Abstract
Context
With better antiretroviral treatments (ART), persons living with HIV (PLWH) are living longer, healthier lives. Therefore, they also experience more medical comorbidities that come with normal aging, as well as side effects of multiple treatments and long-term sequelae of HIV. It can be hard to know whether symptoms reported by PLWH are related to comorbidities or are signs of HIV disease progression and possible treatment failure.
Objectives
The current study was designed to disentangle these issues by examining within-person symptom changes in data collected from a cohort of PLWH before the advent of highly efficacious ART.
Methods
This study was a secondary analysis of symptom reports in longitudinal data collected from 246 PLWH in 1992–1994. Multilevel modeling was used to test for changes over time in HIV-related symptom clusters. Analyses also tested the effects of person-level demographic covariates and co-occurring mental health symptoms on HIV symptoms, and examined the magnitude of within-person versus between-person variations in reported symptom severity.
Results
Two of six HIV-related symptom clusters, malaise/fatigue and nausea/vomiting, increased over time in the context of HIV disease progression, while the other four did not. Changes were independent of baseline disease severity or psychological covariates. There was substantial within-person variability in absolute symptom severity.
Conclusion
Relatively small but consistent changes in symptoms related to nausea or fatigue may suggest HIV disease progression, while changes in other HIV symptom clusters may instead be related to comorbidities or normal aging. Further research is recommended on symptom progression in PLWH.
Keywords: Aging, fatigue, HIV, nausea, symptom clusters
Introduction
With improvements in antiretroviral therapy (ART), the expected course of HIV has substantially changed since 1997. On one hand, persons living with HIV (PLWH) may now enjoy a life expectancy similar to their non-infected peers; on the other hand, they now face a range of chronic disorders associated with aging, which are further complicated by HIV disease processes, treatment side effects, and drug interactions [1, 2]. HIV treatment is, therefore, moving to a chronic care model that requires expertise in primary care, disease management, and health behavior in addition to knowledge of HIV [3]. PLWH for 20 years or more may have exceptionally complex clinical presentations. It can be difficult for practitioners to know whether patients’ reported symptoms are related to HIV infection, comorbid illnesses, or the side effects of ART or other treatments.
One potential source of evidence to help practitioners disentangle the effects of HIV infection from other symptoms comes from data on the course of HIV in the absence of highly effective treatment. Such data are available in the AIDS Time-Oriented Health Outcome Study Databank (ATHOS), a large dataset with HIV symptom data collected before the advent of highly effective ART in the late 1990s. The ATHOS dataset is a longitudinal, observational database of PLWH who received care from community-based providers. Data were collected from medical records and patient questionnaires at three private practices in the San Francisco Bay area, two private practices in Los Angeles, and five community clinics in San Diego. PLWH who provided information for this dataset experienced HIV disease that was minimally treated by today’s standards, because the available drug therapy at the time (zidovudine) did not have a strong effect on disease progression or survival [4]. Using the ATHOS dataset, the current study was designed to describe symptom changes in the context of HIV disease progression.
Symptoms in HIV May Have Multiple Etiologies
Symptoms are subjective health-related experiences that may be described in terms of their intensity, duration, interference with daily activities, or degree of change over time [5]. Although HIV can produce flu-like symptoms of fever, sore throat, weakness, or rash, these usually appear only 2–6 weeks after infection with the virus and tend to resolve in 1–2 weeks once the body begins producing antibodies to HIV. After this period of —primary HIV infection, the virus remains in the body but is largely asymptomatic [6].
Later, if HIV is not effectively treated, symptoms result from disease progression as the amount of HIV in the bloodstream (viral load) increases and the number of CD4+ cells in the bloodstream decreases, indicating diminished immune system functioning. As the immune system progressively weakens, PLWH become more vulnerable to opportunistic infections (OIs) that do not usually cause illness in non-immunocompromised individuals. OIs can cause disease and illness in most major body systems, resulting in diverse symptoms such as mouth pain or sensitivity, genital pain or discomfort, digestion problems, neuropathic pain, muscle pain, breathing problems, vision problems, or cognitive disturbance. Further complicating the clinical picture is the fact that a given OI can produce multiple symptoms. For instance, cytomegalovirus (CMV) is a common virus that is held in check by the immune systems of most healthy individuals, but in immunocompromised persons can lead to retinal damage, breathing problems, and/or colitis. Untreated HIV itself can cause a wasting syndrome as immune functioning decreases and viral load increases; this can include weight loss, weakness, fever, nutritional problems, and diarrhea [6]. The advanced stage of HIV infection, also referred to as acquired immune deficiency syndrome (AIDS), is defined by a CD4+ cell count below a certain level (< 200 cells/mm3 based on current treatment guidelines, but other values in past definitions), or by the presence of one or more OIs [7].
Progression to AIDS is much less likely with current ART medications. Other sources of symptoms in PLWH may be related to normal aging. Dementia, physical wasting, and serious illnesses are more common overall in older individuals. However, HIV disease progression also occurs more quickly among older than younger PLWH, so causality can be difficult to determine. In addition, older PLWH have more comorbid illnesses that may produce symptoms, including diabetes, cardiovascular disease, arthritis, asthma, genitourinary conditions, and headache conditions [6]. Older PLWH actually tend to report that comorbid illnesses create more symptoms and greater functional impairment than their HIV [6]. As illustrated by this list of potential problems, a given symptom reported by a person living with HIV may have many possible etiologies.
