Abstract
There are four types of gastric carcinoid tumors, classified according to their histology and malignant potential. Only a few cases of carcinoid tumors in patients infected with Helicobacter pylori (H. pylori) have been reported so far. We report a patient infected with H. pylori presenting with a small solitary gastric carcinoid tumor with very low proliferative rate and normal gastrin levels. The tumor was endoscopically removed and the patient received an eradication therapy against H. pylori. No signs of metastatic disease have been found so far during more than 3 year of follow-up. Infection with H. pylori may cause chronic gastritis with normal or elevated gastrin levels, leading to the development of gastric carcinoids by mechanisms unrelated to gastrin. Enterochromaffin-like cell tumors related to a chronic H. pylori infection may be considered as a distinct type of gastric carcinoid tumors.
Keywords: Gastric carcinoids, Gastrin, Gastritis, Helicobacter pylori
INTRODUCTION
Gastric carcinoids are rare neuroendocrine tumors of the stomach that arise from the enterochromaffin-like (ECL) cells[1]. Initially, three types of gastric carcinoids were reported[2,3]. The first two types, which are multiple, are related to high gastrin levels; type I arise in patients with autoimmune chronic atrophic gastritis type A and type II occur in patients with the Zollinger-Ellison Syndrome. Type III is a solitary tumor with no known correlation to gastrin production. More recently a highly aggressive variant has been described, named type IV gastric carcinoid tumor[1].
Helicobacter pylori (H. pylori) has been reported to cause chronic atrophic gastritis[4] and alteration of the gastric secretion[5]. Chronic gastritis caused by H. pylori can be a risk factor for gastric cancer[4], but the occurrence of ECL cell tumors in the stomach of patients infected with H. pylori is rare[6]. We here present a patient infected with H. pylori presenting with a solitary gastric carcinoid tumor.
CASE REPORT
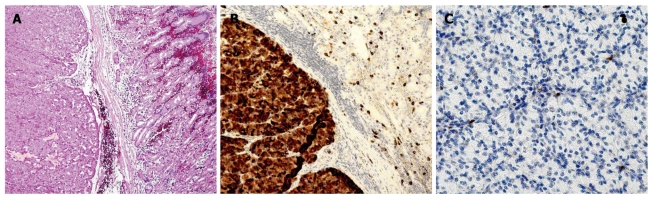
A 60-yr-old woman from Sweden had been suffering from abdominal pain for several years and flushing since 2003. She had no family history for MEN I, Zollinger-Ellison syndrome or autoimmune gastritis. Gastroscopy in May 2006 due to oral lichen showed a polyp-like lesion in the gastric body near the cardia. Microscopic examination showed a neuroendocrine tumor positive for chromogranin A, VMAT-2 and synaptophysin, and with serotonin positivity in the majority of the cells. Ki67 was positive in < 1% of the tumor cells (Figure 1). The tumor was considered to be a type III ECL-oma. Inflammation and H. pylori were present in the gastric mucosa (Figure 2). The patient was referred to our Department and a new gastroscopy in September 2006 showed inflammation in the antrum, corpus and fundus, atrophy in the antrum and corpus, and ECL-cell hyperplasia in the corpus, where a polyp considered as ECL-oma of type I was found. Gastroscopy in November 2006 showed gastritis and positivity for H. pylori but no ECL hyperplasia. Gastric pH was 3.5. The patient had normal urinary histamine metabolites, normal U-5’HIAA, normal fasting serum gastrin and normal plasma chromogranin A and B. She received eradication treatment against H. pylori. A gastroscopy in February 2007 showed chronic inflammation without atrophy in the mucosa. There was a 0.5 cm polyp in the upper corpus surrounded by ECL hyperplasia. The tumor cells were positive for chromogranin A and VMAT 2, but negative for serotonin. Ki67 was < 1%. The tumor was considered to be a type III ECL-oma due to lack of mucosal atrophy. The patient underwent an endoscopic mucosal resection of the polyp in April 2007. The pathology report showed a 7 mm ECL cell carcinoid with 5 mm depth that did not invade the muscularis propria. The tumor cells were positive for chromogranin A, synaptophysin and VMAT-2 but negative for gastrin and serotonin; Ki67 was < 1%. The tumor was considered as a type III ECL-oma. A gastroscopy in September 2007 showed inflammation in the antrum with focal metaplasia but no signs of H. pylori, and another gastroscopy in December 2008 showed no inflammation or atrophy. The patient has not had any signs of metastatic disease in the liver or elsewhere. Repeated CT scans and ultrasounds, as well as an octreoscan in 2006 and a 5-HTP PET scan in January 2008, have been negative. Urinary 5-HIAA, plasma chromogranin A and B and serum gastrin and pancreatic polypeptide have been normal at all control visits. She has no evidence of pernicious anemia and thyroid hormone levels have been normal. At the latest control visit in January 2010, gastroscopy was macro- and microscopically normal. Staining for H. pylori was negative.
Figure 1.
