Abstract
Squamous cell carcinoma of the anus (SCCA) is a common cancer in the human immunodeficiency virus (HIV)-infected population, and its incidence continues to increase in male homosexuals. Combined chemoradiation with mitomycin C and 5-fluorouracil was poorly tolerated by severely immunocompromised patients in the early 1990s. In the era of highly active antiretroviral therapy (HAART), however, recent data indicate that: (1) most HIV patients with anal cancer can tolerate standard chemotherapy regimens; and (2) this approach is associated with survival rates similar to those of HIV-negative patients. However, HIV-positive patients with SCCA are much younger, more likely to develop local tumor recurrence, and ultimately die from anal cancer than immune competent patients. Taken together, these findings suggest that anal cancer is an often fatal neoplasia in middle-aged HIV-positive male homosexuals. In this population, SCCA is an opportunistic disease resulting in patients with suboptimal immune function from persistent infection and prolonged exposition to oncogenic human papillomaviruses (HPVs). Large-scale cancer-prevention strategies (routine anuscopy and anal papanicolaou testing) should be implemented in this population. In addition, definitive eradication of oncogenic HPVs within the anogenital mucosa of high-risk individuals might require a proactive approach with repeated vaccination.
Keywords: Anal cancer, Chemoradiation, Highly active antiretroviral therapy, Human immunodeficiency virus, Human papillomaviruse, Outcome
INTRODUCTION
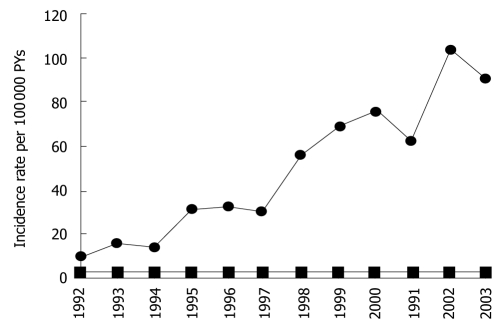
As human immunodeficiency virus (HIV)-infected individuals continue to benefit from highly active antiretroviral therapy (HAART), their risk of dying from neoplasia, including non-AIDS-defining cancers (NADC) is increased[1]. The incidence of squamous cell carcinoma of the anus (SCCA) is not only higher in the HIV-positive population, but continues to increase in the United States[2] (Figure 1). In Australia, anal cancer is now the third most common cancer in the HIV-infected population[3]. SCCA is a sexually transmitted disease clinically related to infection with oncogenic human papillomaviruses (HPV 16-18)[4,5]. Long before the AIDS epidemics, the pivotal role of immune suppression in anal carcinogenesis was highlighted by the high incidence of these tumors in solid organ transplant patients, irrespective of sexual practice[6,7]. In a large French HIV cohort study, the risk of anal cancer increased with the time during which the CD4 count was < 200 cells/microL and viral load was > 100 000 copies/mL[8]. Thus, both compromised immune function and HPV infection play a role in the development of anal intra-epithelial neoplasia (AIN), the precursor lesion of invasive SCCA.
Figure 1.
Annual incidence rates of anal cancer among HIV-infected persons (circles) and the general population (squares), USA 1992-2003.
On a therapeutic standpoint, SCCA has served as a paradigm for the successful application of chemoradiation to solid tumors[9]. Since 1974, it is admitted that: (1) A majority of anal cancers can be cured with chemoradiation therapy (CRT), using 5-fluorouracil (5-FU) and mitomycin C (MMC); and (2) Surgical excision should be restricted to patients who fail to respond to CRT[10,11]. While treatment protocols have remained virtually unchanged during the past three decades, the patients who benefit from this approach nowadays are very different from those who were treated in the 70 s and 80 s. In the 1990s, CRT was poorly tolerated by HIV-positive patients[12,13]. Today, in the Western world, up to 50% of patients with SCCA are relatively young (40-60 years) male homosexuals under HAART[14]. The aim of this paper is to review the clinical data pertaining to clinical outcome of anal cancer in HIV-positive individuals before and after the introduction of HAART.
