Abstract
Objectives.
To examine the association of engagement in cognitively stimulating activities with cognitive and functional decline in a population-based sample of incident Alzheimer's disease (AD).
Method.
After diagnosis, 187 participants (65% females) were followed semiannually for a mean 2.7 (SD = 0.4) years. Mean age and education were 84.6 (SD = 5.8) and 13.2 (SD = 2.9) years. Caregivers enumerated cognitively stimulating leisure activities via the Lifestyle Activities Questionnaire. Cognition was assessed using the Mini-Mental State Examination and functional ability via the Clinical Dementia Rating sum of boxes. Linear mixed models tested the association between stimulating activities and change over time in each outcome. Covariates were demographic factors, estimated premorbid IQ, presence/absence of the APOE ϵ4 allele, duration of dementia, level of physical activity, and general health.
Results.
At initial assessment, 87% of participants were engaged in one or more stimulating activities, with mean (SD) activities = 4.0 (3.0). This number declined to 2.4 (2.0) at the final visit. There was a statistical interaction between dementia duration and number of activities in predicting rate of cognitive decline (p = .02) and overall functional ability (p = .006).
Discussion.
Active involvement in cognitively stimulating pursuits may be beneficial for persons with AD.
Keywords: Alzheimer's disease, Cognitive activity, Cognitive decline, Dementia
ALZHEIMER'S disease (AD) is a neurodegenerative disorder that leads to cognitive and functional deterioration. Despite its progressive course, there is considerable variability in the rates of cognitive and functional decline among those with the condition (Tschanz et al., in press). Little is known about the factors that influence the progression of the illness, but some studies have suggested that educational and occupational attainment predict rates of decline in cognition or functional ability (e.g., Andel, Vigen, Mack, Clark, & Gatz, 2006; Fritsch et al., 2001; Schooler, Mulatu, & Oates, 1999; Stern, Albert, Tang, & Tsai, 1999). Such observations are consistent with the notion of brain or cognitive reserve, which has been postulated to explain resilience against the effects of brain damage (e.g., Brickman et al., 2009; Scarmeas et al., 2003). Two distinctions raised in the literature concern passive versus active reserve. The former may reflect brain size or synaptic density so that clinical symptoms emerge when a certain threshold of damage is reached, whereas the latter may reflect greater efficiency of neural networks or the recruitment of alternative networks when compensatory strategies are used (Stern, 2006). Both brain and cognitive reserve are malleable, reflecting a lifetime of cognitively enriching experiences. In the case of AD, it is theorized that individuals with greater reserve are better able to compensate for the advancing pathology of the condition, resulting in a delay in the clinical expression of dementia (Scarmeas & Stern, 2003; Stern et al., 1999).
It is difficult to directly assess the construct of cognitive reserve, and most studies have relied on proxy indicators such as educational or occupational attainment, literacy, or socioeconomic status (Stern, 2006). This approach, particularly in relation to older retired persons, relies on past achievements, which does not take into account current leisure time activities that arguably may also contribute to reserve. The use of multiple indicators from the past as well as the present may provide a better overall indicator and may also allow one to test the relevance of past versus more recent activities in providing resilience to brain pathology.
Recent studies suggest that ongoing experiences help maintain or enhance cognitive reserve. Several authors have posited, for instance, that later life engagement in intellectually and socially stimulating activities (e.g., reading books or newspapers, working on crossword puzzles, engaging in volunteer work, traveling, or playing card games) may enhance thinking, memory, and attention control processes, thereby maintaining or increasing brain reserve capacity (e.g., Carlson et al., 2009; Fabrigoule et al., 1995; Schooler et al., 1999; Staff, Murray, Deary, & Whalley, 2004; Wang, Karp, Winblad, & Fratiglioni, 2002).
The discovery of neural plasticity in older animals offers insight into the role of late-life activity on cognitive health. Animal models of exercise and enriched environment demonstrate that brain plasticity through the reorganization and development of new neurons (neurogenisis) is possible in the adult brain (Dong & Greenough, 2004; Ming & Song, 2005; Pham, Winblad, Granholm, & Mohammed, 2002; Rakic, 2002). Exercise in mice improves both learning and neurogenesis well into late life (Farmer et al., 2004; van Praag, Christie, Sejnowski, & Gage, 1999). Rats of various ages raised in complex environments have more synapses per nerve cell than rats raised in standard laboratory cages (Briones, Klintosova, & Greenough, 2004; Turner & Greenough, 1985).
In humans, increased engagement in late-life cognitive activity has been associated with slower cognitive decline (e.g., Hall et al., 2009; Wilson et al., 2003). Other studies have examined the effect of cognitive engagement on risk for AD, suggesting that higher levels of reserve may delay the onset of symptoms (e.g., Akbaraly et al., 2009; Friedland et al., 2001; Scarmeas et al., 2003; Verghese et al., 2003; Wilson et al., 2002). By contrast, little attention has been paid to the association between cognitive activity among individuals diagnosed with AD. Two studies have examined the association between cognitively stimulating activities prior to the onset of dementia and rate of cognitive decline thereafter. In a study of 417 individuals with incident AD, Wilson and colleagues (2000) reported that more frequent premorbid reading predicted faster decline on a global cognitive measure. However, Helzner, Scarmeas, Cosentino, Portet, and Stern (2007) found no relationship between premorbid engagement in intellectually stimulating activities and cognitive decline after AD onset. These conflicting findings may reflect differences in methods for assessment of cognitive activity (reading only vs. a broader measure of activities). Both studies examined stimulating activity prior to the onset of dementia, possibly overlooking its effects across the course of the illness.
Here, we report on the association between ongoing cognitive stimulation and the rate of cognitive or functional decline in AD in a population-based sample of persons with incident AD. We also test whether other indicators of cognitive reserve (education, estimated premorbid IQ, and occupational attainment) are associated with these outcomes.