Finally, drug toxicity is an issue in all treatments for HIV and is more likely among older adults. Two common and problematic HIV treatment side effects are osteoporosis and lipodystrophy, which involves a redistribution of body fat from the face and extremities to the abdomen and back, as well as an increase in total cholesterol, an increase in triglycerides, and a decrease in glucose tolerance [6]. In addition, psychosocial stresses are common in HIV and can be severe, including anxiety over disease management and possible outcomes, disruptions in interpersonal relationships, and the possible experience of stigma, overt discrimination, or violence [8]. Because of these potential consequences, many PLWH carefully manage information about their HIV and attempt to limit disclosure of their HIV serostatus; maintaining secrecy about HIV infection may then create additional sources of distress. In part because of these stressors, depression is common in HIV, and may exacerbate existing physical symptoms, result in treatment nonadherence, or be expressed as somatic complaints [9, 10].
Symptom Clusters in Minimally Treated HIV
Recent research on several chronic diseases suggests that symptoms often occur in groups known as symptom clusters [5]. A symptom cluster can be defined as two or more symptoms that occur together and are statistically related; symptoms in a cluster may or may not have a common etiology [11]. HIV symptom clusters have been identified in the ATHOS dataset through a theory-based approach using confirmatory factor analysis. This research identified six symptom clusters in PLWH: malaise/fatigue, confusion/distress, fever/chills, gastrointestinal discomfort, shortness of breath, and nausea/vomiting. A model using these clusters provided an excellent fit to PLWH’s symptom reports in the ATHOS data, RMSEA = .036, CFI = .99 [12]. The existence of symptom clusters in HIV suggests important commonalities of experience among minimally treated PLWH. In cancer research, the severity of clustered symptoms has also been found to predict functional disability [13] and impairments in quality of life (QOL) [14]; such predictive relationships may also exist but have not yet been reported in HIV. Understanding these experiences may be useful in also understanding the symptoms reported by current cohorts of more effectively treated PLWH as they age and cope with comorbid illnesses.
Because symptoms are subjective experiences, they depend not only on physiological processes but also on patients’ recognition and interpretation of those processes. The Symptom Interpretation Model (SIM) [15] provides a framework for understanding patients’ symptom experiences across a variety of chronic diseases, including HIV. The model suggests that symptoms can be understood in terms of 1) input, the process by which a patient recognizes a physiological disturbance and distinguishes it as an experience that is —different from normal; 2) interpretation, including conceptual identification of a symptom through a cognitive process, knowledge structure activation in which symptoms are compared to expectations and past experiences, and reasoning in which judgment heuristics and affective responses serve as filters for the symptom experience; and 3) outcome, a step in which patients respond to their symptom experience through care-seeking or self-management behaviors. In the input stage of the model, sensory experiences can be influenced by one’s experience with a disease, which continues to evolve over time. At this stage, baseline disease severity might be expected to have an impact on patients’ symptom perceptions by providing an overall —frame of health or illness against which new physiological sensations are judged. For patients with more severe disease, minor changes in symptom severity might not even reach the level of awareness because the patient’s attention is focused on other problems. Changes in patients’ symptom reports over time are also dependent on patients’ perceptual awareness of a —difference in their underlying physiological state, which may be either lessened or heightened after long experience with a disease. In the interpretation stage of the model, additional sensory, cognitive, and affective responses come into play, including the patient’s description of the name, cause, consequences, and timeline or duration of a symptom. Because of the role of interpretation, patients’ reports of some symptoms may not change over time even when the underlying disease worsens, while others might show a corresponding progression. Parallel emotional processes can also affect patients’ symptom interpretations [16]: For instance, psychological distress can amplify patients’ reports of symptom experiences such as pain [17]. The outcome stage involves symptom management behaviors that were not evaluated in this study, but that are predicted to depend on the input and interpretation stages.
The current study was a secondary analysis of the ATHOS dataset, in which multilevel modeling (MLM) was used to evaluate within-person symptom changes over time for each of the six HIV symptom clusters. MLM is a newer data analytic approach ideally suited to answer questions about changes in symptom reports over time. Symptoms that change over time in minimally treated PLWH are likely to be signs of disease progression, and potentially important warning signs of treatment failure. However, if patients receiving modern ART regimens experience changes in symptom clusters that are unrelated to disease progression, these symptom changes may instead be signs of drug toxicity or comorbid illness. In this way, describing the natural history of symptoms in a past cohort of minimally treated PLWH can provide important clinical insights for current practitioners.
Methods
Participants
Data were drawn from the records of PLWH (n = 917) who were participants in a longitudinal cohort study from January 1992 through October 1994 and who had complete data on an HIV symptom scale. All participants had a CD4+ count < 500 cells/mm3, indicating AIDS progression, by the time of study enrollment. All participants in the original study signed an informed consent, and de-identified analysis of symptom cluster data was approved by the Arizona State University Institutional Review Board. From the total cohort, 246 participants had at least five data points after their CD4+ cell count dropped below 500 cells/mm3, and these participants constituted the analysis sample. MLM analysis requires a minimum of five data points per participant; therefore, only about 1/3 of the original sample could be included in the analysis. Multilevel models with as few as five observations per participant have been tested in simulation studies and produce results comparable to those obtained with 30 or 50 observations per participant [18], but analyses based on fewer than five observations per participant are potentially unreliable. There were no exclusion criteria other than the number of observations per participant, complete data on symptoms and the other variables of interest, and the original ATHOS study requirements.
