Abstract
Objectives.
To investigate whether 3 positive psychological characteristics, related to sense of control, modify the associations of physical performance levels with subsequent functional decline and institutionalization.
Method.
One thousand five hundred and thirty-two men and women participating in the Longitudinal Aging Study Amsterdam and not living in an institution in 2005–2006 were included. Mastery, self-efficacy, investment in independence, and objective physical performance scores were ascertained in 2005–2006. Functional decline and institutionalization were assessed after 3 years of follow-up.
Results.
The association between lower physical performance levels and increased odds of functional decline was modified by investment in independence, with a weaker association found among people with higher investment in independence scores than in people with lower scores even after adjustment for covariates. The association between lower physical performance levels and higher odds of institutionalization was marginally weaker among those people with above median levels of mastery (test of interaction p = .08). In men, an association between general self-efficacy and functional decline was found and maintained after adjustments.
Conclusions.
Positive psychological characteristics, related to sense of control, play a role in the transition between stages in the disablement process. Specific psychological characteristics may be associated with different stages of the disablement process and may in turn be affected by disablement.
Keywords: Cohort analysis, Disability, Epidemiology, Independence, Mastery, Self-efficacy
THERE is increasing interest in the role that positive psychological characteristics may play in influencing the health of older people. However, the specific disease processes that are associated with these characteristics and the underlying explanations of such associations remain to be elucidated.
Control is an important aspect of psychological functioning and is associated with a broad range of positive psychological and health outcomes (Skinner, 1996). Two widely studied positive psychological characteristics representing different aspects of control are mastery, the sense of control over the circumstances in their own lives that a person considers themselves to have (as opposed to being fatalistically ruled; Pearlin, Nguyen, Schieman, & Milkie, 2007; Pearlin & Schooler, 1978), and self-efficacy, the belief a person has in their ability to organize and execute certain behaviors in order to achieve a desired goal (Bandura, 1977). Although not all findings are consistent, associations have been found in community-based older populations between lower levels of mastery and self-efficacy and higher risk of a range of poor health outcomes including shorter survival times (Penninx et al., 1997; Surtees, Wainwright, Luben, Khaw, & Day, 2006). Differential associations of mastery and self-efficacy with health outcomes including disability have been found (G. I. Kempen, Ranchor, van Sonderen, van Jaarsveld, & Sanderman, 2006; G. I. Kempen, van Heuvelen, et al., 1999; G. I. Kempen, van Sonderen, & Ormel, 1999). This is likely to be due to the fact that even though these characteristics are related concepts, self-efficacy, much more than mastery, pertains to specific sets of behaviors—to perseverance in the face of unexpected problems and setbacks and to confidence in one’s ability to stick to plans and decisions. There is therefore value in considering these two characteristics separately.
In addition to mastery and self-efficacy, another psychological characteristic, investment in independence, which is also related to control and may along with the other two characteristics be a prerequisite for autonomy, is explored. Investment in independence is a much less well-studied concept than mastery or self-efficacy and is a measure of the level of importance of independent living to a person. This factor might thus be especially important in relation to outcomes such as functional decline and institutionalization, which affect independence. Although studies show that older people value their independence and consider mobility limitations as a threat to the loss of this (Gabriel & Bowling, 2004), it cannot be assumed that all older people will place the same value in maintaining their independence as all others. People who attach greater value to their independence may be more likely to exhibit behavior that, in their belief, will ensure they attain their goal of remaining independent than people who attach less value to their independence.
It has long been held that psychosocial factors, including positive psychological characteristics related to sense of control, may influence disablement. It has been proposed that as part of the disablement process impairments in anatomical, physiological, or emotional functions, which often occur with increasing age, detrimentally affect physical performance levels. Once levels of physical performance fall below a critical threshold, the likelihood that a person will experience limitations in the ability to perform defined roles and tasks (including bathing, using transport, and dressing) increases. This in turn affects ability to maintain independence (van Gool et al., 2005; Verbrugge & Jette, 1994). The focus in this article is on a single part of one proposed pathway, that is, the link between objectively measured physical performance levels and subsequent limitations in the ability to perform defined roles and tasks and loss of independence. Physical performance levels can be measured objectively using walking, balance, and other mobility tests, a strength of which is that variations in functioning across the whole spectrum of ability can then be examined.
Moderation of disablement by psychological factors is explicitly assumed in many disablement models. A number of studies have thus explored the associations of positive psychological characteristics with outcomes at different stages on the disablement pathway (Femia, Zarit, & Johansson, 1997; Hardy & Gill, 2005; G. I. Kempen, van Sonderen, et al., 1999; G. I. J. M. Kempen et al., 2005; G. I. Kempen et al., 2006; Mackenbach, Borsboom, Nusselder, Looman, & Schrijvers, 2001; Mendes de Leon, Seeman, Baker, Richardson, & Tinetti, 1996; Nusselder, Looman, & Mackenbach, 2005; Seeman, Unger, McAvay, & Mendes de Leon, 1999). Most recently, a study of community-dwelling older Italian adults reported an association between lower levels of mastery and greater subsequent declines in lower extremity performance levels over six years of follow-up (Milaneschi et al., 2010). However, findings are not entirely consistent, for example, one study has identified gender differences in association where others have not (Seeman et al., 1999). Furthermore, the role positive psychological characteristics may play in influencing the transition between different stages on the disablement pathway has not been fully explored, and additional work is required to elucidate underlying explanations.
It has been suggested that there could be a “direct effect” of positive psychological characteristics on subsequent health through their influence on physiological processes such as neuroendocrine and immune responses (Penninx et al., 1997). A second possibility is that positive psychological characteristics influence health through their moderating effect on other pathways. In the case of disablement, when faced with declining physical performance levels, those older people with higher levels of positive psychological characteristics may be better able than people with lower levels to utilize resources, initiate healthy behaviors, and undertake preventative strategies that reduce the likelihood that their low physical performance levels will lead to functional decline and loss of independence. Very few studies have examined interactions involving self-efficacy or mastery with factors on the disablement pathway (G. I. Kempen et al., 1999; Mendes de Leon et al., 1996; Seeman et al., 1999; ), and most of these studies (Mendes de Leon et al., 1996; Seeman et al., 1999) explored the modifying effect of physical performance on associations between self-efficacy and functional decline rather than the modifying effect of psychological characteristics.