METHOD
Participant Screening and Diagnosis
The Cache County Dementia Progression Study (DPS) is an ongoing longitudinal cohort study of persons with incident AD and other dementias identified from the Cache County Study on Memory in Aging (CCSMA). Since 2002, the DPS has followed 328 persons with dementia and their caregivers (Norton et al., 2009) using semiannual measures of cognition, function, health, and behavior.
The CCSMA enrolled 90% of 5,677 residents of Cache County, Utah, who were aged 65 years or older on January 1, 1995. Through four waves (1995–1996; 1998–2000; 2002–2004; and 2005–2007), the CCSMA examined the prevalence and incidence of dementia in the county. The multistage screening and assessment procedures of the study have been reported in detail elsewhere (Breitner et al., 1999; Miech et al., 2002). Briefly, participants were screened for dementia at each wave using a revision of the Modified Mini-Mental State Examination (Tschanz et al., 2002). Individuals whose sensory and education adjusted screening scores fell below 87 of 100 were studied further using the Dementia Questionnaire (DQ; Silverman, Breitner, Mohs, & Davis, 1986). Persons identified as having moderate to severe cognitive impairment by a study neuropsychologist or who were members of a designated subsample selected to complete all stages of assessment underwent a comprehensive clinical assessment (CA) conducted by a trained research nurse and psychometric technician. The CA included a neurological exam, neuropsychological testing (Tschanz et al., 2000), and a clinical interview to ascertain medical/psychiatric history and demographic information.
The results of the CA were reviewed by a geriatric psychiatrist and neuropsychologist who assigned preliminary diagnoses of dementia using DSM-III-R criteria (American Psychiatric Association, 1987). Dementia severity was rated by stage or severity using the Clinical Dementia Rating Scale (CDR; Hughes, Berg, Danziger, Coben, & Martin, 1982; Morris, 1993), and age of onset was assigned as the age the individual unambiguously met criteria for dementia. Individuals with dementia underwent follow-up laboratory testing, neuroimaging, and an examination by a geriatric psychiatrist. A panel of experts in neurology, geropsychiatry, and neuropsychology then reviewed all available data and assigned final diagnoses of dementia. Diagnosis of Possible or Probable AD followed NINCDS-ADRDA criteria (McKhann et al., 1984). In the present analyses, we included incident cases of AD that occurred singly or mixed cases of AD that occurred with other dementias (e.g., AD with vascular dementia; Breitner et al., 1999). Study procedures remained identical in each wave, except that more sensitive screening cut-off scores were used in later waves, and we omitted use of the DQ in the screening process for Waves 3 and 4. All CCSMA procedures were approved by the Institutional Review Boards (IRBs) of Duke University, Utah State University, the Johns Hopkins University, and the University of Washington.
Procedures of the DPS
We investigated individuals with incident AD and enrolled in the DPS (n = 187). Participants were visited semiannually by a research nurse and a psychometric technician for up to three assessments over three years. Interviews with caregivers provided information regarding participants’ current medical conditions, medications, nutrition, neuropsychiatric symptoms, and participation in leisure activities. Participants also completed a neuropsychological test battery. All procedures were approved by the IRBs of Utah State University and Johns Hopkins University. Participant assent and caregiver informed consent were obtained at each visit.
Exposure Measurement: Lifestyle Activities Questionnaire
At the initial (baseline) and alternate follow-up visits, caregivers were queried about participants’ engagement in physical and leisure pursuits using an adaptation of the Lifestyle Activities Questionnaire (Carlson et al., in press). This instrument enumerates 31 activities with varying levels of cognitive processing demand (e.g., working on crossword puzzles, reading, attending cultural events, and listening to music). Frequency of these activities was recorded as: 1 = never or less than once per month; 2 = once per month; 3 = 2–3 times per month; 4 = once per week; 5 = a few times per week; 6 = every day. We discriminated activities involving novel information processing from other cognitively passive or receptive activities, as recently described (Carlson et al., in press). Active leisure pursuits were defined as those requiring explicit processing or an action relying on novel information (e.g., doing crossword puzzles, reading, taking a course). Passive activities involved predominantly receptive processing demands, such as watching television and listening to the radio or to music. “Intermediate” activities included visiting with friends and relatives, driving or using public transportation, and cooking or baking. Two trained judges (a neuropsychologist [J. T. Tschanz] and the first author, K. A. Treiber) rated each activity. Interrater agreement was 84%, corrected for chance by a kappa score of .75. An expert judge (cognitive neuropsychologist, M.C. Carlson) was consulted when needed to arrive at consensus among the raters.
We analyzed activities endorsed at least weekly. At baseline, the majority of participants engaged in only one or two such active pursuits. Because of a moderate correlation between the number of intermediate and more demanding baseline activities (r = .46, p < .001), the two categories were summed to an aggregate variable for number of cognitively stimulating activities endorsed (of 24 possible).
Other Indicators of Cognitive Reserve
Other indicators of cognitive reserve examined in these analyses included years of formal education, occupational attainment, years of occupation, and estimated premorbid IQ. Data on educational and occupational history were obtained from the CCSMA prior to the onset of dementia. Participants’ primary occupation was categorized as professional, technical, office/business manager, clerical, skilled labor, semiskilled labor, unskilled, and never employed. For analyses, the categories were summarized as: professional/technical, office/business/managerial, clerical, skilled labor, unskilled/semi-skilled labor, and never/minimally employed. Premorbid IQ was also estimated from the CCSMA's Vocabulary subtest of the Shipley Institute of Living Scale (Shipley, 1967), administered at the initial CCSMA diagnostic assessment.
