Abstract
Urban living is associated with an increase in cardiometabolic risks, but the speed at which these risks are accrued over time is unknown. Using a cross-sectional sibling-pair design, the authors surveyed migrant factory workers and their spouses from 4 cities in India together with their rural-dwelling siblings and examined the associations between urban life-years and cardiometabolic risk factors. Data on 4,221 participants (39% women; mean age = 41 years) were available (2005–2007). In regression models, a 2-slope pattern for body fat (with a marked shift at 10 years) was found, whereas a common slope could be accepted for other risk factors. In men, the regression coefficients (per decade of urban life) were 2.5% in the first decade and 0.1% thereafter for body fat; 1.4 mm Hg for systolic blood pressure; and 7% for fasting insulin. Age, gender, marital status, household structure, and occupation did not influence the patterns appreciably; however, stronger gradients for adiposity were noted in migrants from lower socioeconomic positions. The findings suggest that body fat increases rapidly when one first moves to an urban environment, whereas other cardiometabolic risk factors evolve gradually. Public health interventions focused on the control of obesity in newer migrants to urban areas, particularly those from lower socioeconomic positions, may be beneficial.
Keywords: cardiovascular diseases; diabetes mellitus, type 2; obesity; residential mobility; risk factors; urbanization
Rapid urbanization is a key driver in the current epidemic of noncommunicable diseases in developing countries (1, 2). Despite this fact, urban living as an exposure has not been well studied. Simple comparisons of rural and urban populations have suggested that there are higher levels of cardiometabolic risk factors in urban populations (1, 3–6) but do not provide insights into how these risks evolve over time (7), which is clearly important because cardiometabolic conditions typically develop over prolonged periods of time, if not over the entire life course (8, 9). A better understanding of the accumulation of cardiometabolic risks over time after urban migration and the sociodemographic patterning of these risks would improve knowledge of the natural history of the epidemiologic transition and would also suggest high-risk populations and windows of opportunity for disease control (7, 10, 11).
Few have tried to quantify the speed at which cardiometabolic risks accrue after one moves to an urban environment (7). The studies that have been conducted have generally been small and inconsistent in their findings (12–16). Furthermore, they were conducted in settings with limited relevance to the current epidemic of noncommunicable disease (e.g., sub-Saharan Africa (14, 15), islander migrants to New Zealand after a hurricane (16), or Ethiopian Jews airlifted to Israel (13)). This epidemic is unfolding primarily in countries in Asia and Latin America and is driven largely by urbanization and economic development rather than by emergency displacement (1, 2, 6).
We used a cross-sectional sibling-pair design to collect data on migrant factory workers and their spouses who were living in 4 cities across India, as well as on their nonmigrant rural siblings, in the Indian Migration Study (17). We examined the associations between levels of cardiometabolic risk factors and years of urban life estimated from lifetime residential history. We hypothesized that cardiometabolic risks increased linearly, and at broadly similar rates, with time spent in an urban environment. We further hypothesized that the trajectories of risk accrual did not vary substantially by age, marital status, household structure, occupation, and socioeconomic position of the migrants.
MATERIALS AND METHODS
Study population
Using the framework of a cardiovascular risk factor-screening study conducted in factories across India (18), we designed a sibling-pair comparison study in which we recruited urban factory workers who had migrated from rural areas and their rural-dwelling siblings who had not migrated, thereby creating sibling pairs. Details of the study design and the preliminary findings have been reported elsewhere (17).
Briefly, the study was conducted in factories in 4 cities in India that were chosen to represent the northern (Lucknow), central (Nagpur), and southern (Hyderabad and Bangalore) regions of the country. Factory workers and their coresident spouses were recruited if they were rural-urban migrants. We used employer records as the sampling frame. Each participant was asked to invite 1 nonmigrant same-sex full sibling who was closest to him/her in age and still residing in his/her rural place of origin. The fieldwork took place between March of 2005 and December of 2007.
Measurements
We interviewed participants to collect sociodemographic and residential-history data. Data on socioeconomic position were collected through the use of a subset of questions used to derive the Standard of Living Index, which is a household-level, asset-based scale devised for use in India (19, 20). The full Standard of Living Index has a large number of items (29 in total), but we used the 14 items (quality of house; toilet facilities; sources of lighting and drinking water; land ownership; and possession of a clock, radio, television, bicycle, motorcycle, car, tractor, refrigerator, or telephone) that we believed to be most informative for our study population. To estimate urban life-years, respondents were asked to provide information on each place they had lived (from birth to interview) for longer than a year, including whether the place was a village, town, small city, or large city (21).
We weighed the participants while they were wearing light indoor clothing by using a digital personal scale with 100-g accuracy (Beurer Model PS16, Ulm, Germany). Each participant's height was measured using a stadiometer accurate to 1 mm (Leicester height measure; Chasmors Ltd., London, United Kingdom) while the participant was in bare feet. Waist circumference was measured twice at the narrowest anterior part of the waist (Chasmors metallic tape; Chasmors Ltd., London, United Kingdom). Skinfold thickness was measured 3 times in the triceps and subscapular areas using Holtain calipers. Blood pressure was measured on the right upper arm with the participant in the sitting position after a rest of 5 minutes. Two readings were taken using an appropriate-sized cuff connected to a digital device (model M5-I; Omron-Matsusaka Company, Matsusaka City, Japan). Fasting (>8 hours) blood samples were centrifuged immediately, stored locally at −20°C, and transported monthly to a central laboratory for biochemical assays. Serum high density lipoprotein cholesterol level was estimated directly using the elimination method, total cholesterol level was estimated using an enzymatic endpoint method, triglyceride level was estimated using the glycerol-3-phosphate oxidase method, and glucose was estimated using the glucose oxidase glycerol-3-phosphate oxidase method using kits from Randox Laboratory Ltd. (Crumlin City, United Kingdom). We estimated insulin concentrations with radioimmunoassay in batches within 4–6 weeks.
Quality assurance of measurements
We tested all instruments and protocols before the start of the study. Every 6 months, field-workers at the 4 study sites underwent a retraining session in which their techniques were examined to ensure consistency. After they asked questions and took physical measurements of volunteer subjects, their results were compared with those of an expert measurer; all had to be within a 5% margin of error. The anthropometric equipment was calibrated at the start of every clinic. The Cardiac Biochemistry Lab was part of the United Kingdom National External Quality Assessment program for quality assurance of biochemical assays.