Participants’ average age was 39.8 years (standard deviation [SD] = 8.8, 95% confidence interval [CI] 22.6, 57.0) at the time they entered the study. One-hundred percent of the participants were male. As was typical of PLWH in the early stages of the HIV epidemic, 83% of participants were White, non-Hispanic. The remainder were Hispanic (10%), African American (4%), or other racial/ethnic groups (3%). Participants were relatively well-educated, with 40% having some college, 21% with a four-year college degree, and 23% with education beyond the baccalaureate degree. Participants had been living with HIV for an average of 3.6 years (SD = 2.1 years, 95% CI 0, 7.7) at the time of enrollment.
Measures
Baseline Demographic and Clinical Characteristics
Participants’ age, baseline CD4+ cell count, and number of years living with HIV were collected at the time of enrollment.
Sign and Symptom Checklist for Persons with HIV (SSC-HIV)
The SSC-HIV [19] assesses the six HIV symptom clusters described above, with 23 items (3–5 symptoms per cluster). Each item is scored as 1 = yes or 0 = no, and items are summed to create a score within each of the six symptom clusters. Possible scores, therefore, range from 0–3, 0–4, or 0–5, depending on the number of symptoms in the cluster. SSC-HIV symptom cluster subscales have shown good internal consistency, with alphas ranging from 0.77 (nausea/vomiting) to 0.90 (malaise/fatigue and confusion/distress) [12].
Psychological Covariates
The measures of mental health, health distress, and QOL in the ATHOS data bank were derived from general health status scales [20–24]. Mental health questions were designed to measure psychological distress and well-being [25]. The five mental health questions were (1) Do you feel calm and peaceful? (2) Do you feel downhearted and blue? (3) Do you feel very happy? (4) Do you feel very nervous? and (5) Do you feel so down in the dumps that nothing could cheer you up? These questions’ item-scale correlations in the Medical Outcomes Study baseline sample (n = 2,862) ranged from 0.65 to 0.81 [23]. Health worry is defined as the extent to which health problems cause people to worry or be greatly concerned about their health. The following questions were used to measure health worry in the ATHOS dataset: (1) Are you frustrated about your health? (2) Are you afraid because of your health? and (3) Is your health a worry in your life? The item-scale correlations for these questions ranged from 0.76 to 0.87 in the Medical Outcomes Study baseline sample [23]. In the current study, mental health and health worry scales were used as covariates in some models to determine whether psychological variables could account for variations in HIV symptom experiences among PLWH. Patients’ scores on a QOL scale from the ATHOS dataset also provided a QOL measure to validate our initial assumption that ATHOS participants did experience HIV disease progression over the time they provided data for the ATHOS study. The composition and psychometrics of this QOL scale have been extensively reported by Sousa and Chen [26].
Data Analysis
Within-person changes on each of the SSC-HIV’s six subscales—malaise/fatigue, confusion/distress, fever/chills, gastrointestinal discomfort, shortness of breath, and nausea/vomiting—were modeled as a function of time since participants’ first CD4+ cell count < 500 cells/mm3. The time variable was based on exact dates, and measured in years carried to two decimal places. We hypothesized that participants would have higher scores in each symptom category over time (i.e., as their HIV disease progressed). Secondary research questions were whether psychological distress could account for the relationship between HIV disease progression and reported symptoms, and whether symptom reports varied to a greater degree between participants or within individual participants’ scores over time.
All analyses were conducted using the program HLM 6.03 [27]. The following basic equations were used to model symptom change over time:
The six HIV-SSC symptom cluster scales were entered as the —symptom variable in separate models. Participants’ age, CD4+ cell count, and number of years living with HIV at the time of study entry were tested as possible level 2 covariates, because these were considered potentially important clinical and demographic confounds that might affect participants’ HIV symptom experiences. Only CD4+ cell count was sufficiently predictive for inclusion in the final equations. In some analyses presented below, psychological covariates (mental health and health distress) also were entered as within-person covariates in the level-1 equations to control for the possible effects of psychological states on patients’ reported symptom experiences. All level 1 predictors were group-mean centered, and level 2 predictors were grand-mean centered [28]. Because the dataset was limited to participants with at least five data points, computational techniques with robust standard errors were used for all analyses. Unstandardized betas are reported as effect size estimates, interpretable as average increase per year in the original scale units (e.g., points on a 1–4 scale).
Results
Tests for Sampling Bias
Because the requirement of at least five symptom data points per participant resulted in a large number of original participants being excluded from this secondary analysis, tests were performed to compare the study sample to the original population of 917 participants on all available demographic characteristics. Results (Table 1) showed no significant differences between the analysis sample and the original population of PLWH in the ATHOS dataset.
Table 1.