Cognitive Outcome Measurement: Mini-Mental State Examination
Global cognitive ability was assessed at each visit using the Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975). The MMSE assesses five areas of cognition, including orientation, registration of new information, attention, constructional praxis, recall, and language. Scores range from 0 to 30. When ≤10% of test items (up to three points) were invalidated by sensory or motor impairment, we used percent correct to calculate an adjusted score that ignored these items and rescaled the overall result on a 30-point scale. If more than 10% of items were unavailable, we instead categorized the entire score as “missing” (Tschanz et al., in press).
Functional Outcome Measurement: CDR Scale
Functional ability was measured using the CDR (Hughes et al., 1982; Morris, 1993), as completed by a trained research nurse at each DPS follow-up visit. The CDR is a 5-point scale used to characterize six domains of cognitive and functional performance applicable to AD and related dementias: memory, orientation, judgment, community activities, hobbies, and personal care. Scored using a specified algorithm, the CDR yields a score of dementia severity ranging from questionable (CDR = .5) to mild (CDR = 1), moderate (CDR = 2), severe (CDR = 3), profound (CDR = 4), or terminal (CDR = 5). Following recent practice (O’Bryant et al., 2008), we used the instrument to assess functional impairment by summing the ratings in each of the six interview domains. Because these ratings are entered into six individual boxes on the instrument, their sum is commonly described as the CDR Sum of Boxes or CDR-SB. The CDR-SB can range from 0 (no impairment) to 30 (maximum impairment in all domains).
Selection and Assessment of Covariates
Factors that previously had been shown to affect dementia progression or those that could confound the relationship between engagement in cognitively stimulating activity and dementia progression were selected a priori and tested as covariates. These included demographic factors (age and sex), duration of dementia at the initial DPS visit, genotype at the polymorphic genetic locus for apolipoprotein E (APOE), physical health, and frequency of physical activity. Buccal DNA, obtained at the initial CCSMA visit, was treated by polymerase chain reaction amplification and restriction isotyping (Richards et al., 1993; Saunders et al., 1993) for APOE genotyping. Genotype information was simplified to a binary classification based on presence of one or more ϵ4 alleles at this locus (an important AD risk factor).
Data from the CCSMA dementia diagnostic visit indicated each participant's overall health from consensus ratings of the examining research nurse and study neuropsychologist and geropsychiatrist using the General Medical Health Rating (GMHR; Lyketsos et al., 1999). Thereafter, in the DPS, the GMHR was completed by the research nurse in consultation with a neuropsychologist or geropsychiatrist. The GMHR is a measure of general medical comorbidity developed specifically for use with dementia patients and results in a rating on an ordinal scale of 1 (poor), 2 (fair), 3 (good), and 4 (excellent). Caregivers provided information about each participant's level of physical activity during the previous year. Information included the frequency and duration of activities that included walking, lifting weights, gardening, and using an exercise machine. We summed frequency and duration of participation across such activities to compute an estimate of physical exercise hours per month.
Statistical Analyses
Exploratory analyses assessed sample characteristics, cognitive and functional trajectories, survival across time points, and differences between individuals who completed all visits and those who dropped out prior to the final visit. Using a two-tailed alpha of .05 throughout, we examined marginal associations between each independent variable, covariate, and outcome variable to identify variables that confounded the primary variables of interest or modified their relationships to other independent variables. Relationships between categorical variables were examined using chi-square tests of independence, whereas Pearson correlations were used to probe associations between continuous variables. Group differences were examined using t tests. We used linear mixed effects models to examine the association between cognitively stimulating activities and rate of decline across time. This type of analysis can model mean response as a combination of population characteristics (fixed effects) and subject-specific characteristics (random effects). Linear mixed effects models are also flexible in accommodating imbalance in longitudinal data and thus incorporating all available data from participants with incomplete participation at follow-up (Fitzmaurice, Laird, & Ware, 2004). Additionally, we tested whether random intercepts and slopes best fit the data, retaining both terms if there was a significant improvement in model fit (likelihood ratio test, p < .05). Number of stimulating activities, physical activity, and general health rating were treated as time-varying variables, testing an interaction with time to determine differential rate of decline. As with the primary predictors of interest, covariates were entered sequentially, and each new model was compared with the previous simpler model using the likelihood ratio test.
RESULTS
Sample characteristics are displayed in Table 1. We analyzed data from 187 individuals with a diagnosis of AD (87.2% with AD only, 8.6% with AD and VaD, and 4.3% with AD and another form of dementia). The sample was predominantly Caucasian (99%) and female (64.7%). Males had completed more years of education (M = 13.9, SD = 3.3) than females, M = 12.8, SD = 2.6; t(111) = −2.3, p = 0.02. Mean age of AD onset was 82.4 (SD = 6.0), and severity of dementia at diagnosis was generally mild with mean CDR of 1.4 (SD = 0.9). Mean duration of dementia at the initial DPS visit was 4.0 (SD = 2.0) years. Most participants (84.4%) were rated as either in good or in better physical health on the GMHR.
Table 1.
Sample Characteristics at the Initial Dementia Progression Study Visit
| Group/demographic data | M | SD | Minimum/maximum |
| Dementia duration in years | 4.0 | 2.0 | 1/11 |
| Dementia severity (CDR; range: 0.5–5) | 1.4 | 0.9 | 0/4 |
| Age at baseline | 84.6 | 5.8 | 73/99 |
| Age of AD onset | 82.4 | 6.0 | 68/95 |
| Education in years | 13.2 | 2.9 | 3/20 |
| Shipley scores (estimated premorbid IQ; T-scores) | 49.7 | 9.7 | 17/69 |
| Aggregate years workeda | 33.1 | 26.4 | 0/103 |
| Physical activity (hr/month) | 11.2 | 20.5 | |
| N | % | ||
| Sex (male) | 66 | 35.3 | |
| Sex (female) | 121 | 64.7 | |
| APOE genotype (no e4) | 97 | 51.9 | |
| APOE genotype (≥1 e4) | 90 | 48.1 | |
| Health status (GMHR) | |||
| Excellent | 36 | 19.5 | |
| Good | 120 | 64.9 | |
| Fair | 29 | 15.7 | |
| Poor | 0 | 0.0 | |
| Occupational attainment | |||
| Never/minimally employed | 39 | 20.9 | |
| Unskilled/semi-skilled labor | 26 | 13.9 | |
| Skilled labor | 21 | 11.2 | |
| Clerical | 41 | 21.9 | |
| Office/business/managerial | 6 | 3.2 | |
| Professional/technical | 54 | 28.9 | |
| Number of cognitive activities | |||
| 0 activities | 24 | 12.8 | |
| 1 activity | 19 | 10.2 | |
| 2–5 activities | 89 | 47.6 | |
| >5 activities | 55 | 29.4 |
Notes: AD = Alzheimer's disease; CDR = Clinical Dementia Rating; GMHR = General Medical Health Rating.