Statistical analyses
We calculated the Standard of Living Index by applying the standard weights to subsets of questions and rescaling them to the full score (19). The score was then categorized into low (score = 0–14), middle (score = 15–24), and high (score = 25–67) categories, as recommended. Subscapular and tricep skinfold measurements were averaged and used to calculate percentage of body fat using an equation previously validated for use in Indian populations (22). Systolic blood pressure was based on a mean of 2 readings. Low density lipoprotein cholesterol level was estimated using the Friedewald-Fredrickson formula (23). Insulin resistance was estimated according to the homeostasis model assessment (HOMA), excluding participants with a fasting glucose level ≥7 mmol/L (24). We used the lifetime residential history to calculate urban life-years (classifying the place of residence as urban if it was reported as town, small city, or big city) (21).
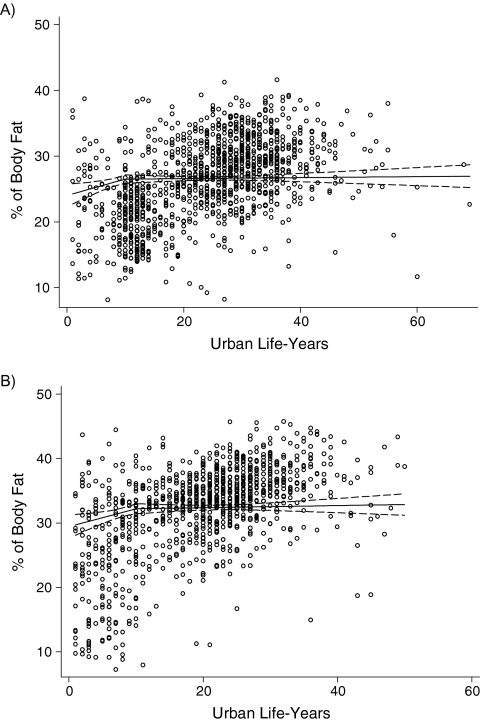
The adjusted mean values for cardiometabolic risk factors were calculated in urban life-years categories (0, >0–10, >10–20, >20–30, or >30 years). Trend tests were carried out to score each category with the median, as the prior expectation was that the levels of risk factors would increase with urban life-years. All statistical analyses were carried out separately for men and women and were adjusted for age and factory. To take into account the correlation between the siblings, we used multilevel models for the analyses, introducing a random effect shared by the siblings into the regression models. Random-effects (multilevel) models were preferred over analyses of sibling-pair differences because they give equivalent estimates but are generally more efficient and allow greater flexibility in handling of covariates. Data on participants with siblings of opposite sex or those with wider differences in age, which would have otherwise been lost, could be retained in analyses using random-effects models. Subsequently, analyses were restricted to migrant participants only (i.e., unpaired data), fitting linear regression models to estimate the linear effect of urban life-years on risk factor levels. Because the initial analyses suggested a 2-slope pattern for some of the risk factors (distinctly steeper in the first decade), we fitted a piecewise linear function, prespecifying a knot at 10 years. Using likelihood ratio tests, we formally tested the fit of this model against models with alternative breakpoints, and the 10-year breakpoint showed a good fit. Because there were fewer participants with less than 10 urban life-years, we probably lacked adequate study power to detect small differences in the timing of the breakpoint. We formally assessed the fit of the 2-slope models against models with categorical effects and models with a single slope using likelihood ratio tests. Models with more than 1 breakpoint were also considered, but they could not be distinguished from models with 1 breakpoint.
To check the stability of the results, we repeated the main analyses using the percentage of life spent in the urban environment instead of urban life-years. Socioeconomic position could be a potential confounder of the association between urban life-years and cardiometabolic risk (25, 26) or be on the causal pathway because urban migration is often associated with improvements in socioeconomic position (1, 27, 28). We therefore examined the associations with and without adjustment for socioeconomic position. The primary outcome measures were percentage of body fat, systolic blood pressure, and fasting insulin level, which were chosen to be broadly representative of the main categories of cardiometabolic risk. We also examined effect modification for these 3 outcome measures by age category (<45 years or ≥45 years), marital status (currently married or not), household structure (nuclear/single or joint-extended), socioeconomic position (low/middle or high), and occupation (manual/housework, skilled manual, or nonmanual/professional). Analyses were conducted using STATA, version 10 (Stata Corporation LLC, College Station, Texas).
Ethics approval and role of the study sponsor
Ethical approval for the study was obtained from a central institutional review board, as well as from institutional review boards at each of the study sites. Written informed consent (witnessed thumbprint if illiterate) was obtained from the participants.
RESULTS
Across the 4 factories, a total of 15,596 potential participants (workers and spouses) were identified and contacted, of which 13,695 (88%) completed the initial assessment for study eligibility. From this assessment, 4,649 were identified as rural-urban migrants and invited to participate in the study; 4,277 agreed in principle to participate together with their rural-dwelling sibling. By the close of fieldwork, 2,108 sibling pairs had visited the clinic, along with an additional 7 unpaired participants (4 factory workers and 3 rural-dwelling siblings), making a total of 4,223 participants (for recruitment flow chart, see Web Figure 1, available at http://aje.oxfordjournals.org/). Failure to participate was largely due to a change of mind by the factory worker, unwillingness of rural siblings to travel, and competing time pressures (school examinations, harvest season, etc.). Persons who participated were broadly similar to those who did not (Web Table 1). The 2,108 sibling pairs comprised 1,152 (55%) factory worker sibling pairs and 956 (45%) spouse sibling pairs. Nearly all (95%) of the factory workers were male. The final sample of 4,221 (2 women were excluded because of missing data on urban life-years) used for analyses represented 45% of all eligible rural-urban migrant sibling pairs.
Table 1 presents the characteristics of the study population by decade of urban life-years. The mean ages of men and women were 41.9 years (range, 17–76 years) and 40.3 years (range, 17–70 years), respectively. Among the migrant participants, the majority (60% of men and 66% of women) had migrated only once in their lifetimes. The migrant men had spent, on average, 24.2 years in an urban area, and the migrant women had spent 19.9 years. Among those who had migrated recently (i.e., within the past decade), the median time since migration was 8 years for men and 6 years for women, which indicated that the participants in the study were long-term migrants.
Table 1.