Participant Demographics
| Characteristic | Sample Included in Analysis (n = 246) | Original Population of ATHOS Dataset (n = 917) | Evidence of Sampling Bias |
|---|---|---|---|
| Age at Start of Study | 40.2 years (SD = 8.5, 95% CI [23.5, 56.9]) | 39.8 years (SD = 8.8, 95% CI [22.6, 57.0]) | No: t (1159) = 0.05, P = 0.96, d = 0.05 |
| Sex | 100% Male | 100% Male | No: χ2 = 0.00, P = 1.00, φ = 0.00 |
| Minority Race/Ethnicity | 13% non-White | 17% non-White | No: χ2 = 2.16, P = 0.14, φ = 0.04 |
| Level of Education | 57.9% college or higher | 60.4% college or higher | No: χ2 = 0.55, P = 0.46, φ = 0.02 |
| Years Living with HIV at Start of Study | 4.03 years (SD = 2.07, 95% CI [0, 8.09]) | 3.61 years (SD = 2.10, 95% CI [0, 7.73]) | No: t (1159) = 0.20, P = 0.84, d = 0.20 |
| Baseline CD4+ Cell Count | M = 312.6 (SD = 138.3, 95% CI [41.5, 583.7]) | M = 288.6 (SD = 146.0, 95% CI [2.4, 574.8]) | No: t (1159) = 0.17, P = 0.87, d = 0.17 |
| Number of Study Visits Completed | M = 9.16 (SD = 2.10, 95% CI [5.04, 13.28]) | M = 6.97 (SD = 3.63, 95% CI [0, 14.08]) | No: t (1159) = 0.65, P = 0.52, d = 0.65 |
Evidence of HIV Disease Progression
CD4+ cell count values were not collected at each study visit, so the pattern of disease progression over time could not be tested directly based on physiological data. However, time from study enrollment predicted declining QOL, T (2233) = −2.64, P = 0.009, β1 = −0.032, suggesting that disease progression did in fact occur over the course of participants’ time in the study. The requirement of an initial CD4+ cell count below 500 cells/mm3 as a study inclusion criterion and the fact that most participants eventually died of AIDS-related illnesses also support the argument that time since enrollment can be used as a proxy for disease progression.
Effects of Person-Level Covariates on Symptom Reports
Clinical and demographic variables at the input stage of the SIM – participant’s age, baseline CD4+ cell count, and years living with HIV before progression to AIDS – were entered as person-level predictors of β0, with each of these potential covariates tested in separate MLM analyses. For all symptom cluster scales except confusion/distress, lower baseline CD4+ cell count predicted significantly higher symptom severity, all Ps < 0.01. This finding suggests that the severity of reported symptoms in most clusters was related to baseline disease severity. Therefore, initial CD4+ cell count was entered as a level 2 covariate predicting β0 (random-intercepts approach) in all subsequent models. However, number of years living with HIV had no significant effect on any of the six symptom clusters, all Ps > 0.06. Similarly, age at study enrollment had no effect on five of the six symptom clusters, Ps > 0.20, with a significant effect only on the shortness of breath subscale, T (2230) = 2.17, P = 0.03, β1 = 0.007. This finding could be due to chance, given the large number of screening analyses conducted on demographic and clinical predictors.
Change in Reported Symptoms over Time
In the context of HIV disease progression, reported symptoms increased over time on two of six HIV-SSC symptom clusters. Significant increases were seen on malaise/fatigue, T (2232) = 3.55, P = 0.001, β1 = 0.110, and nausea/vomiting, T (2232) = 2.49, P = 0.01, β1 = 0.053. However, there were no significant changes over time on fever/chills, T (2232) = 1.83, P = 0.07, β1 = 0.039, confusion/distress, T (2232) = 1.52, P = 0.13, β1 = 0.044, shortness of breath, T (2232) = 1.29, P = 0.20, β1 = 0.016, or gastrointestinal discomfort, T (2232) = 0.26, P = 0.80, β1 = 0.007. Exploratory analyses not presented here, with baseline CD4+ cell count entered as a level 2 moderator of β1 (random-slopes approach), did not change the results. Because all analyses controlled for initial disease severity via participants’ baseline CD4+ cell count, the changes in malaise/fatigue and nausea/vomiting appear to relate specifically to disease progression rather than to participants’ overall health.
Effects of Psychological States on Symptom Reports
Beyond the impact of initial health, it was considered important to control for mental health symptoms at each point in time because the experience of physical symptoms can be exacerbated by psychological distress (e.g., [17]). Therefore, reported symptom increases might be due to increased psychological distress as HIV disease progresses – in the framework of the SIM, a difference in symptom interpretation rather than an actual change in symptom intensity. Both mental health, T (2233) = −5.90, P < 0.001, β1 = −0.363, and health worry, T (2233) = −6.62, P < 0.001, β1 = −0.345, had significant effects on malaise/fatigue. However, the observed effects of disease progression on malaise/fatigue remained significant after controlling for either mental health, T (2231) = 3.59, P = 0.001, β1 = 0.008, or health worry, T (2231) = 3.61, P = 0.001, β1 = 0.106. Similarly, both mental health, T (2233) = −6.19, P < 0.001, β1 = −0.225, and health worry, T (2233) = −7.33, P < 0.001, β1 = −0.217, individually predicted nausea/vomiting symptom severity, but the effects of time on nausea/vomiting also remained significant after controlling for either mental health, T (2231) = 1.99, P = 0.046, β1 = 0.003, or health worry, T (2231) = 2.46, P = 0.01, β1 = 0.050.