Gastric carcinoid. A: Infiltration of the muscularis mucosae; Hematoxylin-eosin stain. Magnification, × 50; B: Tumor and normal mucosa adjacent to tumor immunostained for VMAT-2. Virtually all tumor cells positive. Magnification, × 100; C: Tumor immunostained for Ki-67. < 1% tumor cells positive. Magnification, × 200.
Figure 2.
Signs of Helicobacter pylori infection in biopsy from antral mucosa. Magnification, × 200.
DISCUSSION
We report a patient with normal gastrin levels presenting with a small solitary gastric carcinoid with very low proliferative rate and without evidence of metastatic disease during more than 3 years of follow-up. The normal gastrin levels suggest that the carcinoid tumor was not type I or II. The absence of metastatic disease and the small dimension of the polyp, together with the low proliferative rate, indicate that it was not a type III carcinoid. The patient was infected with H. pylori and had signs of chronic gastritis, gastric atrophy and ECL cell hyperplasia, which resolved after eradication of the Helicobacter infection. There have been no recurrences after the eradication treatment and endoscopic polypectomy. Although careful interpretation is needed, a causal relationship seems plausible. It is well known that chronic acid suppression may induce ECL cell proliferation[7]. However, our patient did not receive any proton pump inhibitors or other acid suppressive therapy, neither before the development of the carcinoid tumor nor during the follow-up period. It has previously been shown that longstanding H. pylori infection causes chronic inflammation of the gastric mucosa in animals[8]. A long-term H. pylori infection is also associated with atrophy of the gastric mucosa, and atrophy is a risk factor for malignancy[4]. H. pylori-induced gastritis may play an important role in the development of gastric adenocarcinoma in humans[4] and animal models[9]. Development of gastric carcinoid tumors in subjects infected with H. pylori is believed to be rare[6], but has been described in animals[9-11] and, more rarely, in humans. Five humans infected with H. pylori without atrophic gastritis or Zollinger-Ellison syndrome who developed gastric carcinoids have been reported in Japan[12]. In Europe, Solcia reported four cases[13] of gastric carcinoids in H. pylori-infected humans, of whom all had chronic atrophic gastritis type A. Infection with H. pylori was, however, found to be much more common in patients with early gastric carcinomas than in carcinoid patients[13]. H. pylori thus seems more likely to cause neoplasms with higher malignant potential than the indolent carcinoids. Since the chronic gastritis in our patient resolved and no tumor recurrences have occurred after eradication treatment, it is nevertheless possible that her gastric carcinoid was actually caused by H. pylori-induced chronic gastritis.
H. pylori may affect the acid secretion of the parietal cells by causing mucosal inflammation[14]. Gastric acid secretion depends on the localization and the degree of the inflammation[14]. Acute infection with H. pylori results in hypochlorhydria, whereas chronic infection can cause either hypo- or hyperchlorhydria, depending on the distribution of the infection and the degree of corpus gastritis[5]. Recent studies suggest that inflammatory cytokines, produced in response to the bacteria, can play a role in the perturbations in acid and gastrin secretion induced by H. pylori [5]. Gastrin is associated with enterochromaffin-like (ECL) cell proliferation and is a factor implicated in the pathogenesis of ECL-cell tumors type I and II[3]. The patients in Japan with H. pylori-associated gastric carcinoids mentioned above all had high gastrin levels. Our patient, however, developed ECL-cell hyperplasia and a gastric carcinoid tumor despite normal gastrin levels. This observation suggests that H. pylori may facilitate gastric ECL cell proliferation by other mechanisms, independent of gastrin hypersecretion. The mucosal inflammation induced by H. pylori has been shown to cause excessive apoptosis, which in turn leads to proliferation[15,16]. Lipopolysaccharides also appear to influence tumor ECL cell proliferation[16,17]. Another factor involved in ECL cell proliferation is REG protein, which may be produced by H. pylori infection[18].
In conclusion, we postulate that H. pylori may lead to chronic gastritis, with normal or elevated gastrin levels, and cause the development of gastric carcinoids by mechanisms unrelated to gastrin. ECL cell tumors related to a chronic H. pylori infection may be considered as a distinct type of gastric carcinoid tumors, as they seem to have distinct histopathological, pathogenetic and clinical characteristics compared to the other types of gastric carcinoids.