The objective of this article was thus to examine whether positive psychological characteristics, related to sense of control (mastery, self-efficacy, and investment in independence), modified the associations of physical performance levels at baseline with functional decline and institutionalization over three years. This was done using data from the two most recent waves of the Longitudinal Aging Study Amsterdam (LASA), a nationally representative cohort of Dutch older people.
METHOD
The LASA is a longitudinal study of the predictors and consequences of changes in physical, cognitive, emotional, and social functioning in older people in the Netherlands (D. J. Deeg, van Tilburg, Smit, & de Leeuw, 2002; Huisman et al., in press; Smit, De Vries, & Poppelaars, 1998). The original cohort consisted of a random sample of 3,107 men and women aged 55–85 years, with birth years 1908–1937, stratified by age and gender according to expected mortality within 5 years, drawn from population registers of 11 municipalities in three geographical areas in the Netherlands in 1992. Since baseline, the main assessments of the cohort have taken place every 3 years, most recently in 2008–2009. Using the same sampling frame, an additional 1,002 men and women, with birth years 1938–1947, were recruited into the study in 2002–2003. These analyses utilize data on the merged samples collected in the two most recent waves of LASA, in 2005–2006 (subsequently referred to as T1) and 2008–2009 (T2). All relevant ethical approval has been obtained.
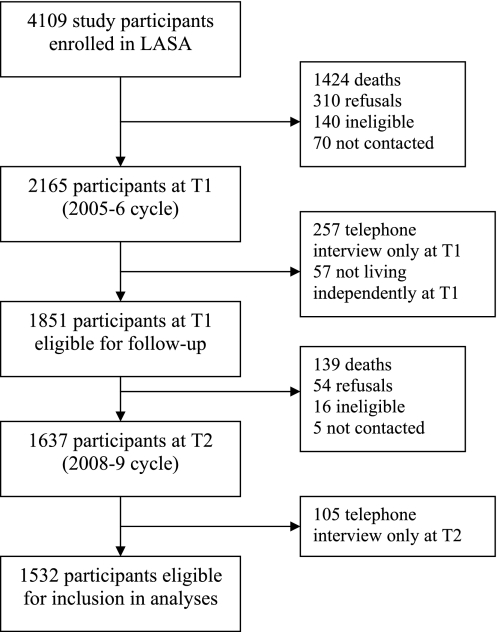
Of the 4,109 participants enrolled in LASA, 2,165 (52.7%) participated in the data collection during the T1 wave in 2005–2006. Of those who did not participate at T1, 1,424 (34.7%) had died, 310 (7.5%) had previously refused to participate, 140 (3.4%) had been classified as ineligible, and 70 (1.7%) could not be contacted. Of the 2,165 who contributed any data at T1, 257 (11.9%) had participated in a telephone interview only and so did not provide the necessary information for inclusion. A further 57 (2.6%) participants from T1 were excluded because they were living in an institution. Of the 1,851 participants remaining at T1 after exclusions, by T2 139 (7.5%) had died, 54 (2.9%) refused to participate, 16 (0.9%) were ineligible, and 5 (0.3%) could not be contacted. Of the remaining 1,637, 105 (6.4%) had participated in a telephone interview only at T2. There were therefore a maximum of 1,532 participants eligible for inclusion (Figure 1).
Figure 1.
Flow diagram showing selection of sample for inclusion in analyses.
Measurements
Change in functional limitations.—
Functional limitations were ascertained by self-report. At both waves, participants were asked to report the degree to which they had difficulty performing seven daily tasks (including some standard activities of daily living): going up and down a staircase of 15 steps without having to stop; walking for 5 min outside the house; getting undressed; sitting down and rising from a chair; cutting own toenails; bathing or showering; and using own or public transportation, with five response options: no difficulty; some difficulty; much difficulty; only with help; and unable. These response categories were coded as 0, 1, 2, 3, and 4, respectively, and sums of scores were calculated (range 0–28) with higher scores indicating greater levels of functional limitations (Cronbach’s α at T1 = .82 and at T2 = .85).
To identify those participants who had experienced a relevant change in total functional limitation score between T1 and T2 (i.e., change which is unlikely to be accounted for by regression to the mean) the Edwards–Nunnally (EN) Index was used (Speer & Greenbaum, 1995). The change in total functional limitation scores between T1 and T2 was computed for each study participant using the formula:
where Cronbach’s α is the scale reliability of the seven items at T1 (0.82), FLT1 is the individual’s functional limitation score at T1, mean T1 is the mean functional limitation score at T1 (2.39, SD 4.11), 1.645 represents the 90% confidence interval, and SE is the 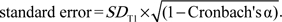 People were categorized as having experienced an increase in functional limitation score (i.e., functional decline) if their functional limitation score at T2 (FLT2) > (Cronbach’s α × (FLT1 − mean T1) + mean T1 + 1.645 × SE), those for whom FLT2 < (Cronbach’s α × (FLT1 − mean T1) + mean T1 − 1.645 × SE) were categorized as having experienced a decrease in functional limitation score (i.e., improvement) with all those people whose value of FLT2 fell between these two parameters categorized as experiencing no change. A distinction was therefore made between no change (N = 1,210 [81.2%], mean [SD] difference in total functional limitation scores between T1 and T2 = 0.04 [1.06]), functional decline (N = 240 [16.1%], mean [SD] difference = 5.92 [3.88]), and improvement (N = 40 [2.7%], mean [SD] difference = −6.28 [2.86]). For the purposes of these analyses, those who had experienced no relevant change and those who had improved were grouped together to create a binary variable distinguishing those who had experienced functional decline from all others.
