawrence J, Cooper N, Clark CR, Kukla M, Mulligan R, Gordon E. Relative contributions of cerebellar vermis and prefrontal lobe volumes on cognitive function across the adult lifespan. Neurobiology of Aging. 2009;30:457–465. doi: 10.1016/j.neurobiolaging.2007.07.017. http://dx.doi.org/10.1016/j.neurobiolaging.2007.07.017. [DOI] [PubMed]
- Paul RH, Haque O, Gunstad J, Tate DF, Grieve SM, Hoth K, Brickman AM, Cohen R, Lange K, Jefferson AL, MacGregor KL, Gordon E. Subcortical hyperintensities impact cognitive function among a select subset of healthy elderly. Archives of Clinical Neuropsychology. 2005;20:697–704. doi: 10.1016/j.acn.2005.02.004. http://dx.doi.org/10.1016/j.acn.2005.02.004. [DOI] [PMC free article] [PubMed]
- Penke L, Deary IJ. Some guidelines for structural equation modeling in cognitive neuroscience: The case of Charlton et al.’s study on white matter integrity and cognitive ageing. Neurobiology of Aging. 2010;31:1656–1660. doi: 10.1016/j.neurobiolaging.2009.10.019. http://dx.doi.org/10.1016/j.neurobiolaging.2009.10.019. [DOI] [PubMed]
- Penke L, Maniega SM, Murray C, Gow A, Hernandez MV, Clayden J, Starr J, Wardlaw J, Bastin M, Deary I. A general factor of brain white matter integrity predicts information processing speed in healthy older people. Journal of Neuroscience. 2010;30:7569–7574. doi: 10.1523/JNEUROSCI.1553-10.2010. http://dx.doi.org/10.1523/JNEUROSCI.1553-10.2010. [DOI] [PMC free article] [PubMed]
- Perry ME, McDonald CR, Hagler DJ, Gharapetian L, Kuperman JM, Koyama AK, Dale AM, McEvoy LK. White matter tracts associated with set-shifting in healthy aging. Neuropsychologia. 2009;47:2835–2842. doi: 10.1016/j.neuropsychologia.2009.06.008. http://dx.doi.org/10.1016/S1053-8119(09)71936-6. [DOI] [PMC free article] [PubMed]
- Persson J, Nyberg L, Lind J, Larsson A, Nilsson L-G, Ingvar M, Buckner RL. Structure-function correlates of cognitive decline in aging. Cerebral Cortex. 2006;16:907–915. doi: 10.1093/cercor/bhj036. http://dx.doi.org/10.1093/cercor/bhj036. [DOI] [PubMed]
- Petkov CI, Wu CC, Eberling JL, Mungas D, Zrelak PA, Yonelinas AP, Haan MN, Jagust WJ. Correlates of memory function in community-dwelling elderly: The importance of white matter hyperintensities. Journal of the International Neuropsychological Society. 2004;10:371–381. doi: 10.1017/S1355617704103056. http://dx.doi.org/10.1017/S1355617704103056. [DOI] [PubMed]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. NeuroImage. 2005;26:891–899. doi: 10.1016/j.neuroimage.2005.02.034. http://dx.doi.org/10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed]
- Pfefferbaum A, Sullivan EV. Increased brain white matter diffusivity in normal adult aging: Relationship to anisotropy and partial voluming. Magnetic Resonance in Medicine. 2003;49:953–961. doi: 10.1002/mrm.10452. http://dx.doi.org/10.1002/mrm.10452. [DOI] [PubMed]
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Archives of General Psychiatry. 1998;55:905–912. doi: 10.1001/archpsyc.55.10.905. http://dx.doi.org/10.1001/archpsyc.55.10.905. [DOI] [PubMed]
- Pieperhoff P, Homke L, Schneider F, Habel U, Shah NJ, Zilles K, Amunts K. Deformation field morphometry reveals age-related structural differences between the brains of adults up to 51 years. Journal of Neuroscience. 2008;28:828–842. doi: 10.1523/JNEUROSCI.3732-07.2008. http://dx.doi.org/10.1523/JNEUROSCI.3732-07.2008. [DOI] [PMC free article] [PubMed]
- Posthuma D, de Geus EJC, Baare WFC, Hulshoff Pol HE, Kahn RS, Boomsma DI. The association between brain volume and intelligence is of genetic origin. Nature Neuroscience. 2002;5:83–84 . doi: 10.1038/nn0202-83. http://dx.doi.org/10.1038/nn0202-83. [DOI] [PubMed]
- Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Jolles J, Koudstaal PJ, Hofman A, Breteler MMB. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128:2034–2041. doi: 10.1093/brain/awh553. http://dx.doi.org/10.1093/brain/awh553. [DOI] [PubMed]