MANAGEMENT AND OUTCOME OF SCCA IN HIV-NEGATIVE PATIENTS
Combined chemoradiation with MMC and 5-FU is poorly tolerated by immunocompromised patients, and is associated with considerable toxicity in immune competent patients. Many HIV-negative patients with SCCA require radiotherapy breaks and/or chemotherapy dose reduction. In the Memorial Sloan-Kettering Cancer Center series, > 40% (all HIV negative) of patients needed chemotherapy dose reduction of at least one agent, and 77% had at least one radiotherapy break[15]. Data from four prospective randomized trials in HIV-negative patients[16-19] also indicate: (1) a male: female ratio of 1:2; (2) a median age > 60 years; (3) a local failure rate of 30%; and (4) a 3-year overall survival rate of 70%-75% (Table 1).
Table 1.
Clinical characteristics and outcome of human immunodeficiency virus-negative patients with squamous cell carcinoma of the anus
| Author | Trial | Yr | n | Male (%) | Age (range) | Local failure (%) | Overall survival |
| Northover et al[16] | UKCCCR | 1987-1991 | 577 | 45 | 64 (26-88) | 39 | 65% at 3 yr |
| Bartelink et al[17] | EORTC | 1987-1994 | 103 | 29 | 60 | 29 | 69% at 3 yr |
| Flam et al[18] | RTOG 87-04 | 1987-1991 | 291 | 30 | 62 (29-85) | 24 | |
| Ajani et al[19] | RTOG 98-11 | 1998-2005 | 644 | 31 | 55 (25-88) | 25 | 84% at 3 yr |
A closer analysis of data reveals, however, that HIV-negative individuals with SCCA represent a relatively old population of patients who rarely succumb to anal cancer. In the UKCCCR trial[16], 54% of deaths in the chemoradiation group were due to co-morbid conditions or second malignancies, and thus were not related to SCCA. In the RTOG trial[18], out of 146 HIV-negative patients who were treated with MMC-based chemoradiation, there were 32 deaths, but only 15 (46%) were attributed to anal cancer progression. In the MD Anderson Cancer Center series, out of 167 (161 HIV-negative) patients, there were 42 deaths, and only 21 (50%) were due to anal cancer[20]. In summary, 5-year overall survival of HIV-negative patients with SCCA who undergo MMC-based chemoradiation is close to 70%, but > 50% of deaths are unrelated to anal cancer. In accordance with the initial experience of Norman Nigro reported 30 years ago[21], these data indicate that SCCA in this population has limited metastatic potential and is ultimately responsible for the death of < 20% of patients.
MANAGEMENT AND OUTCOME OF SCCA IN HIV-POSITIVE PATIENTS IN THE HIV ERA (1982-1995)
In the pre-HAART era, HIV-positive individuals demonstrated poor tolerance to MMC-based chemoradiation protocols for anal cancer. Nonetheless, it was recommended that HIV-positive patients with CD4+ > 200/mm3 should be treated with the standard chemoradiation regimen, whenever possible[22]. In at least seven small series[23-29], clinicians were struck by the fact that HIV-positive and HIV-negative SCCA patients differed by age (40-45 years vs 60-65 years), male gender (90%-95% vs 35%-40%), and homosexuality. Thus, the experience of treating HIV-positive patients with anal cancer prior to the development of HAART was essentially witnessing the emergence of a high-risk population (Table 2).
Table 2.