Characteristics of Participants by Urban Life-Years and Gender, Indian Migration Study, 2005–2007
| Urban Life-Years, Men | Urban Life-Years, Women | |||||||||||||||||||||||||||||||
| 0a | >0–10 | >10–20 | >20–30 | >30 | Ptrend | 0a | >0–10 | >10–20 | >20–30 | >30 | Ptrend | |||||||||||||||||||||
| No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | |||
| No. of participants | 1,357 | 152 | 299 | 404 | 374 | 590 | 240 | 263 | 402 | 140 | ||||||||||||||||||||||
| Age, years | 39.7 (11.5) | 34.5 (7.7) | 36.8 (7.0) | 45.9 (5.7) | 52.3 (4.6) | <0.001 | 41.4 (11.6) | 29.6 (7.4) | 37.6 (5.3) | 43.4 (4.9) | 50.3 (4.3) | <0.001 | ||||||||||||||||||||
| Socioeconomic positionb | ||||||||||||||||||||||||||||||||
| Low | 130 | 9.6 | 2 | 1.3 | 0 | 0.0 | 0 | 0.0 | 2 | 0.5 | <0.001 | 92 | 15.6 | 3 | 1.3 | 0 | 0.0 | 1 | 0.3 | 1 | 0.7 | <0.001 | ||||||||||
| Middle | 399 | 29.4 | 9 | 5.9 | 11 | 3.7 | 2 | 0.5 | 3 | 0.8 | 156 | 26.4 | 13 | 5.4 | 4 | 1.5 | 4 | 1.0 | 0 | 0.0 | ||||||||||||
| High | 828 | 61.0 | 141 | 92.8 | 288 | 96.3 | 402 | 99.5 | 369 | 98.7 | 342 | 58.0 | 224 | 93.3 | 259 | 98.5 | 397 | 98.8 | 139 | 99.3 | ||||||||||||
| Marital status | ||||||||||||||||||||||||||||||||
| Not married | 254 | 18.7 | 16 | 10.5 | 7 | 2.3 | 11 | 2.7 | 6 | 1.6 | <0.001 | 139 | 23.6 | 8 | 3.3 | 8 | 3.0 | 9 | 2.2 | 14 | 10.0 | <0.001 | ||||||||||
| Currently married | 1,103 | 81.3 | 136 | 89.5 | 292 | 97.7 | 393 | 97.3 | 368 | 98.4 | 451 | 76.4 | 232 | 96.7 | 255 | 97.0 | 393 | 97.8 | 126 | 90.0 | ||||||||||||
| Occupation | ||||||||||||||||||||||||||||||||
| Manual/housework | 841 | 62.0 | 20 | 13.2 | 22 | 7.4 | 24 | 5.9 | 24 | 6.4 | <0.001 | 528 | 89.5 | 221 | 92.1 | 251 | 95.4 | 369 | 91.8 | 115 | 82.4 | 0.002 | ||||||||||
| Skilled manual | 230 | 17.0 | 98 | 64.5 | 232 | 77.6 | 194 | 48.0 | 151 | 40.4 | 19 | 3.2 | 8 | 3.3 | 6 | 2.3 | 7 | 1.7 | 9 | 6.3 | ||||||||||||
| Nonmanual | 286 | 21.1 | 34 | 22.4 | 45 | 15.1 | 186 | 46.0 | 199 | 53.2 | 43 | 7.3 | 11 | 4.6 | 6 | 2.3 | 26 | 6.5 | 16 | 11.4 | ||||||||||||
| Household type | ||||||||||||||||||||||||||||||||
| Nuclear family or singlec | 666 | 49.1 | 115 | 75.7 | 262 | 87.6 | 334 | 82.7 | 289 | 77.3 | <0.001 | 365 | 61.9 | 197 | 82.1 | 211 | 80.2 | 339 | 84.3 | 101 | 72.1 | <0.001 | ||||||||||
| Extended family | 691 | 50.9 | 37 | 24.3 | 37 | 12.4 | 70 | 17.3 | 85 | 22.7 | 225 | 38.1 | 43 | 17.9 | 52 | 19.8 | 63 | 15.7 | 39 | 27.9 | ||||||||||||
| Standing height | 165.6 (6.4) | 166.7 (6.0) | 165.7 (6.2) | 165.9 (5.9) | 165.4 (6.4) | 0.2005 | 152.1 (5.7) | 153.7 (5.0) | 153.1 (5.4) | 152.6 (6.0) | 151.6 (5.4) | 0.0006 | ||||||||||||||||||||
Abbreviation: SD, standard deviation.
These categories included nonmigrants.
Socioeconomic position was calculated by using subsets of the questions from the Standard of Living Index scaled to the full scale score: low = 0–14, middle = 15–24, and high = 25–67.
Only 75 subjects were single.
Table 2 presents the levels of cardiometabolic risk factors for nonmigrants and migrants by time spent in an urban environment, in decades. A general trend for higher levels of risk factors from nonmigrants to migrants, and in successive decades of urban exposure, was seen. The measures of adiposity were lower in those who had not migrated and were higher in those exposed to 1 and 2 decades of urban life, with a plateau for subsequent decades. Blood pressures, fasting insulin levels, and HOMA scores were higher in migrants than in nonmigrants and tended to be higher in each successive decade of urban life exposure. There were marginal differences in height between migrants and nonmigrants, but there was no clear pattern of greater height in successive decades of urban exposure. The results presented were adjusted for socioeconomic position but were not materially different from those without adjustment for socioeconomic position. Repetition of the analyses using percentage of life-years spent in an urban environment as the alternative exposure made no substantive difference in the results (Web Table 2).
Table 2.