- Rabbitt P. Does it all go together when it goes? The Nineteenth Bartlett Memorial Lecture. The Quarterly Journal of Experimental Psychology. 1993;46A:385–434. doi: 10.1080/14640749308401055. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Mogapi O, Scott M, Thacker N, Lowe C, Horan M, Pendelton N, Jackson A. Effects of global atrophy, white matter lesions, and cerebral blood flow on age-related changes in speed, memory, intelligence, vocabulary, and frontal function. Neuropsychology. 2007;21:684–695. doi: 10.1037/0894-4105.21.6.684. http://dx.doi.org/10.1037/0894-4105.21.6.684. [DOI] [PubMed]
- Rabbitt P, Scott M, Lunn M, Thacker N, Lowe C, Pendleton N, Horan M, Jackson A. White matter lesions account for all age-related declines in speed but not in intelligence. Neuropsychology. 2007;21:363–370. doi: 10.1037/0894-4105.21.3.363. http://dx.doi.org/10.1037/0894-4105.21.3.363. [DOI] [PubMed]
- Rabbitt P, Scott M, Thacker N, Lowe C, Jackson A, Horan M, Pendleton N. Losses in gross brain volume and cerebral blood flow account for age-related differences in speed but not in fluid intelligence. Neuropsychology. 2006;20:549–557. doi: 10.1037/0894-4105.20.5.549. http://dx.doi.org/10.1037/0894-4105.20.5.549. [DOI] [PubMed]
- Rajah MN, Kromas M, Han JE, Pruessner JC. Group differences in anterior hippocampal volume and in the retrieval of spatial and temporal context memory in healthy young versus older adults. Neuropsychologia. 2010;48:4020–4030. doi: 10.1016/j.neuropsychologia.2010.10.010. http://dx.doi.org/10.1016/j.neuropsychologia.2010.10.010. [DOI] [PubMed]
- Ranganath C, Johnson MK, D’Esposito M. Prefrontal activity associated with working memory and episodic long-term memory. Neuropsychologia. 2003;41:378–389. doi: 10.1016/s0028-3932(02)00169-0. http://dx.doi.org/10.1016/S0028-3932(02)00169-0. [DOI] [PubMed]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. Handbook of Aging and Cognition. Hillsdale, NJ: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: Regional and individual differences. NeuroImage. 2010;51:501–511. doi: 10.1016/j.neuroimage.2010.03.020. http://dx.doi.org/10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: Evidence from structural magnetic imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. http://dx.doi.org/10.1037//0894-4105.12.1.95. [DOI] [PubMed]
- Raz N, Kennedy KM. A systems approach to the aging brain: Neuroanatomic changes, their modifiers, and cognitive correlates. In: Jagust W, D’Esposito M, editors. Imaging the Aging Brain. New York: Oxford University Press; 2009. pp. 43–70. http://dx.doi.org/10.1093/acprof:oso/9780195328875.003.0004. [Google Scholar]
- Raz N, Lindenberger U, Ghisletta P, Rodrigue KM, Kennedy KM, Acker JD. Neuroanatomical correlates of fluid intelligence in healthy adults and persons with vascular risk factors. Cerebral Cortex. 2008;18:718–726. doi: 10.1093/cercor/bhm108. http://dx.doi.org/10.1093/cercor/bhm108. [DOI] [PMC free article] [PubMed]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences, and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. http://dx.doi.org/10.1093/cercor/bhi044. [DOI] [PubMed]
- Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience and Biobehavioral Reviews. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. http://dx.doi.org/10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: A study of a five-year change. Neurology. 2004;62:433–438. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21:149–157 . doi: 10.1037/0894-4105.21.2.149. http://dx.doi.org/10.1037/0894-4105.21.2.149. [DOI] [PubMed]
- Raz N, Torres IJ, Spencer WD, Millman D, Baertschi JC, Sarpel G. Neuroanatomical correlates of age-sensitive and age-invariant cognitive abilities: An in vivo MRI investigation. Intelligence. 1993;17:407–422. http://dx.doi.org/10.1016/0160-2896(93)90008-S.
