Abstract
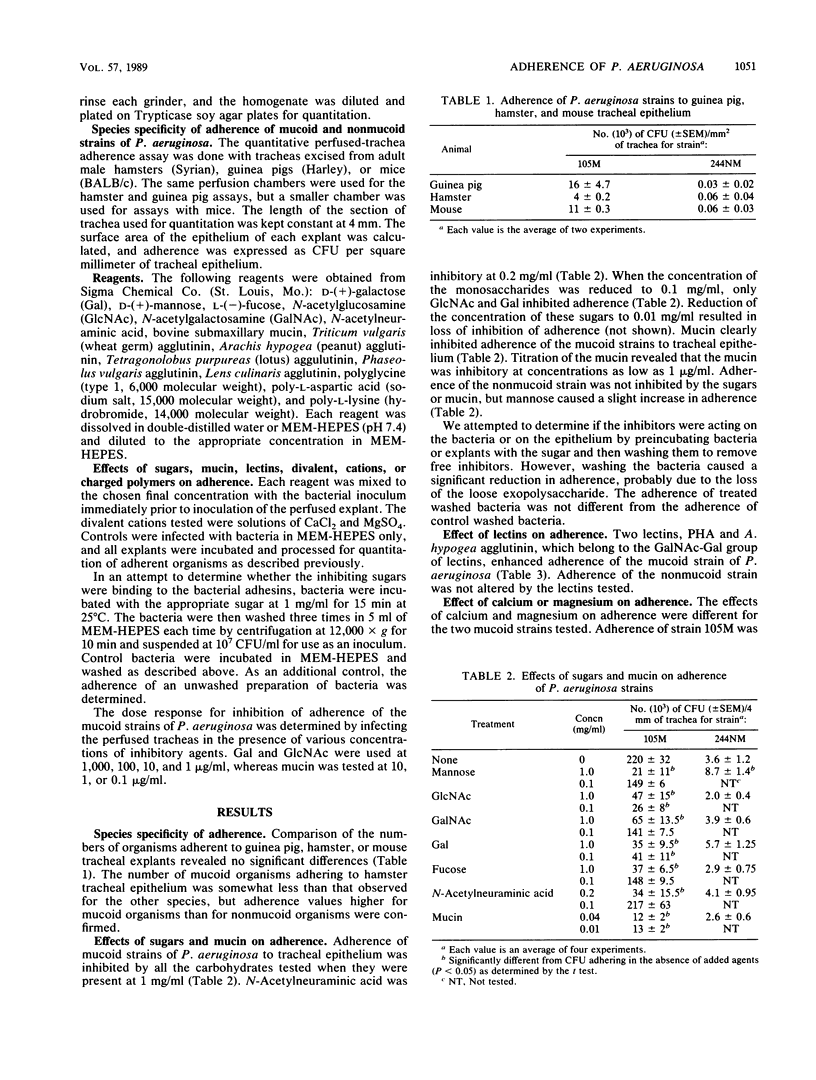
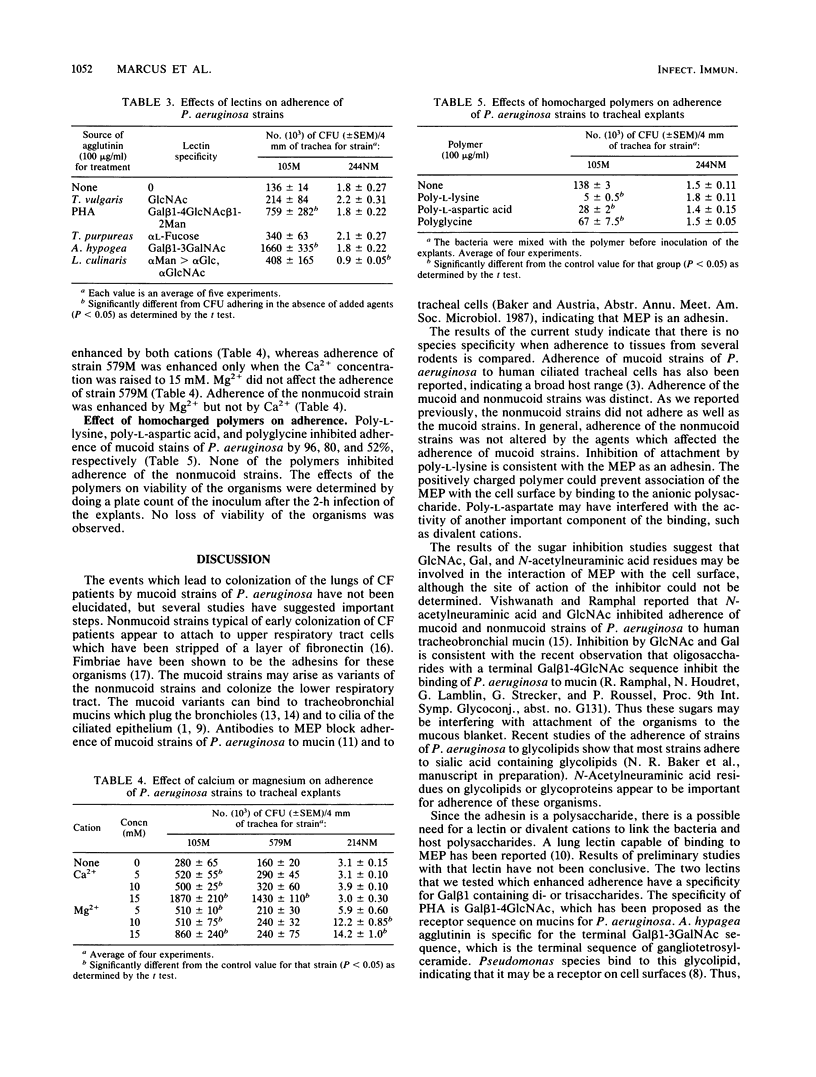
Adherence of mucoid and nonmucoid strains of Pseudomonas aeruginosa to tracheal epithelium was studied with a perfused-trachea model. The species specificity of adherence was studied by infecting tracheas from hamsters, guinea pigs, or mice. Perfused tracheas from hamsters were infected with strains of P. aeruginosa in the presence of various sugars, lectins, cations, or charged polymers. Adherence of mucoid strains of P. aeruginosa was greatest for guinea pigs; that for hamsters and mice was approximately the same. Nonmucoid strains did not adhere well to epithelium from any of the species tested. N-Acetylglucosamine, galactose, and N-acetylneuraminic acid were the best inhibitors of adherence of mucoid strains of P. aeruginosa. Phaseolus vulgaris agglutinin and Arachis hypogaea agglutinin enhanced adherence of mucoid strains. Adherence of mucoid strains was also enhanced by the presence of Ca2+ in the incubation medium. Poly-L-lysine, poly-L-aspartic acid, and polyglycine inhibited adherence of a mucoid strain by 96, 86, and 52%, respectively. In general, the adherence of nonmucoid strains was not affected. The results indicate that carbohydrates are involved in the interaction of mucoid strains of P. aeruginosa with tracheal cells and that divalent cations may enhance this interaction. The lectin data show that lectins can interact with the mucoid organisms and the host and suggest that lectins may play a role in the adhesion process.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evans L. R., Linker A. Production and characterization of the slime polysaccharide of Pseudomonas aeruginosa. J Bacteriol. 1973 Nov;116(2):915–924. doi: 10.1128/jb.116.2.915-924.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin A. L., Todd T., Gurman G., Black D., Mankinen-Irvin P. M., Irvin R. T. Adherence of Pseudomonas aeruginosa to cilia of human tracheal epithelial cells. Infect Immun. 1987 Jun;55(6):1523–1525. doi: 10.1128/iai.55.6.1523-1525.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan J. R., Fyfe J. A. Mucoid Pseudomonas aeruginosa and cystic fibrosis: resistance of the mucoid from to carbenicillin, flucloxacillin and tobramycin and the isolation of mucoid variants in vitro. J Antimicrob Chemother. 1978 May;4(3):233–240. doi: 10.1093/jac/4.3.233. [DOI] [PubMed] [Google Scholar]