Clinical characteristics and outcome of human immunodeficiency virus-positive patients with squamous cell carcinoma of the anus before the era of highly active antiretroviral therapy
| Author | Yr | n | Male (%) | Age (range) | Toxicity 3-4 (%) | Local failure (%) | Overall survival |
| Kim et al[23] | 1985-1998 | 13 | 92 | 42 | 80 | 61 | 34% at 5 yr |
| Holland et al[24] | 1980-1993 | 7 | 100 | 41 | 100 | 43 | 29% at 2 yr |
| Peddada et al[25] | 1987-1995 | 8 | 100 | 48 (37-70) | 100 | 12 | 50% at 3 yr |
| Hoffman et al[26] | 1991-1997 | 17 | 64 | 25 | |||
| Cleator et al[27] | 1989-1999 | 12 | 100 | 43 (30-53) | 50 | 25 | 60% at 2 yr |
| Place et al[28] | 1980-1999 | 14 | 100 | 42 (28-58) | 50 | 57 | 20% at 5 yr |
| Efron et al[29] | 1988-1999 | 6 | 100 | 40 (29-46) | 67 |
Many of these young homosexuals would eventually die of AIDS, with or without evidence of residual anal cancer - but the latter was rarely considered the primary cause of death at a time when median survival with a diagnosis of AIDS was only 17 mo[30]. In the series from Kaiser Permanente Medical Center in Los Angeles[25], after a median follow-up of 38 mo, half of patients were alive and disease-free, while the other half had died from complications of AIDS. Results in terms of local recurrence were disappointing, but many patients did not receive standard chemotherapy for fear of significant hematologic toxicity. Nonetheless, acute toxicity was quite frequent (> 50%), and local tumor recurrence rates were elevated (40%-50%). In addition, Kim et al[23] were the first to note that: (1) HIV-positive patients were more likely to die from SCCA than HIV-negative patients, who often succumbed to other, cancer-unrelated causes; and (2) the median time to cancer-related death in HIV-positive individuals was 1.4 years vs 5.3 years for HIV-negative patients. Since AIN progresses more quickly towards SCCA in HIV-positive patients, it was logical to hypothesize that the molecular biology of anal cancer might differ between the two groups[31,32].
MANAGEMENT AND OUTCOME OF SCCA IN HIV-POSITIVE PATIENTS IN THE HAART ERA (1996-)
HAART does neither prevent the development of AIN, nor the progression of AIN towards SCCA[33,34]. The rising incidence of anal cancer in the HIV-positive population during 1996-2004 is well documented[35]. HAART certainly had a positive impact on patients’ ability to tolerate chemoradiation treatment; accordingly, many radiologists strongly caution against scaling back treatment of anal cancer in HIV-positive individuals[36]. This is also motivated by the recent recognition that SCCA is the greatest threat to these patients’ lives. We have summarized, in Table 3, the results of eleven studies published since 2004, which evaluated the outcome of HIV-positive patients with SCCA in the HAART era[37-47]. With two exceptions[40,42], these small series are underpowered, and inadequate to detect survival differences between HIV-positive and HIV-negative individuals.
Table 3.
Clinical characteristics and outcome of human immunodeficiency virus-positive patients with squamous cell carcinoma of the anus during the era of highly active antiretroviral therapy
| Author | Yr | n | Male (%) | Age (range) | Local failure (%) | Overall survival |
| Stadler et al[37] | 1998-2002 | 8 | 100 | 44 (34-61) | 50 | 67% at 2 yr |
| Blazy et al[38] | 1997-2001 | 9 | 100 | 36 (35-49) | 11 | 100% at 2 yr |
| Bower et al[39] | 1996-2003 | 26 | 100 | 42 (28-56) | 23 | 47% at 5 yr |
| Chiao et al[40] | 1998-2004 | 175 | 99.5 | 49 (43-55) | 66% at 4 yr | |
| Wexler et al[41] | 1997-2005 | 32 | 94 | 45 (31-68) | 16 | 65% at 5 yr |