Within-Person versus Between-Person Variability
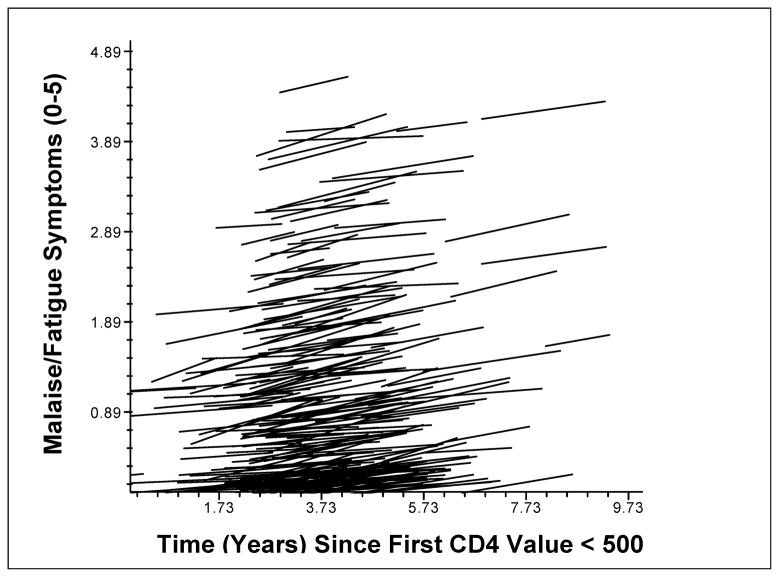
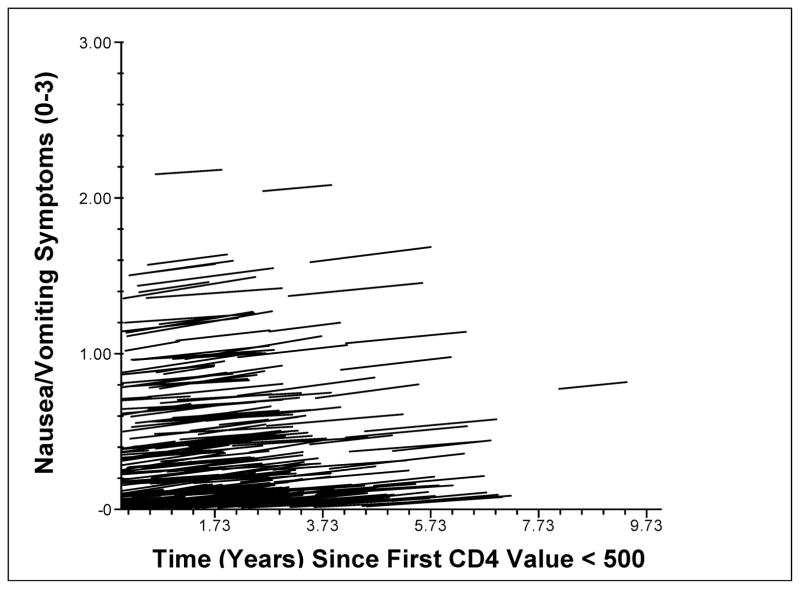
Standardized regression lines were calculated for individual participants’ changes on the malaise/fatigue (Fig. 1) and nausea/vomiting (Fig. 2) symptom clusters over time, with higher scores indicating greater reported symptom severity. The models shown include baseline CD-4 as both a covariate (effect on β0) and possible moderator of symptom change over time (effect on β1), in addition to time as a predictor of symptom scores. As shown in these figures, PLWH had substantial between-person variability in their absolute symptom severity levels (i.e., different intercepts), but similar symptom progression over time shown by the similar slopes of the lines. Regardless of the level of symptoms from which participants started, disease progression was associated with an average increase of 0.77 of five possible units on the malaise/fatigue symptom cluster, and with an average increase of 0.36 of three possible units on the nausea/vomiting symptom cluster over a study duration of 1–5 years.
Figure 1. Change in Malaise/Fatigue with HIV Disease Progression.
Note. Lines represent fitted regression models for individual participants’ symptom changes over time, with time entered as an uncentered level-1 predictor of malaise/fatigue, and using a random-slopes and random-intercepts approach with participants’ baseline CD4 values as a level-2 predictor. Differences in the y-intercept for each line represent between-person variability in the overall severity of symptom reports. The positive slope of each line show within-person increases in symptom severity over time.
Figure 2. Change in Nausea/Vomiting with HIV Disease Progression.
Even though regression lines appeared similar across patients, there could still be random variability not captured in these equations, which tend to make patterns look more regular than might truly be the case. The level of within-person random variability in multilevel models can be quantified using three different metrics. The most common is the intra-class correlation (ICC), which describes between-person variability as a proportion of total variability. In this dataset, ICC = 0.62 for the malaise/fatigue subscale and ICC = 0.38 for the nausea/vomiting subscale, indicating that only one-third to two-thirds of the total variability in symptom severity could be accounted for by considering differences among the multiple reports from individual participants. An alternate measure of within-person variability, the mean squared successive difference (MSSD), measures average change from one data point to the next data point in time [29]. In this study, MSSD = 1.12 for malaise/fatigue and MSSD = 0.07 for nausea/vomiting, indicating small changes from one data point to the next: an average of about one of five possible points for malaise/fatigue and one-tenth of a point from a total of three possible points for nausea/vomiting. Finally, the probability of acute change (PAC) is defined as the likelihood of a dramatic difference between one data point and the next [29]. A movement of more than one point on a symptom cluster was considered an acute change in this dataset. In this sample, PAC = 0.14 for malaise/fatigue and PAC = 0.00 for nausea/vomiting, again suggesting that within-person changes were small and consistent rather than large or dramatic.