- Raz N, Williamson A, Gunning-Dixon F, Head D, Acker JD. Neuroanatomical and cognitive correlates of adult age differences in acquisition of a perceptual-motor skill. Microscopy Research and Technique. 2000;51:85–93. doi: 10.1002/1097-0029(20001001)51:1<85::AID-JEMT9>3.0.CO;2-0. http://dx.doi.org/10.1002/1097-0029(20001001)51:1<85::AID-JEMT9>3.0.CO;2-0. [DOI] [PubMed]
- Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, Bryan RN, Zonderman AB. One-year age changes in MRI brain volumes in older adults. Cerebral Cortex. 2000;10:464–472. doi: 10.1093/cercor/10.5.464. http://dx.doi.org/10.1093/cercor/10.5.464. [DOI] [PubMed]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. The Journal of Neurosciences. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettmann ME, Kraut MA, Prince JL, Resnick SM. Cross-sectional and longitudinal analyses of anatomical sulcal changes associated with aging. Cerebral Cortex. 2006;16:1584–1594 . doi: 10.1093/cercor/bhj095. http://dx.doi.org/10.1093/cercor/bhj095. [DOI] [PubMed]
- Rodrigue KM, Raz N. Shrinkage of the entorhinal cortex over five years predicts memory performance in healthy adults. Journal of Neuroscience. 2004;24:956–963. doi: 10.1523/JNEUROSCI.4166-03.2004. http://dx.doi.org/10.1523/JNEUROSCI.4166-03.2004. [DOI] [PMC free article] [PubMed]
- Ronnlund M, Nilsson L-G. Cross-sectional versus longitudinal age gradients and relations to demographic factors. Intelligence. 2006;34:63–78. http://dx.doi.org/10.1016/j.intell.2005.06.004.
- Ronnlund M, Nyberg L, Backman L, Nilsson L-G. Stability, growth, and decline in adult life span development of declarative memory: Cross-sectional and longitudinal data from a population-based study. Psychology and Aging. 2005;20:3–18 . doi: 10.1037/0882-7974.20.1.3. http://dx.doi.org/10.1037/0882-7974.20.1.3. [DOI] [PubMed]
- Rosen AC, Gabrieli JDE, Stoub T, Prull MW, O’Hara R, Yesavage J, de Toledo-Morrell L. Relating medial temporal lobe volume to frontal fMRI activation for memory encoding in older adults. Cortex. 2005;41:595–602. doi: 10.1016/s0010-9452(08)70199-0. http://dx.doi.org/10.1016/S0010-9452(08)70199-0. [DOI] [PubMed]
- Rovaris M, Iannucci G, Cercignani M, Sormani MP, de Stefano N, Gerevini S, Coml G, Fillippi M. Age-related changes in conventional, magnetization transfer, and diffusion-tensor MR imaging findings: Study with whole-brain tissue histogram analysis. Radiology. 2003;227:731–738. doi: 10.1148/radiol.2273020721. http://dx.doi.org/10.1148/radiol.2273020721. [DOI] [PubMed]
- Rushton JP, Ankney CD. Whole brain size and general mental ability: A review. International Journal of Neuroscience. 2009;119:692–732. doi: 10.1080/00207450802325843. http://dx.doi.org/10.1080/00207450802325843. [DOI] [PMC free article] [PubMed]
- Sachdev P, Wen W, Chen X, Brodaty H. Progression of white matter hyperintensities in elderly individuals over 3 years. Neurology. 2007;68:214–222. doi: 10.1212/01.wnl.0000251302.55202.73. http://dx.doi.org/10.1212/01.wnl.0000251302.55202.73. [DOI] [PubMed]
- Saczynski JS, Jonsdottir MK, Sigurdsson S, Eiriksdottir G, Jonsson PV, Garcia ME, Kjartansson O, van Buchem MA, Gudnason V, Launer LJ. White matter lesions and cognitive performance; The role of cognitively complex leisure activity. Journal of Gerontology: Medical Science. 2008;63A:848–854. doi: 10.1093/gerona/63.8.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, Ebisu Y, Ikeda A, Nakaya M, Nishizaki T. Hippocampal size may contribute to prospective diagnosis of age-related dementia. Psychogeriatrics. 2007;7:76–80. http://dx.doi.org/10.1111/j.1479-8301.2007.00192.x.