Levels of Cardiometabolic Risk Factors by Decade of Urban Life-Years, Indian Migration Study, 2005–2007a
| Risk Factor and No. of Urban Life-Years | Men (n = 2,586) | Women (n = 1,635) | ||
| Mean | 95% Confidence Interval | Mean | 95% Confidence Interval | |
| Body fat, %b,** | ||||
| 0 urban life-years | 21.71 | 21.42, 22.00 | 29.48 | 29.00, 29.97 |
| >0–10 urban life-years | 24.08 | 23.25, 24.90 | 30.54 | 29.73, 31.35 |
| >10–20 urban life-years | 24.64 | 24.04, 25.25 | 31.88 | 31.19, 32.57 |
| >20– 30 urban life-years | 25.56 | 25.03, 26.08 | 32.33 | 31.76, 32.91 |
| >30 urban life-years | 25.91 | 25.35, 26.47 | 32.64 | 31.67, 33.61 |
| Systolic blood pressure, mm Hg** | ||||
| 0 urban life-years | 123.11 | 122.21, 124.01 | 120.14 | 118.69, 121.59 |
| >0–10 urban life-years | 123.72 | 121.15, 126.29 | 118.49 | 116.04, 120.94 |
| >10–20 urban life-years | 124.13 | 122.23, 126.03 | 120.41 | 118.35, 122.47 |
| >20–30 urban life-years | 124.87 | 123.24, 126.50 | 118.73 | 117.01, 120.45 |
| >30 urban life-years | 126.52 | 124.78, 128.26 | 120.55 | 117.66, 123.44 |
| Diastolic blood pressure, mm Hg** | ||||
| 0 urban life-years | 77.07 | 76.47, 77.68 | 76.88 | 75.99, 77.77 |
| >0–10 urban life-years | 78.53 | 76.80, 80.25 | 76.80 | 75.29, 78.30 |
| >10–20 urban life-years | 78.45 | 77.18, 79.73 | 77.60 | 76.34, 78.87 |
| >20–30 urban life-years | 79.22 | 78.12, 80.31 | 76.85 | 75.79, 77.91 |
| >30 urban life-years | 79.88 | 78.71, 81.05 | 77.70 | 75.93, 79.48 |
| Body mass indexb,c,** | ||||
| 0 urban life-years | 22.03 | 21.84, 22.21 | 22.97 | 22.61, 23.34 |
| >0–10 urban life-years | 23.02 | 22.49, 23.55 | 23.46 | 22.85, 24.08 |
| >10–20 urban life-years | 23.23 | 22.84, 23.62 | 24.85 | 24.34, 25.37 |
| >20–30 urban life-years | 23.68 | 23.35, 24.02 | 25.64 | 25.21, 26.07 |
| >30 urban life-years | 24.07 | 23.72, 24.43 | 25.13 | 24.41, 25.86 |
| Waist circumference, cmb,** | ||||
| 0 urban life-years | 81.75 | 81.23, 82.28 | 76.19 | 75.31, 77.06 |
| >0–10 urban life-years | 84.67 | 83.18, 86.16 | 78.20 | 76.72, 79.68 |
| >10–20 urban life-years | 84.74 | 83.64, 85.84 | 79.50 | 78.26, 80.74 |
| >20–30 urban life-years | 86.94 | 85.99, 87.88 | 80.90 | 79.87, 81.94 |
| >30 urban life-years | 87.52 | 86.52, 88.53 | 80.74 | 78.99, 82.48 |
| Height, cm | ||||
| 0 urban life-years | 165.72 | 165.37, 166.07 | 152.70 | 152.21, 153.19 |
| >0–10 urban life-years | 166.03 | 165.06, 166.99 | 152.78 | 151.96, 153.61 |
| >10–20 urban life-years | 165.27 | 164.56, 165.97 | 152.89 | 152.20, 153.59 |
| >20–30 urban life-years | 166.06 | 165.45, 166.68 | 152.42 | 151.84, 153.01 |
| >30 urban life-years | 165.55 | 164.90, 166.20 | 151.99 | 151.02, 152.97 |
| Total cholesterol, mmol/L* | ||||
| 0 urban life-years | 4.58 | 4.52, 4.65 | 4.77 | 4.66, 4.87 |
| >0–10 urban life-years | 4.59 | 4.41, 4.77 | 4.76 | 4.59, 4.94 |
| >10–20 urban life-years | 4.68 | 4.54, 4.81 | 4.79 | 4.65, 4.94 |
| >20–30 urban life-years | 4.79 | 4.67, 4.90 | 4.79 | 4.67, 4.91 |
| >30 urban life-years | 4.67 | 4.55, 4.79 | 4.76 | 4.56, 4.97 |
| Low density lipoprotein cholesterol, mmol/L | ||||
| 0 urban life-years | 2.78 | 2.72, 2.83 | 2.91 | 2.82, 3.01 |
| >0–10 urban life-years | 2.81 | 2.65, 2.96 | 2.93 | 2.77, 3.08 |
| >10–20 urban life-years | 2.87 | 2.76, 2.99 | 2.96 | 2.83, 3.09 |
| >20–30 urban life-years | 2.92 | 2.82, 3.03 | 2.98 | 2.87, 3.09 |
| >30 urban life-years | 2.83 | 2.72, 2.94 | 2.94 | 2.75, 3.12 |
| High density lipoprotein cholesterol, mmol/L | ||||
| 0 urban life-years | 1.14 | 1.13, 1.16 | 1.20 | 1.18, 1.23 |
| >0–10 urban life-years | 1.13 | 1.09, 1.17 | 1.19 | 1.15, 1.22 |
| >10–20 urban life-years | 1.17 | 1.14, 1.20 | 1.20 | 1.17, 1.23 |
| >20–30 urban life-years | 1.15 | 1.13, 1.18 | 1.19 | 1.16, 1.21 |
| >30 urban life-years | 1.14 | 1.11, 1.17 | 1.18 | 1.14, 1.22 |
| Triglycerides, mmol/Lc,* | ||||
| 0 urban life-years | 1.30 | 1.27, 1.34 | 1.29 | 1.24, 1.34 |
| >0–10 urban life-years | 1.33 | 1.24, 1.43 | 1.29 | 1.21, 1.37 |
| >10–20 urban life-years | 1.29 | 1.22, 1.36 | 1.26 | 1.20, 1.34 |
| >20–30 urban life-years | 1.42 | 1.36, 1.49 | 1.24 | 1.18, 1.30 |
| >30 urban life-years | 1.37 | 1.31, 1.44 | 1.25 | 1.16, 1.35 |
| Homeostasis model assessment scored,** | ||||
| 0 urban life-years | 1.01 | 0.96, 1.07 | 1.14 | 1.05, 1.23 |
| >0–10 urban life-years | 1.08 | 0.94, 1.25 | 1.09 | 0.96, 1.24 |
| >10–20 urban life-years | 1.01 | 0.91, 1.12 | 1.34 | 1.20, 1.50 |
| >20–30 urban life-years | 1.28 | 1.17, 1.41 | 1.29 | 1.18, 1.42 |
| >30 urban life-years | 1.33 | 1.20, 1.47 | 1.13 | 0.97, 1.33 |
| Fasting glucose, mmol/Ld,** | ||||
| 0 urban life-years | 4.98 | 4.95, 5.02 | 4.95 | 4.90, 5.00 |
| >0–10 urban life-years | 4.98 | 4.89, 5.08 | 4.96 | 4.87, 5.04 |
| >10–20 urban life-years | 4.93 | 4.87, 5.01 | 5.00 | 4.93, 5.07 |
| >20–30 urban life-years | 5.09 | 5.02, 5.15 | 5.01 | 4.95, 5.07 |
| >30 urban life-years | 5.13 | 5.06, 5.20 | 4.94 | 4.84, 5.05 |
| Fasting insulin, mU/Lc,** | ||||
| 0 urban life-years | 4.65 | 4.43, 4.88 | 5.24 | 4.85, 5.65 |
| >0–10 urban life-years | 4.93 | 4.30, 5.65 | 5.02 | 4.42, 5.70 |
| >10–20 urban life-years | 4.66 | 4.21, 5.16 | 6.29 | 5.64, 7.00 |
| >20–30 urban life-years | 5.70 | 5.22, 6.22 | 5.90 | 5.40, 6.46 |
| >30 urban life-years | 5.98 | 5.45, 6.57 | 5.18 | 4.46, 6.02 |
* P < 0.05; **P < 0.001 (test for trend in urban life-years).