| Oehler-Jänne et al[42] | 1997-2006 | 40 | 93 | 48 (34-75) | 62 | 61% at 5 yr |
| Abramowitz et al[43] | 1998-2004 | 44 | 100 | 45 | 32 | 85% at 3 yr |
| Seo et al[44] | 1999-2007 | 14 | 93 | 45 (34-59) | 92% at 3 yr | |
| Barriger et al[45] | 1995-2008 | 17 | 100 | 44 (29-53) | 59 | 50% at 5 yr |
| Hogg et al[46] | 1996-2006 | 21 | 100 | 45 | 48 | 73% at 3 yr |
| Fraunholz et al[47] | 1997-2008 | 21 | 90 | 45 (31-68) | 41 | 67% at 5 yr |
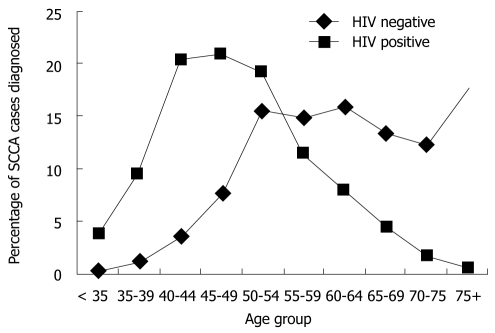
In the Veterans Affairs study[40], the authors concluded that in the HAART era, survival of SCCA is equivalent between HIV-positive and HIV-negative patients (overall 4-year survival 66% vs 62%). However, the age distribution of both groups was quite different; among HIV-positive individuals, patients aged 45-49 represented the largest percentage, whereas among HIV-negative individuals the largest percentage of patients was greater than age 75 (Figure 2). In other words, two populations with an age difference greater than 20 years have the same survival, which strongly suggests that SSCA-related mortality was higher in the HIV-positive group. This hypothesis is supported by the multicenter series reported by Oehler-Jänne et al[42]: five-year overall survival was similar in both groups (61% vs 65%), but HIV-positive individuals had a 4-fold higher risk of locoregional tumor recurrence (62% vs 13%), and the majority of them, unlike HIV-negative individuals with SCCA, died of anal cancer. In summary, and in the HAART era, HIV-individuals with SCCA carry a 50% risk of local relapse and a 33% risk of dying from anal cancer.
Figure 2.
Percentage of anal cancer diagnosed among us veterans by age group (1998-2004). HIV: Human immunodeficiency virus.
CONCLUSION
In some countries, anal cancer is now the third most common cancer in HIV-infected individuals and its incidence continues to increase, despite (or because of) the use of HAART. It is a disease of relatively young male homosexuals, who should be considered candidates for chemoradiation, using standard doses of MMC and 5-FU, as well as pelvic irradiation. There is, however, evidence that HIV-positive patients experience a higher rate of locoregional tumor recurrence and are more likely to die from anal cancer than their HIV-negative counterparts; this explains why both HIV-positive and HIV-negative groups have similar survival, despite a > 20 years difference in age. HIV-positive male homosexuals under HAART are protected from opportunistic infections, but have an increased risk of developing, and eventually succumbing to anal cancer.
SCCA was not a frequent cause of death in HIV-positive patients before 1997-1998, and this affirmation stands true in 2010 for elderly HIV-negative patients. In contrast, for middle-aged male homosexuals under HAART, SCCA is an often fatal, opportunistic cancer which results from the combination of two factors: (1) persistent immune deficiency; and (2) persistent infection with oncogenic HPVs in the anal canal. Cancer-prevention strategies should be implemented in this population: male homosexuals should undergo routine anuscopy and an anal Papanicolaou test to detect and treat precursor lesions of SCCA. This approach, if successful, might hopefully mimic in male homosexuals, the dramatic improvement observed for cancer of the uterine cervix in women. Complete eradication of oncogenic HPVs in the anogenital mucosa might also require a proactive vaccination program for high-risk individuals[48,49]. This approach could also serve an important public health purpose, reducing the pool of susceptible individuals and contributing to the control of re-emerging HPV infection.