The maximum aggregate years worked exceeds expected human capabilities as it reflects a number of individuals who held more than one job simultaneously. Because the data do not support determination of whether an occupation was full or part time, it is not possible to indicate whether multiple occupations were held simultaneously.
Participants in these analyses were examined up to five times, including the initial DPS visit. Of the original 187, 66 (35.3%) completed the fifth visit. Reflecting the ongoing status of the work, an additional 25 participants are pending their fifth assessment. Reasons for losses to follow-up included death (n = 89), moving (n = 1), and refusal (n = 6). Individuals who were pending or who dropped out prior to the final visit were older, M = 87.5, SD = 5.4 for drop-out/pending vs. M = 84.5, SD = 5.9 for completers, t(185) = 3.5, p = .001; in worse physical health (19.2% of pending/drop-out were in fair to poor health vs. 9.2% of completers, χ2(2, N = 185) = 7.7, p = .02; were less physically active, M = 8.3, SD = 16.0 for drop-out/pending versus M = 16.2, SD = 26.0 for completers, t(90) = −2.2, p = .03; had completed fewer years of formal education, M = 12.8, SD = 2.8 for drop-out/pending versus M = 13.8, SD = 3.0 for completers, t(185) = −2.2, p = .03; and engaged in fewer cognitively stimulating activities, M = 3.6, SD = 2.8 for drop-out/pending versus M = 5.2, SD = 3.0 for completers, t(185) = −3.5, p = .001.
At the initial DPS visit, most individuals (87.2%) reported weekly or daily participation in at least one cognitively stimulating activity. A majority reported two or more activities, whereas 29.4% reported engaging in over five. Number of such activities declined with follow-up, from an initial mean of 4.0 (SD = 3.0) to 2.4 (SD = 2.0, t(65) = 8.4, p < .0001) at the final visit. As expected, moderate correlations were found between number of cognitively stimulating activities and dementia duration at each visit, such that increasing duration was associated with fewer activities. For instance, at Visit 1, the association between dementia duration and cognitive activity at the initial DPS visit was −0.34 (p < .0001). At Visit 4, the correlation was −.47 (p < .0001). Initially, mean physical activity totaled 11.2 (SD = 20.5) hrs per month, but this figure also declined over time to 5.8, SD = 11.1, t(60) = 3.9, p < .0001, hrs per month at the last visit, an average of 2.7 years later. Unlike cognitive activity, however, hours of physical activity and dementia duration were not significantly correlated, r(173) = −0.11, p = .16. Participants were relatively highly educated with a mean (SD) of 13.2 (2.9) years.
Prior to modeling the association between engagement in cognitively stimulating activities, other indicators of cognitive reserve, and the other covariates, we examined their correlations with each other as a check for collinearity. Although education was statistically correlated with premorbid IQ, r(187) = .36, p < .0001, and with occupational attainment, r(187) = .40, p < .001, and number of cognitive activities was statistically correlated with occupational attainment, r(187) = .16, p = .037, the magnitude of each association was low, suggesting that each variable would add unique information when added to the linear mixed models.
Lower cognitive activity was associated with increasing duration of dementia, r(180) =−.33, p < .001, and varied by sex, with men engaging in significantly more cognitively stimulating activities than females, t(185) =−2.83, p = .005. Number of cognitive activities was negatively associated with age r(187) = −.18, p = .01, and positively associated with overall health, r(185) = .19, p = .01, and physical activity, r(173) = .29, p < .001. APOE status was not significantly associated with cognitive activity, M = 4.6, SD = 3.2 for APOE E4+ vs. M = 3.8, SD = 2.7 for APOE E4-, t(185) =−1.84, p = .07.
Association of Cognitive Activities, Other Indicators of Cognitive Reserve, and Rate of Cognitive Decline
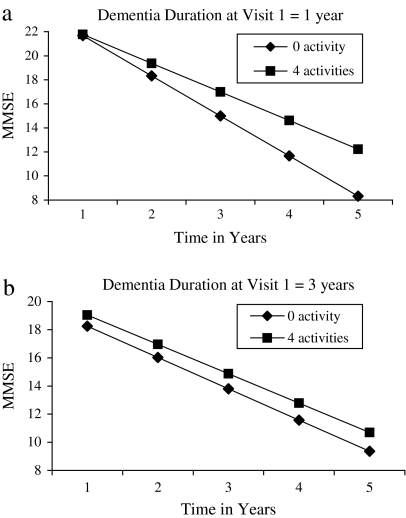
As expected, in linear mixed models significant negative estimates for time indicated deterioration in MMSE scores. A significant three-way interaction between number of activities, dementia duration, and time observed in initial models remained significant with the addition of covariates. In the final model, a significant three-way interaction (F = 5.5, p = .02) indicated a differential effect for stimulating activity on decline in MMSE performance depending upon duration of dementia at the initial DPS visit. Specifically, with shorter illness duration, increased engagement in cognitively stimulating activities was associated with a slower rate of decline. For example, the model predicted that for those with less than one-year dementia duration, engagement in zero activities would be associated with a decline of 3.9 points per year on the MMSE (Figure 1a), whereas those engaged in five stimulating activities would experience a decline of 2.2 points per year, a difference of 1.7 points. By contrast, among those with a three-year dementia duration, the model predicted a decline of 2.2 points per year for those engaged in zero activities and a rate of decline of 2.1 points per year for engagement in five activities (Figure 1b).