People were categorized as having experienced an increase in functional limitation score (i.e., functional decline) if their functional limitation score at T2 (FLT2) > (Cronbach’s α × (FLT1 − mean T1) + mean T1 + 1.645 × SE), those for whom FLT2 < (Cronbach’s α × (FLT1 − mean T1) + mean T1 − 1.645 × SE) were categorized as having experienced a decrease in functional limitation score (i.e., improvement) with all those people whose value of FLT2 fell between these two parameters categorized as experiencing no change. A distinction was therefore made between no change (N = 1,210 [81.2%], mean [SD] difference in total functional limitation scores between T1 and T2 = 0.04 [1.06]), functional decline (N = 240 [16.1%], mean [SD] difference = 5.92 [3.88]), and improvement (N = 40 [2.7%], mean [SD] difference = −6.28 [2.86]). For the purposes of these analyses, those who had experienced no relevant change and those who had improved were grouped together to create a binary variable distinguishing those who had experienced functional decline from all others.
Institutionalization.—
Using information recorded by the interviewer on residence, those 27 people who were not living in an institution at T1 but were living either in a nursing or residential home at T2 were distinguished from those people who were not living in an institution at either wave.
Physical performance.—
During the face-to-face interview at T1, participants were asked to complete four standard physical performance tests: a timed walking test, the time taken to walk 3 m, turn around and walk back as fast as possible; a chair rise test, the time taken to stand up and sit down again from a kitchen chair five times with arms folded as quickly as possible; a tandem stand, the time up to a maximum of 10 s that the participant could stand with one foot placed directly in front of and touching the toes of the other foot; and a cardigan test, the time taken to put on and take off a cardigan provided by the interviewer (D. J. H. Deeg, 1994; Guralnik et al., 1994). The times taken to complete the walking, chair rise, and cardigan tests were categorized into quartiles, using cut-points based on the distribution of performance times in the T1 sample, with those people in the fastest quartile allocated a score of 4 through to those in the slowest quartile being allocated a score of 1. Those people unable to complete the test were allocated a score of 0. For the tandem stand, those unable to hold the position for at least 3 s were allocated a score of 0, those with times of 3–9 s a score of 2, and those able to hold the stand for the maximum time a score of 4. The scores (scaled 0–4) on each of these four tests were then summed to create a total physical performance score (range 0–16) with higher scores indicating better levels of performance.
Mastery.—
Mastery was measured at T1 using a five-item abbreviated version of the Pearlin Mastery Scale (Pearlin & Schooler, 1978). Participants were asked to report how strongly they agreed or disagreed with five statements (e.g., I have little control over the things that happen to me) using the response options strongly disagree, disagree, no disagreement or agreement, agree, or strongly agree. The responses to each item were scored 4, 3, 2, 1, and 0, respectively, and the sum of the five scores calculated (range 0–20) with higher scores indicating greater levels of mastery (Cronbach’s α at T1 = .77).
General self-efficacy.—
Self-efficacy was measured at T1 using a 12-item version of the General Self-Efficacy Scale, which includes three domains, ability to initiate behavior, invest effort, and persevere in the face of misfortune (Sherer et al., 1982). Participants were asked to report how strongly they agreed or disagreed with 12 statements (e.g., if something looks too complicated, I will not even bother to try it) with responses scored from 1 to 5. After reversing the scores of 7 of the items so that they were all scaled in the same direction, the sum of the 12 scores was calculated (range 12–60) with higher total scores indicating greater levels of general self-efficacy (Cronbach’s α at T1 = .73).
Investment in independence.—
Investment in independence was measured using a 16-item version of the meta-memory in adulthood achievement subscale (Dixon & Hultsch, 1983, 1984) modified by Auman, Bosworth, and Hess (2005) to quantify how invested study participants were in being able to function independently in everyday life, which after translation into Dutch was included in the self-completion questionnaire at T1. Each item (e.g., it is important to me to be able to do things for myself) had five response options that were scored 0–4 (with 0 indicating lowest level of investment and 4 indicating the highest level). After excluding two items due to their low correlation with other items and their negative impact on the reliability of the scale, the scores from the other 14 items were summed to create an overall investment score, with values imputed based on their average score on the other items for those people with only one or two missing items (n = 201). The range of this score is 0–56, with higher values indicating greater levels of investment in independence (Cronbach’s α at T1 = .85).
Covariates.—
In addition to age in years and gender, other covariates were selected as potential confounders a priori (Table 1). Partner status was ascertained at T1 and categorized as having a partner or no partner. Highest level of education was categorized into three groups: low (elementary school or less), intermediate (lower vocational, general intermediate, intermediate vocational, or general secondary school), high (higher vocational education, college, or university). Number of chronic diseases at T1, categorized as none, 1, 2, or 3 or more, was based on self-reports of seven major conditions: chronic obstructive pulmonary diseases, cardiovascular disease (myocardial infarction, arrhythmias, congestive heart failure, angina pectoris, and narrowing of the coronary arteries), peripheral arterial disease, diabetes mellitus, cerebrovascular accidents, rheumatoid or osteoarthritis, and cancer. Depressive symptoms and cognitive impairment were assessed at T1 using the Dutch version of the Center for Epidemiological Studies–Depression Scale (CES-D; Beekman, van Limbeek, Deeg, Wouters, & van Tilburg, 1994; Radloff, 1977) and Mini-Mental State Examination (MMSE), respectively (Folstein, Folstein, & McHugh, 1975). As the CES-D and MMSE scores were both highly skewed (i.e., 45% of participants had a CES-D score of 0–5 [range of recorded scores: 0–49] and 66% had an MMSE score of 28–30 [range of recorded scores: 10–30]), binary variables were created using the scores ≥16 and ≤23 to identify those with clinically relevant levels of depressive symptoms (Beekman et al., 1997) and cognitive impairment, respectively.
Table 1.