Adjusted means in urban life-year groups are presented. Nonmigrants are in the zero urban life years group. All models included a random effect of sibling pairs. All models were adjusted for age group, factory, and socioeconomic position.
Weight (kg)/height (m)2.
The test for trend in urban life-years was conducted in both men and women.
Geometric mean.
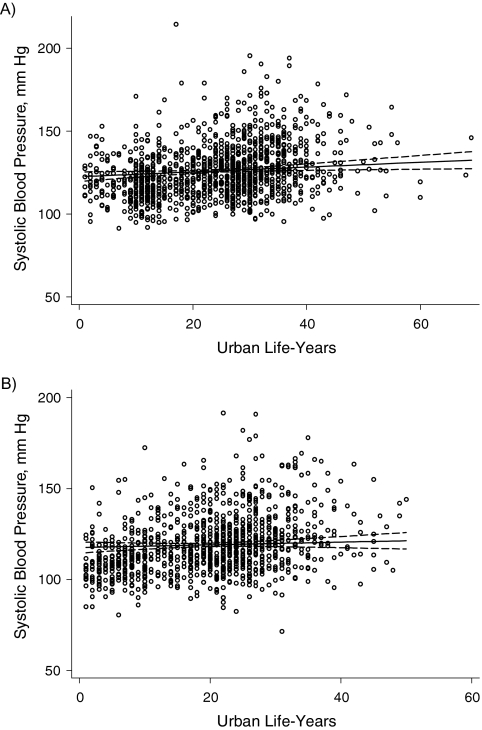
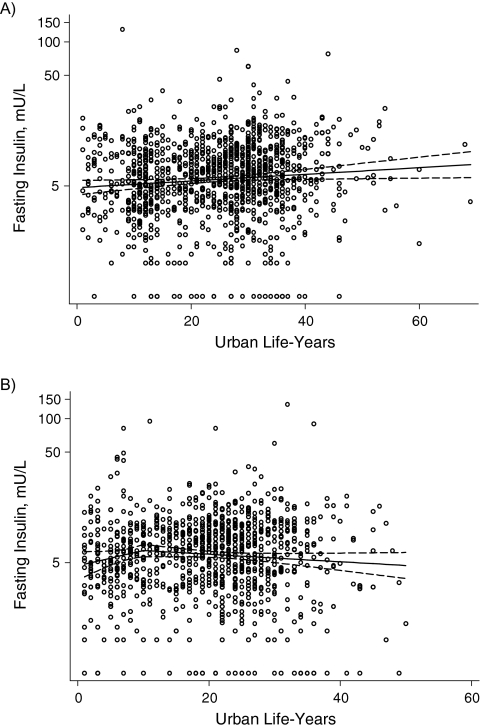
Table 3 presents the regression coefficients for the test of linear association between urban life-years and cardiometabolic risk factors among migrants only. A single slope could be accepted for all of the examined associations except percentage of body fat (men and women) and HOMA score (women only), for which 2 slopes with a break at 10 years provided the best fit to the data. The strongest effects were seen for the 3 primary outcome measures: percentage of body fat, systolic blood pressure, and fasting insulin level (Figures 1, 2, and 3). The proportions of variation explained by the fitted lines (generalized R2) were as follows: for percentage of body fat, 0.66 for males and 0.63 for females; for systolic blood pressure, 0.29 for males and 0.16 for females; and for insulin, 0.22 for males and 0.54 for females. The R2 values appear relatively big, given the scatter plot, because 1) the scatter plots represent raw data whereas the fitted lines (and R2 values) are for adjusted models, and 2) the 2-slope models have more covariates. Because of the lognormal distribution of fasting triglyceride levels, glucose levels, insulin levels, and HOMA score, the regression coefficients represent a relative difference. For example, the 1.07 coefficient for fasting insulin in men equates to a 7% higher insulin level in each successive decade of urban life. The results of the models without adjustment for socioeconomic position were not materially different.
Table 3.
Association of Urban Life-Years (in Decades) and Cardiometabolic Risk in Migrant Siblings, Indian Migration Study, 2005–2007a
| Outcome Variable | Men (n = 2,586) | Women (n = 1,635) | ||
| Mean Change | 95% Confidence Interval | Mean Change | 95% Confidence Interval | |
| Body fat, %b,* | ||||
| In the first decade | 2.48 | 0.62, 4.34 | 2.78 | 0.71, 4.85 |
| After the first decade | 0.08 | −0.31, 0.46 | 0.16 | −0.42, 0.74 |
| Systolic blood pressure, mm Hg | 1.44 | 0.32, 2.57 | 0.74 | −0.72, 2.21 |
| Diastolic blood pressure, mm Hg | 0.63 | −0.13, 1.39 | 0.22 | −0.70, 1.14 |
| Body mass indexc | 0.09 | −0.14, 0.32 | No fitd | |
| Waist circumference, cm | 0.25 | −0.36, 0.86 | 0.52 | −0.42, 1.45 |
| Height, cm | −0.06 | −0.52, 0.39 | ||
| Total cholesterol, mmol/L | −0.03 | −0.11, 0.05 | −0.01 | −0.12, 0.10 |
| Low density lipoprotein cholesterol, mmol/Le* | −0.00 | −0.08, 0.07 | ||
| In the first decade | -0.39 | −0.81, 0.03 | ||
| After the first decade | 0.09 | −0.02, 0.21 | ||
| High density lipoprotein cholesterol, mmol/L | −0.00 | −0.02, 0.01 | -0.02 | −0.04, 0.01 |
| Triglycerides, mmol/Lf | No fit | 0.97 | 0.93, 1.01 | |
| Homeostasis model assessment scoref | 1.07 | 1.00, 1.14 | 0.98 | 0.90, 1.06 |
| Fasting glucose, mmol/Lf | 1.01 | 1.00, 1.03 | 1.01 | 0.99, 1.03 |
| Fasting insulin, mU/Le,f | 1.07 | 1.01, 1.14 | ||
| In the first decade | 1.37 | 0.98, 1.93 | ||
| After the first decade | 0.92 | 0.84, 1.02 | ||
* P < 0.05 (test of no change in effect of urban life-years at 10 years).