Figure 1.
(a) and (b) The graphs display the predicted decline in Mini-Mental State Examination (MMSE) scores for varying numbers of cognitively stimulating activities by dementia duration at Visit 1. Note that the graph is based on the regression equation for the final model and represents mean MMSE scores by frequency of engagement in cognitive activities for one and three years of Alzheimer's disease duration. Dementia durations of one and three years were selected for illustrative purposes.
Estimate of premorbid IQ on average was associated with higher MMSE score (Estimate = 0.11, p = .02) but not with rate of decline (Estimate =−0.01, p = .62). Occupational attainment (Estimate = 0.15, p = .55) and years of education (Estimate = 0.09, p = .61) were not significantly associated with MMSE. However, these were retained in the final model because of their theoretical importance as indicators of cognitive reserve. Engagement in physical activity was not associated with cognitive performance (Estimate = −0.01, p = .54). The results of the final model are displayed in Table 2.
Table 2.
Effect of Engagement in Cognitively Stimulating Activities and Other Indicators of Cognitive Reserve on Mini-Mental State Examination Scores: Results of the Final Model
| Model term | Estimate (SE) | df | t | Significance | 95% Confidence interval |
|
| Lower | Upper | |||||
| Intercept | 19.99 (2.87) | 143.50 | 6.97 | <0.001 | 14.32 | 25.65 |
| Time | −3.90 (0.81) | 193.85 | −4.83 | <0.001 | −5.49 | −2.31 |
| Dementia duration in years | −2.28 (0.28) | 221.36 | −8.27 | <0.001 | −2.82 | −1.73 |
| # Cognitive activities | −0.52 (0.19) | 237.29 | −2.10 | 0.037 | −0.79 | −0.02 |
| Education | 0.09 (0.17) | 140.40 | 0.51 | 0.610 | −0.25 | 0.42 |
| Estimated premorbid IQ | 0.11 (0.05) | 138.84 | 2.36 | 0.020 | 0.02 | 0.21 |
| Occupation | 0.15 (0.26) | 149.73 | 0.60 | 0.55 | −0.35 | 0.66 |
| Dementia Duration × # Cognitive Activities | 0.19 (0.05) | 246.73 | 4.20 | <0.001 | 0.10 | 0.28 |
| Time × Dementia Duration | 0.56 (0.18) | 176.19 | 3.10 | 0.002 | 0.20 | 0.91 |
| Time × # Cognitive Activities | 0.34 (0.16) | 303.13 | 2.11 | 0.035 | 0.02 | 0.66 |
| Time × Dementia Duration × # Cognitive Activities | −0.10 (0.04) | 286.42 | −2.35 | 0.02 | −0.19 | −0.02 |
Association of Cognitive Activities, Other Indicators of Cognitive Reserve, and Rate of Functional Decline
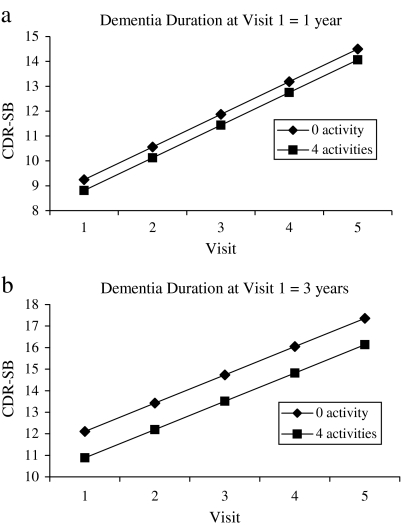
Linear mixed models of the CDR-SB yielded a significant positive estimate for time, indicating that functional impairment increased over time. Models examining the effects of covariates and their interactions with time also revealed a significant main effect for GMHR rating, indicating better functional ability with better physical health. The final model, controlling for physical health, revealed a significant interaction between dementia duration at the initial visit and number of cognitive activities (F = 7.5, p = .006). Specifically, a higher number of cognitive activities was associated, on average, with better functional performance, particularly with longer dementia duration. For every 5-unit increase in dementia duration and cognitive activity, there was a corresponding reduction of 0.5 points on the CDR-SB. Figure 2a and b depicts the relationship between cognitive activities and dementia duration on functional ability. Engagement in physical activity (Estimate = 0.01, p = .24), estimate of premorbid IQ (Estimate =−0.05, p = .15), education (Estimate = 0.06, p = .61), and occupational attainment (Estimate = −0.09, p = .64) were not associated with overall functional ability or rate of decline. However, in view of their theoretical importance as indicators of cognitive reserve, the latter terms were retained in the final model (see Table 3).
Figure 2.
(a) and (b) The graphs 2 display the predicted change in Clinical Dementia Rating sum of boxes (CDR-SB) scores for varying numbers of cognitively stimulating activities by dementia duration at Visit 1. Note that the graph is based on the regression equation for the final model and represents mean CDR-SB scores by frequency of engagement in cognitive activities for one and three years of Alzheimer's disease duration. Dementia durations of one and three years were selected for illustrative purposes.
Table 3.