Baseline Characteristics of LASA Sample by Change in Functional Status and Institutionalization Between Baseline and 3-Year Follow-Up
|
N (%), M (SD), or median (IQR) |
|||||
| Functional status |
Institutionalization |
||||
| No decline | Decline | Not institutionalized | Institutionalized | ||
| Baseline characteristics | Totala | 1,250 (83.9) | 240 (16.1) | 1,505 (98.2) | 27 (1.8) |
| Age (years) | 70.0 (8.5) | 68.4 (7.5) | 76.7 (8.7)*** | 69.8 (8.3) | 82.5 (6.3)*** |
| Gender | |||||
| Male | 693 (45.2) | 604 (88.8) | 76 (11.2) | 685 (98.9) | 8 (1.1) |
| Female | 839 (54.8) | 646 (79.8) | 164 (20.2)*** | 820 (97.7) | 19 (2.3) |
| Physical performance | 10.3 (3.5) | 10.9 (3.1) | 7.2 (3.7)*** | 10.4 (3.4) | 4.7 (3.3)*** |
| Functional limitation | 1 (0–3) | 0 (0–2) | 5 (1–10)*** | 1 (0–3) | 6.5 (4–13)*** |
| Mastery | 12.8 (3.4) | 13.0 (3.4) | 11.7 (3.3)*** | 12.8 (3.4) | 10.1 (3.1)*** |
| Investment in independence | 43.4 (6.7) | 43.4 (6.7) | 43.1 (7.0) | 43.4 (6.6) | 43.4 (7.6) |
| Self-efficacy | 43.0 (5.4) | 43.3 (5.4) | 41.0 (5.3)*** | 43.0 (5.4) | 39.0 (4.8)** |
| Education | |||||
| High | 295 (19.3) | 261 (91.6) | 24 (8.4) | 293 (99.3) | 2 (0.7) |
| Middle | 857 (55.9) | 714 (85.4) | 122 (14.6) | 845 (98.6) | 12 (1.4) |
| Low | 380 (24.8) | 275 (74.5) | 94 (25.5)*** | 367 (96.6) | 13 (3.4)** |
| Partner status | |||||
| Partner | 1094 (71.4) | 948 (87.9) | 130 (12.1) | 1087 (99.4) | 7 (0.6) |
| No partner | 438 (28.6) | 302 (73.3) | 110 (26.7)*** | 418 (95.4) | 20 (4.6)*** |
| MMSE score | |||||
| ≥24 | 1,453 (94.8) | 1,202 (84.8) | 215 (15.2) | 1,434 (98.7) | 19 (1.3) |
| <24 | 79 (5.2) | 48 (65.8) | 25 (34.2)*** | 71 (89.9) | 8 (10.1)*** |
| CES-D score | |||||
| <16 | 1,314 (85.9) | 1,098 (85.7) | 184 (14.3) | 1,294 (98.5) | 20 (1.5) |
| ≥16 | 215 (14.1) | 151 (73.3) | 55 (26.7)*** | 209 (97.2) | 6 (2.8) |
| No. of chronic conditions | |||||
| None | 451 (29.4) | 420 (94.4) | 25 (5.6) | 448 (99.3) | 3 (0.7) |
| 1 | 581 (37.9) | 487 (85.7) | 81 (14.3) | 572 (98.5) | 9 (1.5) |
| 2 | 335 (21.9) | 254 (78.9) | 68 (21.1) | 326 (97.3) | 9 (2.7) |
| 3 or more | 165 (10.8) | 89 (57.4) | 66 (42.6)*** | 159 (96.4) | 6 (3.6)* |
Notes: Range of: physical performance score = 0 (unable to perform any of the four performance tests)–16 (in the best performing quarter of all four tests); functional limitation score = 0 (no reported limitations performing any of the 7 activities)–28 (unable to perform any of the seven activities [i.e., severely limited]); mastery score = 0 (low)–20 (high); self-efficacy score = 12 (low)–60 (high); investment in independence score = 0 (low)–56 (high). CES-D = Center for Epidemiological Studies–Depression Scale; LASA = Longitudinal Aging Study Amsterdam; MMSE = Mini-Mental State Examination.
Maximum N = 1,532, which includes those people not living in an institution at baseline with information on institutionalization at follow-up, but total N varies due to missing data on main explanatory factors and baseline characteristics.
* .05 > p > 0.01; ** .01 ≥ p ≤ .001 ; *** p < .001, where p values are from t tests, chi-squared tests, or Mann–Whitney tests of differences in distribution between outcome groups (as appropriate).
Analyses
The unadjusted associations of physical performance, mastery, self-efficacy, investment in independence, and covariates with functional decline and institutionalization between T1 and T2 were tested using t tests, chi-squared tests, and Mann–Whitney tests, as appropriate.
Using logistic regression models, the associations of standardized physical performance, mastery, self-efficacy, and investment in independence scores with each of the two outcomes were tested. Models were first adjusted for age and gender and then for indicators of baseline health status (including functional limitation score at T1), education, and partner status. Tests of interaction by gender were performed, given evidence of this has been found in some other studies (Seeman et al., 1999) and where there was evidence of this analyses were stratified by gender. No evidence of deviation from linearity was found.
We tested the interactions of physical performance with mastery, self-efficacy, and investment in independence in logistic regression models with functional decline and institutionalization as the outcomes. For the purposes of these analyses, it was decided a priori to model each of the positive psychological characteristics as binary variables. As there are no defined cut-points for these characteristics, a decision was made a priori to use the median values as the cut-point in the first stage of analyses. Likelihood ratio tests were used to compare models including interaction terms between the binary categorizations of each of the psychological characteristics and the standardized physical performance scores with equivalent models with no interaction term. Where evidence of interaction was found, to test the significance of the interaction across the full range of the distribution and improve interpretability, interactions were then formally tested using a prespecified set of different binary categorizations of the psychological characteristics. These binary categorizations involved using deciles as cut-points and examining each combination of categorization from bottom 10% versus top 90% through to bottom 90% versus top 10%.
All models presented were run on the sample with complete data on covariates. Sensitivity analyses were also performed in which those people who had died between T1 and T2 were coded as having experienced functional decline rather than being excluded, but results from these analyses are not presented as there were no changes in findings.