- Salat DH, Greve DN, Pacheco JL, Quinn BT, Helmer KG, Buckner RL, Fischl BG. Regional white matter volume differences in nondemented aging and Alzheimer’s disease. NeuroImage. 2009;44:1247–1258. doi: 10.1016/j.neuroimage.2008.10.030. http://dx.doi.org/10.1016/j.neuroimage.2008.10.030. [DOI] [PMC free article] [PubMed]
- Salat DH, Kaye JA, Janowsky JS. Greater orbital prefrontal volume selectively predicts worse working memory performance in older adults. Cerebral Cortex. 2002;12:494–505. doi: 10.1093/cercor/12.5.494. http://dx.doi.org/10.1093/cercor/12.5.494. [DOI] [PubMed]
- Salat DH, Lee SY, van der Kouwe AJ, Greve DN, Fischl B, Rosas HD. Age-associated alterations in cortical gray matter and white matter signal intensity and gray to white matter contrast. NeuroImage. 2009;48:21–28. doi: 10.1016/j.neuroimage.2009.06.074. http://dx.doi.org/10.1016/j.neuroimage.2009.06.074. [DOI] [PMC free article] [PubMed]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJW, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiology of Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. http://dx.doi.org/10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed]
- Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Annals of the New York Academy of Sciences. 2005;1064:37–49. doi: 10.1196/annals.1340.009. http://dx.doi.org/10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed]
- Salthouse TA. Does the meaning of neurocognitive change change with age? Neuropsychology. 2010a;24:273–278. doi: 10.1037/a0017284. http://dx.doi.org/10.1037/a0017284. [DOI] [PMC free article] [PubMed]
- Salthouse TA. The paradox of cognitive change. Journal of Clinical and Experimental Neuropsychology. 2010b;32:622–629. doi: 10.1080/13803390903401310. http://dx.doi.org/10.1080/13803390903401310. [DOI] [PMC free article] [PubMed]
- Salthouse TA. Influence of age on practice effects in longitudinal neurocognitive change. Neuropsychology. 2010c;24:563–572. doi: 10.1037/a0019026. http://dx.doi.org/10.1037/a0019026. [DOI] [PMC free article] [PubMed]
- Salthouse TA. Major Issues in Cognitive Aging. New York: Oxford University Press; 2010d. [Google Scholar]
- Salthouse TA, Ferrer-Caja E. What needs to be explained to account for age-related effects on multiple cognitive variables? Psychology and Aging. 2003;18:91–110. doi: 10.1037/0882-7974.18.1.91. http://dx.doi.org/10.1037/0882-7974.18.1.91. [DOI] [PubMed]
- Salthouse TA, Nesselroade JR. An examination of the Hofer and Sliwinski (2001) evaluation. Gerontology. 2002;48:18–21. doi: 10.1159/000048919. http://dx.doi.org/10.1159/000048919. [DOI] [PubMed]
- Sasson E, Doniger GM, Pasternak O, Assaf Y. Structural correlates of memory performance with diffusion tensor imaging. NeuroImage. doi: 10.1016/j.neuroimage.2009.12.079. in press. http://dx.doi.org/10.1016/S1053-8119(09)71449-1. [DOI] [PubMed]
- Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Archives of Neurology. 2003;60:989–994. doi: 10.1001/archneur.60.7.989. http://dx.doi.org/10.1001/archneur.60.7.989. [DOI] [PubMed]
- Schaie KW. Developmental influences on adult intelligence: The Seattle Longitudinal Study. New York: Oxford University Press; 2005. [Google Scholar]
- Schiavone F, Charlton RA, Barrick TR, Morris RG, Markus HS. Imaging age-related cognitive decline: A comparison of diffusion tensor and magnetization transfer MRI. Journal of Magnetic Resonance Imaging. 2009;29:23–30 . doi: 10.1002/jmri.21572. http://dx.doi.org/10.1002/jmri.21572. [DOI] [PubMed]
- Schmidt R, Ropele S, Enzinger C, Petrovic K, Smith S, Schmidt H, Matthews PM, Fazekas F. White matter lesion progression, brain atrophy, and cognitive decline: The Austrian Stroke Prevention Study. Annals of Neurology. 2005;58:610–616. doi: 10.1002/ana.20630. http://dx.doi.org/10.1002/ana.20630. [DOI] [PubMed]
- Schretlen D, Pearlson GD, Anthony JC, Aylward EH, Augustine AM, Davis A, Barta P. Elucidating the contribution of processing speed, executive ability, and frontal lobe volume to normal age-related differences in fluid intelligence. Journal of the International Neuropsychological Society. 2000;6:52–61 . doi: 10.1017/s1355617700611062. http://dx.doi.org/10.1017/S1355617700611062. [DOI] [PubMed]
- Schwartz BS, Chen S, Caffo B, Stewart WF, Bolla KI, Yousem D, Davatzikos C. Relations of brain volumes with cognitive function in males 45 years and older with past lead exposure. NeuroImage. 2007;37:633–641. doi: 10.1016/j.neuroimage.2007.05.035. http://dx.doi.org/10.1016/j.neuroimage.2007.05.035. [DOI] [PMC free article] [PubMed]
- Selig JP, Preacher KJ. Mediation models for longitudinal data in developmental research. Research in Human Development. 2009;62:144–164. http://dx.doi.org/10.1080/15427600902911247.