Effect of Engagement in Cognitively Stimulating Activities and Other Indicators of Cognitive Reserve on Clinical Dementia Rating Sum of Boxes Scores: Results of the Final Model
| Model term | Estimate (SE) | df | t | Significance | 95% Confidence interval |
|
| Lower | Upper | |||||
| Intercept | 8.28 (2.19) | 162.50 | 3.78 | <0.001 | 3.95 | 12.61 |
| Time | 1.31 (0.19) | 94.33 | 7.02 | <0.001 | 0.94 | 1.68 |
| Dementia duration | 1.43 (0.20) | 225.41 | 7.27 | <0.001 | 1.04 | 1.82 |
| Estimated premorbid IQ | −0.05 (0.03) | 134.66 | −1.47 | 0.15 | −0.12 | 0.02 |
| Education | 0.06 (0.12) | 138.99 | 0.51 | 0.61 | −0.18 | 0.30 |
| Occupation | −0.09 (0.19) | 144.59 | −0.46 | 0.64 | −0.45 | 0.28 |
| # Cognitive activities | −0.01 (0.13) | 301.47 | −0.07 | 0.95 | −0.26 | 0.24 |
| Overall health (GMHR) | −0.89 (0.25) | 447.80 | −3.62 | <0.001 | −1.37 | −0.41 |
| Dementia Duration × # Cognitive Activities | −0.10 (0.03) | 266.03 | −3.31 | 0.001 | −0.16 | −0.04 |
GMHR = General Medical Health Rating.
DISCUSSION
In this population-based study of incident AD, engagement in cognitively stimulating activities early in the course of AD was associated with slower cognitive decline. This effect was striking, particularly at two and a half years of follow-up where, for example, statistical modeling predicted that those engaged in four or more activities scored approximately four points higher on the MMSE than those engaged in no stimulating activities. Although differences in methodology prohibit direct comparisons, the results differ from those of Helzner and colleagues (2007) and Wilson and colleagues (2000) who reported, respectively, no effect or a faster deterioration in cognitive functioning with greater engagement in premorbid mental activity. The latter study restricted the assessment to reading activity five years prior to dementia onset and did not reassess the activity through the course of dementia. Unlike these studies, we required at least weekly participation in cognitive pursuits, which could suggest that higher levels of engagement are needed to observe a relationship with slower decline. Alternatively, higher levels of engagement in stimulating activities may be a reflection of less severe dementia. Indeed, over time, participants declined in their level of engagement in stimulating activities. Furthermore, the association between stimulating activity and cognition was evident only early in the course of AD.
Engagement in cognitively stimulating activities was also associated with better functional ability, but here, the associations were greater for those with longer dementia duration at assessment. Our lack of observation for similar association in early dementia may reflect the nature of the CDR, a measure that emphasizes functional abilities that are more routinized and exhibit greater declines later in the course of dementia (Hughes et al., 1982; Morris, 1993). There was no association between engagement in cognitive activity and rate of functional decline.
Physical activity was not associated with change in rate of cognitive or functional decline in our participants. Physical activity has known positive benefits in healthy individuals, and engagement throughout mid- and late-life has been shown to be protective against late-life cognitive decline and AD in some studies (Colcombe et al., 2004; Dik, Deeg, Visser, & Jonker, 2003; Laurin, Verreault, Lindsay, MacPherson, & Rockwood, 2001; Weuve et al., 2004; Yaffe, Barnes, Nevitt, Lui, & Covinsky, 2001) but not others (Carlson et al., 2008; Scarmeas, Levy, Tang, Manly, & Stern, 2001; Verghese et al., 2003, 2006). Thus, the effects of physical activity in AD are not well understood. Although our results are negative, it is possible that our crude measurement of total physical activity was insufficient in capturing the effects of potentially neuroprotective aerobic activities. Nonetheless, our results, while observational, suggest limited benefits of physical activity to cognition following the onset of dementia.
Among the strengths of the study were its large population-based sample, systematic assessment of cognitive activity, and examination of both cognitive and functional outcomes. In addition, the six-month follow-up intervals allowed for increased sensitivity in tracking decline. The comprehensive protocol for determining a dementia diagnosis and high participation rates among participants and their caregivers were additional strengths.
Several limitations of this study merit discussion. We did not examine differential effects of specific activities. Previous research has suggested a positive effect for social engagement and the maintenance of cognitive functioning (e.g., Barnes, Mendes de Leon, Wilson, Bienias, & Evans, 2004). Additionally, social disengagement may lead to a reduction in cognitive stimulation (Wang et al., 2002). Given that many leisure activities are both cognitive and social in nature, the relative effect of each represents an area for future research. There was also significant participant attrition (mostly due to death) over the course of the study. This may have reduced statistical power in examining the longitudinal effects of engagement in stimulating activities. Our results may also reflect the effects of differential attrition, for example, if greater attrition had occurred among those with severe dementia or engaged in fewer activities. Finally, our sample, while population based, was 99% Caucasian and relatively well educated. Our results may not generalize to other populations with more diverse ethnic representation or levels of educational attainment.
A worthwhile endeavor may be to explicitly examine whether engagement in cognitively stimulating activities after dementia onset may produce beneficial effects. A randomized controlled intervention trial of stimulating activities in early dementia may clarify this issue. To date, there have been no such trials after the onset of dementia. Studies among the non-demented elderly suggest some benefit of cognitive intervention (Willis et al., 2006). From a cognitive perspective, late-life volunteer activity in cognitively at-risk older adults has been associated with protection (Carlson et al., 2008, 2009). These findings suggest the potential for use-dependent brain plasticity among those with dementia, but their limits remain to be determined.
FUNDING
National Institute of Health grants R01AG21136 and R01AG11380.
Acknowledgments
We acknowledge the contributions of the following individuals whose activities have helped to ensure the success of the project: Cara Brewer, BA; James Burke, MD; Tony Calvert, RN, BA; Eric Christopher, MD; Elizabeth Fauth, PhD; Amber Frye, BA; Jane Gagliardi, MD; Kimberly Graham, BA; Kyle Hess, MS; Carol Leslie, MS; Ronald G. Munger, PhD, MPH; Chiadi U. Onyike, MD; Roxane Pfister, MS; Brenda Plassman, PhD; Pritham Raj, MD; Georgiann Sanborn, MS; Nancy Sassano, PhD; Ingmar Skoog, MD; David C. Steffens, MD; Martin Steinberg, MD; Martin J. Toohill, PhD; Heidi Wengreen, PhD, RD; James Wyatt; and Peter P. Zandi, PhD, MPH. Finally, we thank the participants and their families for their participation and support. This study was presented in preliminary form at the International Conference on Alzheimer's Disease and Other Dementias, July 2010, Honolulu, HI. This project was completed as part of a doctoral dissertation by K. A. Treiber.