Discussion
Understanding patterns of symptom change in minimally treated HIV can help clinicians to differentiate among signs of HIV disease progression, symptoms of comorbid illness, and treatment side effects. The current study was designed to examine symptom changes in the context of HIV disease progression, using a secondary analysis of symptom data collected from a large sample of PLWH whose HIV progressed to AIDS in the era before highly effective ART. Person-level associations were found between PLWH’s initial disease severity (measured by CD4+ cell count at the time of study entry) and their reported level of symptom severity at each follow-up assessment on six symptom clusters identified in previous research with PLWH. After controlling for baseline disease severity, there also were significant within-person changes over time on the malaise/fatigue and nausea/vomiting subscales of the SSC-HIV symptom measure. There were no significant changes over time in participants’ scores for the other four symptom clusters: fever/chills, confusion/distress, shortness of breath, or gastrointestinal discomfort.
Our results support key predictions of the SIM: For example, baseline disease severity did predict patients’ symptom reports, confirming the role of patients’ personal disease history as an influence on symptoms at the input stage of the model. The lack of change over time found in some symptom clusters may be due to patients’ changing interpretations of their symptom experience over time; based on other evidence for disease progression in this sample, the underlying physiological processes likely did change despite patients’ perceptions that their symptoms in most clusters were no worse from the beginning to the end of the study. In this context, the changes observed in two of six symptom clusters are particularly noteworthy: These changes were extreme enough to rise to the level of patients’ awareness and be reported as differences in PLWH’s experiences of their disease. As expected based on Leventhal’s model of parallel cognitive and emotional processes as incorporated in the SIM, psychological distress also predicted patients’ symptom experiences, but changes in psychological distress alone could not account for observed changes over time in nausea/vomiting and malaise/fatigue. The association of symptom changes with a physiological endpoint (mortality) in the current study suggests that changes in patients’ subjective symptom experiences have objective health significance, although self-management or help-seeking behaviors in the output stage of the SIM were not examined in the current research.
Change over time is one way to describe symptom clusters [5], but past research has not examined change over time in HIV symptom clusters. Therefore, the results of the current study are novel descriptive findings that may suggest important areas for further investigation, but they require replication before any definitive conclusions can be drawn. These initial results suggest that HIV disease progression is associated with increases in two specific symptom clusters – malaise/fatigue and nausea/vomiting – but not with changes in other HIV-related symptom clusters. Knowing this may help practitioners to differentiate between symptoms that suggest treatment failure or nonadherence, and those that reflect comorbid illnesses, treatment side effects, or physiological states unrelated to HIV. Such determinations are particularly important for practitioners who treat the growing population of older adults with HIV, because aging and long-term HIV survivorship are each associated with multiple inter-related health problems that may be related or unrelated to HIV infection.
To test a possible alternative interpretation that symptom changes were due to PLWH’s psychological reactions rather than to the physical effects of HIV disease progression, analyses of symptom change over time were repeated with two psychological covariates, the mental health and health worry scales. Based on these analyses, observed changes in the malaise/fatigue and nausea/vomiting symptom clusters were independent of any psychological changes experienced by PLWH in the context of HIV disease progression.
Despite consistent patterns of change in symptom clusters across PLWH, our results also showed substantial between-person variability in the absolute level of symptoms reported. Some PLWH reported very low levels of symptoms that increased by the end of the study but still remained low overall. Other PLWH started out reporting very high levels of symptoms but still had slight increases in reported symptom severity. These findings are also consistent with the SIM, which suggests that patients’ individual perceptions of symptom severity are based on their judgments, emotions, and personal frame of reference. Differences in such person-level characteristics, which were not measured in the current study, are likely responsible for PLWH reporting different absolute levels of symptom severity, and for the finding that these overall symptom severity levels were relatively constant within persons over time. Based on the current findings, the clinical significance of any specific symptom severity level must be evaluated in the context of each PLWH’s baseline symptom experience. It appears that change in symptoms from baseline is more indicative of HIV disease progression than absolute symptom severity level. Again, this is a novel finding from an innovative approach to studying HIV symptom change over time, which requires replication in order to draw definitive rules for practice.
Clinical Implications
Because of the high within-person variability and small overall magnitude of symptom changes, it is unlikely that a simple cutoff score could be used to identify clinically meaningful symptom changes. However, based on our analysis of acute changes, a movement of more than one point from one observation to the next was extremely unlikely on either the malaise/fatigue or nausea/vomiting symptom clusters among PLWH included in the ATHOS dataset. Therefore, any change of this magnitude on either of these HIV-progression-related subscales would be an important clinical rationale (although not the only one possible) for inquiring further about patients’ adherence, the efficacy of ART, or other factors related to HIV disease progression. In some cases, providers might be able to identify medication dispensing errors (e.g., patients given ritonavir instead of Retrovir and, therefore, receiving an inefficacious ART regimen), health literacy concerns (e.g., patients taking —two pills twice daily as one in the morning and one at night rather than two at each dose), or patient nonadherence because of treatment side effects or other concerns [30].