RESULTS
Of the 1,532 participants eligible for inclusion in analyses (Figure 1), 11 were missing data on functional limitations at T1, 28 were missing data at T2, and 3 were missing data at both waves. Of the remaining 1,490 participants, 240 (16.1%) were classified as having experienced functional decline between T1 and T2 (Table 1). Data were available on housing status for all 1,532 participants, and of these, 27 (1.8%) had been institutionalized in the 3 years between T1 and T2. Those participants with data at T1 who were included in analyses were younger, had higher educational levels, were more likely to have a partner, had higher physical performance and positive psychological characteristic scores, and were healthier than those participants with data at T1 who were necessarily excluded (results not shown).
The study sample had a mean age at T1 of 70 years (range 57.7–97.5 years) and approximately 45% were men. In univariate analyses, the majority of factors examined, including older age, lower educational levels, no partner, lower MMSE scores, and greater numbers of chronic conditions at T1 were associated with both functional decline and institutionalization (Table 1). Mastery, self-efficacy, and investment in independence were not or only moderately correlated with each other (correlation coefficients: mastery–self-efficacy = .57; mastery–investment in independence = .01; self-efficacy–investment in independence = .06).
Functional Decline
Those people with higher physical performance scores at T1 (indicating better physical performance) were at lower odds of subsequent functional decline between T1 and T2 than those people with lower physical performance scores (Table 2). Higher levels of mastery were associated with lower odds of functional decline in models adjusted for age and gender, but with further adjustments, this association was attenuated. There was evidence of an interaction by gender in associations between self-efficacy and functional decline (test of interaction p = .03) with no evidence of an association in women but evidence that men with higher levels of general self-efficacy had lower odds of functional decline than men with lower levels of self-efficacy. This association in men was maintained after adjustments. There was no evidence of association between investment in independence and functional decline.
Table 2.
Results From Logistic Regression Models Testing the Associations of Standardized Physical Performance and Positive Psychological Characteristic Scores With Subsequent Functional Decline and Institutionalization
| Per SD increase in | OR of functional decline (95% CI) |
||
| N | Model 1 | Model 2 | |
| Physical performance | 1,425 | 0.46 (0.39–0.55)** | 0.61 (0.49–0.75)** |
| Mastery | 1,433 | 0.83 (0.71–0.97)* | 1.03 (0.86–1.24) |
| Self-efficacy | |||
| Men | 668 | 0.60 (0.45–0.80)** | 0.68 (0.49–0.94)* |
| Women | 768 | 0.91 (0.74–1.13) | 1.09 (0.87–1.38) |
| Investment in independence | 1,412 | 0.89 (0.76–1.05) | 0.92 (0.77–1.09) |
| OR of institutionalization (95% CI) | |||
| Physical performance | 1,451 | 0.39 (0.23–0.65)** | 0.50 (0.27–0.93)* |
| Mastery | 1,459 | 0.62 (0.39–0.98)* | 0.84 (0.50–1.42) |
| Self-efficacy | 1,462 | 0.58 (0.34–0.99)* | 0.79 (0.45–1.37) |
| Investment in independence | 1,437 | 0.97 (0.59–1.60) | 1.01 (0.61–1.70) |
Notes: Model 1: Adjusted for age and gender. Model 2: Model 1 plus indicators of baseline health status (MMSE score, CES-D score, number of chronic conditions, and functional limitations score), education, and partner status. Total N varies between analyses of different independent variables due to variation in the amount of missing data on these four variables. CI = confidence interval; CES-D = Center for Epidemiological Studies–Depression Scale; MMSE = Mini-Mental State Examination; OR = odds ratio.
*.01 < p < .05; **p ≤.01.
There was no evidence to suggest that the association between physical performance and functional decline was modified by mastery or general self-efficacy (Table 3). However, there was evidence to suggest that the association between physical performance and functional decline was weaker in those with investment in independence scores above the median than in those with investment in independence scores below the median, and this was maintained in fully adjusted models (Table 3). There was also weak evidence of interaction when the bottom 30% (cut-point 39) and 40% (cut-point 41) of investment in independence scores were compared with the top 70% and 60%, respectively; however, there was no evidence of interaction when using other deciles as cut-points (results not shown).
Table 3.
Associations of Standardized Physical Performance Scores With Subsequent Functional Decline Stratified by Positive Psychological Characteristics
| Stratified by | OR of functional decline per 1 SD increase in physical performance score (95% CI) |
|||
| N | Model 1 | Model 2 | Model 3 | |
| Mastery | ||||
| Below median (0–12) | 572 | 0.44 (0.34–0.56) | 0.57 (0.42–0.77) | 0.57 (0.42–0.77) |
| Above median (13–20) | 846 | 0.49 (0.38–0.64) | 0.67 (0.50–0.92) | 0.68 (0.50–0.93) |
| p Value | .58 | .69 | .64 | |
| Self-efficacy | ||||
| Below median (22–42) | 643 | 0.54 (0.42–0.67) | 0.68 (0.52–0.90) | 0.67 (0.51–0.89) |
| Above median (43–60) | 778 | 0.40 (0.31–0.52) | 0.57 (0.41–0.79) | 0.56 (0.40–0.79) |
| p Value | .16 | .24 | .25 | |
| Investment in independence | ||||
| Below median (0–42) | 622 | 0.38 (0.28–0.50) | 0.53 (0.37–0.76) | 0.54 (0.37–0.77) |
| Above median (43–56) | 743 | 0.55 (0.44–0.69) | 0.72 (0.55–0.94) | 0.72 (0.55–0.95) |
| p Value | .03 | .04 | .05 | |
Notes: Model 1: Adjusted for age and gender. Model 2: Model 1 plus indicators of baseline health status (MMSE score, CES-D score, number of chronic conditions, and functional limitations score). Model 3: Model 2 plus education and partner status. p Values from likelihood ratio tests of interaction. CI = confidence interval; CES-D = Center for Epidemiological Studies–Depression Scale; MMSE = Mini-Mental State Examination; OR = odds ratio.