References
- Akbaraly TN, Portet F, Fustinoni S, Dartigues JF, Artero S, Rouaud O. Berr C. Leisure activities and the risk of dementia in the elderly: Results from the Three-City Study. Neurology. 2009;73:854–861. doi: 10.1212/WNL.0b013e3181b7849b. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed. Revised Washington, DC: Author; 1987. [Google Scholar]
- Andel R, Vigen C, Mack WJ, Clark LJ, Gatz M. The effect of education and occupational complexity on rate of cognitive decline in Alzheimer's patients. Journal of the International Neuropsychological Society. 2006;12:147–152. doi: 10.1017/S1355617706060206. [DOI] [PubMed] [Google Scholar]
- Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and Whites. Neurology. 2004;63:2322–2326. doi: 10.1212/01.wnl.0000147473.04043.b3. [DOI] [PubMed] [Google Scholar]
- Breitner JC, Wyse BW, Anthony JC, Welsh-Bohmer KA, Steffens DC, Norton MC, Khachaturian A. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: The Cache County Study. Neurology. 1999;53:321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Siedlecki KL, Muraskin J, Manly JJ, Luchsinger JA, Yeung LK, Stern Y. White matter hyperintensities and cognition: Testing the reserve hypothesis. Neurobiology of Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.10.013. published ahead of print, doi: 10.1016/j.neurobiolaging.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones TL, Klintsova AY, Greenough WT. Stability of synaptic plasticity in the adult rat visual cortex induced by complex environment exposure. Brain Research. 2004;1018:130–135. doi: 10.1016/j.brainres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Carlson MC, Erickson KI, Kramer AF, Voss MW, Bolea N, Mielke M, Fried LP. Evidence for neurocognitive plasticity in at-risk older adults: The experience corps program. The Journals of Gerontology, Series A Biological Sciences and Medical Sciences. 2009;64:1275–1282. doi: 10.1093/gerona/glp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson MC, Helms MJ, Steffens DC, Burke JR, Potter GG, Plassman BL. Midlife activity predicts risk of dementia in older male twin pairs. Alzheimer's and Dementia. 2008;4:324–331. doi: 10.1016/j.jalz.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson MC, Parisi JM, Xia J, Xue Q, Rebok GW, Bandeen-Roche K, Fried L. P. Lifestyle activities and aging: Variety may be the spice of life. The Women's Health and Aging Study II. Journal of the International Neuropsychological Society. doi: 10.1017/S135561771100169X. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson MC, Saczynski JS, Rebok GW, Seeman T, Glass TA, McGill S, Fried LP. Exploring the effects of an “everyday” activity program on executive function and memory in older adults: Experience corps. The Gerontologist. 2008;48:793–801. doi: 10.1093/geront/48.6.793. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, cAuley EM, Cohen NJ, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dik M, Deeg DJ, Visser M, Jonker C. Early life physical activity and cognition at old age. Journal of Clinical and Experimental Neuropsychology. 2003;25:643–53. doi: 10.1076/jcen.25.5.643.14583. [DOI] [PubMed] [Google Scholar]
- Dong WK, Greenough WT. Plasticity of nonneuronal brain tissue: Roles in developmental disorders. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:85–90. doi: 10.1002/mrdd.20016. [DOI] [PubMed] [Google Scholar]
- Fabrigoule C, Letenneur L, Dartigues JF, Zarrouk M, Commenges D, Barberger-Gateau P. Social and leisure activities and risk of dementia: A prospective longitudinal study. Journal of the American Geriatrics Society. 1995;43:485–490. doi: 10.1111/j.1532-5415.1995.tb06093.x. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gabe FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method grading the cognitive state of patients for the clinician. Psychiatry Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friedland RP, Fritsch T, Smyth KA, Koss E, Lerner AJ, Petot GJ, Debanne SM. Patients with Alzheimer's disease have reduced activities in midlife compared with healthy control-group members. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3440–3445. doi: 10.1073/pnas.061002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch T, McClendon MJ, Smyth KA, Lerner AJ, Chen CH, Petot GJ, Friedland RP. Effects of educational attainment on the clinical expression of Alzheimer's disease: Results from a research registry. American Journal of Alzheimer's Disease and Other Dementias. 2001;16:369–376. doi: 10.1177/153331750101600606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CB, Lipton RB, Sliwinski M, Katz MJ, Derby CA, Verghese J. Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology. 2009;73:356–361. doi: 10.1212/WNL.0b013e3181b04ae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzner EP, Scarmeas N, Cosentino S, Portet F, Stern Y. Leisure activity and cognitive decline in incident Alzheimer disease. Archives of Neurology. 2007;64:1749–1754. doi: 10.1001/archneur.64.12.1749. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. British Journal of Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Archives of Neurology. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Galik E, Steele C, Steinberg M, Rosenblatt A, Warren A, Brandt J. The General Medical Health Rating (GMHR): A bedside global rating of medical comorbidity in patients with dementia. Journal of the American Geriatrics Society. 1999;47:487–491. doi: 10.1111/j.1532-5415.1999.tb07245.x. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ARDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Miech RA, Breitner JCS, Zandi PP, Khachaturian AS, Anthony JC, Mayer L. Incidence of AD may decline in the early 90s for men, later for women. The Cache County Study. Neurology. 2002;58:209–218. doi: 10.1212/wnl.58.2.209. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annual Review of Neuroscience. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Morris JC. The clinical dementia rating (CDR): Current version and scoring rules. Neurology. 