Limitations and Directions for Future Research
Strengths of the current study included the longitudinal dataset, the state-of-the-art statistical approach used to examine within-person change over time, and the availability of symptom data from a cohort of PLWH not treated with modern ART medications that likely complicate the study of symptom trajectories. However, one substantial limitation of this study was our inability to analyze data from more than one-third of participants in the ATHOS dataset. This was because of the analytic requirements of the multilevel modeling procedure, which so far has been validated only in datasets with five or more observations per participant. In the current dataset, participants with five or more data points also remained in the study for a longer period of time, which may suggest better initial health or more gradual HIV-related disease progression. Although we attempted to address this potential source of selection bias and found no significant differences between the study sample and the overall population of PLWH in the ATHOS dataset, nonsignificant differences on baseline CD4+ cell count and study duration were in the direction that would be expected if participants in the analysis sample did in fact have less severe illness. In addition, it is possible that our analysis sample could have differed from the total ATHOS population in important ways that were not measured or included in these comparisons. Thus, the current findings may not apply to PLWH with very rapid HIV disease progression.
Second, because this was a secondary data analysis, other covariates not included in the original dataset could not be controlled statistically and may have had an impact on our results. For instance, treatment side effects were not systematically assessed. Because CD4+ cell count was recorded only at baseline, it was not possible to directly examine the extent of disease progression over time; however, participants’ declining scores on the QOL scale support our assumption that disease progression occurred. Based on these limitations, it is not possible to definitively conclude that time-related changes in the malaise/fatigue and nausea/vomiting symptom clusters were the direct result of HIV disease progression. It is possible that some other time-dependent covariate could have produced these changes independent of HIV disease progression. In addition, because of the epidemiology of HIV at the time these data were collected, the sample included few minority group members and no women. Therefore, results may not generalize to these groups, which are currently the faster-growing segments of the population of Americans living with HIV [31].
It is difficult today to replicate the type of data contained in the ATHOS dataset, for the simple reason that more effective antiretroviral treatments now exist and it would be unethical to deny patients these treatments in order to observe how their symptoms advance over time. Therefore, it is unlikely that future studies will be able to directly address the limitations noted, such as by adding variables that the original ATHOS investigators did not choose to include. However, future research could make an important contribution by prospectively tracking symptom changes among PLWH with effectively treated HIV, recording eventual disease outcomes, and looking for symptom markers that might have served as early warning signs that a case was headed towards treatment failure. Statistical methods were employed in the current study to control for disease severity and mental health symptoms, which are important covariates to consider in future research; treatment side effects and continuity of care might be additional covariates to measure in future studies of symptom clusters in HIV. A similar methodology could be employed to study symptom trajectories in other chronic illness populations with identified symptom clusters (e.g., cancer), and the same covariates are likely to be important in such research. Future studies of symptom trajectories in any chronic disease also could make an important contribution by implementing more frequent symptom measurements, for example, by using ecological momentary assessment strategies to collect symptom data from patients in the course of their everyday lives. Finally, the relationship between symptom changes and other important disease-related outcomes could be studied by examining secondary measures like functional status and QOL in addition to mortality.
Conclusion
Results of this study suggest that changes in some symptom clusters, but not others, occur in the context of HIV disease progression. This finding complements other data on the existence and prevalence of symptom clusters in HIV, and extends that research by suggesting potential clinical implications of changes in PLWH’s symptom experiences over time. More broadly, the current findings also support components of the SIM as a framework for understanding patients’ symptom experiences in chronic diseases. At the input stage of the model, patients’ perceptions of their symptoms were influenced by their past history of disease, and at the interpretation stage, evidence was found for hypothesized parallel cognitive and emotional processes as determinants of patients’ reported symptom severity. Results may be useful to researchers who study patients’ symptom experiences in HIV and other chronic diseases.
Acknowledgments
This research was partially supported by NIH/NINR grant # R01NR004817 (PI: K.H. Sousa) and by the Biostatistics, Epidemiology, and Research Design Core of the Colorado Clinical and Translational Research Institute (NIH grant #1UL1RR025780-01).