Footnotes
Peer reviewer: Dr. Benjamin Perakath, Professor, Department of Surgery Unit 5, Christian Medical College, Vellore 632004, Tamil Nadu, India
S- Editor Tian L L- Editor Webster JR E- Editor Ma WH
References
- 1.Pantanowitz L, Schlecht HP, Dezube BJ. The growing problem of non-AIDS-defining malignancies in HIV. Curr Opin Oncol. 2006;18:469–478. doi: 10.1097/01.cco.0000239886.13537.ed. [DOI] [PubMed] [Google Scholar]
- 2.Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, Holmberg SD, Brooks JT. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med. 2008;148:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 3.van Leeuwen MT, Vajdic CM, Middleton MG, McDonald AM, Law M, Kaldor JM, Grulich AE. Continuing declines in some but not all HIV-associated cancers in Australia after widespread use of antiretroviral therapy. AIDS. 2009;23:2183–2190. doi: 10.1097/QAD.0b013e328331d384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frisch M, Glimelius B, van den Brule AJ, Wohlfahrt J, Meijer CJ, Walboomers JM, Goldman S, Svensson C, Adami HO, Melbye M. Sexually transmitted infection as a cause of anal cancer. N Engl J Med. 1997;337:1350–1358. doi: 10.1056/NEJM199711063371904. [DOI] [PubMed] [Google Scholar]
- 5.Gervaz P, Allal AS, Villiger P, Bühler L, Morel P. Squamous cell carcinoma of the anus: another sexually transmitted disease. Swiss Med Wkly. 2003;133:353–359. doi: 10.4414/smw.2003.10225. [DOI] [PubMed] [Google Scholar]
- 6.Aigner F, Boeckle E, Albright J, Kilo J, Boesmueller C, Conrad F, Wiesmayr S, Antretter H, Margreiter R, Mark W, et al. Malignancies of the colorectum and anus in solid organ recipients. Transpl Int. 2007;20:497–504. doi: 10.1111/j.1432-2277.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 7.Sunesen KG, Nørgaard M, Thorlacius-Ussing O, Laurberg S. Immunosuppressive disorders and risk of anal squamous cell carcinoma: a nationwide cohort study in Denmark, 1978-2005. Int J Cancer. 2010;127:675–684. doi: 10.1002/ijc.25080. [DOI] [PubMed] [Google Scholar]
- 8.Guiguet M, Boué F, Cadranel J, Lang JM, Rosenthal E, Costagliola D. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10:1152–1159. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 9.Ryan DP, Compton CC, Mayer RJ. Carcinoma of the anal canal. N Engl J Med. 2000;342:792–800. doi: 10.1056/NEJM200003163421107. [DOI] [PubMed] [Google Scholar]
- 10.Buchs NC, Allal AS, Morel P, Gervaz P. Prevention, chemoradiation and surgery for anal cancer. Expert Rev Anticancer Ther. 2009;9:483–489. doi: 10.1586/era.09.4. [DOI] [PubMed] [Google Scholar]
- 11.Silverberg MJ, Abrams DI. AIDS-defining and non-AIDS-defining malignancies: cancer occurrence in the antiretroviral therapy era. Curr Opin Oncol. 2007;19:446–451. doi: 10.1097/CCO.0b013e3282c8c90d. [DOI] [PubMed] [Google Scholar]
- 12.Gervaz P, Allal AS, Roth A, Morel P. Chemotherapeutic options in the management of anal cancer. Expert Opin Pharmacother. 2004;5:2479–2484. doi: 10.1517/14656566.5.12.2479. [DOI] [PubMed] [Google Scholar]
- 13.Clifford GM, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, Rapiti E, Levi F, Jundt G, Fisch T, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97:425–432. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 14.Powles T, Robinson D, Stebbing J, Shamash J, Nelson M, Gazzard B, Mandelia S, Møller H, Bower M. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J Clin Oncol. 2009;27:884–890. doi: 10.1200/JCO.2008.19.6626. [DOI] [PubMed] [Google Scholar]