1993;24:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Norton MC, Piercy KW, Rabins PV, Greene RC, Breitner JC, Ostbye T, Tschanz JT. Caregiver-recipient closeness and symptom progression in Alzheimer disease. The Cache County Dementia Progression Study. The Journals of Gerontology, Series B Psychological Sciences and Social Sciences. 2009;64:560–568. doi: 10.1093/geronb/gbp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryant SE, Waring SC, Cullum C, Hall J, Lacritz L, Massman PJ, Doody R. Staging dementia using clinical dementia rating scale sum of boxes scores. Archives of Neurology. 2008;65:1091–1095. doi: 10.1001/archneur.65.8.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TM, Winblad B, Granholm AC, Mohammed AH. Environmental influences on brain neurotrophins in rats. Pharmacology Biochemistry and Behavior. 2002;73:167–75. doi: 10.1016/s0091-3057(02)00783-9. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurogenesis in adult primates. Progress in Brain Research. 2002;138:3–14. doi: 10.1016/S0079-6123(02)38067-1. [DOI] [PubMed] [Google Scholar]
- Richards B, Skoletsky J, Shuber AP, Balfour R, Stern RC, Dorkin HL, Klinger KW. Multiplex PCR amplification from the CFTR gene using DNA prepared from buccal brushes/swabs. Human Molecular Genetics. 1993;2:159–163. doi: 10.1093/hmg/2.2.159. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Roses AD. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer's disease. Neurology. 2001;57:2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Stern Y. Cognitive reserve and lifestyle. Journal of Clinical and Experimental Neuropsychology. 2003;25:625–633. doi: 10.1076/jcen.25.5.625.14576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Zarahn E, Anderson KE, Habeck CG, Hilton J, Flynn J, Stern Y. Association of life activities with cerebral blood flow in Alzheimer disease: implications for the cognitive reserve hypothesis. Archives of Neurology. 2003;60:359–365. doi: 10.1001/archneur.60.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooler C, Mulatu MS, Oates G. The continuing effects of substantively complex work on the intellectual functioning of older workers. Psychology and Aging. 1999;14:483–506. doi: 10.1037//0882-7974.14.3.483. [DOI] [PubMed] [Google Scholar]
- Shipley WS. Shipley institute of living scale. Los Angeles, CA: Western Psychological Services; 1967. [Google Scholar]
- Silverman JM, Breitner JCS, Mohs RC, Davis KL. Reliability of the family history method in genetic studies of Alzheimer's disease and related dementias. American Journal of Psychiatry. 1986;143:1279–1282. doi: 10.1176/ajp.143.10.1279. [DOI] [PubMed] [Google Scholar]
- Staff RT, Murray AD, Deary IJ, Whalley L. What provides cerebral reserve? Brain. 2004;127:1191–1199. doi: 10.1093/brain/awh144. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Disease and Associated Disorders. 2006;20(Suppl. 2):S69–S74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- Stern Y, Albert S, Tang M, Tsai W. Rate of memory decline in AD is related to education and occupation: Cognitive reserve? Neurology. 1999;53:1942–1947. doi: 10.1212/wnl.53.9.1942. [DOI] [PubMed] [Google Scholar]
- Tschanz JT, Corcoran C, Schwartz S, Treiber K, Norton MN, Green RC, Lyketsos CG. Progression in cognition, function and neuropsychiatric symptoms in a population cohort with Alzheimer's dementia. The Cache County Dementia Progression Study. American Journal of Geriatric Psychiatry. published ahead of print, doi: 10.1097/JGP.0b013e3181faec23. [Google Scholar]
- Tschanz JT, Welsh-Bohmer KA, Plassman BL, Norton MC, Wyse BW, Breitner JC. An adaptation of the modified mini-mental state examination: Analysis of demographic influences and normative data: The Cache County Study. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 2002;15:28–38. [PubMed] [Google Scholar]
- Tschanz JT, Welsh-Bohmer KA, Skoog I, West N, Norton MC, Wyse BW, Breitner JC. Dementia diagnoses from clinical and neuropsychological data compared: The Cache County Study. Neurology. 2000;54:1290–1296. doi: 10.1212/wnl.54.6.1290. [DOI] [PubMed] [Google Scholar]
- Turner AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. I. Synaptic and neuronal density and synapses per neuron. Brain Research. 1985;329:195–203. doi: 10.1016/0006-8993(85)90525-6. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, LeValley A, Derby C, Kuslansky G, Katz M, Hall C, Lipton RB. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology. 2006;66:821–827. doi: 10.1212/01.wnl.0000202520.68987.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G, Buschke H. Leisure activities and the risk of dementia in the elderly. New England Journal of Medicine. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- Wang H, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: A longitudinal study from the Kungsholmen project. American Journal of Epidemiology. 2002;155:1081–1087. doi: 10.1093/aje/155.12.1081. [DOI] [PubMed] [Google Scholar]
- Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. Journal of the American Medical Association. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke K. M ACTIVE Study Group. Long-term effects of cognitive training on everyday functional outcomes in older adults. Journal of the American Medical Association. 2006;296:2852–2854. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Bennett DA, Bienias JL, Mendes de Leon CF, Morris MC, Evans DA. Cognitive activity and cognitive decline in a biracial community population. Neurology. 2003;61:812–816. doi: 10.1212/01.wnl.0000083989.44027.05. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Bennett DA, Gilley DW, Beckett LA, Barnes LL, Evans DA. Premorbid reading activity and patterns of cognitive decline in Alzheimer disease. Archives of Neurology. 2000;57:1718–1723. doi: 10.1001/archneur.57.12.1718. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Mendes de Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. Journal of the American Medical Association. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: Women who walk. Archives of Internal Medicine. 2001;161:1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]