The effect per 10 urban life-years on the risk factors for migrant men and women are presented. Nonmigrants were in the zero life-years group. All models were adjusted for age group, factory, and socioeconomic position.
The test of no change in effect of urban life-years at 10 years was conducted in both men and women.
Weight (kg)/height (m)2.
Neither linear nor piecewise linear models fit the data compared with categorical model using a likelihood ratio test.
P < 0.05 for women.
Relative change per 10 urban life-years.
Figure 1.
Observed percentage of body fat in A) men and B) women by urban life-years, Indian Migration Study, 2005–2007. Percentage of body fat was adjusted for age group, factory, and socioeconomic position.
Figure 2.
Observed systolic blood pressure in A) men and B) women by urban life-years, Indian Migration Study, 2005–2007. Percentage of body fat was adjusted for age group, factory, and socioeconomic position.
Figure 3.
Observed fasting insulin in A) men and B) women by urban life-years, Indian Migration Study, 2005–2007. Percentage of body fat was adjusted for age group, factory, and socioeconomic position.
In tests for interactions, no clear evidence for effect modification was noted when stratifying by age, marital status, household structure, or occupation of the migrants. However, evidence for effect modification by socioeconomic position was found for the outcome of percentage of body fat; stronger gradients were noted in migrants from lower socioeconomic positions (Web Table 3). The regression coefficients for percentage of body fat in migrants from the low/middle and high socioeconomic positions over the first decade were 12% and 1%, respectively, in men (Pinteraction < 0.01) and 5% and 3%, respectively, in women (Pinteraction < 0.05).
DISCUSSION
These cross-sectional data support our hypothesis that levels of cardiometabolic risk factors increase with time spent in an urban environment. However, the patterns and magnitude of these changes were not uniform across risk factors. The change in adiposity was strongest in the first decade of urban life and then appeared to level off, whereas systolic blood pressure and fasting insulin (in men) showed progressively greater values up to the fourth decade of urban living. The change in adiposity appeared to be particularly marked in persons from lower socioeconomic positions. Patterns for men and women were broadly similar, although a decline in fasting insulin level was noted in women after the first decade.
Strengths and weaknesses
Migrant populations offer a unique model in which to study the effects of urbanization because of their clearly demarcated timings of onset and cessation of exposure to urban life (7, 11). True longitudinal studies with prospectively collected premigration data are rare because of difficulties in predicting who will migrate. We used a sibling-pair design with the counterfactual reasoning that the rural nonmigrant sibling provided a control for the migrant sibling, thereby dissecting out the effect of urban life exposures from any general secular drift in environmental exposures and universal changes in health behaviors (17). The use of a sibling as a control allows partial matching on genes and early life circumstances, improving the limited inferences that can be drawn from unselected rural-urban comparisons.
The main limitation of the study is that data were cross-sectional, and we can only infer that the observed differences represent risk factor increases with duration of urban life. The potential for selection bias is another limitation, because persons who migrated may have been different from those who did not. We did not have premigration data with which to examine this bias; however, the absence of remarkable differences in height (a useful global marker of selection and health, particularly in low-income settings) between migrants and nonmigrants provides some reassurance (29). Generalizability of the data from rural siblings could be a concern, as they all had an urban sibling. Furthermore, their decision to travel to the study site could have been influenced by existing illness of their own or of someone in the family. The broadly comparable levels of risk factors reported in other recent surveys from rural India argue against a strong role of such bias (6, 30, 31).
Comparisons with previous research
In the only true pre-/postmigration study, Tokelau islanders who moved to New Zealand after a hurricane were examined pre- and postmigration (after 5 years) and compared with nonmigrants (16). Systolic blood pressure rose 1 mm Hg/year faster in migrant men than in nonmigrant men; however, no increase was noted in women. Ethiopian immigrants airlifted to Israel were measured once in the first week of arrival and again at 1 year; no change was noted in systolic blood pressure, although participants gained weight (13). A temporary rise in blood pressure in the initial weeks due to the stress associated with airlift may have masked any subsequent rise in blood pressure. In Japanese Americans living in Hawaii, a decade of life spent in Japan (compared with none) was associated with decreased likelihood (odds ratio = 0.93) of age-adjusted prevalence of diabetes (12). In a sample of 200 urban residents of Benin, age- and sex-adjusted odds ratios for hypertension were 1.3 and 2.8 among those who had spent 21–33 years and >34 years in the city, respectively, when compared with those who had spent <20 years in the city (15). In another African study, this time conducted in Cameroon, time spent in an urban environment was associated with increased cardiometabolic risk: The odds ratios for obesity were 0.8 for 1–10 years of urban living and 2.3 for >10 years of urban living, and for impaired fasting glucose/diabetes, the odds were 1.4 for 1–10 years of urban living and 2.7 for >10 years of urban living in comparison with no urban exposure (14). Data presented in this report were adjusted for lifestyle changes, which precluded direct comparisons with the effect estimates of our study. In a systematic review of blood pressure changes after acculturation, Steffen et al. (4) reported an average systolic blood pressure that was 4 mm Hg higher in more acculturated individuals. This was broadly comparable to our estimate of approximately 1 mm Hg change per decade, because most migrants had lived in the urban areas for 2–3 decades. The rapid initial rise in adiposity in those from lower socioeconomic positions is consistent with the unexpectedly high levels of cardiometabolic risk reported in urban slums populated by poorer, new migrants (32, 33).
Interpretation and areas for further research
The triad of findings—a rapid rise in adiposity upon first moving to an urban environment coupled with more gradual but sustained rises in blood pressure and insulin—can be explained by rapid initial weight gain due to sudden lifestyle changes (i.e., increased caloric intake, particularly of sugary and fatty foods, and decreased physical activity) (1, 2), followed by equilibration of adiposity to the prevailing norm for the host population (a level potentially determined by the nature of the urban environment). The higher levels of adiposity in turn lead to greater insulin resistance, thereby initiating a feedback cycle of increasing cardiometabolic risks mediated through insulin (2, 34, 35). Stress associated with migration and acculturation could potentially play a role in the rapid cardiometabolic changes seen in early stages of urban migration, and this needs to be examined further.