- 15.Roohipour R, Patil S, Goodman KA, Minsky BD, Wong WD, Guillem JG, Paty PB, Weiser MR, Neuman HB, Shia J, et al. Squamous-cell carcinoma of the anal canal: predictors of treatment outcome. Dis Colon Rectum. 2008;51:147–153. doi: 10.1007/s10350-007-9125-z. [DOI] [PubMed] [Google Scholar]
- 16.Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet. 1996;348:1049–1054. [PubMed] [Google Scholar]
- 17.Bartelink H, Roelofsen F, Eschwege F, Rougier P, Bosset JF, Gonzalez DG, Peiffert D, van Glabbeke M, Pierart M. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040–2049. doi: 10.1200/JCO.1997.15.5.2040. [DOI] [PubMed] [Google Scholar]
- 18.Flam M, John M, Pajak TF, Petrelli N, Myerson R, Doggett S, Quivey J, Rotman M, Kerman H, Coia L, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527–2539. doi: 10.1200/JCO.1996.14.9.2527. [DOI] [PubMed] [Google Scholar]
- 19.Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB 3rd, Thomas CR Jr, Mayer RJ, Haddock MG, Rich TA, Willett C. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008;299:1914–1921. doi: 10.1001/jama.299.16.1914. [DOI] [PubMed] [Google Scholar]
- 20.Das P, Bhatia S, Eng C, Ajani JA, Skibber JM, Rodriguez-Bigas MA, Chang GJ, Bhosale P, Delclos ME, Krishnan S, et al. Predictors and patterns of recurrence after definitive chemoradiation for anal cancer. Int J Radiat Oncol Biol Phys. 2007;68:794–800. doi: 10.1016/j.ijrobp.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 21.Nigro ND, Seydel HG, Considine B, Vaitkevicius VK, Leichman L, Kinzie JJ. Combined preoperative radiation and chemotherapy for squamous cell carcinoma of the anal canal. Cancer. 1983;51:1826–1829. doi: 10.1002/1097-0142(19830515)51:10<1826::aid-cncr2820511012>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 22.Berry JM, Palefsky JM, Welton ML. Anal cancer and its precursors in HIV-positive patients: perspectives and management. Surg Oncol Clin N Am. 2004;13:355–373. doi: 10.1016/j.soc.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, Sarani B, Orkin BA, Young HA, White J, Tannebaum I, Stein S, Bennett B. HIV-positive patients with anal carcinoma have poorer treatment tolerance and outcome than HIV-negative patients. Dis Colon Rectum. 2001;44:1496–1502. doi: 10.1007/BF02234605. [DOI] [PubMed] [Google Scholar]
- 24.Holland JM, Swift PS. Tolerance of patients with human immunodeficiency virus and anal carcinoma to treatment with combined chemotherapy and radiation therapy. Radiology. 1994;193:251–254. doi: 10.1148/radiology.193.1.8090901. [DOI] [PubMed] [Google Scholar]
- 25.Peddada AV, Smith DE, Rao AR, Frost DB, Kagan AR. Chemotherapy and low-dose radiotherapy in the treatment of HIV-infected patients with carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 1997;37:1101–1105. doi: 10.1016/s0360-3016(96)00596-2. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman R, Welton ML, Klencke B, Weinberg V, Krieg R. The significance of pretreatment CD4 count on the outcome and treatment tolerance of HIV-positive patients with anal cancer. Int J Radiat Oncol Biol Phys. 1999;44:127–131. doi: 10.1016/s0360-3016(98)00528-8. [DOI] [PubMed] [Google Scholar]