Footnotes
Peer reviewer: Cuong D Tran, PhD, Research Fellow, Affiliate Lecturer, University of Adelaide, Gastroenterology Unit, Children, Youth and Women’s Health Service, 72 King William Rd, North Adelaide, SA 5006, Australia
S- Editor Tian L L- Editor Logan S E- Editor Ma WH
References
- 1.Oberg K, Astrup L, Eriksson B, Falkmer SE, Falkmer UG, Gustafsen J, Haglund C, Knigge U, Vatn MH, Välimäki M. Guidelines for the management of gastroenteropancreatic neuroendocrine tumours (including bronchopulmonary and thymic neoplasms). Part II-specific NE tumour types. Acta Oncol. 2004;43:626–636. doi: 10.1080/02841860410018584. [DOI] [PubMed] [Google Scholar]
- 2.Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993;104:994–1006. doi: 10.1016/0016-5085(93)90266-f. [DOI] [PubMed] [Google Scholar]
- 3.Solcia E, Rindi G, Silini E, Villani L. Enterochromaffin-like (ECL) cells and their growths: relationships to gastrin, reduced acid secretion and gastritis. Baillieres Clin Gastroenterol. 1993;7:149–165. doi: 10.1016/0950-3528(93)90035-q. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi S. Long-term Helicobacter pylori infection and the development of atrophic gastritis and gastric cancer in Japan. J Gastroenterol. 2002;37 Suppl 13:24–27. doi: 10.1007/BF02990095. [DOI] [PubMed] [Google Scholar]
- 5.Schubert ML. Gastric secretion. Curr Opin Gastroenterol. 2002;18:639–649. doi: 10.1097/00001574-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Solcia E, Villani L, Luinetti O, Fiocca R. Proton pump inhibitors, enterochromaffin-like cell growth and Helicobacter pylori gastritis. Aliment Pharmacol Ther. 1993;7 Suppl 1:25–28, discussion 29-31. doi: 10.1111/j.1365-2036.1993.tb00584.x. [DOI] [PubMed] [Google Scholar]
- 7.Klinkenberg-Knol EC, Festen HP, Jansen JB, Lamers CB, Nelis F, Snel P, Lückers A, Dekkers CP, Havu N, Meuwissen SG. Long-term treatment with omeprazole for refractory reflux esophagitis: efficacy and safety. Ann Intern Med. 1994;121:161–167. doi: 10.7326/0003-4819-121-3-199408010-00001. [DOI] [PubMed] [Google Scholar]
- 8.Sun YQ, Petersson F, Monstein HJ, Söderholm JD, Rehfeld JF, Borch K. Long-term morpho-functional development of Helicobacter pylori-induced gastritis in Mongolian gerbils. Scand J Gastroenterol. 2005;40:1157–1167. doi: 10.1080/00365520510023378. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998;115:642–648. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- 10.Chiba T. One more new gastric disease induced by Helicobacter pylori infection, enterochromaffin-like (ECL) cell carcinoid tumor. J Gastroenterol. 1999;34:545–546. doi: 10.1007/s005350050313. [DOI] [PubMed] [Google Scholar]
- 11.Kagawa J, Honda S, Kodama M, Sato R, Murakami K, Fujioka T. Enterocromaffin-like cell tumor induced by Helicobacter pylori infection in Mongolian gerbils. Helicobacter. 2002;7:390–397. doi: 10.1046/j.1523-5378.2002.00115.x. [DOI] [PubMed] [Google Scholar]
- 12.Sato Y, Iwafuchi M, Ueki J, Yoshimura A, Mochizuki T, Motoyama H, Sugimura K, Honma T, Narisawa R, Ichida T, et al. Gastric carcinoid tumors without autoimmune gastritis in Japan: a relationship with Helicobacter pylori infection. Dig Dis Sci. 2002;47:579–585. doi: 10.1023/a:1017972204219. [DOI] [PubMed] [Google Scholar]
- 13.Solcia E, Rindi G, Fiocca R, Villani L, Buffa R, Ambrosiani L, Capella C. Distinct patterns of chronic gastritis associated with carcinoid and cancer and their role in tumorigenesis. Yale J Biol Med. 1992;65:793–804; discussion 827-829. [PMC free article] [PubMed] [Google Scholar]
- 14.Schubert ML. Gastric secretion. Curr Opin Gastroenterol. 2007;23:595–601. doi: 10.1097/MOG.0b013e3282f03462. [DOI] [PubMed] [Google Scholar]
- 15.Lamarque D, Tran Van Nhieu J, Breban M. [What are the gastric modifications induced by acute and chronic Helicobacter pylori infection?] Gastroenterol Clin Biol. 2003;27:391–400. [PubMed] [Google Scholar]
- 16.Kidd M, Miu K, Tang LH, Perez-Perez GI, Blaser MJ, Sandor A, Modlin IM. Helicobacter pylori lipopolysaccharide stimulates histamine release and DNA synthesis in rat enterochromaffin-like cells. Gastroenterology. 1997;113:1110–1117. doi: 10.1053/gast.1997.v113.pm9322505. [DOI] [PubMed] [Google Scholar]
- 17.Kidd M, Tang LH, Schmid S, Lauffer J, Louw JA, Modlin IM. Helicobacter pylori lipopolysaccharide alters ECL cell DNA synthesis via a CD14 receptor and polyamine pathway in mastomys. Digestion. 2000;62:217–224. doi: 10.1159/000007819. [DOI] [PubMed] [Google Scholar]
- 18.Kinoshita Y, Ishihara S, Kadowaki Y, Fukui H, Chiba T. Reg protein is a unique growth factor of gastric mucosal cells. J Gastroenterol. 2004;39:507–513. doi: 10.1007/s00535-004-1354-5. [DOI] [PubMed] [Google Scholar]