The socioeconomic differences in effects of migration on cardiometabolic risk may be explained by differences in behavior (greater awareness or prior habituation to an urban lifestyle among the affluent) or potential “programming” of those raised in deprived early living conditions (i.e., the developmental origins of adult disease hypothesis) (36, 37). The relation between socioeconomic position and noncommunicable disease tends to be direct in low-income countries and then inverts as the country progresses through the stages of epidemiologic transition (25, 26). There is emerging evidence that this reversal may already be happening in India (26); the strong gradients in obesity seen in poorer migrants suggests that they may be at the vanguard of this change. The comparable levels of risk-factor changes between men and women are inconsistent with the current differences in disease prevalence (higher in men) but may portend a future rise in disease prevalence in women (6).
The results from this cross-sectional study suggest important areas for further research. The findings need to be confirmed, preferably in longitudinal studies. The lifestyle changes associated with urban migration and the role of early life circumstances and their potential interaction with exposures to urban environment at different stages of the life course need to be examined in greater detail (7, 11, 38).
Public health implications
The findings from this study offer useful pointers for noncommunicable disease-control programs in developing countries. Programs focused on preventing obesity in new migrants to urban areas and tailored to the needs of those in lower socioeconomic positions could deliver long-term health benefits. Between one-third to one-half of the urban population of big cities in India and elsewhere now live in urban slums (28); these areas, with their high proportion of poorer new migrants, suggest a clearly defined setting for targeted public health interventions (39, 40). Bridging the rapidly expanding rural-urban divide in economic and other circumstances could contribute importantly to the control of noncommunicable disease epidemics in developing countries such as India. The findings from the present study may also be informative in understanding the health risks to migrants from developing countries to developed countries, which is of increasing public health importance.
Supplementary Material
Acknowledgments
Author affiliations: Faculty of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, London, United Kingdom (Sanjay Kinra, Elisabeth Andersen, Liza Bowen, Shah Ebrahim); School of Social and Community Medicine, University of Bristol, Bristol, United Kingdom (Yoav Ben-Shlomo, George Davey Smith); Public Health Foundation of India, New Delhi, India (Tanica Lyngdoh, Kolli Srinath Reddy); Centre for Chronic Disease Control, New Delhi, India (Dorairaj Prabhakaran); Department of Cardiac Biochemistry, All India Institute of Medical Sciences, New Delhi, India (Lakshmy Ramakrishnan); and St. John's Research Institute, Bangalore, India (Ankalmadagu Bharathi, Mario Vaz, Anura Kurpad).
This work was funded by Wellcome Trust project grant GR070797MF.
The authors thank the field staff and local investigators who conducted this study.
The Indian Migration Study group comprises Prof. K. Srinath Reddy, Dr. Dorairaj Prabhakaran, Prof. Tulsi Patel, Dr. Lakshmy Ramakrishnan, Dr. Ruby Gupta, and Dr. Tanica Lyngdoh (New Delhi); Prof. R. C. Ahuja and Prof. R. K. Saran (Lucknow); Dr. Prashant Joshi and Dr. N. M. Thakre (Nagpur); Dr. K. V. R. Sarma, Prof. S. Mohan Das, Dr. R. K. Jain, and Dr. S. S. Potnis (Hyderabad); Prof. Anura V. Kurpad, Dr. Mario Vaz, A.V. Barathi, and Dr. Murali Mohan (Bangalore); Dr. Chittaranjan Yajnik (Pune); Prof. George Davey Smith and Prof. Yoav Ben Shlomo (Bristol); and Professor Shah Ebrahim and Dr. Sanjay Kinra (London School of Hygiene and Tropical Medicine).
The findings and conclusions in this report are those of the authors and do not represent the views of the funders, who had no role in the design, data collection, or publication of the manuscript.
Conflict of interest: none declared.
Glossary
Abbreviation
- HOMA
homeostasis model assessment
References
- 1.Fall CH. Non-industrialised countries and affluence. Br Med Bull. 2001;60:33–50. doi: 10.1093/bmb/60.1.33. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Reddy S, Ounpuu S, et al. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104(22):2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 3.Torun B, Stein AD, Schroeder D, et al. Rural-to-urban migration and cardiovascular disease risk factors in young Guatemalan adults. Int J Epidemiol. 2002;31(1):218–226. doi: 10.1093/ije/31.1.218. [DOI] [PubMed] [Google Scholar]
- 4.Steffen PR, Smith TB, Larson M, et al. Acculturation to Western society as a risk factor for high blood pressure: a meta-analytic review. Psychosom Med. 2006;68(3):386–397. doi: 10.1097/01.psy.0000221255.48190.32. [DOI] [PubMed] [Google Scholar]
- 5.Ramachandran A, Snehalatha C, Dharmaraj D, et al. Prevalence of glucose intolerance in Asian Indians. Urban-rural difference and significance of upper body adiposity. Diabetes Care. 1992;15(10):1348–1355. doi: 10.2337/diacare.15.10.1348. [DOI] [PubMed] [Google Scholar]
- 6.Gupta R, Joshi P, Mohan V, et al. Epidemiology and causation of coronary heart disease and stroke in India. Heart. 2008;94(1):16–26. doi: 10.1136/hrt.2007.132951. [DOI] [PubMed] [Google Scholar]
- 7.Kinra S. Commentary: can conventional migration studies really identify critical age-period effects? Int J Epidemiol. 2004;33(6):1226–1227. doi: 10.1093/ije/dyh340. [DOI] [PubMed] [Google Scholar]
- 8.Strong JP, Malcom GT, McMahan CA, et al. Prevalence and extent of atherosclerosis in adolescents and young adults: implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA. 1999;281(8):727–735. doi: 10.1001/jama.281.8.727. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31(2):285–293. [PubMed] [Google Scholar]
- 10.Kinra S. Commentary: beyond urban-rural comparisons: towards a life course approach to understanding health effects of urbanization. Int J Epidemiol. 2004;33(4):777–778. doi: 10.1093/ije/dyh161. [DOI] [PubMed] [Google Scholar]