Institutionalization
Lower physical performance scores at T1 were associated with higher odds of institutionalization between T1 and T2, and this was maintained after adjustment for covariates (Table 2). Although both higher levels of mastery and general self-efficacy were associated with lower odds of institutionalization in models adjusted for age and gender, further adjustments attenuated these associations. There was no evidence of association between investment in independence and institutionalization.
There was weak evidence to suggest that mastery modifies the association between physical performance and institutionalization in models adjusted for age and gender, with the association found to be weaker in those people with mastery scores above the median than in people with mastery scores below the median (Table 4). There was also weak evidence of interaction when examining other deciles as cut-points (results not shown). There was no evidence that either general self-efficacy or investment in independence modified the association between physical performance and institutionalization (Table 4).
Table 4.
Associations of Standardized Physical Performance Scores With Subsequent Institutionalization Stratified by Positive Psychological Characteristics
| Stratified by | OR of institutionalization per 1 SD increase in physical performance score (95% CI) |
|||
| N | Model 1 | Model 2 | Model 3 | |
| Mastery | ||||
| Below median (0–12) | 585 | 0.28 (0.12–0.65) | 0.43 (0.17–1.08) | 0.53 (0.21–1.36) |
| Above median (13–20) | 859 | 0.63 (0.26–1.52) | 0.53 (0.19–1.49) | 0.49 (0.16–1.53) |
| p Value | .08 | .09 | .10 | |
| Self-efficacy | ||||
| Below median (22–42) | 654 | 0.47 (0.25–0.89) | 0.60 (0.28–1.25) | 0.63 (0.30–1.33) |
| Above median (43–60) | 793 | 0.30 (0.10–0.89) | 0.48 (0.12–2.00) | 0.49 (0.11–2.19) |
| p Value | .58 | .62 | .60 | |
| Investment in independence | ||||
| Below median (0–42) | 633 | 0.37 (0.13–1.06) | 0.50 (0.16–1.58) | 0.50 (0.15–1.61) |
| Above median (43–56) | 755 | 0.57 (0.29–1.15) | 0.51 (0.22–1.20) | 0.52 (0.21–1.28) |
| p Value | .32 | .34 | .34 | |
Notes: Model 1: Adjusted for age and gender. Model 2: Model 1 plus indicators of baseline health status (number of chronic conditions and functional limitations score). Model 3: Model 2 plus education and partner status. p Values from likelihood ratio tests of interaction. CI = confidence interval; OR = odds ratio.
DISCUSSION
In a study of older Dutch people, there was some evidence to suggest that the association between lower physical performance levels and increased odds of functional decline over three years is modified by investment in independence, with this association found to be stronger among people with lower investment in independence scores than in those with higher scores. There was also evidence to suggest that mastery may modify the association between physical performance levels and institutionalization over three years of follow-up in models adjusted for age and gender. Although there was no evidence that general self-efficacy modified the associations between physical performance levels and either functional decline or institutionalization, in men, an association between general self-efficacy and subsequent functional decline was found and maintained after adjustments.
Explanation of Findings
The finding of a stronger association between lower physical performance levels and increased odds of functional decline in those with below median levels of investment in independence could be explained by a link between investment in independence and factors that influence functional decline including utilization of resources. If people with lower levels of investment in independence are less likely to utilize strategies, which prevent decline in the face of challenges to their future independence, this could explain the interaction found. That there was only evidence of interaction when examining the lower cut-points of investment in independence suggests that this is not a dose–response relationship. It may therefore be that not only is having a low level of investment in independence detrimental but that having the highest levels of investment is not most beneficial. People with the highest levels of a characteristic such as investment in independence may have more unrealistic expectations of their ability to maintain their independence in older age and therefore be less flexible when faced with challenges to this (Brandtstädter, 2009).
That the direct associations of mastery with functional decline and institutionalization were attenuated after adjustments for health status at baseline suggests that associations of mastery with health outcomes, including those on the pathway to disablement, may be bidirectional or confounded by health status. If, as others have proposed, current levels of mastery are influenced by contemporaneous circumstances and prior health and life experiences (Pearlin et al., 2007), it may be that at the stages of disablement examined in our study, mastery had been influenced by prior experience of declines in physical performance and the onset of functional limitations. It is also possible that mastery levels changed during follow-up. As it has been suggested that mastery is not a fixed trait (D. J. Deeg & Huisman, 2010; Pearlin et al., 2007), it may therefore be difficult to establish the temporal nature of associations of this characteristic with others. It would therefore be beneficial to investigate whether mastery earlier in life, assessed prior to the onset of declines in functioning and health status, is associated with outcomes including those on the disablement pathway.
Self-efficacy could be associated with functional decline in men through its effect on their uptake and maintenance of health behaviors, utilization of resources, and use of support networks that influence risk of functional decline. Gender differences are observed in a wide number of relevant behaviors and processes including health care utilization and social support (Oksuzyan, Juel, Vaupel, & Christensen, 2008). As women are more likely to utilize health care and receive support from others than men (Oksuzyan et al., 2008), it may be that such processes are influenced less by levels of general self-efficacy among women than in men where such behaviors are less common and higher levels of general self-efficacy are required in order to increase the likelihood that such protective pathways will be followed.
The three psychological characteristics examined were selected as they each represent aspects of control and are prerequisites for autonomy. However, the difference in findings by these psychological characteristics suggests that despite being related, these factors represent different and distinct aspects of control. Mastery and self-efficacy correlated strongly with one another but neither correlated with investment in independence. This might be because mastery and self-efficacy refer to experienced control or confidence in one’s ability to take control, whereas investment in independence reflects a personal need for control. People who feel the need to be independent may not actually experience control if they are unable to remain so. This confirms the value in studying these characteristics separately.