Footnotes
Disclosures
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Long M, Whirry R. Persons 75 years and older living with HIV/AIDS: their lives and their needs. Presentation at the Ryan White HIV/AIDS Program Grantee Meeting and 11th Clinical Update; Washington DC. August 2008. [Google Scholar]
- 2.Wu J, Avalos E. Are we ready for the aging HIV-positive populations? PLWH/A living with HIV for over 20 years and those 50 years of age and older in Los Angeles. Presentation at the Ryan White HIV/AIDS Program Grantee Meeting and 11th Clinical Update; Washington DC. August 2008. [Google Scholar]
- 3.Clanon KA. The chronic care model: implications for HIV care and training. Presentation at the Mountain-Plains AIDS Education and Training Center Faculty Development Conference; Sioux Falls, SD. August 2006. [Google Scholar]
- 4.Lucas GM. Antiretroviral adherence, drug resistance, viral fitness and HIV disease progression: a tangled web is woven. J Antimicrob Chemother. 2005;55(4):413–416. doi: 10.1093/jac/dki042. [DOI] [PubMed] [Google Scholar]
- 5.Miaskowski C, Meek P. Opportunities and challenges in symptom clusters research. Communicating Nursing Research. 2009;41:29–38. [Google Scholar]
- 6.Nichols JE, Speer DC, Watson BJ, et al. Aging with HIV: Psychological, social, and health issues. New York: Academic Press; 2002. [Google Scholar]
- 7.U.S. Department of Health & Human Services. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. 2008 Available from: http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
- 8.Scott-Sheldon LAJ, Kalichman SC, Carey MP, Fielder RL. Stress management interventions for HIV+ adults: a meta-analysis of randomized controlled trials, 1989 to 2006. Health Psychol. 2008;27:129–139. doi: 10.1037/0278-6133.27.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barfod TS, Gerstoft J, Rodkjaer L, et al. Patients' answers to simple questions about treatment satisfaction and adherence and depression are associated with failure of HAART: a cross-sectional survey. AIDS Patient Care and STDs. 2005;19:317–325. doi: 10.1089/apc.2005.19.317. [DOI] [PubMed] [Google Scholar]
- 10.Janda LH, Markowski E, Derlega VJ, Nezlek J, McCain N. Daily events and mood state among individuals living with HIV: examination of the within-persons approach to data collection using daily diary methodology. J Nurs Meas. 2006;14:116–128. doi: 10.1891/jnm-v14i2a004. [DOI] [PubMed] [Google Scholar]
- 11.Kim H-J, Abraham IL. Statistical approaches to modeling symptom clusters in cancer patients. Cancer Nurs. 2008;31:E1–E10. doi: 10.1097/01.NCC.0000305757.58615.c8. [DOI] [PubMed] [Google Scholar]
- 12.Sousa KH, Kwok O-M, Tann SS. Testing of a measurement model for HIV-AIDS symptom status. J Assoc Nurses AIDS Care. 2006;17:36–46. doi: 10.1016/j.jana.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001;28:465–470. [PubMed] [Google Scholar]
- 14.Dodd MJ, Cho MH, Cooper BA, Miaskowski C. The effect of symptom clusters on functional status and quality of life in women with breast cancer. Eur J Oncol Nurs. 2010;14(2):101–110. doi: 10.1016/j.ejon.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teel CS, Meek P, McNamara AM, Watson L. Perspectives unifying symptom interpretation. Image J Nurs Sch. 1997;29(2):175–181. doi: 10.1111/j.1547-5069.1997.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 16.Leventhal H, Diefenbach M, Leventhal EA. Illness cognition: using common sense to understand treatment adherence and affect cognition interactions. Cognitive Therapy & Research. 1992;16:143–163. [Google Scholar]
- 17.Feldman SI, Downey G, Schaffer-Neitz R. Pain, negative mood, and perceived support in chronic pain patients: a daily diary study of people with reflex sympathetic dystrophy syndrome. J Consult Clin Psychol. 1999;67:776–785. doi: 10.1037//0022-006x.67.5.776. [DOI] [PubMed] [Google Scholar]
- 18.Maas CJM, Hox JJ. Sufficient sample sizes for multilevel modeling. Methodology. 2007;1(3):86–92. [Google Scholar]
- 19.Holzemer WL, Henry SB, Nokes KM, et al. Validation of the sign and symptom check-list for persons with HIV disease (SSC-HIV) J Adv Nurs. 1999;30:1041–1049. doi: 10.1046/j.1365-2648.1999.01204.x. [DOI] [PubMed] [Google Scholar]
- 20.Lubeck DP, Fries JF. Health status among persons infected with human immunodeficiency virus. Med Care. 1993;31:269–276. doi: 10.1097/00005650-199303000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Hays RD, Stewart AL. The structure of self-report health in chronic disease patients. J Consult Clin Psychol. 1990;2:22–30. [Google Scholar]
- 22.McHorney CA, Ware JE, Lu JFR, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Stewart AL, Ware JE. Measuring functioning and well-being: The Medical Outcomes Study approach. Durham, NC: Duke University; 1992. [Google Scholar]
- 24.Ware JE, Davies-Avery A, Brook RH. Analysis of relationships among health status measures. Publication no. R-1987/6-HEW. Vol. 6. Santa Monica, CA: RAND; 1980. Conceptualization and measurement of health for adults in the Health Insurance Study. [Google Scholar]
- 25.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcomes in arthritis. Arthritis Rheum. 1980;23:137–145. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 26.Sousa KH, Chen FF. A theoretical approach to measuring quality of life. J Nurs Meas. 2002;10:47–58. doi: 10.1891/jnum.10.1.47.52545. [DOI] [PubMed] [Google Scholar]
- 27.HLM 6: Hierarchical linear and nonlinear modeling [computer program] Lincolnwood, IL: Scientific Software International; 2006. [Google Scholar]
- 28.Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychol Methods. 2007;12:121–138. doi: 10.1037/1082-989X.12.2.121. [DOI] [PubMed] [Google Scholar]
- 29.Jahng S, Wood PK, Trull TJ. Analysis of affective instability in ecological momentary assessment: Indices using successive difference and group comparison via multilevel modeling. Psychol Methods. 2008;13:354–375. doi: 10.1037/a0014173. [DOI] [PubMed] [Google Scholar]
- 30.Balano K, Kindrick A, Tulsky J. A team approach to medication errors in HIV/AIDS. Presentation at the Mountain-Plains AIDS Education and Training Center Faculty Development Conference; Denver, CO. August 2009. [Google Scholar]
- 31.Centers for Disease Control and Prevention (CDC) Cases of HIV infection and AIDS in the United States and dependent areas, 2005. HIV/AIDS Surveillance Report. 2007;17 (Revised). Available from: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2005report/