- 27.Cleator S, Fife K, Nelson M, Gazzard B, Phillips R, Bower M. Treatment of HIV-associated invasive anal cancer with combined chemoradiation. Eur J Cancer. 2000;36:754–758. doi: 10.1016/s0959-8049(00)00009-5. [DOI] [PubMed] [Google Scholar]
- 28.Place RJ, Gregorcyk SG, Huber PJ, Simmang CL. Outcome analysis of HIV-positive patients with anal squamous cell carcinoma. Dis Colon Rectum. 2001;44:506–512. doi: 10.1007/BF02234322. [DOI] [PubMed] [Google Scholar]
- 29.Efron JE, Pikarsky AJ, Gervaz P, Locker G, Weiss EG, Wexner SD, Nogueras JJ. The efficacy of chemoradiation therapy in HIV seropositive patients with squamous cell carcinoma of the anus. Colorectal Dis. 2001;3:402–405. doi: 10.1046/j.1463-1318.2001.00281.x. [DOI] [PubMed] [Google Scholar]
- 30.Survival for women and men with AIDS, San Francisco 1981-90. San Francisco Epidemiol Bull. 1992;12:47–49. [Google Scholar]
- 31.Gervaz P, Hahnloser D, Wolff BG, Anderson SA, Cunningham J, Beart RW Jr, Klipfel A, Burgart L, Thibodeau SN. Molecular biology of squamous cell carcinoma of the anus: a comparison of HIV-positive and HIV-negative patients. J Gastrointest Surg. 2004;8:1024–1030; discussion 1031. doi: 10.1016/j.gassur.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 32.Gervaz P, Hirschel B, Morel P. Molecular biology of squamous cell carcinoma of the anus. Br J Surg. 2006;93:531–538. doi: 10.1002/bjs.5376. [DOI] [PubMed] [Google Scholar]
- 33.Palefsky JM, Holly EA, Efirdc JT, Da Costa M, Jay N, Berry JM, Darragh TM. Anal intraepithelial neoplasia in the highly active antiretroviral therapy era among HIV-positive men who have sex with men. AIDS. 2005;19:1407–1414. doi: 10.1097/01.aids.0000181012.62385.4a. [DOI] [PubMed] [Google Scholar]
- 34.Crum-Cianflone NF, Hullsiek KH, Marconi VC, Ganesan A, Weintrob A, Barthel RV, Agan BK. Anal cancers among HIV-infected persons: HAART is not slowing rising incidence. AIDS. 2010;24:535–543. doi: 10.1097/QAD.0b013e328331f6e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009;101:1120–1130. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myerson RJ, Outlaw ED, Chang A, Birnbaum EH, Fleshman JW, Grigsby PW, Kodner IJ, Malayapa RS, Mutch MG, Parikh P, et al. Radiotherapy for epidermoid carcinoma of the anus: thirty years’ experience. Int J Radiat Oncol Biol Phys. 2009;75:428–435. doi: 10.1016/j.ijrobp.2008.11.047. [DOI] [PubMed] [Google Scholar]
- 37.Stadler RF, Gregorcyk SG, Euhus DM, Place RJ, Huber PJ, Simmang CL. Outcome of HIV-infected patients with invasive squamous-cell carcinoma of the anal canal in the era of highly active antiretroviral therapy. Dis Colon Rectum. 2004;47:1305–1309. doi: 10.1007/s10350-004-0584-1. [DOI] [PubMed] [Google Scholar]
- 38.Blazy A, Hennequin C, Gornet JM, Furco A, Gérard L, Lémann M, Maylin C. Anal carcinomas in HIV-positive patients: high-dose chemoradiotherapy is feasible in the era of highly active antiretroviral therapy. Dis Colon Rectum. 2005;48:1176–1181. doi: 10.1007/s10350-004-0910-7. [DOI] [PubMed] [Google Scholar]