- Seshadri S, Wolf PA, Beiser A, Elias MF, Au R, Kase CS, D’Agostino RB, DeCarli C. Stroke risk profile, brain volume, and cognitive function. The Framingham Offspring Study. Neurology. 2004;63:1591–1599. doi: 10.1212/01.wnl.0000142968.22691.70. http://dx.doi.org/10.1080/03610730490484380. [DOI] [PubMed]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychological Methods. 2002;7:422–445. http://dx.doi.org/10.1037//1082-989X.7.4.422. [PubMed]
- Soderlund H, Nyberg L, Nilsson LG. Cerebral atrophy as predictor of cognitive function in old, community-dwelling individuals. Archives of Neurology Scandinavia. 2004;109:398–406 . doi: 10.1111/j.1600-0404.2004.00239.x. http://dx.doi.org/10.1111/j.1600-0404.2004.00239.x. [DOI] [PubMed]
- Soderlund H, Nilsson L-G, Berger K, Breteler MM, Dufouil C, Fuhrer R, Giampaoli S, Hofman A, Pajak A, de Ridder M, Sans S, Schmidt R, Launer LJ. Cerebral changes on MRI and cognitive function: The CASCADE study. Neurobiology of Aging. 2006;27:16–23. doi: 10.1016/j.neurobiolaging.2004.12.008. http://dx.doi.org/10.1016/j.neurobiolaging.2004.12.008. [DOI] [PubMed]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. http://dx.doi.org/10.1038/nn1008. [DOI] [PubMed]
- Stadlbauer A, Salomonowitz E, Strunk G, Hammen T, Ganslandt O. Age-related degradation in the central nervous system: Assessment with diffusion-tensor imaging and quantitative fiber tracking. Radiology. 2008;247:179–188. doi: 10.1148/radiol.2471070707. http://dx.doi.org/10.1148/radiol.2471070707. [DOI] [PubMed]
- Staff RT, Murray AD, Deary IJ, Whalley LJ. Generality and specificity in cognitive aging: A volumetric brain analysis. NeuroImage. 2006;30:1433–1440. doi: 10.1016/j.neuroimage.2005.11.004. http://dx.doi.org/10.1016/j.neuroimage.2005.11.004. [DOI] [PubMed]
- Stone-Romero EF, Rosopa PJ. The relative validity of inferences about mediation as a function of research design characteristics. Organizational Research Methods. 2008;11:326–352. http://dx.doi.org/10.1177/1094428107300342.
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cerebral Cortex. 2006;16:1030–1039. doi: 10.1093/cercor/bhj045. http://dx.doi.org/10.1093/cercor/bhj045. [DOI] [PubMed]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff’s syndrome: Relation to ataxia. Neuropsychology. 2000;14:341–352. doi: 10.1037//0894-4105.14.3.341. http://dx.doi.org/10.1037//0894-4105.14.3.341. [DOI] [PubMed]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neuroscience and Biobehavioral Reviews. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. http://dx.doi.org/10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed]
- Sullivan EV, Pfefferbaum A, Adalsteinsson E, Swan GE, Carmelli D. Differential rates of regional brain change in callosal and ventricular size: A 4-year longitudinal MRI study of elderly men. Cerebral Cortex. 2002;12:438–445. doi: 10.1093/cercor/12.4.438. http://dx.doi.org/10.1093/cercor/12.4.438. [DOI] [PubMed]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: Relations to timed performance. Neurobiology of Aging. 2010;31:464–481. doi: 10.1016/j.neurobiolaging.2008.04.007. http://dx.doi.org/10.1016/j.neurobiolaging.2008.04.007. [DOI] [PMC free article] [PubMed]
- Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, Carmelli D. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51:986–993. doi: 10.1212/wnl.51.4.986. [DOI] [PubMed] [Google Scholar]
- Taki Y, Goto R, Evans A, Zijdenbos A, Neelin P, Lerch J, Sato K, Ono S, Kinomura S, Nakagawa M, Sugiura M, Watanabe Y, Kawashima R, Fukuda H. Voxel-based morphometry of human brain with age and cerebrovascular risk factors. Neurobiology of Aging. 2004;25:455–463. doi: 10.1016/j.neurobiolaging.2003.09.002. http://dx.doi.org/10.1016/j.neurobiolaging.2003.09.002. [DOI] [PubMed]
- Taki Y, Kinomura S, Sato K, Goto R, Kawashima R, Fukuda H. A longitudinal study of gray matter volume decline with age and modifying factors. Neurobiology of Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.05.003. (Advance Publication). http://dx.doi.org/10.1016/j.neurobiolaging.2009.05.003. [DOI] [PubMed]