- Govan J. R. Mucoid strains of Pseudomonas aeruginosa: the influence of culture medium on the stability of mucus production. J Med Microbiol. 1975 Nov;8(4):513–522. doi: 10.1099/00222615-8-4-513. [DOI] [PubMed] [Google Scholar]
- Krivan H. C., Ginsburg V., Roberts D. D. Pseudomonas aeruginosa and Pseudomonas cepacia isolated from cystic fibrosis patients bind specifically to gangliotetraosylceramide (asialo GM1) and gangliotriaosylceramide (asialo GM2). Arch Biochem Biophys. 1988 Jan;260(1):493–496. doi: 10.1016/0003-9861(88)90473-0. [DOI] [PubMed] [Google Scholar]
- Marcus H., Baker N. R. Quantitation of adherence of mucoid and nonmucoid Pseudomonas aeruginosa to hamster tracheal epithelium. Infect Immun. 1985 Mar;47(3):723–729. doi: 10.1128/iai.47.3.723-729.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur H. A., Ceri H. Interaction of a rat lung lectin with the exopolysaccharides of Pseudomonas aeruginosa. Infect Immun. 1983 Nov;42(2):574–578. doi: 10.1128/iai.42.2.574-578.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Guay C., Pier G. B. Pseudomonas aeruginosa adhesins for tracheobronchial mucin. Infect Immun. 1987 Mar;55(3):600–603. doi: 10.1128/iai.55.3.600-603.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Sadoff J. C., Pyle M., Silipigni J. D. Role of pili in the adherence of Pseudomonas aeruginosa to injured tracheal epithelium. Infect Immun. 1984 Apr;44(1):38–40. doi: 10.1128/iai.44.1.38-40.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzmann S., Boring J. R. Antiphagocytic Effect of Slime from a Mucoid Strain of Pseudomonas aeruginosa. Infect Immun. 1971 Jun;3(6):762–767. doi: 10.1128/iai.3.6.762-767.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwanath S., Ramphal R. Adherence of Pseudomonas aeruginosa to human tracheobronchial mucin. Infect Immun. 1984 Jul;45(1):197–202. doi: 10.1128/iai.45.1.197-202.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwanath S., Ramphal R. Tracheobronchial mucin receptor for Pseudomonas aeruginosa: predominance of amino sugars in binding sites. Infect Immun. 1985 May;48(2):331–335. doi: 10.1128/iai.48.2.331-335.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Bass J. A. Role of fibronectin in the prevention of adherence of Pseudomonas aeruginosa to buccal cells. J Infect Dis. 1981 Jun;143(6):784–790. doi: 10.1093/infdis/143.6.784. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Berry V. K., Bass J. A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980 Sep;29(3):1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