Methodological Considerations
Only a proportion of the original LASA sample was included in analyses. The initial sample selection and subsequent attrition could have introduced bias and affected the representativeness of the study population and generalizability of findings. However, a large proportion of the losses to follow-up in LASA are due to unmodifiable causes, most importantly death (Figure 1). As high mortality is a characteristic of older populations, losses to follow-up for this specific reason will not necessarily affect the representativeness of the study population or bias results. For instance, there were no changes in findings when those people who had died between T1 and T2 were included in sensitivity analyses rather than being excluded (results not shown). Although sample attrition may introduce bias, in another study where factors similar to those shown to be associated with losses to follow-up in LASA (i.e., age, education, and health status), the impact of attrition on the associations between psychological attributes and disability was not strong (G. I. Kempen & van Sonderen, 2002).
Due to losses to follow-up, the sample size for our analyses was limited. In particular, only a small proportion of the population were institutionalized during follow-up. This could mean that we had limited statistical power to detect associations when examining institutionalization as our outcome.
The investment in independence scale used in these analyses has not been validated, and while Cronbach’s α suggests that this scale has good internal reliability, further work is required to confirm its validity. However, mastery and general self-efficacy were assessed using widely used and validated instruments. In assessing functional decline study, participants self-reported their abilities to undertake the specified tasks. It is possible that these self-reports are influenced by positive psychological characteristics whereby those people with lower levels are more likely to perceive difficulties in performing tasks and report this than those people with similar levels of functional ability but higher levels of positive psychological characteristics. Such effects would be expected to dilute the size of associations found. However, in assessing functional decline, we used the EN Index that allows for regression to the mean and we also adjusted for baseline functional limitation scores.
Future Research and Conclusions
In trying to identify how positive psychological characteristics are associated with health outcomes on the disablement pathway, it may be beneficial to test the interactions of mastery, self-efficacy, and investment in independence with other related personality characteristics and factors that are associated with people’s ability to successfully translate their beliefs into appropriate actions. For example, it may be that higher levels of positive psychological characteristics are only beneficial if they are accompanied by other traits such as high educational levels, cognitive reserve, or income that ensure that the person’s perceptions of their ability to perform tasks can actually be translated into beneficial actions such as seeking social support and adopting more health-promoting behaviors. If there is a mismatch between mastery, self-efficacy, or investment in independence and other characteristics, it may be that this is detrimental.
Our findings suggest that positive psychological characteristics related to sense of control play a role in the transition between stages in the disablement process. Different psychological characteristics may be associated with different stages of the disablement process in different ways and associations may be bidirectional. There is thus a clear need for further research investigating the impact of psychological characteristics on performance at different stages of the disablement process that takes account of this.
FUNDING
LASA is funded by the Netherlands Ministry of Health Welfare and Sports, Department of Long Term Care. R. Cooper is supported by the New Dynamics of Ageing (RES-353-25-0001) and received a Royal Society travel award (TG091023) to undertake this project. M. Huisman is supported by the Dutch Ministry of Health, Welfare and Sport. D. Kuh is supported by the UK Medical Research Council.
References
- Auman C, Bosworth HB, Hess TM. Effect of health-related stereotypes on physiological responses of hypertensive middle-aged and older men. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2005;60:3–10. doi: 10.1093/geronb/60.1.p3. [DOI] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy—Toward a unifying theory of behavioral change. Psychological Review. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- Beekman AT, Deeg DJH, van Limbeek J, Braam AW, De Vries MZ, van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): Results from a community-based sample of older subjects in the Netherlands. Psychological Medicine. 1997;27:231–235. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- Beekman AT, van Limbeek J, Deeg DJ, Wouters L, van Tilburg W. [A screening tool for depression in the elderly in the general population: The usefulness of Center for Epidemiological Studies Depression Scale (CES-D)] Tijdschrift voor Gerontologie en Geriatrie. 1994;25:95–103. [PubMed] [Google Scholar]
- Brandtstädter J. Goal pursuit and goal adjustment: Self-regulation and intentional self-development in changing developmental contexts. Advances in Life Course Research. 2009;14:52–62. [Google Scholar]
- Deeg DJH. Performance tests of physical ability. In: Deeg DJH, Westendorp-de Seriere M, editors. Autonomy and well-being in the aging population I: Report from the Longitudinal Aging Study Amsterdam 1992–1993. Amsterdam, The Netherlands: VU University; 1994. pp. 21–29. [Google Scholar]
- Deeg DJH, Huisman M. Cohort differences in 3-year adaptation to health problems among Dutch middle-aged, 1992–1995 and 2002–2005. European Journal of Ageing. 2010;7:157–165. doi: 10.1007/s10433-010-0157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeg DJH, van Tilburg T, Smit JH, de Leeuw ED. Attrition in the Longitudinal Aging Study Amsterdam. The effect of differential inclusion in side studies. Journal of Clinical Epidemiology. 2002;55:319–328. doi: 10.1016/s0895-4356(01)00475-9. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Hultsch DF. Structure and development of metamemory in adulthood. Journal of Gerontology. 1983;38:682–688. doi: 10.1093/geronj/38.6.682. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Hultsch DF. The Metamemory in Adulthood (MIA) instrument. Psychological Documents. 1984;14:3. [Google Scholar]