- Terribilli D, Schaufelberger MS, Duran FLS, Zanetti MV, Curiati PK, Menezes PR, Scazufca M, Amaro E, Leite CC, Busatto GF. Age-related gray matter volume changes in the brain during non-elderly adulthood. Neurobiology of Aging. 2011;32:354–368. doi: 10.1016/j.neurobiolaging.2009.02.008. http://dx.doi.org/10.1016/j.neurobiolaging.2009.02.008. [DOI] [PMC free article] [PubMed]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen V-P, Huttunen M, Lonnqvist J, Standertskjold-Nordenstam C-G, Kaprio J, Khaledy M, Dail R, Zoumalan CI, Toga AW. Genetic influences on brain structure. Nature Neuroscience. 2001;4:1253–1258. doi: 10.1038/nn758. http://dx.doi.org/10.1038/nn758. [DOI] [PubMed]
- Thomsen T, Specht K, Hammar A, Nyttingnes J, Ersland L, Hugdahl K. Brain localization of attentional control in different age groups by combining functional and structural MRI. NeuroImage. 2004;22:912–919. doi: 10.1016/j.neuroimage.2004.02.015. http://dx.doi.org/10.1016/j.neuroimage.2004.02.015. [DOI] [PubMed]
- Tisserand DJ, Pruessner JC, Santz Arigita EJ, van Boxtel MPJ, Evans AC, Jolles J, Uylings HBM. Regional frontal cortical volumes decrease differentially in aging: An MRI study to compare volumetric approaches and voxel-based morphometry. NeuroImage. 2002;17:657–669. http://dx.doi.org/10.1006/nimg.2002.1173. [PubMed]
- Tisserand DJ, van Boxtel MPJ, Pruessner JC, Hofman P, Evans AC, Jolles J. A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cerebral Cortex. 2004;14:966–973. doi: 10.1093/cercor/bhh057. http://dx.doi.org/10.1093/cercor/bhh057. [DOI] [PubMed]
- Tisserand DJ, Visser PJ, van Boxtel MPJ, Jolles J. The relation between global and limbic brain volumes on MRI and cognitive performance in healthy individuals across the age range. Neurobiology of Aging. 2000;21:569–576. doi: 10.1016/s0197-4580(00)00133-0. http://dx.doi.org/10.1016/S0197-4580(00)00133-0. [DOI] [PubMed]
- Tuch DS, Salat DH, Wisco JJ, Zaleta AK, Hevelone ND, Rosas HD. Choice reaction time performance correlates with diffusion anisotropy in white matter pathways supporting visual attention. Proceedings of the National Academy of Sciences. 2005;102:12212–12217. doi: 10.1073/pnas.0407259102. http://dx.doi.org/10.1073/pnas.0407259102. [DOI] [PMC free article] [PubMed]
- Tullberg M, Fletcher E, DeCarli C, Mungas D, Reed BR, Harvey DJ, Weinder MW, Chui HC, Jagust WJ. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2005;63:246–253. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupler LA, Krishnan KRR, Greenberg DL, Marcovina SM, Payne ME, MacFall JR, Charles HC, Doraiswamy PM. Predicting memory decline in normal elderly: Genetics, MRI, and cognitive reserve. Neurobiology of Aging. 2007;28:1644–1656. doi: 10.1016/j.neurobiolaging.2006.07.001. http://dx.doi.org/10.1016/j.neurobiolaging.2006.07.001. [DOI] [PubMed]
- Turken AU, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JDE. Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. NeuroImage. 2008;42:1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. http://dx.doi.org/10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed]
- Tyler LK, Shafto MA, Randall B, Wright P, Marslen-Wilson WD, Stamatakis EA. Preserving syntactic processing across the adult life span: The modulation of the frontotemporal language system in the context of age-related atrophy. Cerebral Cortex. 2010;20:352–364. doi: 10.1093/cercor/bhp105. http://dx.doi.org/10.1093/cercor/bhp105. [DOI] [PMC free article] [PubMed]
- Ullen F, Forsman L, Blom O, Karabanov A, Madison G. Intelligence and variability in a simple timing task share neural substrates in the prefrontal white matter. Journal of Neuroscience. 2008;28:4238–4243. doi: 10.1523/JNEUROSCI.0825-08.2008. http://dx.doi.org/10.1523/JNEUROSCI.0825-08.2008. [DOI] [PMC free article] [PubMed]
- Van den Heuvel DMJ, ten Dam VH, de Craen AJM, Admiraal-Behloul F, Olofsen H, Bollen EKEM, Jolles J, Murray HM, Blauw GJ, Westendorp RGJ, van Buchem MA. Increase in periventricular white matter hyperintensities parallels decline in mental processing speed in a non-demented elderly population. Journal of Neurology, Neurosurgery and Psychiatry. 2007;77:149–153. doi: 10.1136/jnnp.2005.070193. http://dx.doi.org/10.1136/jnnp.2005.070193. [DOI] [PMC free article] [PubMed]