- 39.Bower M, Powles T, Newsom-Davis T, Thirlwell C, Stebbing J, Mandalia S, Nelson M, Gazzard B. HIV-associated anal cancer: has highly active antiretroviral therapy reduced the incidence or improved the outcome? J Acquir Immune Defic Syndr. 2004;37:1563–1565. doi: 10.1097/00126334-200412150-00004. [DOI] [PubMed] [Google Scholar]
- 40.Chiao EY, Giordano TP, Richardson P, El-Serag HB. Human immunodeficiency virus-associated squamous cell cancer of the anus: epidemiology and outcomes in the highly active antiretroviral therapy era. J Clin Oncol. 2008;26:474–479. doi: 10.1200/JCO.2007.14.2810. [DOI] [PubMed] [Google Scholar]
- 41.Wexler A, Berson AM, Goldstone SE, Waltzman R, Penzer J, Maisonet OG, McDermott B, Rescigno J. Invasive anal squamous-cell carcinoma in the HIV-positive patient: outcome in the era of highly active antiretroviral therapy. Dis Colon Rectum. 2008;51:73–81. doi: 10.1007/s10350-007-9154-7. [DOI] [PubMed] [Google Scholar]
- 42.Oehler-Jänne C, Huguet F, Provencher S, Seifert B, Negretti L, Riener MO, Bonet M, Allal AS, Ciernik IF. HIV-specific differences in outcome of squamous cell carcinoma of the anal canal: a multicentric cohort study of HIV-positive patients receiving highly active antiretroviral therapy. J Clin Oncol. 2008;26:2550–2557. doi: 10.1200/JCO.2007.15.2348. [DOI] [PubMed] [Google Scholar]
- 43.Abramowitz L, Mathieu N, Roudot-Thoraval F, Lemarchand N, Bauer P, Hennequin C, Mitry E, Romelaer C, Aparicio T, Sobhani I. Epidermoid anal cancer prognosis comparison among HIV+ and HIV- patients. Aliment Pharmacol Ther. 2009;30:414–421. doi: 10.1111/j.1365-2036.2009.04026.x. [DOI] [PubMed] [Google Scholar]
- 44.Seo Y, Kinsella MT, Reynolds HL, Chipman G, Remick SC, Kinsella TJ. Outcomes of chemoradiotherapy with 5-Fluorouracil and mitomycin C for anal cancer in immunocompetent versus immunodeficient patients. Int J Radiat Oncol Biol Phys. 2009;75:143–149. doi: 10.1016/j.ijrobp.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 45.Barriger RB, Calley C, Cárdenes HR. Treatment of anal carcinoma in immune-compromised patients. Clin Transl Oncol. 2009;11:609–614. doi: 10.1007/s12094-009-0412-0. [DOI] [PubMed] [Google Scholar]
- 46.Hogg ME, Popowich DA, Wang EC, Kiel KD, Stryker SJ, Halverson AL. HIV and anal cancer outcomes: a single institution’s experience. Dis Colon Rectum. 2009;52:891–897. doi: 10.1007/DCR.0b013e31819eefa6. [DOI] [PubMed] [Google Scholar]
- 47.Fraunholz I, Weiss C, Eberlein K, Haberl A, Rödel C. Concurrent chemoradiotherapy with 5-fluorouracil and mitomycin C for invasive anal carcinoma in human immunodeficiency virus-positive patients receiving highly active antiretroviral therapy. Int J Radiat Oncol Biol Phys. 2010;76:1425–1432. doi: 10.1016/j.ijrobp.2009.03.060. [DOI] [PubMed] [Google Scholar]
- 48.Geretti AM, Doyle T. Immunization for HIV-positive individuals. Curr Opin Infect Dis. 2010;23:32–38. doi: 10.1097/QCO.0b013e328334fec4. [DOI] [PubMed] [Google Scholar]
- 49.Anderson JS, Hoy J, Hillman R, Barnden M, Eu B, McKenzie A, Gittleson C. A randomized, placebo-controlled, dose-escalation study to determine the safety, tolerability, and immunogenicity of an HPV-16 therapeutic vaccine in HIV-positive participants with oncogenic HPV infection of the anus. J Acquir Immune Defic Syndr. 2009;52:371–381. doi: 10.1097/QAI.0b013e3181b7354c. [DOI] [PubMed] [Google Scholar]