- Femia EE, Zarit SH, Johansson B. Predicting change in activities of daily living: A longitudinal study of the oldest old in Sweden. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 1997;52:294–302. doi: 10.1093/geronb/52b.6.p294. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gabriel Z, Bowling A. Quality of life from the perspectives of older people. Ageing & Society. 2004;24:675–691. [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Wallace RB. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. Journal of Gerontology. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Hardy SE, Gill TM. Factors associated with recovery of independence among newly disabled older persons. Archives of Internal Medicine. 2005;165:106–112. doi: 10.1001/archinte.165.1.106. [DOI] [PubMed] [Google Scholar]
- Huisman M, Poppelaars J, Van der Horst M, Beekman ATF, Brug J, Van Tilburg TG, Deeg DJH. Cohort profile: The Longitudinal Aging Study Amsterdam. International Journal of Epidemiology. in press doi: 10.1093/ije/dyq219. doi:10.1093/ije/dyq219. [DOI] [PubMed] [Google Scholar]
- Kempen GI, Ranchor AV, van Sonderen E, van Jaarsveld CH, Sanderman R. Risk and protective factors of different functional trajectories in older persons: Are these the same? The Journals of Gerontology: Psychological Sciences and Social Sciences. 2006;61:95–101. doi: 10.1093/geronb/61.2.p95. [DOI] [PubMed] [Google Scholar]
- Kempen GI, van Heuvelen MJ, van Sonderen E, van den Brink RH, Kooijman AC, Ormel J. The relationship of functional limitations to disability and the moderating effects of psychological attributes in community-dwelling older persons. Social Science & Medicine. 1999;48:1161–1172. doi: 10.1016/s0277-9536(98)00427-4. [DOI] [PubMed] [Google Scholar]
- Kempen GI, van Sonderen E. Psychological attributes and changes in disability among low-functioning older persons: Does attrition affect the outcomes? Journal of Clinical Epidemiology. 2002;55:224–229. doi: 10.1016/s0895-4356(01)00474-7. [DOI] [PubMed] [Google Scholar]
- Kempen GI, van Sonderen E, Ormel J. The impact of psychological attributes on changes in disability among low-functioning older persons. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 1999;54:23–29. doi: 10.1093/geronb/54b.1.p23. [DOI] [PubMed] [Google Scholar]
- Kempen GIJM, Ranchor AV, Ormel J, van Sonderen E, van Jaarsveld CHM, Sanderman R. Perceived control and long-term changes in disability in late middle-aged and older persons: An eight-year follow-up study. Psychology & Health. 2005;20:193–206. [Google Scholar]
- Mackenbach JP, Borsboom GJJM, Nusselder WJ, Looman CWN, Schrijvers CTM. Determinants of levels and changes of physical functioning in chronically ill persons: Results from the GLOBE Study. Journal of Epidemiology and Community Health. 2001;55:631–638. doi: 10.1136/jech.55.9.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes de Leon CF, Seeman TE, Baker DI, Richardson ED, Tinetti ME. Self-efficacy, physical decline, and change in functioning in community-living elders: A prospective study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 1996;51:183–190. doi: 10.1093/geronb/51b.4.s183. [DOI] [PubMed] [Google Scholar]
- Milaneschi Y, Bandinelli S, Corsi AM, Vazzana R, Patel KV, Ferrucci L, Guralnik JM. Personal mastery and lower body mobility in community-dwelling older persons: The Invecchiare in Chianti Study. Journal of the American Geriatrics Society. 2010;58:98–103. doi: 10.1111/j.1532-5415.2009.02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusselder WJ, Looman CW, Mackenbach JP. Nondisease factors affected trajectories of disability in a prospective study. Journal of Clinical Epidemiology. 2005;58:484–494. doi: 10.1016/j.jclinepi.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Oksuzyan A, Juel K, Vaupel JW, Christensen K. Men: Good health and high mortality. Sex differences in health and aging. Aging Clinical and Experimental Research. 2008;20:91–102. doi: 10.1007/bf03324754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlin LI, Nguyen KB, Schieman S, Milkie MA. The life-course origins of mastery among older people. Journal of Health and Social Behaviour. 2007;48:164–179. doi: 10.1177/002214650704800205. [DOI] [PubMed] [Google Scholar]
- Pearlin LI, Schooler C. The structure of coping. Journal of Health and Social Behaviour. 1978;19:2–21. [PubMed] [Google Scholar]
- Penninx BW, van Tilburg T, Kriegsman DM, Deeg DJ, Boeke AJ, van Eijk JT. Effects of social support and personal coping resources on mortality in older age: The Longitudinal Aging Study Amsterdam. American Journal of Epidemiology. 1997;146:510–519. doi: 10.1093/oxfordjournals.aje.a009305. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Seeman TE, Unger JB, McAvay G, Mendes de Leon CF. Self-efficacy beliefs and perceived declines in functional ability: MacArthur studies of successful aging. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 1999;54:214–222. doi: 10.1093/geronb/54b.4.p214. [DOI] [PubMed] [Google Scholar]
- Sherer M, Maddux JE, Mercandante B, Prenticedunn S, Jacobs B, Rogers RW. The Self-efficacy Scale—Construction and validation. Psychological Reports. 1982;51:663–671. [Google Scholar]
- Skinner EA. A guide to constructs of control. Journal of Personality and Social Psychology. 1996;71:549–570. doi: 10.1037//0022-3514.71.3.549. [DOI] [PubMed] [Google Scholar]
- Smit JH, De Vries MZ, Poppelaars JL. Data collection and field-work procedures. In: Deeg DJH, Beekman ATF, Kriegsman DMW, Westendorp-de Seriere M, editors. Autonomy and well-being in the aging population II. Report from the Longitudinal Aging Study Amsterdam 1992–1996. Amsterdam, The Netherlands: VU University Press; 1998. pp. 9–20. [Google Scholar]
- Speer DC, Greenbaum PE. Five methods for computing significant individual client change and improvement rates: Support for an individual growth curve approach. Journal of Consulting and Clinical Psychology. 1995;63:1044–1048. doi: 10.1037//0022-006x.63.6.1044. [DOI] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NW, Luben R, Khaw KT, Day NE. Mastery, sense of coherence, and mortality: Evidence of independent associations from the EPIC-Norfolk Prospective Cohort Study. Health Psychology. 2006;25:102–110. doi: 10.1037/0278-6133.25.1.102. [DOI] [PubMed] [Google Scholar]
- van Gool CH, Kempen GIJM, Penninx BWJH, Deeg DJH, Beekman ATF, van Eijk JTM. Impact of depression on disablement in late middle aged and older persons: Results from the Longitudinal Aging Study Amsterdam. Social Science & Medicine. 2005;60:25–36. doi: 10.1016/j.socscimed.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Verbrugge LM, Jette AM. The disablement process. Social Science & Medicine. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]