- Van Der Werf YD, Tisserand DJ, Visser PJ, Hofman PAM, Vurrman E, Uylings HBM, Jolles J. Thalamic volume predicts performance on tests of cognitive speed and decreases in healthy aging: A magnetic resonance imaging-based volumetric analysis. Cognitive Brain Research. 2001;11:377–385. doi: 10.1016/s0926-6410(01)00010-6. http://dx.doi.org/10.1016/S0926-6410(01)00010-6. [DOI] [PubMed]
- Vannorsdall TD, Waldstein SR, Kraut M, Pearlson GD, Schretlen DJ. White matter abnormalities and cognition in a community sample. Archives of Clinical Neuropsychology. 2009;24:209–217. doi: 10.1093/arclin/acp037. http://dx.doi.org/10.1093/arclin/acp037. [DOI] [PMC free article] [PubMed]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: Review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Plante E, Davidson PSR, Kuo TY, Bajuscak L, Glisky EL. Memory and executive function in older adults: Relationships with temporal and prefrontal gray matter volumes and white matter hyperintensities. Neuropsychologia. 2004;42:1313–1335. doi: 10.1016/j.neuropsychologia.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Venkatraman VK, Aizenstein H, Guralnik J, Newman AB, Glynn NW, Taylor C, Studenski S, Launer L, Pahor M, Williamson J, Rosano C. Executive control function, brain activation and white matter hyperintensities in older adults. NeuroImage. 2010;49:3436–3442. doi: 10.1016/j.neuroimage.2009.11.019. http://dx.doi.org/10.1016/j.neuroimage.2009.11.019. [DOI] [PMC free article] [PubMed]
- Vernooij MW, de Groot M, van der Lugt A, Ikram MA, Krestin GP, Hofman A, Niessen WJ, Breteler MMB. White matter atrophy and lesion formation explain the loss of structural integrity of white matter in aging. NeuroImage. 2008;43:470–477. doi: 10.1016/j.neuroimage.2008.07.052. http://dx.doi.org/10.1016/j.neuroimage.2008.07.052. [DOI] [PubMed]
- Voineskos AN, Rajji TK, Lobaugh NJ, Miranda D, Shenton ME, Kennedy JL, Pollock BG, Mulsant BH. Age-related decline in white matter tract integrity and cognitive performance: A DTI tractography and structural equation modeling study. Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2010.02.009. in press. http://dx.doi.org/10.1016/j.neurobiolaging.2010.02.009. [DOI] [PMC free article] [PubMed]
- Walhovd KB, Fjell AM. White matter volume predicts reaction time instability. Neuropsychologia. 2007;45:2277–2284. doi: 10.1016/j.neuropsychologia.2007.02.022. http://dx.doi.org/10.1016/j.neuropsychologia.2007.02.022. [DOI] [PubMed]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiology of Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. http://dx.doi.org/10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Fischl B, Quinn BT, Dale AM. Size does matter in the long run: Hippocampal and cortical volume predict recall across weeks. Neurology. 2004;63:1193–1197. doi: 10.1212/01.wnl.0000140489.33249.95. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Fjell AM. Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiology of Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.05.013. (Advance Publication). http://dx.doi.org/10.1016/j.neurobiolaging.2009.05.013. [DOI] [PMC free article] [PubMed]
- Westlye LT, Walhovd KB, Dale AM, Bjornerud A, Due-Tonnessen P, Engvig A, Grydeland H, Tamnes CK, Ostby Y, Fjell AM. Life-span changes of the human brain white matter: Diffusion tensor imaging (DTI) and volumetry. Cerebral Cortex. 2010;20:2055–2068. doi: 10.1093/cercor/bhp280. http://dx.doi.org/10.1093/cercor/bhp280. [DOI] [PubMed]
- Wickett JC, Vernon PA, Lee DH. Relationships between factors of intelligence and brain volume. Personality and Individual Differences. 2000;29:1095–1122. http://dx.doi.org/10.1016/S0191-8869(99)00258-5.
- Willerman L, Schultz R, Rutledge JN, Bigler ED. In vivo brain size and intelligence. Intelligence. 1991;15:223–228. http://dx.doi.org/10.1016/0160-2896(91)90031-8.