- 11.Elford J, Ben-Shlomo Y. Geography and migration with special reference in cardiovascular disease. In: Kuh D, Ben-Shlomo Y, editors. A Life Course Approach to Chronic Disease Epidemiology. Oxford, United Kingdom: Oxford University Press; 2004. pp. 144–164. [Google Scholar]
- 12.Huang B, Rodriguez BL, Burchfiel CM, et al. Acculturation and prevalence of diabetes among Japanese-American men in Hawaii. Am J Epidemiol. 1996;144(7):674–681. doi: 10.1093/oxfordjournals.aje.a008980. [DOI] [PubMed] [Google Scholar]
- 13.Goldbourt U, Khoury M, Landau E, et al. Blood pressure in Ethiopian immigrants: relationship to age and anthropometric factors, and changes during their first year in Israel. Isr J Med Sci. 1991;27(5):264–267. [PubMed] [Google Scholar]
- 14.Sobngwi E, Mbanya JC, Unwin NC, et al. Exposure over the life course to an urban environment and its relation with obesity, diabetes, and hypertension in rural and urban Cameroon. Int J Epidemiol. 2004;33(4):769–776. doi: 10.1093/ije/dyh044. [DOI] [PubMed] [Google Scholar]
- 15.Sodjinou R, Agueh V, Fayomi B, et al. Obesity and cardio-metabolic risk factors in urban adults of Benin: relationship with socio-economic status, urbanisation, and lifestyle patterns. BMC Public Health. 2008;8:84. doi: 10.1186/1471-2458-8-84. doi: 10.1186/1471-2458-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salmond CE, Joseph JG, Prior IA, et al. Longitudinal analysis of the relationship between blood pressure and migration: the Tokelau Island Migrant Study. Am J Epidemiol. 1985;122(2):291–301. doi: 10.1093/oxfordjournals.aje.a114101. [DOI] [PubMed] [Google Scholar]
- 17.Ebrahim S, Kinra S, Bowen L, et al. The effect of rural-to-urban migration on obesity and diabetes in India: a cross-sectional study. Indian Migration Study group. PLoS Med. 2010;7(4):e1000268. doi: 10.1371/journal.pmed.1000268. doi: 10.1371/journal.pmed.1000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy KS, Prabhakaran D, Chaturvedi V, et al. Methods for establishing a surveillance system for cardiovascular diseases in Indian industrial populations. Bull World Health Organ. 2006;84(6):461–469. doi: 10.2471/blt.05.027037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Family Health Survey (NFHS-2), 1998–99. Mumbai, India: International Institute for Population Sciences; 2000. International Institute for Population Sciences and ORC Macro. [Google Scholar]
- 20.Subramanian SV, Nandy S, Irving M, et al. The mortality divide in India: the differential contributions of gender, caste, and standard of living across the life course. Am J Public Health. 2006;96(5):818–825. doi: 10.2105/AJPH.2004.060103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Registrar General and Census Commissioner, India. Census of India. New Delhi, India: Office of the Registrar General, India; 2006. [Google Scholar]
- 22.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32(1):77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993;88(4):1973–1998. doi: 10.1161/01.cir.88.4.1973. [DOI] [PubMed] [Google Scholar]
- 26.Reddy KS, Prabhakaran D, Jeemon P, et al. Educational status and cardiovascular risk profile in Indians. Proc Natl Acad Sci U S A. 2007;104(41):16263–16268. doi: 10.1073/pnas.0700933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Globalization, Diets and Noncommunicable Diseases. Geneva, Switzerland: World Health Organization; 2002. [Google Scholar]
- 28.Ramachandran R. Urbanisation and Urban Systems in India. New Delhi, India: Oxford University Press; 2001. [Google Scholar]
- 29.Miura K, Nakagawa H, Greenland P. Invited commentary: height-cardiovascular disease relation: where to go from here? Am J Epidemiol. 2002;155(8):688–689. doi: 10.1093/aje/155.8.688. [DOI] [PubMed] [Google Scholar]
- 30.Chow C, Cardona M, Raju PK, et al. Cardiovascular disease and risk factors among 345 adults in rural India—the Andhra Pradesh Rural Health Initiative. Int J Cardiol. 2007;116(2):180–185. doi: 10.1016/j.ijcard.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 31.Chow CK, Naidu S, Raju K, et al. Significant lipid, adiposity and metabolic abnormalities amongst 4535 Indians from a developing region of rural Andhra Pradesh. Atherosclerosis. 2008;196(2):943–952. doi: 10.1016/j.atherosclerosis.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 32.Misra A, Pandey RM, Devi JR, et al. High prevalence of diabetes, obesity and dyslipidaemia in urban slum population in northern India. Int J Obes Relat Metab Disord. 2001;25(11):1722–1729. doi: 10.1038/sj.ijo.0801748. [DOI] [PubMed] [Google Scholar]
- 33.Mohan V, Mathur P, Deepa R, et al. Urban rural differences in prevalence of self-reported diabetes in India—the WHO-ICMR Indian NCD risk factor surveillance. Diabetes Res Clin Pract. 2008;80(1):159–168. doi: 10.1016/j.diabres.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 34.Reaven GM. Relationship between insulin resistance and hypertension. Diabetes Care. 1991;14(suppl 4):33–38. doi: 10.2337/diacare.14.4.33. [DOI] [PubMed] [Google Scholar]
- 35.McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991;337(8738):382–386. doi: 10.1016/0140-6736(91)91164-p. [DOI] [PubMed] [Google Scholar]
- 36.Barker DJP. Mothers, Babies and Health in Later Life. Edinburgh, United Kingdom: Churchill Livingstone; 1998. [Google Scholar]
- 37.Kinra S, Rameshwar Sarma KV, Ghafoorunissa, et al. Effect of integration of supplemental nutrition with public health programmes in pregnancy and early childhood on cardiovascular risk in rural Indian adolescents: long term follow-up of Hyderabad Nutrition Trial. BMJ. 2008;337 doi: 10.1136/bmj.a605. :a605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schooling M, Leung GM, Janus ED, et al. Childhood migration and cardiovascular risk. Int J Epidemiol. 2004;33(6):1219–1226. doi: 10.1093/ije/dyh221. [DOI] [PubMed] [Google Scholar]
- 39.Willett WC, Koplan JP, Nugent R, et al. Prevention of chronic disease by means of diet and lifestyle changes. In: Jamison DT, Breman JG, Measham AR, et al., editors. Disease Control Priority in Developing Countries. New York, NY: Oxford University Press/World Bank; 2006. pp. 833–850. [PubMed] [Google Scholar]
- 40.Siegel K, Narayan KM, Kinra S. Finding a policy solution to India's diabetes epidemic. Health Aff (Millwood) 2008;27(4):1077–1090. doi: 10.1377/hlthaff.27.4.1077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.