- Woodard JL, Seidenberg M, Nielson KA, Smith JC, Antuono P, DUrgerian S, Guidotti L, Zhang Q, Butts A, Hantke N, Lancaster M, Rao SM. Prediction of cognitive decline in healthy older adults using fMRI. Journal of Alzheimer’s Disease. 2010;21:871–885. doi: 10.3233/JAD-2010-091693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CB, Festa JR, Paik MC, Schmiedigen A, Brown TR, Yoshita M, DeCarli C, Sacco R, Stern Y. White matter hyperintensities and subcliical infarction: associations with psychomotor speed and cognitive flexibility. Stroke. 2008;39:800–805. doi: 10.1161/STROKEAHA.107.484147. http://dx.doi.org/10.1161/STROKEAHA.107.484147. [DOI] [PMC free article] [PubMed]
- Ylikoski R, Salonen O, Mantyla R, Ylikoski A, Keskivaara P, Leskelia M, Erkinjuntti T. Hippocampal and temporal lobe atrophy and age-related decline in memory. Acta Neurology Scandinavia. 2000;101:273–278. doi: 10.1034/j.1600-0404.2000.101004273.x. http://dx.doi.org/10.1034/j.1600-0404.2000.101004273.x. [DOI] [PubMed]
- Ylikoski R, Ylikoski A, Erkinjuntti T, Sulkava R, Raininko R, Tilvis R. White matter changes in healthy elderly persons correlate with attention and speed of mental processing. Archives of Neurology. 1993;50:818–824. doi: 10.1001/archneur.1993.00540080029009. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Widaman K, Mungas D, Reed B, Weinder MW, Chui HC. Memory in the aging brain: Doubly dissociating the contribution of the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1134–1140. doi: 10.1002/hipo.20341. http://dx.doi.org/10.1002/hipo.20341. [DOI] [PMC free article] [PubMed]
- Yoon B, Shim Y-S, Lee K-S, Shon Y-M, Yang D-W. Region-specific changes of cerebral white matter during normal aging: A diffusion-tensor analysis. Archives of Gerontology and Geriatrics. 2008;47:129–138. doi: 10.1016/j.archger.2007.07.004. http://dx.doi.org/10.1016/j.archger.2007.07.004. [DOI] [PubMed]
- Ystad MA, Lundervold AJ, Wehling E, Espeseth T, Rootwelt H, Westlye LT, Andersson M, Adolfsdottir S, Geitung JT, Fjell AM, Reinvang I, Lundervold A. Hippocampal volumes are important predictors of memory function in elderly women. BMC Medical Imaging. 2009;9:17 . doi: 10.1186/1471-2342-9-17. http://dx.doi.org/10.1186/1471-2342-9-17. [DOI] [PMC free article] [PubMed]
- Yu C, Li J, Liu Y, Qin W, Li Y, Shu N, Jiang T, Li K. White matter tract integrity and intelligence in patients with mental retardation and healthy adults. NeuroImage. 2008;40:1533–1541. doi: 10.1016/j.neuroimage.2008.01.063. http://dx.doi.org/10.1016/j.neuroimage.2008.01.063. [DOI] [PubMed]
- Zahr NM, Rohlfing T, Pfefferbaum A, Sullivan EV. Problem solving, working memory, and motor correlates of association and commissural fiber bundles in normal aging: A quantitative fiber tracking study. NeuroImage. 2008;44:1050–1062. doi: 10.1016/j.neuroimage.2008.09.046. http://dx.doi.org/10.1016/j.neuroimage.2008.09.046. [DOI] [PMC free article] [PubMed]
- Zelinski EM, Burnight KP. Sixteen-year longitudinal and time lag changes in memory and cognition in older adults. Psychology and Aging. 1997;12:503–513. doi: 10.1037//0882-7974.12.3.503. http://dx.doi.org/10.1037//0882-7974.12.3.503. [DOI] [PubMed]
- Ziegler DA, Piguet O, Salat DH, Prince K, Connally E, Corkin S. Cognition in healthy aging is related to regional white matter integrity but not cortical thickness. Neurobiology of Aging. 2010;31:1912–1926. doi: 10.1016/j.neurobiolaging.2008.10.015. http://dx.doi.org/10.1016/j.neurobiolaging.2008.10.015. [DOI] [PMC free article] [PubMed]
- Zimmerman ME, Brickman AM, Paul RH, Grieve SM, Tate DF, Gunstad J, Cohen RA, Aloia MS, Williams LM, Clark CR, Whitford TJ, Gordon E. The relationship between frontal gray matter volume and cognition varies across the healthy adult lifespan. American Journal of Geriatric Psychiatry. 2006;14:823–833. doi: 10.1097/01.JGP.0000238502.40963.ac. http://dx.doi.org/10.1097/01.JGP.0000238502.40963.ac. [DOI] [PubMed]